Abstract
Significance
Little is known about how the preferred retinal locus (PRL) develops in patients with macular disease. We found that acuity is worse at the PRL than at other retinal locations around the scotoma, suggesting that the selection of the PRL location is unlikely to be based on optimizing acuity.
Purpose
Following the onset of bilateral macular disease, most patients adopt a retinal location outside the central scotoma, the preferred retinal locus (PRL), as their new retinal location for visual tasks. Very little information is known about how the location of a PRL is chosen. In this study, we tested the hypothesis that the selection of the location for a PRL is based on optimizing visual acuity, which predicts that acuity is the best at the PRL, compared with other retinal locations.
Methods
Using a scanning laser ophthalmoscope that allowed us to position visual targets at precise retinal locations, we measured acuity psychophysically using a four-orientation Tumbling-E presented at the PRL, and at multiple (range between 23 and 36 across observers) locations around the scotoma, for five observers with bilateral macular disease.
Results
For all five observers, the acuity at the PRL was never the best among all testing locations. Instead, acuities were better at 15–86% of the testing locations other than the PRL, with the best acuity being 17–58% better than that at the PRL. The locations with better acuities did not cluster around the PRL, and did not necessarily lie at the same distance from the fovea or the PRL.
Conclusions
Our finding that acuity is worse at the PRL than at other locations around the scotoma implies that the selection of the PRL location is unlikely to be based on optimizing acuity.
Keywords: low vision, macular disease, central vision loss, preferred retinal locus, visual acuity
With normal healthy eyes, we automatically and effortlessly move our eyes to put visual objects of interest on the fovea, the small region of the retina that supports fine, detailed vision. When the fovea or the macular region in both eyes of an individual are damaged due to macular diseases such as age-related macular degeneration, these regions will not be able to process any visual input, thus the individual would need to rely on an alternate retinal area outside the macular lesioned area for seeing. This alternate retinal area is known as the preferred retinal locus.1,2 Previous research suggested that patients with macular disease may use multiple preferred retinal loci for different tasks,3,4 or sometimes, even for the same task, such as reading.5 In addition to serving as the reference locus for spatial tasks, our recent work suggested that the preferred retinal locus also serves as the locus for eye movements (unpublished data from our laboratory). Unfortunately, literature related to the development of the preferred retinal locus is scarce. To our knowledge, there has been only one report providing some hint on the time course of development of the preferred retinal locus. In this report, Crossland et al6 found that patients could reliably and consistently use a preferred retinal locus within six months after the onset of the bilateral macular loss, even when no intervention was provided.
Given the importance of the preferred retinal locus, it would be a significant clinical breakthrough for low vision rehabilitation if we could predict the location of the preferred retinal locus for a patient with recent onset bilateral macular loss, so that we could shorten the amount of time for the patient to consistently adopt the location as his/her preferred retinal locus, such as through training. To be able to predict the location of the preferred retinal locus, we would need to first understand how a preferred retinal locus develops, or how a location around a scotoma is chosen that eventually develops as the preferred retinal locus. To date, we have very little knowledge about how a preferred retinal locus evolves. Earlier studies on the location of preferred retinal locus primarily categorized the locations relative to the scotoma as above, below, to the left or right of the scotoma.2,6–8 This traditional classification of the location of preferred retinal locus has provided us with some insight into the interaction between a scotoma and reading but the classification into only four directions is too crude for us to understand what determines the location of a preferred retinal locus. In this study, we tested the hypothesis that the selection of the location of the preferred retinal locus is based on optimizing visual performance. This hypothesis predicts that the preferred retinal locus should correspond to the location with the best visual performance, at least for some measurements. Although this hypothesis has been suggested previously,9 there has not been any systematic investigation to test the hypothesis, or published data supporting or refuting the hypothesis, leading to split opinions as to whether the preferred retinal locus corresponds to the retinal location that supports the best visual performance.10 Clearly, there are different types of visual performance to consider; in this study, we chose to examine visual acuity, because acuity is the most widely-accepted clinical measurement of an individual’s vision, and is a measurement used in all eye clinics as well as an outcome measure in almost all clinical trials that involve patients with eye disorders or diseases. Hence, our specific hypothesis was that the preferred retinal locus corresponds to the location with the best acuity.
METHODS
Five observers with macular disease (four with age-related macular degeneration and one with Stargardt disease) and 11 older adults with normal vision serving as control observers participated in this study. The visual characteristics of the five observers with macular disease are summarized in Table 1. All observers gave written informed consent before the commencement of data collection. This research adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at the University of California, Berkeley.
Table 1.
Demographic characteristics and acuities (measured using the Bailey-Lovie Visual Acuity Chart) of the five observers with macular disease. Acuity values shown in bold represent the tested eyes.
| Observer | Sex | Age (y) | Diagnosis | Letter-chart Acuity logMAR | Years since onset | |
|---|---|---|---|---|---|---|
| Right eye | Left eye | |||||
| S1 | F | 86 | Age-related macular degeneration | 0.52 | 0.56 | 13 |
|
| ||||||
| S2 | F | 76 | Age-related macular degeneration | 0.66 | 0.54 | 10 |
|
| ||||||
| S3 | M | 72 | Age-related macular degeneration | 0.80 | 0.80 | 1 |
|
| ||||||
| S4 | F | 75 | Age-related macular degeneration | 0.54 | 1.12 | 7 |
|
| ||||||
| S5 | M | 57 | Stargardt | 1.10 | 1.10 | 40 |
The experiment was performed using a Rodenstock scanning laser ophthalmoscope, under the control of custom-written software that allowed us to position the acuity target at specific locations on the retina. We used a gaze-contingent paradigm to ensure that the acuity stimulus was presented at the intended testing retinal location, as in MacKeben and Gofen.11 To summarize briefly, before each trial, observers were asked to fixate at a fixation cross. As soon as the experimenter initiated a trial, the fundus image was captured and compared with the reference frame (representing the “ground truth” of the fundus image) of that observer. The intended testing location was then calculated taking into account the difference in eye positions between the moment that we initiated the trial and the reference frame.
The field of view of the scanning laser ophthalmoscope is 30° × 24°. The spatial resolution of each “pixel” of the scanning laser ophthalmoscope is 0.05° (3 arc min). For observers with macular disease, the smallest letter size and the size of our microperimetry stimuli were 0.5°, which ensured that there were at least 2 “pixels” per letter stroke of the stimuli. This did not pose a problem in our acuity measurement for observers with macular disease because there was no need to present acuity stimuli smaller than 0.5° for these observers. For our older adults with normal vision tested at 5° eccentricity, stimulus sizes smaller than 0.5° were used (the smallest one we could display was 0.25°, which comprised of Tumbling Es made up of 1 pixel per stroke). Based on the systematic changes in acuity with eccentricity in the normal periphery (filled gray triangles) shown in Figure 2, we believe that the spatial resolution of the scanning laser ophthalmoscope did not pose a serious limitation for our measurements of acuities in the normal periphery. In addition, our stimulus presentation was corrected for the trapezoid distortions inherent to the Rodenstock scanning laser ophthalmoscope. For our scanning laser ophthalmoscope, the difference in width between the top and the bottom of the raster was 10.6%. This distortion or correction factor was determined according to the procedures described by Timberlake et al.12 before the experiment. Another characteristic of the scanning laser ophthalmoscope is that the imaging of the fundus is accomplished by an infra-red laser (780 nm) while the stimulus is presented using a Helium-Neon laser (632.8 nm). Because of the difference in wavelengths, there could be small shifts in stimulus locations. However, these shifts are likely to be small compared with the sizes of letters used in this study.
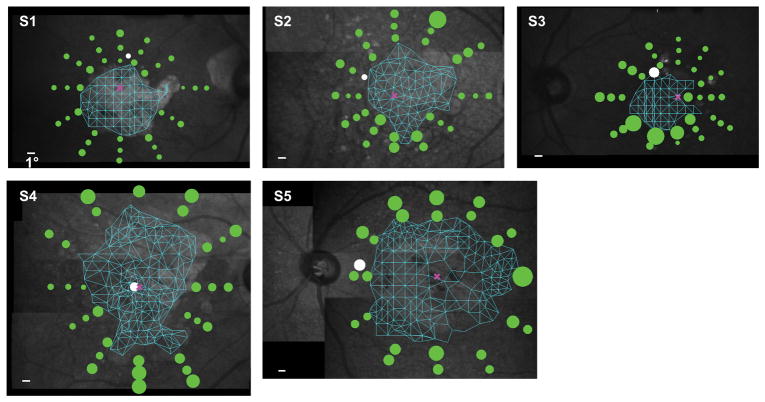
Figure 2.
Acuity thresholds are plotted as green circles around the scotoma of the five observers. For comparison, the acuity threshold at the preferred retinal locus is also plotted, as represented by the white circle. The size of each circle scales with the acuity threshold (the white calibration bar in each panel represents 1°). The mesh plot in cyan represents the results of the Delaunay triangulation that we used to determine the scotoma map. The pink cross in each panel represents the location of the fovea (based on standard measurements, see text for details). Note that observer S4 still had foveal sparing, resulting in a small island that she used to process small stimuli, thus her preferred retinal locus appeared to be very close to the fovea. The background fundus image of each observer was obtained from only a single frame of video.
Retinal images were digitally recorded at a frame rate of 30 Hz for the duration of each trial by interfacing the video output of the scanning laser ophthalmoscope with a frame grabber (Matrox Imaging Adapter: Meteor-II PCI Frame Grabber), via a TV-One CORIO scan converter (CS-450 Eclipse, Erlanger, KY). The experiment was performed and controlled using a ViSaGe system (Cambridge Research Systems, UK), with software custom-written in MATLAB 7.3.0 (The MathWorks, MA).
In this study, all visual stimuli, including the small dots for microperimetry measurement, fixation cross and Tumbling-E stimuli, were presented as a positive-contrast target (target brighter than the background). As seen by the observers, the luminances of these stimuli and the background were approximately 205 and 22 cd/m2, yielding a Weber contrast of 8.32 (832%). These luminances were calculated and converted based on the method described by Nygaard and Schuchard.13
Testing was performed monocularly on the preferred eye of each observer. Except for observer S2, the testing eye corresponded to the eye with acuity better than, or equal to, the other eye (see Table 1). To identify the retinal locations for testing for each observer, we first mapped the central scotoma of the observer using our custom-written software before any acuity measurement. We presented a small dot (0.5°) at random locations inside and outside the macular lesion area, using the macular lesions visible in the retinal images as the guide initially, and refined based on the seeing or not-seeing responses of the observers (Figure 1A). Observers were asked to indicate whether or not they saw the dot. We then used a custom written MATLAB program that utilized the Delaunay triangulation method to define the shape and extent of the non-seeing central scotoma (Figure 1B). Next, we identified the anatomical fovea for each observer based on the “standard” distances (horizontal and vertical) between the center of the optic nerve head and the anatomical fovea. These standard distances were empirical measurements obtained in our laboratory from 18 young and 18 older adults with normal vision, and averaged 15.12±1.04° (range: 12.84° to 17.36°) horizontally and –1.37±0.84° (range: –3.47° to 0.48°) vertically (the anatomical fovea is inferior to the center of the optic nerve head in most eyes).14 These measurements were highly comparable with data reported by other labs with sample sizes of 50 to 104 eyes.15–17 Once the anatomical fovea was defined, we identified between 23 and 36 locations (depending on the extent of the central scotoma and the availability of the observers) around the scotoma for acuity measurement. These locations were positioned along 12 meridians originating from the anatomical fovea (30 angular degrees apart, see Figure 1C), with 2 or 3 locations along each meridian (Figure 1D). The order of testing these 23–36 locations, together with the testing at the preferred retinal locus, was randomized.
Figure 1.
An example of how we determined the testing locations around the scotoma of an observer with macular disease. (A) Results of microperimetry from observer S1. Blue dots represent locations where she did not see the stimulus dot, and white dots represent locations where she saw the stimulus dot. (B) Based on the seeing/not-seeing data, we used the Delaunay triangulation to determine the scotoma map, represented by the mesh plot. (C) The location corresponding to the anatomical fovea (represented by the pink rectangle) was identified (see text for details) and 12 meridians (30 angular degrees apart) were added with the origins placed at the fovea, shown as white dashed lines. (D) Three locations (represented by the yellow dots) were identified along each meridian as the testing locations. For other observers, depending on availability of the observers and the specific shape of the scotoma, often two or three locations were tested along a meridian. The background fundus image of the observer was obtained from only a single frame of video.
Acuity was measured using a four-orientation Tumbling-E task. Presentation duration was 300 ms. At each location, we used a two-down-one-up staircase procedure to change the letter size and determine the performance accuracy of identifying the orientation of the Tumbling E stimulus as a function of letter size. Step size used was 0.1 log units, with the letter size being presented based on the integer of the calculated value. Each staircase terminated after 10 reversals. The number of trials presented in each staircase averaged between 37 and 92 across the five observers. When testing at retinal locations that were not the preferred retinal locus, observers were asked to keep a fixation cross visible at all time using their preferred retinal locus. Although it has been reported that patients with macular disease may use multiple preferred retinal loci or different preferred retinal loci for the same or different tasks, it is not the universal rule. In this study, we allowed observers to use any retinal location to view the fixation target (a cross) and the Tumbling-E targets, without forcing them to use the same retina location. All of them demonstrated that they used essentially the same location for both fixation and acuity tasks, at least for the characteristics (spectral, contrast and luminance etc.) of our targets. This was confirmed with the scanning laser ophthalmoscope image before each trial was initiated. For testing at the preferred retinal locus, observers were first asked to keep the fixation cross visible, then upon initiation of the trial, the fixation cross disappeared and was replaced by the Tumbling-E stimulus. Retinal images were continuously recorded for the entire duration of each trial for offline analysis. Each retinal location was tested multiple times (between 2 and 5 times, although occasionally only one block of trials was useful after we reviewed the video images, see below) across multiple sessions (over a course of 4–6 weeks) and each acuity threshold reported in this paper represents the average of the repeated testings. Inspections of the multiple measurements at each testing location and for each observer did not reveal any systematic improvement of acuity with time with repeated measurements.
Data Analysis
To further ensure that the acuity stimuli were presented at the intended retinal location, we performed offline analysis and reviewed each frame of the recorded video of each trial to identify frames in which the center of the stimulus was located >0.5° away from the intended retinal location. Note that this criterion of 0.5° is much smaller than the mean amplitude of microsaccade (~1.1°) of most patients with macular disease.18 This was accomplished using a custom-written program in MATLAB that utilized cross-correlation algorithms to determine the relative position of the target stimulus with respect to the intended location on the retina for each frame. Trials containing such frames were excluded for analysis. Then we fit a cumulative-Gaussian function to the remaining trials of the block to relate identification accuracy with letter size. Acuity threshold was defined as the letter size that corresponded to 62.5% correct (50% correct after correction for guessing) on the cumulative-Gaussian function. As stated earlier, each reported acuity threshold in this paper represents the average of repeated testings.
RESULTS
Figure 2 plots the acuity threshold obtained at each tested retinal location as a green circle for each of the five observers. The diameter of the green circle scales with the acuity threshold, defined as the whole letter size of the Tumbling-E target that yielded our performance criterion (62.5% correct) on a psychometric function. A smaller circle represents better acuity. To facilitate comparison, the acuity threshold obtained at the preferred retinal locus is plotted as a white circle. According to our hypothesis that acuity is the best at the preferred retinal locus, we expected the white circle to be smaller than all of the green circles. For all observers, the size of the white circle was larger than at least a few of the green circles, implying that the acuity at the preferred retinal locus was worse than that at some other retinal locations around a scotoma. To quantify the effect, we list in Table 2 the total number of tested retinal locations, and the number of tested locations at which the acuity thresholds were smaller than the threshold at the preferred retinal locus. Across observers, between 15.2% and 85.7% of the tested retinal locations yielded acuities better than that at the preferred retinal locus. The best acuity at these locations was 16.8% to 57.5% better than that at the preferred retinal locus. Note that the locations with acuities better than that at the preferred retinal locus did not cluster around the preferred retinal locus (Figure 2).
Table 2.
Number of tested retinal locations around the scotoma of the five observers, and number of retinal locations at which acuity threshold was smaller than that at the preferred retinal locus.
| Observer | Total number of retinal locations tested | Number of retinal locations at which acuity threshold was smaller than that at the preferred retinal locus | Percentage of retinal locations at which acuity threshold was smaller than that at the preferred retinal locus |
|---|---|---|---|
| S1 | 36 | 10 | 27.8% |
| S2 | 33 | 5 | 15.2% |
| S3 | 35 | 30 | 85.7% |
| S4 | 32 | 13 | 40.6% |
| S5 | 23 | 14 | 60.9% |
An interesting observation is that for all observers, the acuity measured at the preferred retinal locus using the scanning laser ophthalmoscope was worse than that measured using a letter chart, as reported in Table 1. It is well known that acuity measurements depend on many factors, such as the specific targets, luminance and contrast of the targets, psychophysical methods, number of trials and presentation duration. In this study, the letter chart acuities were measured using the standard Bailey-Lovie Visual Acuity Chart, with black-on-white letters under standard office illumination, and using the standard Method of Limit (read until the observer could no longer read any more letters correctly) with unlimited duration for viewing and responding. All these factors favor a better acuity measurement than that measured using the scanning laser ophthalmoscope. In addition, it is also likely that observers used different retinal locations other than their preferred retinal locus when reading letters on a letter chart. Indeed, at least for observers S3 and S5, their letter chart acuities were highly comparable with the best acuities (not at the preferred retinal locus, however) measured using the scanning laser ophthalmoscope.
Does Acuity Correlate with the Distance from the Fovea?
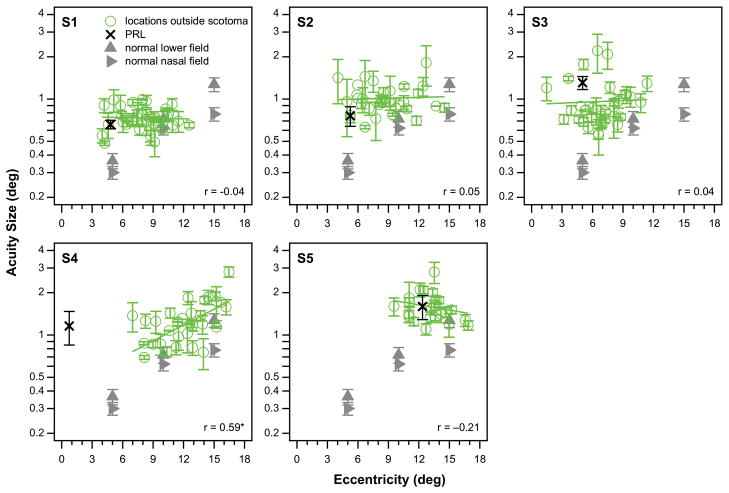
In normal periphery, performance for many spatial tasks, including acuity, worsens with increased distance from the fovea.19–23 In the presence of macular loss, does such a relationship still hold? To provide data for comparison, we measured acuity at 5°, 10° and 15° eccentricity in the lower and nasal field of a randomly chosen eye in 11 older adults with normal vision (age, 62 – 77 years, mean = 69.3 years). All these observers had best-corrected distance acuity of 20/20 or better in each eye and no detectable ocular disease or abnormality. Acuity was measured using the same equipment and psychophysical methods as in observers with macular disease. For reporting purpose, acuities were averaged across the 11 control (eyes) at each eccentricity. These data are plotted as gray triangles in Figure 3. As expected, acuity threshold worsens systematically with eccentricity in the normal periphery, as shown by the gray triangles in Figure 3. Also shown in Figure 3 are the acuity thresholds plotted as a function of the eccentricity of the retinal location from the fovea, for the five observers (green circles). For each observer, the correlation coefficient of acuity threshold with the eccentricity of the retinal location from the fovea is given in the respective panel of plot. With the exception of observer S4 who still had a small foveal island and thus had a preferred retinal locus very close to the fovea (0.74° eccentricity by our estimation), across the other four observers, there is no significant relationship between acuity threshold and eccentricity of the testing locations closely surrounding their respective scotomas.
Figure 3.
Acuity thresholds are plotted (green circles) for each observer as a function of the eccentricity of the testing locations from the fovea, with the green solid line representing the best-fit regression line to the data-set. The correlation coefficient of the regression line is given in the bottom right corner in each panel. The black cross in each panel plots the acuity threshold at the preferred retinal locus eccentricity. For these measurements, the associated error bars represent ± 1 standard error of the mean. Data points without error bars mean that we only had one useable block of trials to determine the acuity threshold for that location. For comparison, acuity thresholds obtained at 5, 10 and 15° eccentricity from 11 older adults with normal vision are given as gray triangles (two visual fields were tested), with the error bars representing ±95% confidence intervals. Only observer S4 (with a small foveal island and a preferred retinal locus close to the fovea) showed a significant correlation between acuity and eccentricity of the testing locations, as denoted by an asterisk next to the correlation coefficient.
The lack of a relationship between acuity threshold and eccentricity in observers with macular disease could be due to the following interesting observation. At small eccentricities, acuity thresholds for observers with macular disease are consistently worse than those in the normal periphery, for matched eccentricity. This observation is consistent across the five observers with macular disease. At larger eccentricities, the acuity difference between observers with macular disease and the normal periphery is negligible. Smaller eccentricities are locations closer to the edge of the scotoma, therefore, our finding could be due to the fact that the edge of the scotoma was not healthy in our observers, resulting in relative scotomas, and leading to poorer-than-expected acuity measurements. Interestingly, preferred retinal locus is usually located very close to the edge of scotoma. However, a plot of acuity as a function of the distance of the testing location from the edge of the scotoma (based on the Delaunay triangulation method, see Methods section) reveals that there is a lack of a relationship between acuity and the distance from the edge of the scotoma (Appendix Figure A1, available at [LWW insert link]).
In each panel in Figure 3, the vertical position of the black cross (representing acuity threshold at the preferred retinal locus) is not close to the lowest limit of the cluster of green circles, further illustrating our main result that the acuity at the preferred retinal locus is not the best. Besides this observation, it is also clear that the acuity at the preferred retinal locus, as well as at other retinal locations closely surrounding a scotoma, are worse than the predicted acuity based on the normal periphery (comparing the cross with the trend shown by the triangles).
Does Acuity Correlate with the Distance from the Preferred Retinal Locus?
We also examined if acuity threshold exhibits a relationship with the distance from the preferred retinal locus. Figure 4 plots the acuity threshold as a function of the distance of the retinal location from the preferred retinal locus. Similar to the data we observed in Figure 3, except for observer S4, there exists no correlation between acuity threshold and the distance from the preferred retinal locus. The combined results of Figures 3 and 4 imply that acuity thresholds immediately around a central scotoma cannot be predicted based on the distance from the fovea or the preferred retinal locus.
Figure 4.
Acuity thresholds are plotted (green circles) for each observer as a function of the distance of the testing locations from the preferred retinal locus, with the green solid line representing the best-fit regression line to the data-set. The correlation coefficient of the regression line is given in the bottom right corner in each panel. The black cross in each panel plots the acuity threshold at the preferred retinal locus. As is the case for the relationship between acuity and eccentricity, only observer S4 showed a significant correlation between acuity and the distance of the testing locations from the preferred retinal locus, as denoted by an asterisk next to the correlation coefficient. Error bars represent ± 1 standard error of the mean.
DISCUSSION
The specific goal of this study was to examine the hypothesis that the choice of a preferred retinal locus in people with bilateral macular disease is based on the location that offers the best visual acuity, which predicts that acuity should be the best at the preferred retinal locus. We acknowledge that there are many other visual tasks that could be important for patients with macular disease, but given that acuity is an important clinical measurement of vision, as well as a default outcome measures in clinical trials that involve patients with ocular disorders or diseases, we chose it as our measurement in this study.
By measuring acuities at multiple retinal locations around the central scotoma, we showed that acuity is not the best at the preferred retinal locus for all five observers with long-standing bilateral macular disease. The central scotomas of these five observers were of different sizes and shapes, and the preferred retinal loci were also at different locations relative to the scotoma. Yet, in each case, acuities were found to be better in several other retinal locations than at the preferred retinal locus. This finding contradicts the prediction of our hypothesis and suggests that the selection of the preferred retinal locus may not be based on optimizing visual acuity.
There are a couple of important implications of this finding. First, if the selection of the preferred retinal locus is not based on optimizing visual acuity, then upon what is it based? As we acknowledged earlier, there are many visual tasks that are equally important to the daily activities of patients, such as reading or mobility. It is also possible that a stronger relationship may exist for lower-contrast stimuli. Future studies may wish to examine if there exists a relationship between performance on other tasks and the retinal locations around the scotoma. Another characteristic to consider could be the structural integrity of the retinal locations. As clearly illustrated in Figure 3, acuities at many retinal locations around each of the scotoma are worse than what we expected based solely on the eccentricity of the retinal location. Considering that retinal locations immediately surrounding a scotoma are likely to be neither normal nor healthy,24,25 this might account for higher acuity thresholds. Therefore, it may be important to examine the structural characteristics at these retinal locations as well to see if there exists a structure-function relationship at these locations. Clearly, it is also entirely possible that the preferred retinal locus is determined by multiple factors, such as those aforementioned (structure of the retinal locations, eccentricity, tasks etc.), and that the weighting for each of the factors in governing the location of the preferred retinal locus may differ for individual observers.
The second implication is whether or not another retinal location with better visual capability should be trained to replace the preferred retinal locus. Indeed, there were attempts to train another location, sometimes referred to as the trained retinal location to replace the preferred retinal locus, with mixed success.26–28 However, without a solid understanding on how a preferred retinal locus evolves and the potential consequences (both positive and negative) of patients having to switch between two or more retinal locations for visual tasks, the endeavor of training a trained retinal location should be treated with caution. Further research is needed to fully understand how a preferred retinal locus evolves, or how a location for the preferred retinal locus is chosen, either through an active or a passive process, to ensure more successful training results. A longitudinal study following patients who just lose their vision would be useful.
What could account for the finding that acuity is better at other retinal locations than at the preferred retinal locus? The most logical explanation is that retinal locations with acuities better than that at the preferred retinal locus are closer to the fovea than the preferred retinal locus. In other words, even in the presence of a central scotoma, acuity is completely limited by the eccentricity effect, as in the normal retina.17–19,21 This seems plausible since after all, acuity or resolution limit is limited by the photoreceptor spacing and also the photoreceptor-to-ganglion convergence,29–31 structural limitations that may not change for retinal locations away from the macular lesion area. However, our results in Figure 3 show that acuities in the near vicinity around the edge of a central scotoma show a weak, if any, dependence on eccentricity. The only exception was for observer S4 who had some foveal sparing and thus could use a near-foveal region as her preferred retinal locus. We attribute the weak dependence of acuity on eccentricity at retinal locations immediately around a scotoma to the possibility that the retinal area surrounding a scotoma is not healthy.24,25
Recent evidence suggests that there is remapping at the preferred retinal locus such that some spatial properties such as the shape of the spatial interaction zone demonstrates changes following years of macular disease,14 and that the reference locus for fixational eye movements shifts from the fovea to the preferred retinal locus (unpublished data from our laboratory). If so, perhaps the dependence of acuity on eccentricity should also be referenced with respect to the preferred retinal locus instead of the non-functional fovea. However, our results in Figure 4 refute this hypothesis — acuity does not depend on the distance from the preferred retinal locus, again, except for observer S4 whose preferred retinal locus was very close to the fovea.
Several caveats should be taken into account while interpreting our finding. First, since our observers already had macular loss for a while, we did not know whether the initial preferred retinal locus immediately following the onset of bilateral macular loss was at the same location as what we observed in this study. If we assume that the preferred retinal locus has not changed (provided that the macular lesions have not changed in size or shape significantly), then there is a possibility that the acuity at the preferred retinal locus was the best initially, but with time, the disease encroached on the preferred retinal locus causing a reduction in acuity. However, one would expect that with constant usage, the functional capability at the preferred retinal locus should improve with time, akin to perceptual learning.32 Also, if the original preferred retinal locus no longer supports the most optimal visual acuity, then based on the hypothesis that the selection of the location of the preferred retinal locus is based on optimizing visual acuity, another retinal location with better acuity should replace the original preferred retinal locus. It is also possible that there is a lag between the location of the preferred retinal locus and the progression of the disease. A longitudinal study following patients with macular disease and how their preferred retinal locus change with progression of the disease could shed light on these questions. In addition, even though in this study, we found that our observers invariably relied on a single preferred retinal locus for several visual tasks tested in our laboratory, it is possible that more than one location could be candidates for the preferred retinal locus initially. Regardless of whether the preferred retinal locus has changed with time, our finding still stands that the preferred retinal locus used by people with macular disease might not be the location that gives the person the best visual acuity. After ruling out the hypothesis that the selection of the preferred retinal locus is based on optimizing visual acuity, future studies may wish to test if the choice of the preferred retinal locus is based on optimizing other visual tasks, and/or to focus on testing other plausible hypotheses for how a retinal location becomes the preferred retinal locus in patients with bilateral macular disease.
Acknowledgments
This study was supported by research grant R01-EY012810 from the National Eye Institute, National Institutes of Health.
APPENDIX
Appendix Figure A1.
Acuity thresholds are plotted (green circles) for each observer as a function of the distance of the testing locations from the edge of the scotoma, with the green solid line representing the best-fit regression line to the data-set. The correlation coefficient of the regression line is given in the bottom right corner in each panel. None of the correlation coefficients are significant. The black cross in each panel plots the acuity threshold at the preferred retinal locus. Error bars represent ± 1 standard error of the mean.
References
- 1.Cummings RW, Whittaker SG, Watson GR, Budd JM. Scanning Characters and Reading with a Central Scotoma. Am J Optom Physiol Opt. 1985;62:833–43. doi: 10.1097/00006324-198512000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Timberlake GT, Mainster MA, Peli E, et al. Reading with a Macular Scotoma. I. Retinal Location of Scotoma and Fixation Area. Invest Ophthalmol Vis Sci. 1986;27:1137–47. [PubMed] [Google Scholar]
- 3.Lei H, Schuchard RA. Using Two Preferred Retinal Loci for Different Lighting Conditions in Patients with Central Scotomas. Invest Ophthalmol Vis Sci. 1997;38:1812–8. [PubMed] [Google Scholar]
- 4.Crossland MD, Crabb DP, Rubin GS. Task-Specific Fixation Behavior in Macular Disease. Invest Ophthalmol Vis Sci. 2011;52:411–6. doi: 10.1167/iovs.10-5473. [DOI] [PubMed] [Google Scholar]
- 5.Duret F, Issenhuth M, Safran A. Combined Use of Several Preferred Retinal Loci in Patients with Macular Disorders when Reading Single Words. Vision Res. 1999;39:873–9. doi: 10.1016/s0042-6989(98)00179-5. [DOI] [PubMed] [Google Scholar]
- 6.Crossland MD, Culham LE, Kabanarou SA, Rubin GS. Preferred Retinal Locus Development in Patients with Macular Disease. Ophthalmology. 2005;112:1579–85. doi: 10.1016/j.ophtha.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Guez JE, Le Gargasson JF, Rigaudiere F, O’Regan JK. Is There a Systematic Location for the Pseudo-Fovea in Patients with Central Scotoma? Vision Res. 1993;33:1271–9. doi: 10.1016/0042-6989(93)90213-g. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher DC, Schuchard RA. Preferred Retinal Loci Relationship to Macular Scotomas in a Low-Vision Population. Ophthalmology. 1997;104:632–8. doi: 10.1016/s0161-6420(97)30260-7. [DOI] [PubMed] [Google Scholar]
- 9.Timberlake GT, Peli E, Essock EA, Augliere RA. Reading with a Macular Scotoma. II. Retinal Locus for Scanning Text. Invest Ophthalmol Vis Sci. 1987;28:1268–74. [PubMed] [Google Scholar]
- 10.Crossland MD, Engel SA, Legge GE. The Preferred Retinal Locus in Macular Disease: Toward a Consensus Definition. Retina. 2011;31:2109–14. doi: 10.1097/IAE.0b013e31820d3fba. [DOI] [PubMed] [Google Scholar]
- 11.MacKeben M, Gofen A. Gaze-Contingent Display for Retinal Function Testing by Scanning Laser Ophthalmoscope. J Opt Soc Am A. 2007;24:1402–10. doi: 10.1364/josaa.24.001402. [DOI] [PubMed] [Google Scholar]
- 12.Timberlake GT, Sharma MK, Gobert DV, Maino JH. Distortion and Size Calibration of the Scanning Laser Ophthalmoscope (SLO) Laser-Beam Raster. Optom Vis Sci. 2003;80:772–7. doi: 10.1097/00006324-200311000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Nygaard RW, Schuchard RA. SLO Radiant Power and Brightness. J Rehab Res Dev. 2001;38:123–8. [PubMed] [Google Scholar]
- 14.Chung STL. Cortical Reorganization after Long-Term Adaptation to Retinal Lesions in Humans. J Neurosci. 2013;33:18080–6. doi: 10.1523/JNEUROSCI.2764-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohrschneider K. Determination of the Location of the Fovea on the Fundus. Invest Ophthalmol Vis Sci. 2004;45:3257–8. doi: 10.1167/iovs.03-1157. [DOI] [PubMed] [Google Scholar]
- 16.Timberlake GT, Sharma MK, Grose SA, et al. Retinal Location of the Preferred Retinal Locus Relative to the Fovea in Scanning Laser Ophthalmoscope Images. Optom Vis Sci. 2005;82:177–85. doi: 10.1097/01.opx.0000156311.49058.c8. [DOI] [PubMed] [Google Scholar]
- 17.Tarita-Nistor L, González EG, Markowitz SN, Steinbach MJ. Fixation Characteristics of Patients with Macular Degeneration Recorded with the MP-1 Microperimeter. Retina. 2008;28:125–33. doi: 10.1097/IAE.0b013e3180ed4571. [DOI] [PubMed] [Google Scholar]
- 18.Kumar G, Chung STL. Characteristics of Fixational Eye Movements in People with Macular Disease. Invest Ophthalmol Vis Sci. 2014;55:5125–33. doi: 10.1167/iovs.14-14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weymouth FW. Visual Sensory Units and the Minimum Angle of Resolution. Am J Ophthalmol. 1958;46:102–13. doi: 10.1016/0002-9394(58)90042-4. [DOI] [PubMed] [Google Scholar]
- 20.Westheimer G. The Spatial Grain of the Perifoveal Visual Field. Vision Res. 1982;22:157–62. doi: 10.1016/0042-6989(82)90177-8. [DOI] [PubMed] [Google Scholar]
- 21.Phelps CD. Acuity Perimetry and Glaucoma. Trans Am Ophthalmol Soc. 1984;82:753–91. [PMC free article] [PubMed] [Google Scholar]
- 22.Levi DM, Klein SA, Aitsebaomo AP. Vernier Acuity, Crowding and Cortical Magnification. Vision Res. 1985;25:963–77. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- 23.Strasburger H, Rentschler I, Jüttner M. Peripheral Vision and Pattern Recognition: A Review. J Vis. 2011;11:13. doi: 10.1167/11.5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunness JS, Applegate CA, Haselwood D, Rubin GS. Fixation Patterns and Reading Rates in Eyes with Central Scotomas from Advanced Atrophic Age-Related Macular Degeneration and Stargardt Disease. Ophthalmology. 1996;103:1458–66. doi: 10.1016/s0161-6420(96)30483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maia-Lopes S, Silva ED, Silva MF, et al. Evidence of Widespread Retinal Dysfunction in Patients with Stargardt Disease and Morphologically Unaffected Carrier Relatives. Invest Ophthalmol Vis Sci. 2008;49:1191–9. doi: 10.1167/iovs.07-1051. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson UL, Frennesson C, Nilsson SEG. Patients with AMD and a Large Absolute Central Scotoma can be Trained Successfully to Use Eccentric Viewing, as Demonstrated in a Scanning Laser Ophthalmoscope. Vision Res. 2003;43:1777–87. doi: 10.1016/s0042-6989(03)00219-0. [DOI] [PubMed] [Google Scholar]
- 27.Déruaz A, Goldschmidt M, Whatham AR, et al. A Technique to Train New Oculomotor Behavior in Patients with Central Macular Scotomas during Reading Related Tasks Using Scanning Laser Ophthalmoscopy: Immediate Functional Benefits and Gains Retention. BMC Ophthalmol. 2006;6:35. doi: 10.1186/1471-2415-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson GR, Schuchard RA, De l’Aune WR, Watkins E. Effects of Preferred Retinal Locus Placement on Text Navigation and Development of Advantageous Trained Retinal Locus. J Rehabil Res Dev. 2006;43:761–70. doi: 10.1682/jrrd.2005.07.0120. [DOI] [PubMed] [Google Scholar]
- 29.Rossi EA, Roorda A. The Relationship between Visual Resolution and Cone Spacing in the Human Fovea. Nat Neurosci. 2010;13:156–7. doi: 10.1038/nn.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson SJ, Mullen KT, Hess RF. Human Peripheral Spatial Resolution for Achromatic and Chromatic Stimuli: Limits Imposed by Optical and Retinal Factors. J Physiol. 1991;442:47–64. doi: 10.1113/jphysiol.1991.sp018781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkinson MO, Anderson RS, Bradley A, Thibos LN. Neural Bandwidth of Veridical Perception across the Visual Field. J Vis. 2016;16(2):1.1–1.17. doi: 10.1167/16.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung STL. Improving Reading Speed for People with Central Vision Loss through Perceptual Learning. Invest Ophthalmol Vis Sci. 2011;52:1164–70. doi: 10.1167/iovs.10-6034. [DOI] [PMC free article] [PubMed] [Google Scholar]