Summary
A redox-mediated decrease in N-methyl-D-aspartate (NMDA) receptor function contributes to psychiatric diseases and impaired cognition during aging. Inflammation provides a potential source of reactive oxygen species for inducing NMDA receptor hypofunction. The current study tested the hypothesis that the non-steroidal anti-inflammatory drug indomethacin, which improves spatial episodic memory in aging rats, would enhance NMDA receptor function through a shift in the redox state. Male F344 young and aged rats were prescreened using a one-day version of the water maze task. Animals were then treated with the indomethacin or vehicle, delivered in a frozen milk treat (orally, twice per day, 18 days) and re-tested on the water maze. Indomethacin treatment enhanced water maze performance. Hippocampal slices were prepared for examination of CA3-CA1 synaptic responses, long-term potentiation (LTP), and NMDA receptor-mediated synaptic responses. No effect of treatment was observed for the total synaptic response. LTP magnitude and NMDA receptor input-output curves were enhanced for aged indomethacin treated animals. To examine redox regulation of NMDA receptors, a second group of aged animals was treated with indomethacin or vehicle, and the effect of the reducing agent, dithiothreitol (DTT, 0.5 mM) on NMDA receptor mediated synaptic responses was evaluated. As expected, DTT increased the NMDA receptor response and the effect of DTT was reduced by indomethacin treatment. The results indicate that indomethacin acted to diminish the age-related and redox-mediated NMDA receptor hypofunction and suggests that inflammation contributes to cognitive impairment through an increase in redox stress.
Keywords: Aging, indomethacin, spatial memory, NMDA receptor, LTP, redox
1. INTRODUCTION
An increase in oxidative stress during aging contributes to age-related cognitive decline due in part to a redox induced hypofunction of N-methyl-D-aspartate (NMDA)(Bodhinathan, et al., 2010,Kumar and Foster, 2013,Kumar, et al., 2018,Lee, et al., 2014,Robillard, et al., 2011,Yang, et al., 2010). NMDA receptor activation contributes to the induction of synaptic plasticity, thought to underlie rapidly acquired and flexible episodic memory (Foster, 2012). NMDA receptor hypofunction and impaired episodic memory emerge in middle age, along with an increase in oxidative stress. The mechanisms for increased oxidative stress in the brain during aging are varied and complex, but likely involve inflammation and activated microglial cells.
Aging can be characterized by a persistent low level increase in serum markers of inflammation, and this rise in systemic inflammation is thought to contribute to age-related diseases, including age-related cognitive decline (Chen, et al., 2008,Gimeno, et al., 2008,Murray, et al., 2012,Rafnsson, et al., 2007,Scheinert, et al., 2015,Teeling, et al., 2007). Microglia are the primary immune cell of the brain. Microglial activation can increase during aging (Blalock, et al., 2003,Ianov, et al., 2017,Ianov, et al., 2016,Wong, 2013,Zeier, et al., 2011) resulting in an increased production of reactive oxygen species (ROS) (Streit, et al., 2008).
Interestingly, non-steroidal anti-inflammatory drugs (NSAIDs) can ameliorate age-related cognitive deficits and impaired NMDA receptor-dependent synaptic plasticity (Casolini, et al., 2002,Jain, et al., 2002,Marquez Loza, et al., 2017,McGuiness, et al., 2017,Melnikova, et al., 2006,Mesches, et al., 2004,Small, et al., 2008). However, whether NSAIDs act on the redox-mediated NMDA receptor hypofunction is unknown. The current study was designed to determine if NSAID treatment would improve NMDA receptor function in a redox-dependent manner. For this study, we employed indomethacin, which can improve cognition for conditions associated with microglial activation and oxidative stress, including aging (Bruce-Jones, et al., 1994,Kanno, et al., 2012,McGuiness, et al., 2017,Stephan, et al., 2003,Yang, et al., 2015). The results of the current study confirms that indomethacin treatment rescued synaptic plasticity and improved age-related performance on a spatial episodic memory task. Furthermore, indomethacin treatment had effects specific to NMDA receptor function, increasing the NMDA receptor component of synaptic transmission and reversing the redox mediated NMDA receptor hypofunction.
2. EXPERIMENTAL PROCEDURES
2.1. Subjects
Young (3–5 months, n = 24) and aged (18–20 months, n = 48) male Fisher 344 rats were obtained from the National Institute on Aging colony (Taconic) through the University of Florida Animal Care and Service facility. Animals were paired housed (2 per cage) and maintained on a 12:12 hr reverse light cycle with ad libitum access to water and food. All procedures involving animal subjects were reviewed and approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
2.2. Behavioral characterization
2.2.1. Cue discrimination training
Prior to behavioral testing, rats were trained to voluntarily consume frozen strawberry milk vehicle treats. Methods employed to assess sensory-motor and memory deficits on the water maze have been published previously (McGuiness, et al., 2017). The experimental timeline is provided in Figure 1. Young rats (n = 24) and aged rats (n = 34) were trained on a cue version followed 3 day later by the spatial episodic memory version of the water maze task. This initial testing was employed as a prescreen for matching performance of aged animals in assigning treatment groups. Briefly, rats were first trained on the cue discrimination version of the water escape task. Animals were habituated to the pool by allowing for a 30 sec free swim and 4-guided attempts to climb onto the escape platform from the 4 different cardinal directions. The platform was extended approximately 1 cm above the water level and a white Styrofoam flag was attached. Training consisted of five blocks comprised of three trials with all training massed into one day. Inter-trial intervals were 20 sec and inter-block intervals were approximately 15 minutes. For each trial, a rat was placed in the water in one of four equally spaced start locations (N, S, E, and W) and was allowed 60 sec to escape onto the platform. If an animal did not escape the water maze within the allotted time, the rat was gently guided to the platform. Rats remained on the platform between trials. After each trial block, the rats were placed in home cages under warming fans in order to prevent hypothermia. The platform position and start locations were randomized and relocated prior to the start of each subsequent trial. Rats that failed to find the visible platform at least two times during the last three trials were removed from the study and were not included in the analysis.
Figure 1.
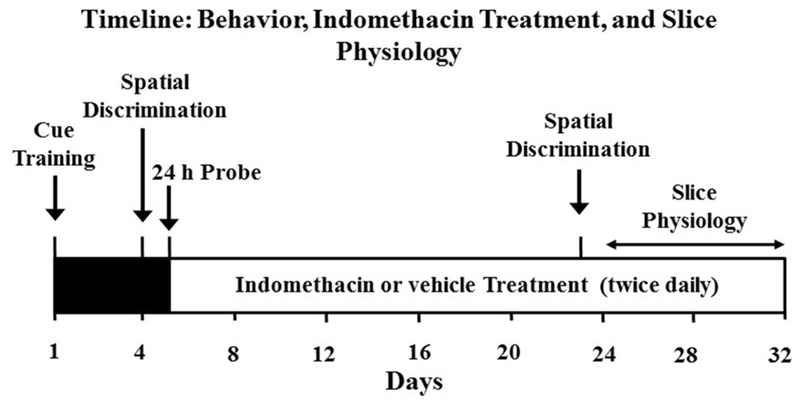
Male F344 young (3–5 months, n = 24) and aged (18–20 months, n = 34) were handled daily and trained to eat frozen milk treats. All rats underwent to the cue discrimination version of the water escape on day one of the experiment. Three days following cue training, animals were trained on the episodic spatial discrimination task. Following behavior characterization, animals were matched for performance and treated with indomethacin, delivered in a frozen strawberry milk treat or vehicle (orally, twice per day). Starting 18 days after initiation of indomethacin treatment (36 treatments), animals were retested on the episodic spatial discrimination task. A subset of rats continued to receive the drug treatment twice a day for up to 12 additional days, until the hippocampus was prepared for slice electrophysiology studies.
2.2.2. Spatial Discrimination Training
Three days following cue training, animals were trained on the episodic spatial discrimination task (Ianov, et al., 2017,Kumar and Foster, 2013,McGuiness, et al., 2017). For spatial discrimination, the escape platform was hidden approximately 1.5 cm beneath the water level and remained in the same location relative to the distal cues in the room for the duration of spatial training. Training procedures for spatial learning were similar to the cue discrimination task, consisting of four blocks of three trials with all training massed into a single day. Inter-trial intervals were 20 sec and inter-block intervals were approximately 15 minutes. On each trial, the rat was placed in the water from one of four start locations. Subjects had 60 sec to escape during each trial; if they did not escape within the allotted time, they were gently guided to the platform. Rats remained on the platform between trials and in cages under the heat lamp after each block. Start locations were randomized across each trial. Escape latency and escape path-length was measured. Fifteen min following the end of block 3 training, a free-swim probe trial was administered in order to test learning, followed by a final training block. For probe trials, the platform was removed and the animal placed in the tank for one minute. A spatial discrimination index score was computed according to the formula (G – O)/(G + O) where G and O represent the percent of time spent in the goal quadrant and quadrant opposite the goal, respectively.
2.3. Indomethacin Treatment
Young rats were randomized to treatment groups. Due to the high variability of aged animals, aged rats matched by their discrimination index scores were assigned to the vehicle (Veh) or indomethacin (Indo) treatment groups. The indomethacin (Sigma) was added to strawberry flavored milk to produce the required dose (2.0 mg/ml) of indomethacin and 500 μl of the frozen strawberry milk treat was voluntarily consumed, twice per day, for 18 days, starting at the end of the initial behavioral testing (McGuiness, et al., 2017,Ormerod, et al., 2013). At the end of the 18 days of treatment, animals were retested on spatial episodic memory task. In this case, the platform location was changed to a new quadrant. Rats continued to receive the drug treatment twice a day for up to 10–12 additional days until killed for electrophysiology studies.
2.4. Hippocampal Slice Preparation
The methods for hippocampal slice preparation and recording have been published previously (Bean, et al., 2015,Bodhinathan, et al., 2010,Guidi, et al., 2015,Kumar and Foster, 2013). Briefly, rats were anesthetized with isoflurane (Halocarbon Laboratories, River Edge, NJ) and swiftly decapitated. The brains were rapidly removed and the hippocampi were dissected. Hippocampal slices (~ 400 μm) were cut parallel to the alvear fibers using a tissue chopper. The slices were incubated in a holding chamber (room temperature) containing standard artificial cerebrospinal fluid (aCSF) (in mM): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and glucose 10. Thirty to sixty min before recording, 2–3 slices were transferred to a standard interface recording chamber (Harvard Apparatus, Boston, MA); the chamber was continuously perfused with standard oxygenated (95% O2, 5% CO2) aCSF at a flow rate of 2 ml/min. The pH and temperature were maintained at 7.4 and 30 ± 0.5oC, respectively. Humidified air (95% O2, 5% CO2) was continuously blown over the slices.
2.5. Extracellular Recordings
Extracellular synaptic field potentials from CA3-CA1 hippocampal synaptic contacts were recorded with glass micropipettes (4–6 MΩ) filled with aCSF. Concentric bipolar stimulating electrodes (outer pole: stainless steel, 200 μm; inner pole: platinum/iridium, 25 μm, Fredrick Haer & Co, Bowdoinham, ME) were positioned on either side (approximately 1 mm) of a recording electrode localized to the middle of stratum radiatum to stimulate CA3 inputs onto CA1. Using a SD9 stimulator (Grass Instruments), filed potentials were induced by single biphasic stimulus pulses (100 μs), and were alternated between pathways such that each pathway was activated at 0.033 Hz. One stimulating electrode activated a control pathway to ensure that the effects of pattern stimulation were specific to activated synapses rather than a change in slice health. Stimulation intensity was set to elicit a response that was 50% of maximum excitatory postsynaptic potential (EPSP), and a stable baseline synaptic response was recorded for at least 10 min before pattern stimulation using the same stimulation intensity. Theta burst stimulation (TBS, four sets of five bursts of 4 pulses at100 Hz with 200 msec intervals between bursts and the four sets separated by a 10 sec interval) was used for induction of threshold long-term potentiation (LTP), and LTP was recorded for 60 min after TBS. The signals were amplified, filtered between 1 Hz and 1 kHz and stored on computer disk for off-line analysis (Data Wave Technologies, Longmont, CO). Two cursors were placed around the initial descending phase of the waveform and the maximum slope (mV/ms) of the EPSP was determined by a computer algorithm that found the maximum change across all sets of consecutively recorded points (20 kHz sampling rate) between the two cursors. To obtain the NMDA receptor-mediated component of synaptic transmission (NMDA receptor-EPSP) slices were incubated in aCSF containing low Mg2+ (0.5 mM), 6,7-dinitroquinoxaline-2,3-dione (DNQX, 30 μM), and picrotoxin (PTX, 10 μM). Input-output curves for the total and NMDA receptor-EPSP (mV/mS) were constructed for increasing stimulation intensities.
Because water maze training may affect the NMDA receptor response and redox state of the hippocampus, we tested the effects of indomethacin on redox regulation of the NMDA-mediated synaptic response in cage control aged animals. Aged animals randomly assigned to vehicle (age-Veh, n = 6) or indomethacin (age-Indo, n = 6) treatment groups received at least 32 total treatments (16 days) before hippocampal slices were collected for electrophysiology. The NMDA receptor component of synaptic transmission was isolated and a baseline of at least 10 min was collected before application of dithiothreitol (DTT, 0.5 mM). The response was followed for an additional 60 min.
2.6. Statistical Analysis
Analyses of variance (ANOVAs) and regression analyses were carried out using StatView 5.0 (SAS Institute Inc, NC). Post-hoc ANOVAs within age groups or Fisher’s protected least significant difference (PLSD) post-hoc tests, with p set at 0.05, were employed to localize differences. Initially, ANOVAs for age and treatment were conducted to determine if treatment influenced young animals. If no treatment effect was observed for young, then young-Indo and young-Veh were combined for further analysis. In the case, that analyses revealed an effect of treatment that included young animals, post-hoc comparisons were performed comparing each group (young-Veh; young-Indo; age-Veh; age-Indo) to the other three groups. For electrophysiological measures, in many cases, responses were recorded from multiple slices. Therefore, the number of animals and number of slices are reported and statistics were conducted using slice data. A one-tailed t-test was employed to test the effects of indomethacin on the DTT-induced increase in the NMDA receptor synaptic response. Indomethacin was predicted to reduce redox stress, such that the increase in the NMDA receptor response would be greater in slices from the age-Veh relative to the age-Indo treatment group.
3. RESULTS
3.1. Cue discrimination
One aged animal from the indomethacin treatment group and one aged animal from the vehicle treatment group did not complete the treatment and were excluded from the analysis. For prescreening, animals (young = 24 and aged = 32) were first trained on the cue version of the water maze. A repeated measures ANOVA on escape pathlength indicated a significant effect of training [F(4,216) = 41.28, p < 0.0001] and age [F(1,216) = 21.27, p < 0.0001], in the absence of an interaction [F(4,216) = 1.67, p = 0.16] due to shorter escape pathlength for young rats relative to the aged group (Fig 2A). ANOVAs within each age group indicated a training effect (p < 0.0001) with a decrease in pathlength in each group. Examination of swim speed indicated a training effect [F(4,216) = 53.67, p < 0.0001] and an age difference [F(1,216) = 24.72, p < 0.0001], with young rats exhibiting faster swim speeds than aged rats, in the absence of an interaction [F(4,216) = 0.55, p = 0.7] (Fig 2B). Post-hoc ANOVAs within each age group indicated that aged rats increased their swim speed over the course of training (p < 0.0001) and young animals exhibited a tendency (0.057) to increase swim speed with training. All animals were able to escape from the pool within 60 s during the final training block.
Figure 2.
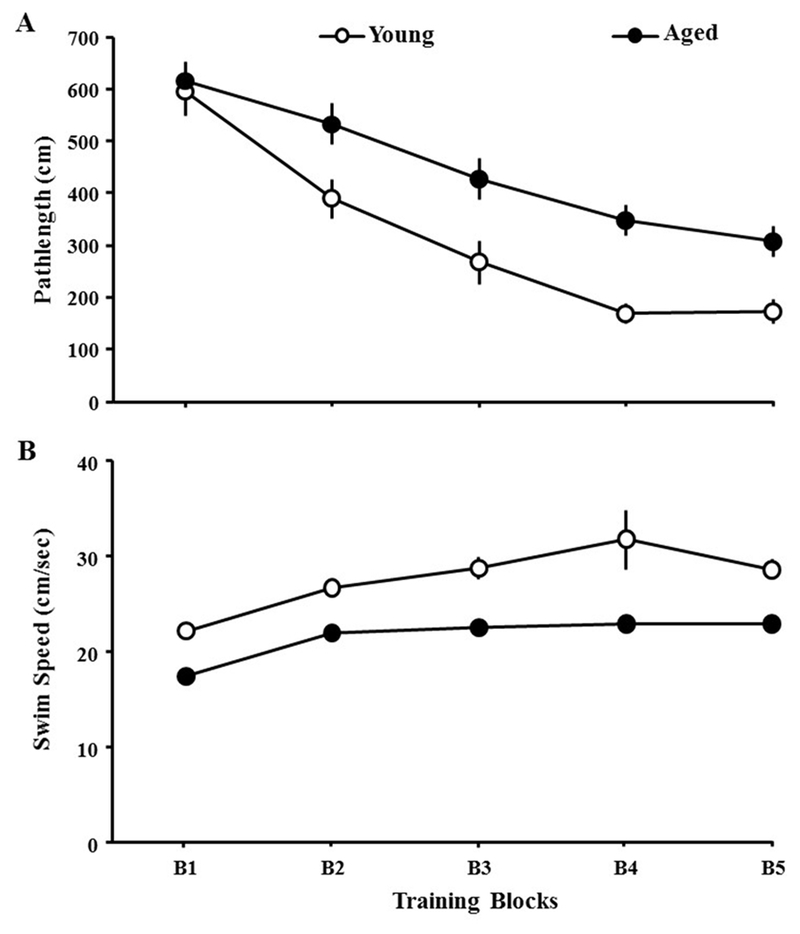
Animal’s performance on the cue version of the water maze. A) Pathlength (±SEM) to reach the escape platform and B) swim speed on each of the five training blocks (B1–B5) during cue discrimination training. Each point represents the mean (±SEM) for young (open circle) and aged (filled circle) animals.
3.2. Spatial discrimination
Three days following cue training, animals were trained on a short version of the spatial water maze involving three training blocks followed by a probe trial and a final training block. An ANOVA indicated an age x training block interaction for escape pathlength [F(3,162) = 8.5, p < 0.001]. ANOVAs within each age group indicated that the pathlengths decreased for each age group (p < 0.0001). Post-hoc ANOVAs for age effects within each block indicated age differences on blocks 3–4 (p < 0.001 in each case) due to shorter pathlength for younger rats (Fig 3A). An ANOVA for swim speed on the spatial task indicated an age x training block interaction [F(3,162) = 3.44, p < 0.05]. Subsequent ANOVAs indicate that each group exhibited a decrease in swim speed over the course of training (p < 0.001). An ANOVA for each block indicated faster swim speed by young relative to aged rats for each training block (p < 0.05) (Fig 3B).
Figure 3.
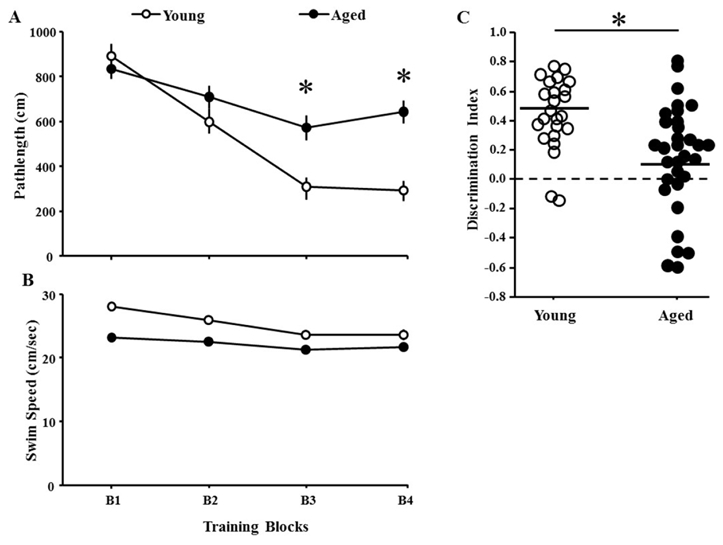
Performance during spatial discrimination prescreening. Mean (±SEM) pathlength A) and swim speed B) for the four training blocks (B1–B4) of the spatial discrimination task for young (open circle) and aged (filled circle) animals. (C) The scatter diagram illustrates variability in discrimination index scores of the probe trail for young (open) and aged (filled) animals. In each case, the solid line indicates the average discrimination index score for young and aged animals. The asterisk indicates a significant age difference.
Examination of the discrimination index scores for the probe trial indicated an age effect [F(1,54) = 11.77, p < 0.005], with young rats exhibiting better performances than aged rats (Fig 3C). Indeed, nearly a third of the aged animals exhibited discrimination index scores near or below chance. Despite the age difference, average discrimination index scores for both groups were > 0 (chance levels) confirming the acquisition of a spatial search strategy, which is in line with our observation that pathlength decreased across training blocks in both groups. Regression analysis within each age group was used to examine the relationship between spatial behavior (pathlength for the last block of spatial training) and performance on the cue discrimination task (last block of cue training). No correlation was observed indicating that differences in sensory motor performance did not contribute to variability in spatial behavior.
3.3. Influence of indomethacin treatment on spatial discrimination performance
Due to the fact that a large proportion of aged animals performed near chance on the probe trial, aged rats were pseudorandomly assigned to the vehicle or indomethacin treatment groups matched by their discrimination index scores (age-Veh = 16; age-Indo = 16). Young animals were randomly assigned to treatment groups (young-Veh = 11; young-Indo = 13) and no difference was observed for prescreening behavior between the treatment assignment groups. Following 18 days of indomethacin or vehicle treatment, a second round of spatial training was conducted using a different platform location. Since no treatment effect on pathlength or swim speed was found across training blocks in young rats, they were collapsed into one group (n = 24) for analysis. A repeated measures ANOVA on escape pathlength indicated an effect of training [F(3,159) = 50.29, p < 0.0001] and a group difference [F(2,159) = 5.56, p < 0.01] (Fig 4A). There was a trend for an interaction of group and training block [F(6, 159) = 1.95, p = 0.077]. Fisher’s PLSD post-hoc tests confirmed young rats outperformed aged-Veh rats (p < 0.005) and tended to outperform aged-Indo rats (p = 0.062) on all trial blocks combined. Post-hoc ANOVAs within each trial block indicated that young rats outperformed aged-Veh on blocks 1, 2, and 4 and only outperformed aged-Indo rats on block 3. Interestingly, aged-Indo rats outperformed aged-Veh rats on block 1. A repeated measures ANOVA revealed a decrease in swim speeds across training blocks [F(3,156) = 5.00, p < 0.005] in the absence of a group difference (p = 0.11) (Fig 4B).
Figure 4.
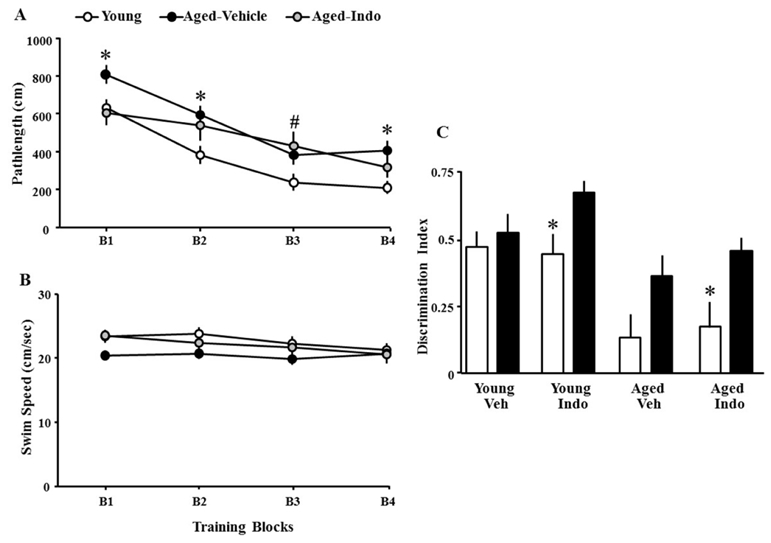
Indomethacin effects on performance of the spatial discrimination task. A) Mean escape pathlength (±SEM) for young (open circles), aged-Veh (filled circles) and aged-Indo (gray circles) animals. Asterisks indicate difference between young and aged-Veh animals. Pound sign indicates difference between young and aged-Indo animals. B) Swim speed decreased during training for young and aged-Indo groups and the swim speed of aged-Veh was reduced relative to young. C) Mean (+SEM) discrimination index scores of the prescreening probe trail (open) and following treatment (filled) for young and aged animals treated with vehicle (Veh) or indomethacin (Indo). Asterisks indicate difference in discrimination index scores between prescreening and following treatment.
For the probe trials, a repeated measures ANOVA was conducted on the discrimination index scores during the prescreening and following treatment. The results indicated an effect of age [F(1,52) = 24.22, p < 0.0001] and repeated testing [F(1,52) = 12.31, p < 0.001], in the absence of a main effect of treatment [F(1,52) = 1.74, p = 0.19] or interactions (all p-values > 0.3). However, post hoc ANOVAs for effects of repeated testing within each age and treatment group indicated that a significant improvement in the discrimination index score was limited to young-Indo [F(1,12) = 7.54, p < 0.05] and aged-Indo [F(1,15) = 4.65, p < 0.05] groups, with a tendency (p = 0.07) for improved performance in the aged-Veh group (Fig 4C).
3.4. Effect of indomethacin treatment on synaptic function
To examine influence of indomethacin treatment on glutamatergic synaptic transmission at CA3-CA1 synapses, total field excitatory postsynaptic potentials were recorded from the CA3-CA1 hippocampal synapses in slices obtained from indomethacin and vehicle treated animals. Input-output curves were generated by plotting the fiber potential amplitude or slope of total synaptic response across different stimulation intensities for young (vehicle = 12/6 slices/animals, indomethacin = 9/5 slices/animals) and aged (vehicle = 22/9 slices/animals, indomethacin = 21/10 slices/animals) animals treated with indomethacin or vehicle. Because a repeated measures ANOVA across stimulation intensities indicated that treatment did not affect the fiber potential or EPSP in young rats (p = 0.5), young animals were collapsed into one group for analysis. A repeated measures ANOVA on the fiber potential indicated an effect of stimulation intensity [F(5,305) = 111.85, p < 0.00001] in the absence of a group effect (Fig 5B). Similarly, for the slope of the synaptic response, a repeated measures ANOVA indicated an effect of stimulation intensity [F(5,305) = 72.76, p < 0.00001] in the absence of a group effect (Fig 5C). Relative to aged animals, a modest increase in the synaptic response was observed for young animals, but this difference was not significant (p = 0.14).
Figure 5.
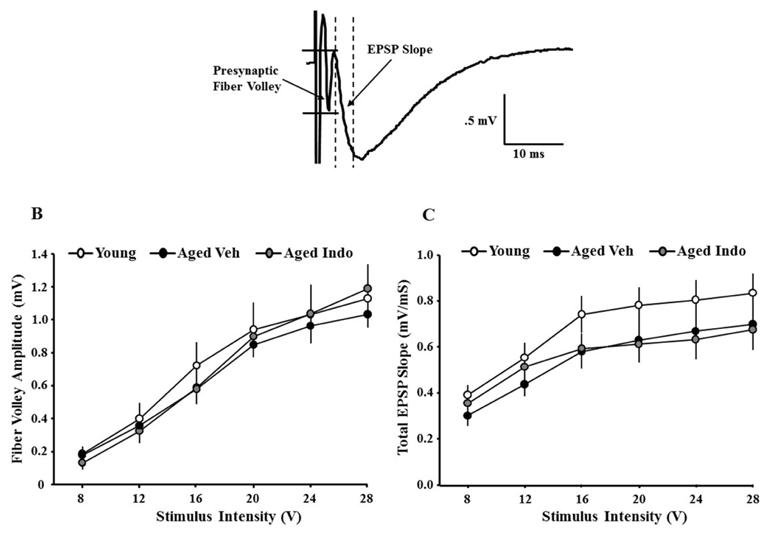
Slope of excitatory postsynaptic potentials (EPSPs) recorded from hippocampal CA3-CA1 synapses. A) Example of an EPSP, illustrating the initial descending slope measured between the dashed lines and the fiber potential amplitude measured between the solid horizontal lines. B) The amplitude of presynaptic fiber volley was measured for increasing stimulation intensity and input-output curves were generated for young (open circles), aged vehicle (aged-Veh, filled circles), and aged indomethacin treated (aged-Indo, gray circles) animals. C) The initial slope of the EPSP was measured for increasing stimulation intensity and input-output curves were generated for young (open circles), aged vehicle (aged-Veh, filled circles), and aged indomethacin treated (aged-Indo, gray circles) animals. Each point represents the mean (±SEM) for the given stimulation intensity.
For some slices, stimulation was set to evoke a response ~50% of maximum. Following baseline recording, TBS was delivered to induce LTP. For statistical analysis, the average response during the last 5 min of 60 min recording was used to calculate the percent change relative to the averaged baseline response collected 10 min prior to pattern stimulation. An ANOVA for TBS-induced LTP indicated no effect of treatment on LTP in young (Veh: 10/6 slices/animals; indo 7/6 slices/animals) animals (p = 0.19); therefore; young groups were combined for further analysis. An ANOVA on the three groups indicated a group difference [F(2, 39) = 3.55, p < 0.05]. Fisher’s PLSD post-hoc tests indicated decreased LTP amplitude in aged-Veh (9/6 slices/animals) compared to aged-Indo (16/11 slices/animals) and young (17/6 slices/animals) (Fig 6).
Figure 6.
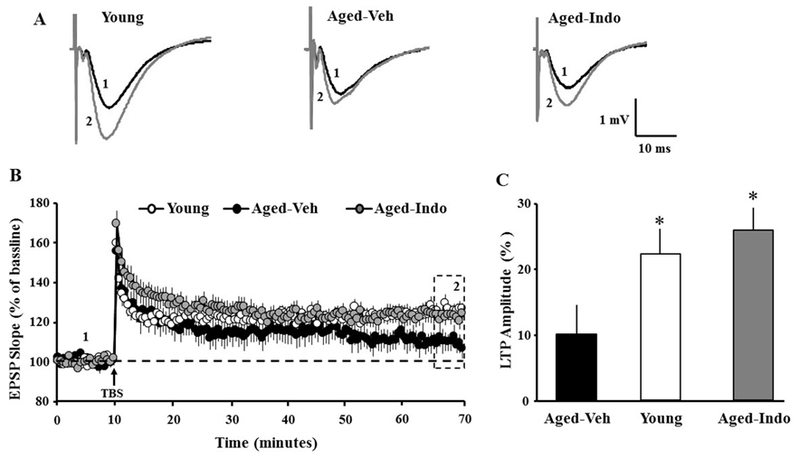
Indomethacin treatment increases the amplitude of LTP in aged animals. A) Examples of LTP for the three conditions at the indicated time points during baseline recording (1, black trace) and 60 min after induction of LTP (2, gray trace). B) Time course of mean (±SEM) EPSP slope normalized to the baseline. TBS (arrow) was delivered after a 10 min stable baseline recording (time point 1). C) Mean percent increase in the synaptic response 60 min following TBS (dotted box, time point 2). Asterisks indicate difference relative to aged-Veh animals.
For other slices, following the assessment of the total synaptic response, the NMDA receptor component of the synaptic response was pharmacologically isolated. Input-output curves for the fiber potential amplitude and NMDA receptor mediated synaptic response were recorded from CA3-CA1 hippocampal slices obtained from young (Veh: 11/6 slices/animals; indo: 9/6 slices/animals) and aged (Veh: 16/9 slices/animals; Indo: 13/9 slices/animals) rats. A repeated measures ANOVA across stimulation intensities indicated no treatment effect on the fiber potential amplitude or the NMDA receptor response in young rats; therefore, young groups were combined for further analysis. Subsequent repeated measures ANOVAs indicated an increase in the fiber potential amplitude with increasing stimulation intensity [(F5,230) = 105.83, p < 0.0001], in the absence of a group differences (Fig 7B). Examination of the NMDA receptor synaptic response indicated an interaction of stimulation intensity and group [F(10,230) = 7.65, p < 0.0001]. Fisher’s PLSD post-hoc tests indicated a decreased in the synaptic responses in aged-Veh animals relative to young and aged-Indo animals (Fig 7C).
Figure 7.
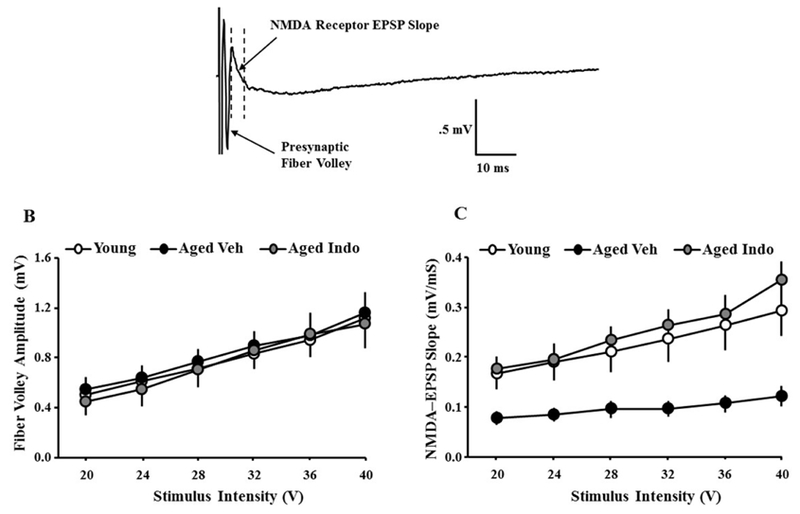
Slope of NMDA receptor-mediated excitatory postsynaptic potentials (EPSPs) recorded from hippocampal CA3-CA1 synapses. A) Example of NMDA receptor-mediated EPSP, illustrating the presynaptic fiber volley measured from the solid horizontal lines, and initial descending slope of the EPSP measured between the dashed lines. B) The amplitude of the fiber potential was measured for increasing stimulation intensity generated for young (open circles), aged-Veh (filled circles), and aged-Indo treated (gray circles) animals. C) Input-output curves plotting the mean (±SEM) slope of the NMDA receptor-EPSP relative to stimulation intensity. The NMDA response was increased for young vehicle and aged-Indo relative to aged-vehicle.
3.5. Indomethacin treatment influences redox-mediated NMDA receptor hypofunction
We hypothesized that indomethacin may decrease redox stress-mediated NMDA receptor hypofunction. However, differences in the NMDA receptor component could result from differences in learning or the stress associated with water maze training (Foster and Dumas, 2001,Foster, et al., 1996,Nasca, et al., 2015,Quinlan, et al., 2004,Zinebi, et al., 2003). To remove learning or stress as potential confounds and, in order to examine the possibility that indomethacin was acting to influence the redox regulation of the NMDA response, another group of aged animals was treated for at least 18 days with indomethacin (n = 6) or vehicle (n = 6) and NMDA receptor mediated synaptic responses were collected from two slices per animal. Similar to aged animals trained in the water maze, input-output curves indicated no difference in the fiber potential amplitude, and NMDA receptor mediated synaptic responses were increased [F(1, 110) = 9.61, p < 0.01] for the animals treated with indomethacin (Fig 8A & B). The NMDA receptor-EPSP slope was set a ~50% of maximum and recorded for 10 min, followed by application of reducing agent, DTT (0.5 mM). Consistent with previous reports, DTT increased the synaptic response in slices from aged-Veh animals and the increase was highly variable, which has been associated with variability in cognitive function (Guidi, et al., 2015,Kumar and Foster, 2013,Lee, et al., 2014). If the effect of indomethacin is due to decreased redox stress, then the growth of the response due to application of DTT should be reduced in the indomethacin treatment group. As predicted, a one-tailed t-test confirmed that the growth of the NMDA response was reduced (t(22) = 1.77, p < 0.05) for indomethacin treated (118.27+ 5.53) relative to vehicle treated animals (161.46+ 23.74) (Fig 8).
Figure 8.
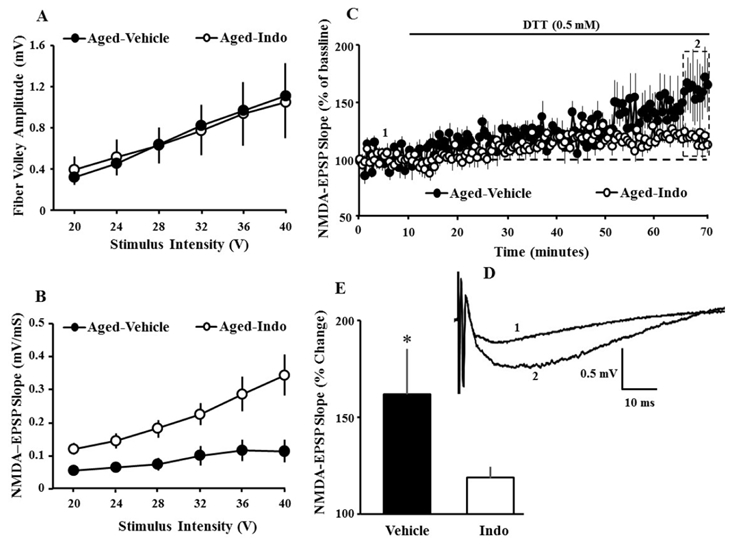
Indomethacin treatment influences redox regulation of NMDA receptor function. A) Input-output curves plotting the mean (±SEM) amplitude of the fiber potential for aged-Veh (filled circles) and aged-Indo (gray circles) treated animals. B) Input-output curves plotting the mean (±SEM) slope of the NMDA receptor-EPSP relative to increasing stimulation intensity for aged-Veh (filled circles) and aged-Indo treated (gray circles) animals. C) Time course of changes cand aged-Indo (open circles) treated animals. D) Example of the NMDA receptor component of synaptic transmission recorded from an aged-vehicle animal during baseline (1) and 60 min following application of the reducing agent DTT (2). E) Bar diagram showing the mean (+SEM) percentage increase in the amplitude of the NMDA-mediated synaptic response during the last 5 min of recording (dotted box in C). The asterisk indicates a significant (p < 0.05) difference between vehicle and indomethacin treated animals.
4. DISCUSSION
The current study found that indomethacin treatment improved NMDA receptor function in aged animals. Furthermore, the mechanism for improved NMDA receptor function involved redox signaling. In the case of behavior, indomethacin minimized age differences in pathlength to find the escape platform and increased the discrimination index. The improvement in water maze performance, while modest, is consistent with several studies demonstrating that NSAID treatment can enhance cognition, particularly during aging (Casolini, et al., 2002,Jain, et al., 2002,McGuiness, et al., 2017,Melnikova, et al., 2006,Mesches, et al., 2004,Rogers, et al., 2017,Small, et al., 2008).
The mechanism for NSAID effects on cognition have been linked to enhanced synaptic plasticity and NMDA receptor function (Mesches, et al., 2004,Rogers, et al., 2017,Stephan, et al., 2003). The increased NMDA receptor function and improved cognition may be due to indomethacin acting to inhibit microglia. Microglia exhibit complement activation and phagocytic activity associated with neuroinflammation and lipopolysaccharide treatment (Furst, et al., 2005,Vilalta and Brown, 2017,Zhang, et al., 2014). However, in this case, we would have expected differences in the total-EPSP due to AMPA receptor internalization following activation of the complement receptors or phagocytosis of synapses. The absence of a treatment effect on the total-EPSP suggests that indomethacin did not influence AMPA receptor function.
Microglial activation is associated with increased oxidative stress and indomethacin decreases oxidative stress through peroxisome proliferator-activated receptors gama activation and inhibition of cyclooxygenase-1 (COX1) and COX2 (Ajmone-Cat, et al., 2010,Goncalves, et al., 2010,Huong, et al., 2011,Klegeris and McGeer, 2002,Pepicelli, et al., 2002,Widmer, et al., 2007). The current study provides evidence that indomethacin treatment increased NMDA receptor function through a redox mechanism. First, previous work demonstrates that redox effects are specific to NMDA receptors and not observed for the AMPA receptor component of synaptic transmission (Bodhinathan, et al., 2010). In the current study, the effects of indomethacin were specific to the NMDA receptor component of synaptic transmission, consistent with a redox mechanism. Second, during oxidative stress, hydrogen peroxide acts as a signaling mechanism, generating disulfide bonds that alter the function of proteins, including NMDA receptors. DTT breaks disulfide bonds, enhancing NMDA receptor function under conditions of elevated hydrogen peroxide (i.e. redox stress) (Kumar, et al., 2018,Lee, et al., 2014). In the current study, indomethacin reduced the ability of DTT to enhance NMDA receptor synaptic responses, providing strong evidence for a redox mechanism. Finally, the enhancement of NMDA receptor function contributes to enhanced induction of LTP (Bodhinathan, et al., 2010,Foster, 2012) and indomethacin was associated with an increase in the NMDA receptor component of synaptic transmission and rescue of LTP in aged animals.
Variability in DTT effects on the NMDA receptor response are linked to the level of redox stress and cognitive function, such that DTT enhances the NMDA receptor response to a greater extent in cognitively impaired animals (Guidi, et al., 2015,Kumar and Foster, 2013). However, it is also possible that learning or stress associated with training on the water maze could induce changes in synaptic function and redox state. Therefore, in order to examine the effects on indomethacin on redox regulation of NMDA receptors, it was important to employ naïve animals. As predicted, DTT had a larger and more variable effect on vehicle treated animals. Indeed, for indomethacin treated animals, the increase in the NMDA receptor response was similar to that reported for young animals and cognitively intact middle-age animals (Bodhinathan, et al., 2010,Kumar and Foster, 2013). The higher variability of vehicle treated animals is likely due to variability in redox stress and possibly variability in cognitive function.
The results are consistent with work indicating that indomethacin reduces oxidative stress in the hippocampus (Goncalves, et al., 2010,Horakova, et al., 1997,Huong, et al., 2011,Miyamoto, et al., 2003). A decrease in oxidative stress may contribute to effects that have been described for other NSAID on the aging hippocampus. For example, oxidative stress influences hippocampal neurogenesis and indomethacin treatment increases neurogenesis in aged animals (Huang, et al., 2012,McGuiness, et al., 2017,Yang, et al., 2015). Similarly, glial fibrillary acidic protein (GFAP) is an important component of astrocyte response to hydrogen peroxide (de Pablo, et al., 2013,Morgan, et al., 1997) and, depending on the activating signal, NSAIDs can reduce GFAP (Bates, et al., 2007,Heneka, et al., 2005,Rogers, et al., 2017).
An important aspect of the current research is that it strengthens the link between inflammation and NMDA receptor hypofunction through redox signaling. Stress induced activation of immune responses and NMDA receptor hypofunction is characteristic of several neurological diseases. A redox-mediated NMDA receptor hypofunction within the prefrontal cortex may shift the balance of excitatory/inhibitory synaptic interactions during development leading to schizophrenia (Steullet, et al., 2016). In adults, a stressor-induced NMDA receptor hypofunction contributes to depressive-like behavior (Ibi, et al., 2017). During aging, the redox mediated NMDA receptor hypofunction in the prefrontal cortex and hippocampus influence executive function and episodic memory, respectively (Guidi, et al., 2015,Kumar and Foster, 2013,Kumar, et al., 2018). Indeed, systemic inflammation in young animals can mimic aging, inducing microglial activation and impairing NMDA receptor-dependent synaptic plasticity (Di Filippo, et al., 2013,Maggio, et al., 2013).
In the case of normal aging and the onset of cognitive impairment, NMDA receptor hypofunction may be treatable, since receptor hypofunction emerges early as a component of reversible redox signaling rather than irreversible oxidative damage. In contrast, oxidative damage is more apparent with advanced age and neurodegenerative disease (Kumar, et al., 2018). However, it is important to recognize that systemic inflammation and neuroinflammation play an important role in tissue repair, the response to toxic agents, and likely influences resilience to neurodegenerative disease. Other considerations include the increased use of NSAIDs by the elderly for pain relief and obstacles for NSAID treatment due to problems with efficacy and possible adverse effects that NSAIDs may create for the elderly (Barkin, et al., 2010,Johnson, 1998,Sarchielli, et al., 2006,Savage, 2005). Thus, there may be better approaches than chronic anti-inflammatory treatments or NSAIDs may be combined with other anti-inflammatory treatments. Environmental toxins, obesity, and lifestyle factors such as diet and exercise influence the aging process through several mechanisms including epigenetics and feed forward and feedback mechanisms for regulating oxidative stress and the inflammation response. In each case, it will be interesting to determine if individual or combined manipulations that improve cognition are linked to NMDA receptor redox regulation.
Highlights.
Redox state links N-methyl-D-aspartate (NMDA) receptor hypofunction to memory decline.
Inflammation is a source of reactive oxygen species for NMDA receptor hypofunction.
The anti-inflammatory drug, indomethacin, improved cognition, long-term synaptic plasticity, and NMDA-mediated synaptic responses.
Indomethacin diminished the redox-mediated NMDA receptor hypofunction.
ACKNOWLEDGMENTS
Supported by National Institute of Aging grants R37AG036800, RO11049711, and RO1052258 and the Evelyn F. McKnight Brain Research Foundation. This work was partially supported by the University of Florida Claude D. Pepper Older Americans Independence Center (P30-AG028740). Special thanks to Semir Karic, Erica Willner, Araliya Gunawardene, Ethan Salgado, and Nick Sarantos for preparing and feeding treats to animals and their help in behavior assessment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
None of the authors has any financial conflict of interest related to this manuscript.
REFERENCES
- Ajmone-Cat MA, Bernardo A, Greco A, Minghetti L 2010. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals (Basel) 3(6), 1949–65. doi: 10.3390/ph3061949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkin RL, Beckerman M, Blum SL, Clark FM, Koh EK, Wu DS 2010. Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult? Drugs Aging 27(10), 775–89. doi: 10.2165/11539430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bates KA, Martins RN, Harvey AR 2007. Oxidative stress in a rat model of chronic gliosis. Neurobiol Aging 28(7), 995–1008. doi: 10.1016/j.neurobiolaging.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Bean LA, Kumar A, Rani A, Guidi M, Rosario AM, Cruz PE, Golde TE, Foster TC 2015. Re-Opening the Critical Window for Estrogen Therapy. J Neurosci 35(49), 16077–93. doi: 10.1523/JNEUROSCI.1890-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW 2003. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci 23(9), 3807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC 2010. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci 30(5), 1914–24. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Jones PN, Crome P, Kalra L 1994. Indomethacin and cognitive function in healthy elderly volunteers. Br J Clin Pharmacol 38(1), 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolini P, Catalani A, Zuena AR, Angelucci L 2002. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res 68(3), 337–43. doi: 10.1002/jnr.10192. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW 2008. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 22(3), 301–11. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablo Y, Nilsson M, Pekna M, Pekny M 2013. Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen-glucose deprivation and reperfusion. Histochem Cell Biol 140(1), 81–91. doi: 10.1007/s00418-013-1110-0. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampa C, Costa C, Tantucci M, Zianni E, Boraso M, Siliquini S, de Iure A, Ghiglieri V, Colcelli E, Baker D, Sarchielli P, Fusco FR, Di Luca M, Calabresi P 2013. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis 52, 229–36. doi: 10.1016/j.nbd.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Foster TC 2012. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Prog Neurobiol 96(3), 283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Dumas TC 2001. Mechanism for increased hippocampal synaptic strength following differential experience. J Neurophysiol 85(4), 1377–83. doi: 10.1152/jn.2001.85.4.1377. [DOI] [PubMed] [Google Scholar]
- Foster TC, Gagne J, Massicotte G 1996. Mechanism of altered synaptic strength due to experience: relation to long-term potentiation. Brain Res 736(1–2), 243–50. [DOI] [PubMed] [Google Scholar]
- Furst SM, Komocsar WJ, Khan KN, White KL Jr., Peachee VL, Mennear JH 2005. Screening New Drugs for Immunotoxic Potential: I. Assessment of the Effects of Conventional Nonsteroidal Anti-Inflammatory Drugs and Selective COX-2 Inhibitors on In Vitro and In Vivo Phagocytic Activity. J Immunotoxicol 1(3), 149–58. doi: 10.1080/15476910490916828. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Marmot MG, Singh-Manoux A 2008. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology 33(10), 1322–34. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO, Silva AP 2010. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur J Neurosci 31(2), 315–26. doi: 10.1111/j.1460-9568.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- Guidi M, Kumar A, Foster TC 2015. Impaired attention and synaptic senescence of the prefrontal cortex involves redox regulation of NMDA receptors. J Neurosci 35(9), 3966–77. doi: 10.1523/JNEUROSCI.3523-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE 2005. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain 128(Pt 6), 1442–53. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Horakova L, Stolc S, Chromikova Z, Pekarova A, Derkova L 1997. Mechanisms of hippocampal reoxygenation injury. Treatment with antioxidants. Neuropharmacology 36(2), 177–84. [DOI] [PubMed] [Google Scholar]
- Huang TT, Zou Y, Corniola R 2012. Oxidative stress and adult neurogenesis--effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol 23(7), 738–44. doi: 10.1016/j.semcdb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huong NQ, Nakamura Y, Kuramoto N, Yoneyama M, Nagashima R, Shiba T, Yamaguchi T, Hasebe S, Ogita K 2011. Indomethacin ameliorates trimethyltin-induced neuronal damage in vivo by attenuating oxidative stress in the dentate gyrus of mice. Biol Pharm Bull 34(12), 1856–63. [DOI] [PubMed] [Google Scholar]
- Ianov L, De Both M, Chawla MK, Rani A, Kennedy AJ, Piras I, Day JJ, Siniard A, Kumar A, Sweatt JD, Barnes CA, Huentelman MJ, Foster TC 2017. Hippocampal Transcriptomic Profiles: Subfield Vulnerability to Age and Cognitive Impairment. Front Aging Neurosci 9, 383. doi: 10.3389/fnagi.2017.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianov L, Rani A, Beas BS, Kumar A, Foster TC 2016. Transcription Profile of Aging and Cognition-Related Genes in the Medial Prefrontal Cortex. Front Aging Neurosci 8, 113. doi: 10.3389/fnagi.2016.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi M, Liu J, Arakawa N, Kitaoka S, Kawaji A, Matsuda KI, Iwata K, Matsumoto M, Katsuyama M, Zhu K, Teramukai S, Furuyashiki T, Yabe-Nishimura C 2017. Depressive-Like Behaviors Are Regulated by NOX1/NADPH Oxidase by Redox Modification of NMDA Receptor 1. J Neurosci 37(15), 4200–12. doi: 10.1523/JNEUROSCI.2988-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain NK, Patil CS, Kulkarni SK, Singh A 2002. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res 133(2), 369–76. [DOI] [PubMed] [Google Scholar]
- Johnson AG 1998. NSAIDs and blood pressure. Clinical importance for older patients. Drugs Aging 12(1), 17–27. [DOI] [PubMed] [Google Scholar]
- Kanno T, Yaguchi T, Nagata T, Shimizu T, Tanaka A, Nishizaki T 2012. Indomethacin enhances learning and memory potential by interacting with CaMKII. J Cell Physiol 227(3), 919–26. doi: 10.1002/jcp.22800. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL 2002. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol Aging 23(5), 787–94. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC 2013. Linking redox regulation of NMDAR synaptic function to cognitive decline during aging. J Neurosci 33(40), 15710–5. doi: 10.1523/JNEUROSCI.2176-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yegla B, Foster TC 2018. Redox Signaling in Neurotransmission and Cognition During Aging. Antioxid Redox Signal 28(18), 1724–45. doi: 10.1089/ars.2017.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Kumar A, Rani A, Foster TC 2014. Role of antioxidant enzymes in redox regulation of N-methyl-D-aspartate receptor function and memory in middle-aged rats. Neurobiol Aging 35(6), 1459–68. doi: 10.1016/j.neurobiolaging.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N, Shavit-Stein E, Dori A, Blatt I, Chapman J 2013. Prolonged systemic inflammation persistently modifies synaptic plasticity in the hippocampus: modulation by the stress hormones. Frontiers in molecular neuroscience 6, 46. doi: 10.3389/fnmol.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez Loza A, Elias V, Wong CP, Ho E, Bermudez M, Magnusson KR 2017. Effects of ibuprofen on cognition and NMDA receptor subunit expression across aging. Neuroscience 344, 276–92. doi: 10.1016/j.neuroscience.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuiness JA, Scheinert RB, Asokan A, Stadler VC, Lee CS, Rani A, Kumar A, Foster TC, Ormerod BK 2017. Indomethacin Increases Neurogenesis across Age Groups and Improves Delayed Probe Trial Difference Scores in Middle-Aged Rats. Front Aging Neurosci 9, 280. doi: 10.3389/fnagi.2017.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova T, Savonenko A, Wang Q, Liang X, Hand T, Wu L, Kaufmann WE, Vehmas A, Andreasson KI 2006. Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer’s disease in a sex-dimorphic pattern. Neuroscience 141(3), 1149–62. doi: 10.1016/j.neuroscience.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC 2004. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging 25(3), 315–24. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Miyamoto O, Tamae K, Kasai H, Hirakawa H, Hayashida Y, Konishi R, Itano T 2003. Suppression of hyperemia and DNA oxidation by indomethacin in cerebral ischemia. Eur J Pharmacol 459(2–3), 179–86. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Rozovsky I, Goldsmith SK, Stone DJ, Yoshida T, Finch CE 1997. Increased transcription of the astrocyte gene GFAP during middle-age is attenuated by food restriction: implications for the role of oxidative stress. Free Radic Biol Med 23(3), 524–8. [DOI] [PubMed] [Google Scholar]
- Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, Cunningham C 2012. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging 33(3), 603–16 e3. doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nistico R, McEwen BS 2015. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci U S A 112(48), 14960–5. doi: 10.1073/pnas.1516016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod BK, Hanft SJ, Asokan A, Haditsch U, Lee SW, Palmer TD 2013. PPARgamma activation prevents impairments in spatial memory and neurogenesis following transient illness. Brain Behav Immun 29, 28–38. doi: 10.1016/j.bbi.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli O, Fedele E, Bonanno G, Raiteri M, Ajmone-Cat MA, Greco A, Levi G, Minghetti L 2002. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E(2) extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J Neurochem 81(5), 1028–34. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E 2004. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron 41(2), 185–92. [DOI] [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GD, Fowkes FG 2007. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc 55(5), 700–7. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Robillard JM, Gordon GR, Choi HB, Christie BR, MacVicar BA 2011. Glutathione restores the mechanism of synaptic plasticity in aged mice to that of the adult. PLoS One 6(5), e20676. doi: 10.1371/journal.pone.0020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Liu CC, Zhao N, Wang J, Putzke T, Yang L, Shinohara M, Fryer JD, Kanekiyo T, Bu G 2017. Subacute ibuprofen treatment rescues the synaptic and cognitive deficits in advanced-aged mice. Neurobiol Aging 53, 112–21. doi: 10.1016/j.neurobiolaging.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P, Mancini ML, Calabresi P 2006. Practical considerations for the treatment of elderly patients with migraine. Drugs Aging 23(6), 461–89. [DOI] [PubMed] [Google Scholar]
- Savage R 2005. Cyclo-oxygenase-2 inhibitors: when should they be used in the elderly? Drugs Aging 22(3), 185–200. [DOI] [PubMed] [Google Scholar]
- Scheinert RB, Asokan A, Rani A, Kumar A, Foster TC, Ormerod BK 2015. Some hormone, cytokine and chemokine levels that change across lifespan vary by cognitive status in male Fischer 344 rats. Brain Behav Immun 49, 216–32. doi: 10.1016/j.bbi.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Siddarth P, Silverman DH, Ercoli LM, Miller KJ, Lavretsky H, Bookheimer SY, Huang SC, Barrio JR, Phelps ME 2008. Cognitive and cerebral metabolic effects of celecoxib versus placebo in people with age-related memory loss: randomized controlled study. Am J Geriatr Psychiatry 16(12), 999–1009. doi: 10.1097/JGP.0b013e31818cd3a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan A, Laroche S, Davis S 2003. Learning deficits and dysfunctional synaptic plasticity induced by aggregated amyloid deposits in the dentate gyrus are rescued by chronic treatment with indomethacin. Eur J Neurosci 17(9), 1921–7. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Monin A, Dwir D, O’Donnell P, Cuenod M, Do KQ 2016. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr Res 176(1), 41–51. doi: 10.1016/j.schres.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Miller KR, Lopes KO, Njie E 2008. Microglial degeneration in the aging brain--bad news for neurons? Front Biosci 13, 3423–38. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH 2007. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav Immun 21(6), 836–50. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Vilalta A, Brown GC 2017. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J doi: 10.1111/febs.14323. [DOI] [PubMed] [Google Scholar]
- Widmer R, Engels M, Voss P, Grune T 2007. Postanoxic damage of microglial cells is mediated by xanthine oxidase and cyclooxygenase. Free Radic Res 41(2), 145–52. doi: 10.1080/10715760600978807. [DOI] [PubMed] [Google Scholar]
- Wong WT 2013. Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Front Cell Neurosci 7, 22. doi: 10.3389/fncel.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang M, Kang X, Jiang C, Zhang H, Wang P, Li J 2015. Thrombin-induced microglial activation impairs hippocampal neurogenesis and spatial memory ability in mice. Behav Brain Funct 11(1), 30. doi: 10.1186/s12993-015-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YJ, Wu PF, Long LH, Yu DF, Wu WN, Hu ZL, Fu H, Xie N, Jin Y, Ni L, Wang JZ, Wang F, Chen JG 2010. Reversal of aging-associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and NMDA receptor function. Aging Cell 9(5), 709–21. doi: 10.1111/j.1474-9726.2010.00595.x. [DOI] [PubMed] [Google Scholar]
- Zeier Z, Madorsky I, Xu Y, Ogle WO, Notterpek L, Foster TC 2011. Gene expression in the hippocampus: regionally specific effects of aging and caloric restriction. Mech Ageing Dev 132(1–2), 8–19. doi: 10.1016/j.mad.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Malik A, Choi HB, Ko RW, Dissing-Olesen L, MacVicar BA 2014. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron 82(1), 195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Zinebi F, Xie J, Liu J, Russell RT, Gallagher JP, McKernan MG, Shinnick-Gallagher P 2003. NMDA currents and receptor protein are downregulated in the amygdala during maintenance of fear memory. J Neurosci 23(32), 10283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


