Abstract
Rationale:
Ecological momentary assessment (EMA) of specific events usually focuses more on antecedents and concomitants than on aftermaths.
Objectives:
To examine mental state both before and after discrete episodes of stress and drug use.
Methods:
For up to 16 weeks, outpatients on opioid-agonist treatment carried smartphones on which they initiated entries for stressful events (SEs) or lapses to drug use (DUs), and thrice daily when randomly prompted (RPs). Participants rated their stress, opioid craving, cocaine craving, and moods. RP entries within 5 hours of an event were analyzed and compared to other RPs.
Results:
Stress, negative mood, and craving were generally higher before and after DUs and SEs compared to background levels in participants with at least one DU (n=149) or SE (n=158). Before DUs, there were increases in negative mood, opioid craving, and cocaine craving, but not background stress. Before SEs, there were increases in background stress, opioid craving, and cocaine craving, but not negative mood. These changes were more variable after events than before. Neither DUs nor SEs were significantly related to positive mood.
Conclusions:
Stress increased before stressful-event entries, but was less evident before drug use. Craving increased in the hours before drug use and stressful events—and remained elevated in the hours after either event. These results suggest a stronger link between drug use and craving than between drug use and stress. Lapses to drug use did not improve mood or reduce stress, at least not at our one-hour-bin time resolution, suggesting that if such benefits exist, they are brief.
Keywords: cocaine, opioid, ecological momentary assessment, stress, craving
Introduction
The need for better and expanded access to treatment for opioid use disorders has become increasingly evident as the consequences of the opioid epidemic in the United States have grown (Campbell et al. 2017; Dowell et al. 2017; Rudd et al. 2016; Zibbell et al. 2018). Development of better treatments would be aided by a better understanding of the multiple complex influences on drug use to identify additional therapeutic targets and, perhaps, guide the timing of delivery of existing treatments. A promising approach to investigating an important subset of those influences, the context and precursors of drug use, is ecological momentary assessment (EMA), a research method in which patients use electronic diaries to report on their mood, activities, and surroundings in real time as they go about daily lives (Shiffman et al. 2008). In EMA, participants typically make two types of entries: randomly prompted entries that gather information on the usual, or background levels, of mood and exposure to environmental context; and event-contingent entries that participants initiate at every instance of a target behavior or event, such as drug use or stress.
Some randomly prompted EMA entries will, by chance, occur in close temporal proximity to the target behavior of interest. These can be compared to other randomly prompted entries to study the conditions or contexts that precede that behavior. For example, in smokers attempting to quit, Shiffman and Waters (2004) found that lapses followed rapid rises in negative mood, on the order of hours, but not on the order of whole days. Businelle and colleagues (2016) also found increases in specific risk factors within four hours of patients’ first smoking lapse. Our group has shown that, in cocaine and heroin users on opioid agonist maintenance, exposure to putative risk factors increases in the hours leading up to cocaine use, but less so for heroin use (Epstein et al. 2009).
A limitation of our earlier work lay in a restriction on our randomly prompted entries. In almost all EMA studies, “random” prompts are random only within specified time frames—usually the participant’s self-reported waking hours. In our earlier work, we also programmed the random prompts not to occur within one hour after a drug-use report. We had meant to avoid entries covering overlapping time frames, especially because some of our EMA items in that study asked about “the past hour.” The unintended consequence is that we never collected data on the immediate aftermath of drug use. We have since reversed that decision.
Another new feature of our current EMA studies is that we ask participants to make event-contingent entries for episodes of stress (the instruction we give is “any time you feel stressed, overwhelmed, or anxious, more than usual”) as well as drug use.
These two changes enable us to evaluate temporal trends in background craving and mood (including background stress) in randomly prompted EMA entries occurring shortly before or shortly after drug use or a stressful event. As we note in the discussion section, better understanding of the momentary changes surrounding instances of drug use and stress could inform the optimal timing and content of smartphone-based interventions.
Patients and Methods
Participants:
The participants were in a 46-week natural-history study of stress, geographical location, and drug use at a treatment-research clinic in Baltimore, MD. At enrollment, all were seeking treatment for opioid use disorder. During screening, participants completed the Addiction Severity Index (ASI; McLellan et al. 1985) and the Diagnostic Interview Schedule (DIS IV; Robins et al. 1995) and were given physical examinations and psychological testing. The main inclusion criteria were: age 18 to 75 years, physical dependence on opioids, and (due to the behavioral-geography focus of the parent study) residence in Baltimore City or one of the surrounding counties. The main exclusion criteria were: history of any DSM-IV psychotic disorder, bipolar disorder, or current Major Depressive Disorder; current dependence on alcohol or sedative-hypnotics; cognitive impairment precluding informed consent or valid self-report; conditions that preclude urine collection; or medical illness or medications that would compromise research participation. This study was reviewed and approved by the National Institutes of Health Addictions Institutional Review Board. Participants gave prior written informed consent and were paid for completing the research components of this study.
Procedure:
Upon enrollment in the parent study, participants began treatment with daily methadone or buprenorphine. They attended clinic five to seven days a week; methadone or buprenorphine was administered daily, and individual counseling occurred weekly. Urine was collected under observation and screened for illicit drugs three times weekly. Urine was tested for the presence of heroin and other opioids (through testing for morphine), oxycodone (through a more specific assay), buprenorphine, cocaine metabolite, amphetamines, and cannabinoids.
After two weeks of treatment, each participant was trained to use a smartphone as an electronic diary and then carried it for up to 16 weeks, during weeks 3-18 of the study. The smartphones were programmed with our electronic-diary software (Vahabzadeh et al. 2004; Vahabzadeh et al. 2012) to prompt participants three times per day to answer a series of questions. The timing of the prompts was random, but constrained by the individual participant’s self-reported typical waking hours for each day of the week. In these randomly prompted (RP) entries, participants rated their stress level, their cravings for opioids and cocaine, and, using a list of 28 adjectives, their moods. Ratings were made on a 5-point scale from 1 (not at all) to 5 (extremely). Participants also used dropdown lists to indicate whom they were with, what they were doing, and their location, and were asked to report whether they had recently seen, been offered, or seen others using drugs, alcohol, or tobacco; data from these measures are not reported here.
Participants were asked to initiate an entry for every stress event (SE) and for drug use (DU). SEs were defined as any time the participants felt “more stressed, overwhelmed, or anxious than usual”; the list of questions and additional details are published elsewhere (Preston et al. 2017a). We refer to these stress entries as “events” because each of them represents a discrete occurrence identified by the participant, even when the occurrence was “I just started thinking about stressful things” (one of the choices on our dropdown menu). For drug use, we asked participants to initiate an entry any time they used a drug for nonmedical purposes. Participants were asked to indicate yes or no for use of heroin, cocaine, other opiates (Percocet, oxycodone, etc.), marijuana, methamphetamines, benzodiazepines, “street” methadone or buprenorphine, alcohol, and “other,” followed by questions about the amount, reasons for use, and time since use.
The participants were paid $10 each week for completing at least 82% of their random prompts (23 out of 28), or were given a warning if they did not meet the criterion. Participants who did not meet the 82% completion criterion for 2 weeks in a row were not allowed to continue in the study and assisted with transfer into community-based addiction treatment. To incentivize reporting of drug use, an investigator met with each participant once a week to review the drug-use entries and urine results. Participants received $5 each time they had a urine screen (conducted three times per week) that was negative for both cocaine and heroin/opioids and there was no self-reported use for either drug in their electronic diary. Participants received $3 if their urine drug screen was positive for either cocaine or heroin/opioids and there was self-reported use (on electronic diaries) of the drug for which the participant tested positive (i.e., if the urine tested positive for cocaine, and the participant had self-reported cocaine use in an EMA drug use entry). Payment was made only for corresponding urine screens and drug use self-reports; no payment is made for non-corresponding data.
Data Analysis:
Random-prompt entries provided background levels of stress, craving, and mood, but we separated those entries into those that occurred near drug or stress events and those that did not. To do that, we calculated the amount of time from each random-prompt entry until the next stress-event entry and the next drug-use entry. Random-prompt entries were then classified by the hour, up to 5 hours before a stress or drug entry (1 = 0:00:01 to 1:00:00 before the event, 2 = 1:00:01 to 2:00:00 before the event, and so on.) This process was repeated for the hours since a stress or drug entry. All random prompts that were not within 5 hours of either type of event were used as indices of base levels (i.e., hypothetically unperturbed background levels) of stress, mood, and craving. These prompts include data points that can be days or even weeks away from an event entry.
To reduce the number of analyses, we factor-analyzed the 28 mood adjectives; they loaded onto two factors, one consisting of predominately positive items accounting for 18.5% of variance and the other consisting of predominately negative items accounting for 24.8% of variance. (We had intended to capture both a positive/negative valence factor and a high/low arousal factor, but scree plots offered no support for the latter.) Positive-mood scores were calculated by taking the average score on the following items (loading score): carefree (0.67), happy (0.85), lively (0.76), cheerful (0.87), relaxed (0.8), contented (0.81), pleased (0.87). Negative-mood scores were calculated by taking the average score on the following items: fatigued (0.61), worn out (0.63), afraid (0.73), annoyed (0.66), angry (0.62), hopeless (0.65), on edge (0.73), sad (0.67), discouraged (0.7), resentful (0.63), exhausted (0.66), uneasy (0.72). Not loading at .60 or above on either factor: anxious, good mood/felt like celebrating, afraid, sleepy, vigorous, bored, lonely, desire to be alone, needed a hit, and ill or in pain.
We also reduced the number of analyses (and kept cell sizes large) by combining all drug-use entries, regardless of whether the drug was heroin, another opioid, cocaine, or another drug. Use of drugs other than opioids or cocaine was infrequent in our sample.
Four multilevel models (SAS Proc Mixed, SAS version 9.4, Cary, NC) were constructed for each dependent variable: one assessing the effect of time until a stress event, one assessing the effect of time since a stress event, one assessing the effect of time until a drug event, and one assessing the effect of time since a drug event. We ran exploratory analyses examining cocaine use alone and opioid use alone, to assess the appropriateness of having analyzed them together. (Much of the drug use in our participants was polysubstance use, so events that consisted of both opioid and cocaine use were used in each of the exploratory analyses.) We also ran separate exploratory analyses examining participants taking methadone and those taking buprenorphine, with the underlying limitation that participants were not randomly assigned to either treatment medication. The dependent variables were positive mood, negative mood, stress, opioid craving, and cocaine craving. Each model was limited to participants who reported at least one event of the type being assessed. All models controlled for clock time by including a term classifying each random prompt as being within or outside normal working hours (9 AM to 5 PM). The models used a first-order autoregressive error structure for the modeled effects, and included the subject number for nesting responses. A random intercept was calculated for each subject to control for individual differences in responding. To characterize the most common patterns of change in the entries within 5 hours of an event-contingent entry, we used contrast coding to test for linear effects (i.e., increases or decreases over 5 hours) and quadratic effects (U-shaped or inverted-U-shaped changes over 5 hours). Reports that were made more than 5 hours from an event were not used to examine linear or quadratic effects. Least-squares means were calculated for the effect of time, and pairwise comparisons are presented between the value for each hour bin and the value for base-level reports (reports made more than 5 hours away from any event-contingent entry). The p values for these pairwise comparisons are adjusted using Tukey-Kramer method to control for multiple comparisons; corrections were done separately for each symptom type and for pre- and post-time periods. Alpha for all analyses was set at .05, two-tailed.
A variety of sensitivity analyses were conducted. Because drug use and stressful events often occurred sufficiently close together to be associated with overlapping random-prompt entries, we separately analyzed the subset of drug uses and stressful events that could be isolated with nonoverlapping random prompts. We also assessed whether results would change with time coded continuously by hour of the day rather than with a dichotomous “9:00 to 5:00” variable (results did not change; see online supplement).
Results
Participants and EMA compliance
Data were collected between July 14, 2009 and June 4, 2015. A total of 192 participants made 44,866 random-prompt entries. Of those, 182 provided EMA data for at least two weeks. Among those 182, there were EMA data for a mean (± SD) of 101.0 (± 26.6) days (range 14-156) over 18,379 study days. On average, they completed 2.4 (± 0.4) random-prompt entries per day, for a total of 44,724 entries, with an overall compliance rate of 81%. Six participants were dropped from the study for noncompliance with random prompts. One did not provide adequate data to be considered evaluable (i.e., at least two weeks), and 5 met the criteria for evaluable, providing 3.5 to 14 weeks of EMA data.
There were 149 participants who made at least one drug-use entry; for a total of 2,763 drug-use entries, mean 18.5 (± 17.6) (range 1-95) per participant, mean (± SD) of 0.20 (± .17) per day. These 149 participants provided EMA data for a mean (± SD) of 99.5 (± 28.2) days (range 14-156) over 14,829 study days and completed 2.4 (± 0.4) random-prompt entries per day for total of 35,962 entries, with an overall compliance rate of 79%.
There were 158 participants who made at least one stress-event entry, for a total 1787 stress-event entries. They provided EMA data for a mean (± SD) of 102.2 (± 25.5) days (range 14-156) over 16,152 study days and completed 2.4 (± 0.4) random-prompt entries per day for total of 38,768 entries, with an overall compliance rate of 80%.
The demographic and drug-use histories of all 182 participants who provided EMA data, the 158 who made stress-event entries, and the 149 who made drug use-event entries were similar (Table 1).
Table 1:
Clinical and demographic characteristics
| All Participants | Participants with Stress Events | Participants with Drug Use Events | |
|---|---|---|---|
| N | 182 | 158 | 149 |
| Sex | |||
| males | 135 (74.2%) | 113 (71.5%) | 107 (71.8%) |
| Age mean (SD) years | 41.9 (9.6) | 42.4 (9.7) | 42.0 (9.6) |
| Race | |||
| African American | 118 (64.8%) | 102 (64.6%) | 95 (63.8%) |
| White | 62 (34.0%) | 52 (32.9%) | 52 (34.9%) |
| Education mean (SD) years | 12.1 (1.5) | 12.1 (1.6) | 12.1 (1.5) |
| Marital Status | |||
| Married | 24 (13.3%) | 23 (14.6%) | 20 (13.5%) |
| Never married | 111 (60.8%) | 95 (59.9%) | 94 (62.8%) |
| Separated/divorced/widowed | 47 (26.0%) | 40 (25.5%) | 35 (23.6%) |
| Employment Status | |||
| Full time | 84 (46.4%) | 70 (44.0%) | 69 (46.0%) |
| Part time | 41 (22.6%) | 36 (22.9%) | 31 (21.0%) |
| Unemployed | 46 (25.4%) | 42 (26.8%) | 39 (26.4%) |
| Retired/disability/controlled | 10 (5.5%) | 10 (6.4%) | 10 (6.8%) |
| Drug-Use History | |||
| Days used in last 30 | |||
| Heroin mean (SD) | 19.3 (11.9) | 19.1 (11.9) | 20.8 (11.0) |
| Other opioid mean (SD) | 8.1 (10.3) | 8.0 (10.3) | 7.2 (9.3) |
| Cocaine mean (SD) | 4.5 (8.5) | 4.6 (8.5) | 5.3 (9.1) |
| Years Using | |||
| Heroin mean (SD) | 14.5 (10.2) | 14.5 (10.5) | 14.7 (9.9) |
| Other opioid mean (SD) | 1.4 (2.6) | 1.5 (2.8) | 1.4 (2.6) |
| Cocaine mean (SD) | 6.0 (8.0) | 6.2 (8.1) | 6.5 (8.2) |
| Opioid agonist treatment* | |||
| Methadone | 107 (58.8%) | 91 (57.6%) | 88 (59.1%) |
| Buprenorphine/naloxone | 75 (41.2%) | 67 (42.4%) | 61 (40.9%) |
| Urine drug screen results* | |||
| Opioids mean % negative (SD) | 61.8% (38.2) | 61.0% (36.7) | 53.6% (34.5) |
| Cocaine mean % negative (SD) | 66.2% (39.6) | 65.2% (39.8) | 58.7% (34.5) |
| Cannabis mean % negative (SD) | 85.8% (29.3) | 85.9% (29.0) | 83.4% (29.9) |
Data collected during the study; all other measures were collected during screening on the Addiction Severity Index (McLellan et al., 1985).
Random-prompt responses before and after drug use
The numbers of random-prompt entries in each hour bin used in the analyses were 353, 370, 362, 351, and 363 for the 5, 4, 3, 2, and 1 hr bins before drug-use events, and 331, 432, 359, 271, and 233 in the 1, 2, 3, 4, and 5 hour bins after drug-use events. Of the 1,799 random prompts in the 5 hours before a drug-use entry, 130 (7.2%) were within 5 hours, before or after, a stress entry. Of the 1,626 random prompts 5 hours after a drug-use entry, 138 (8.5%) were within 5 hours, before or after, a stress entry. The analyses also included 31,386 base-level random-prompt entries that occurred more than 5 hours away from any drug-use entry or stress entry.
Patterns of stress, negative mood, opioid craving, and cocaine craving relative to drug use are shown in Figure 1. The associated statistical results are in Table 2.
Fig. 1.
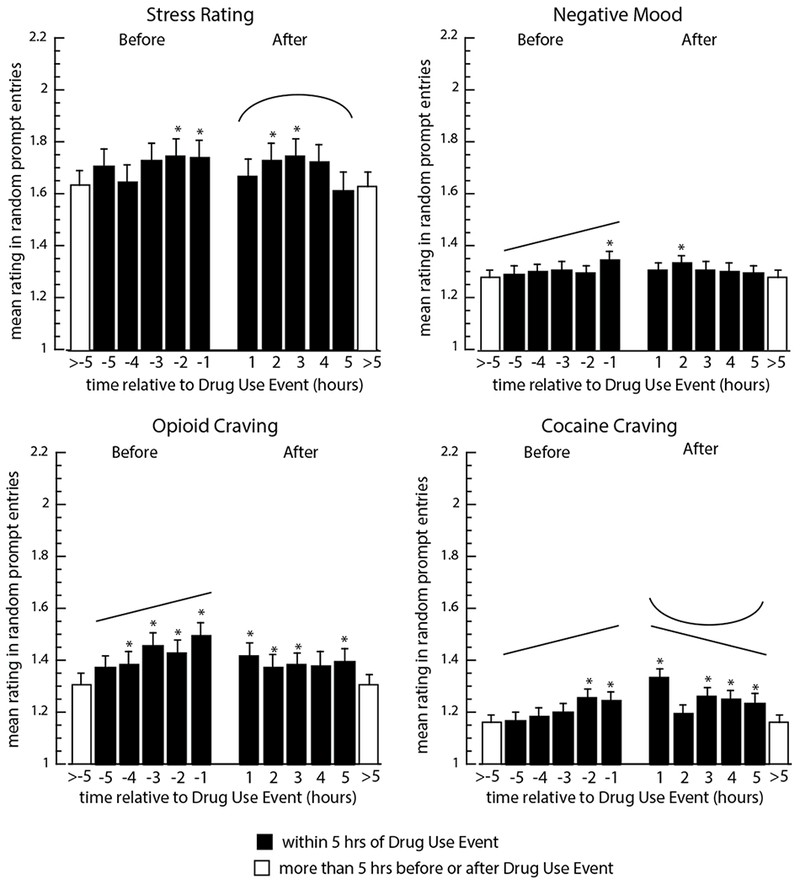
Model-predicted mean random-prompt ratings of stress, negative mood, opioid craving, and cocaine craving in the 5 hours before and after drug-use entries. Filled bars show data for one-hour intervals before (−5 to −1) and after (1 to 5) an instance of drug use; open bars show “base levels” (means for all random prompts not occurring within 5 hours of a drug entry or a stress-event entry). Asterisks indicate significant differences (p<.05) between the marked bar and the base level (open bar), based on Tukey-adjusted pairwise comparisons. Significant linear increases or decreases are indicated by straight angled lines, and significant quadratic effects are indicated by u-shaped lines; F-values and p values are shown in Table 2.
Table 2:
Linear and quadratic trends in ratings of stress, mood, and craving 5 hours before and after drug use and stress events.
| 5 hours before event | 5 hours after event | ||||||
|---|---|---|---|---|---|---|---|
| Trend type | df | F value | p-value | df | F value | p-value | |
|
|
|||||||
| Drug-Use Events | |||||||
| Stress | linear | 1,525 | 2.51 | 0.11 | 1,500 | 0.88 | 0.34 |
| quadratic | 1,525 | 0.13 | 0.73 | 1,500 | 7.61 | 0.006 | |
| Positive mood | linear | 1,525 | 0.56 | 0.46 | 1,500 | 0.31 | 0.58 |
| quadratic | 1,525 | 1.93 | 0.17 | 1,500 | 0.54 | 0.46 | |
| Negative mood | linear | 1,525 | 5.68 | 0.018 | 1,500 | 1.38 | 0.24 |
| quadratic | 1,525 | 1.51 | 0.22 | 1,500 | 0.84 | 0.36 | |
| Opioid Craving | linear | 1,518 | 15.47 | <.0001 | 1,495 | 0.33 | 0.56 |
| quadratic | 1,518 | 0.01 | 0.92 | 1,495 | 1.27 | 0.26 | |
| Cocaine Craving | linear | 1,516 | 12.69 | 0.0004 | 1,495 | 3.94 | 0.048 |
| quadratic | 1,516 | 0.06 | 0.81 | 1,495 | 4.31 | 0.038 | |
| 5 hours before event | 5 hours after event | ||||||
|---|---|---|---|---|---|---|---|
| Trend type | df | F value | p-value | df | F value | p-value | |
|
|
|||||||
| Stress Events | |||||||
| Stress | linear | 1,385 | 0.26 | 0.61 | 1,387 | 6.35 | 0.012 |
| quadratic | 1,385 | 12.38 | 0.0005 | 1,387 | 1.62 | 0.20 | |
| Positive mood | linear | 1,385 | 0.55 | 0.46 | 1,387 | 1.72 | 0.19 |
| quadratic | 1,385 | 0.89 | 0.35 | 1,387 | 0.52 | 0.47 | |
| Negative mood | linear | 1,385 | 2.17 | 0.14 | 1,387 | 0.48 | 0.49 |
| quadratic | 1,385 | 2.32 | 0.13 | 1,387 | 1.24 | 0.27 | |
| Opioid Craving | linear | 1,374 | 6.56 | 0.011 | 1,384 | 0.01 | 0.94 |
| quadratic | 1,374 | 1.96 | 0.16 | 1,384 | 1.90 | 0.17 | |
| Cocaine Craving | linear | 1,373 | 4.71 | 0.031 | 1,381 | 0.03 | 0.86 |
| quadratic | 1,373 | 0.07 | 0.79 | 1,381 | 0.33 | 0.57 | |
df - degrees of freedom; bold text indicates p < .05.
Background stress (Figure 1, upper left) did not linearly increase or quadratically vary in the 5 hours leading up to drug use, though stress in the two hours prior to drug use was significantly higher than the base level (in random prompts more than 5 hours away from a drug-use or stress entry). In sensitivity analyses, this significant increase in stress immediately before drug use was not found when random prompts within 5 hours of a stress report were not included. After drug use, stress varied quadratically (reffect (95% CI) = 0.11 (0.01, 0.21), with an initial increase and then a decrease. Stress was significantly higher than the base level in hours 2 and 3 after drug use. Negative mood (Figure 1, upper right) increased linearly in the 5 hours leading up to drug use (reffect (95% CI) = 0.10 (0.01, 0.18); negative mood in the hour preceding drug use was significantly higher than the base level. After drug use, there were no significant linear or quadratic trends in negative mood, though it was significantly higher than the base level in hour 2 after drug use.
Opioid craving (Figure 1, lower left) increased linearly in the 5 hours leading up to drug use (reffect (95% CI) = 0.17 (0.09, 0.25); it was significantly higher than the base level in each of the four hours prior to drug use. After drug use, there were no significant linear or quadratic trends in opioid craving, but it was significantly higher than the base level in hours 1, 2, 3, and 5 after drug use.
Cocaine craving (Figure 1, lower right) also increased linearly increase in the 5 hours leading up to drug use (reffect (95% CI) = 0.15 (0.06, 0.23); it was significantly higher than the base level in the 2 hours prior to drug use. After drug use, it decreased linearly (reffect (95% CI) = 0.09 (0.00, 0.18), though there was also a significant quadratic effect reflecting especially low levels at hour 2 post-use (reffect (95% CI) = 0.09 (0.00, 0.18). Cocaine craving was significantly higher than the base level in all but hour 2 after drug use.
Positive mood did not systematically change before or after drug use (Table 2; graph not shown). Exploratory analyses are presented in Figure S1 (opioids) and Figure S2 (cocaine). The associated statistical results are presented in Table S2.
Responses appeared largely similar between opioid-use entries and cocaine-use entries. Before opioid use, stress increased over base level (5, 3, 2, and 1 hour before opioid use); before cocaine use, stress increased linearly (F (1,322) = 4.00, p ≤ .05, (reffect (95% CI) = 0.11 (0.00, 0.22). As with the combined drug analyses, these effects were not seen when we removed prompts that were also associated with a stress entry. The quadratic pattern of change in stress after drug use appeared to be driven by the opioid-use entries, as it was present for opioids (F (1,341) = 7.41, p ≤ .01, (reffect (95% CI) = 0.15 (0.04, 0.25), but not for cocaine (F (1,277) = 0.74, p = 0.39.
Cocaine craving diverged from the general pattern in combined drug-use entries. Opioid entries were not associated with increases in cocaine craving, but cocaine entries were preceded by linear increases in cocaine craving (F (1,316) = 17.46, p ≤ .0001, (reffect (95% CI) = 0.23 (0.12, 0.33) with values in the 2 hours immediately preceding use being elevated over base levels. After cocaine use, craving remained elevated over base levels for all 5 hours.
Exploratory analyses, separating buprenorphine-treated participants from methadone-treated participants, are in Figure S3A (buprenorphine) and Figure S4A (methadone). The associated statistical results are in Tables S3 and S4, respectively. Stress ratings did not change systematically in the hours before drug-use events in either medication group, but did change afterwards (a quadratic inverted U for buprenorphine participants; a linear decrease for methadone participants). In buprenorphine participants only, positive mood was increased in the first hour after drug-use events, then decreased to background levels. Opioid craving increased linearly in the hours before (but not after) drug use in both medication groups, as in the total sample. Cocaine craving did not change systematically before drug-use events in the buprenorphine group, but linearly increased before drug-use events in the methadone group.
Random-prompt responses before and after stress events
The numbers of random-prompt entries in each hour bin used in the analyses were 140, 178, 151, 142, and 209 for the 5, 4, 3, 2, and 1 hr bins before stress entries, and 198, 308, 223, 181, and 144 in the 1, 2, 3, 4, and 5 hour bins after stress entries. Of the 820 random prompts in the 5 hours before a stress entry, 146 (17.8 %) were within 5 hours, before or after, a drug-use entry. Of the 1054 random prompts that were in the 5 hours following a stress entry, 124 (11.8 %) were within 5 hours, before or after, a drug-use entry. The analyses also included 34,539 base-level random-prompt entries that occurred more than 5 hours away from any drug-use entry or stress-event entry.
Patterns of stress, negative mood, opioid craving, and cocaine craving relative to stress events are shown in Figure 2. The associated statistical results are in Table 2.
Fig. 2.
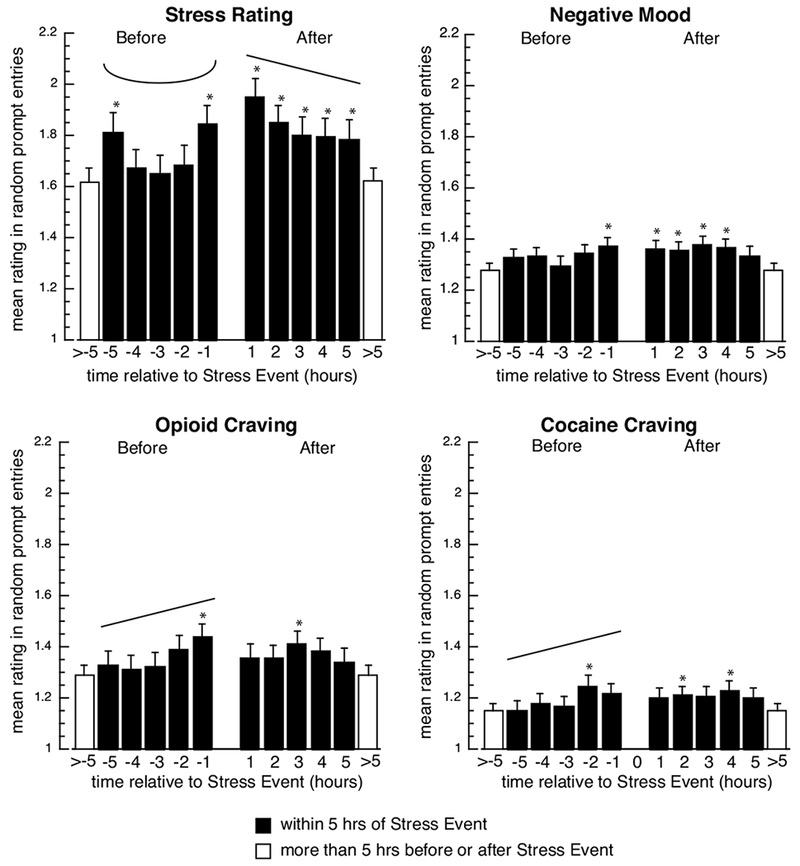
Model-predicted mean random-prompt ratings of stress, negative mood, opioid craving, and cocaine craving in the 5 hours before and after stress events. Filled bars show data for one-hour intervals before (−5 to −1) and after (1 to 5) a stress event; open bars show “base levels” (means for all random prompts not occurring with 5 hours of a drug entry or a stress-event entry). Asterisks indicate significant differences (p<.05) between the marked bar and the base level (open bars), based on Tukey-adjusted pairwise comparisons. Significant linear increases or decreases are indicated by straight angled lines, and significant quadratic effects are indicated by u-shaped lines; degrees of freedom, F-values, and p values are shown in Table 2.
Background stress (Figure 2, upper left) assumed an inverted U-shape in the 5 hours leading up to stress events (reffect (95% CI) = 0.18 (0.08, 0.28); background-stress ratings were significantly higher than the base level in hours 1 and 5 before stress events. In contrast, after stress events, there was a significant linear decrease in background stress (reffect (95% CI) = 0.13 (0.03, 0.23), though it remained significantly higher than base level in all 5 hours.
Negative mood (Figure 2, upper right) did not change linearly or quadratically before or after stress events. However, it was significantly higher than base levels in the first hour before stress events and for the first four hours after stress events.
Opioid craving (Figure 2, lower left) increased linearly in the 5 hours leading up to stress events (reffect (95% CI) = 0.13 (0.03, 0.23); it was significantly higher than the base level in the hour prior to stress events. After stress events, it did not change linearly or quadratically, but was significantly higher than the base level in hour 3.
Cocaine craving (Figure 2, lower right) also increased linearly in the 5 hours leading up to stress entries; it was significantly higher than the base level in hour 2 prior to stress entries. After stress entries, it did not change linearly or quadratically, but was significantly higher than the base level in hours 2 and 4. In sensitivity analyses, removing random prompts that were associated with drug use, these increases in cocaine craving over base level following a stress entry were no longer statistically significant.
Positive mood did not systematically change before or after stress entries (Table 2, graph not shown).
Exploratory analyses of stress events by treatment medication showed no systematic changes in background stress, mood, opioid craving, or cocaine craving in the buprenorphine group (Figure S3B and Table S3). In the methadone group, background stress showed the same temporal patterns as the total sample, i.e., a U-shape before and a decrease after (Figure S4B and Table S4). Negative mood showed the only other systematic change before stress events (an increase) in methadone-treated participants, with no systematic change after stress events.
Discussion
In general, the results showed changes in craving, stress, and negative mood, but not positive mood, in the hours before and after drug use and stressful events, with clearer patterns of change before events than after events. The patterns of change surrounding stress events were different from those surrounding drug use. This was not surprising, because, for one thing, drug uses tend to be discrete events, with pharmacologically determined time courses of effects, whereas stressful events are often less discrete, and the source of the stress may not end at any specific time. Some stressors could be brief, like misplacing and then finding car keys, while others might be quite prolonged, like the illness of a parent or ongoing financial distress. As it turned out, more of the stressful events fell under the latter category (see Preston et al., 2017, for a more complete description).
The hours before events
Opioid craving and cocaine craving each increased linearly in the 5 hours prior to drug use, even though we analyzed the data for uses of all drugs together. The finding for cocaine craving is consistent with our previous work (Preston et al. 2009). Opioid craving and cocaine craving also increased linearly in the 5 hours prior to stress events. This finding seemed to be driven by higher craving in the hour or two prior to the stress event.
Background stress was elevated in the hour immediately prior to stressful events, but during the four hours preceding that, stress actually followed a U shape, with an increase at hours −5 and −1 with a trough in hours −4 through −2. We do not have a firm explanation for this; we tested for quadratic effects only because the graphs suggested their presence, not because there were theoretical reasons to expect them. Future work may need to address whether waxing and waning levels of background stress in a several-hour period set up a greater likelihood of deciding that one has reached the threshold for a stress event.
Background stress did not increase systematically across the full 5 hours prior to drug use, though it was slightly higher than base levels in hours −2 and −1. The comparison of initial analysis to sensitivity analysis may reflect heterogeneity across people and measurement occasions as there was an increase in stress immediately before drug use, but only when we included random prompts that were within 5 hours of a stress report. This mirrors our previous finding that there were increases in the severity of stress reports within 3 days of a drug use, but there was not an increase in the overall likelihood of a drug use report in the days immediately following a stress report (Furnari et al. 2015). In both the present and earlier analyses, the association between background stress and episodes of drug use may also have been limited by the instructions we gave our participants. We told participants to initiate an entry whenever they felt more stressed than usual (the reasons for which, on our dropdown menu, could include “I just started thinking about stressful things”—i.e., no external precipitant was required). Followed faithfully, this instruction would impose a ceiling on the levels of stress that would be reported in randomly prompted entries. Nonetheless, the association between stress and drug use was also small in one of our prior EMA studies with no instruction to report discrete stress events (Preston and Epstein 2011). Together, our findings on stress and drug use are a reminder that aggregate associations (e.g., Sinha et al. 2006) can mask many individual disconfirming instances (Furnari et al. 2015; Preston et al. 2018).
Mood, as measured by ratings of a series of adjectives, was not a particularly sensitive measure—especially not positive mood, which underwent no systematic changes in the hours before stress events or drug use. In a previous EMA study, we found that ratings of mood “right now” (as used in the current analyses) were less sensitive predictors of subsequent cocaine use than reports of mood “in the past hour.” Negative mood “right now” did linearly increase prior to drug use in the current analyses, though the increase was small. For stress events, the buildup in negative mood was even smaller. Different patterns for background stress versus background negative mood in our data are probably explained in part by our inclusion of negative-mood adjectives that reflect low-arousal states (fatigued, worn out, sad, discouraged) that are not prototypical of stress.
The hours after events
In the exploratory analyses separately analyzing data from participants treated with methadone and buprenorphine, we found the only systematic change in positive mood: participants in the buprenorphine group showed a transient increase in positive mood during the first hour after drug use, followed by a decrease to base levels. We do not know why this was seen only in the buprenorphine group, but the brevity of the effect underscores the transience of any mood elevation that accompanies drug use in these participants. Opioid and cocaine craving were higher than base levels in the 5 hours after drug use. Opioid craving remained modestly but steadily elevated during that time; cocaine craving seemed to decrease, but not to base levels. This is consistent with results from one of our prior EMA studies in which cocaine use seemed to drive craving for itself (Epstein et al. 2010) and, more generally, with the idea of priming (Mahoney et al. 2007). In the current study, we did not systematically assess continued drug use over the 5 “after” hours; this presumably adds noise to the data.
Opioid and cocaine craving were also slightly higher than base levels during portions of the 5 hours after stress events. Like the (brief, subtle) increase in background stress prior to actual drug use, this small stress/drug relationship probably reflects considerable heterogeneity across both people and measurement occasions.
Background stress and negative mood after drug use, were never lower than their base levels and were occasionally higher; there were no increases in positive mood. Thus, in our population—people in treatment for opioid- and cocaine-use disorders—we saw no evidence that lapses to cocaine or opioid use provided even short-term reward, though we probably missed effects of intoxication within the first hour. In contrast, background stress clearly decreased from its peak in the hours after stress events, showing that our “after” measurements were sensitive to change.
Implications
EMA usually does not include systematic assessment of the consequences of momentary behaviors. Our own first EMA study, the largest ever done with opioid and cocaine users, was especially limited in that regard because we decided not to issue random prompts within the hour after any event-contingent entry. With our current EMA data, we can now examine this “post-event hour” and the hours that follow it.
An even more powerful approach would be to prompt participants for follow-up entries at specific intervals after each event-contingent entry. To our knowledge, only one group of investigators has done that, with the target behavior being alcohol drinking (Treloar et al. 2015). Emulating their approach with drugs such as opioids and cocaine may not be straightforward, because dose units are less standardized—there may be no interpretable equivalent to the hourly “How many drinks have you had?” follow-ups that enabled them to correlate responses with estimated blood alcohol concentrations. Nonetheless, systematic follow-up of EMA entries would provide more complete within-person time-course data, which is needed to parcel heterogeneous drug effects into person-level differences versus situation-level associations.
Our analyses of random-prompt responses in the hours preceding events are a continuation of work we have done before (Epstein et al. 2009), but this work is taking on more practical relevance as treatment begins to be delivered via text messaging (Abroms et al. 2017; Lin et al. 2015; Scott-Sheldon et al. 2016) and smartphone apps (Gustafson et al. 2014; Scott et al. 2017). Interventions tailored to individual patients’ needs or delivered at just the right moment might have enhanced benefits. Researchers have been investigating the use of EMA data to predict future drug use. For example, Businelle and colleagues (2016) found that risk factors were higher within four hours of patients’ first smoking lapse and that EMA was promising as a tool to estimate the likelihood of lapse and automate delivery of tailored smoking cessation treatment. Mobile treatment might provide a path to improved treatment efficacy as well as making treatment available to areas where treatment programs are geographically remote and extend treatment beyond the usual duration of clinician contact. Knowing the time course of changes in mood, stress and craving in the lives of people with substance-use disorders could help inform mobile “just in time” treatment, and, perhaps, predict when patients are at risk for drug use (Businelle et al. 2016).
One limitation of this study is that the findings might not generalize to people who are not in maintenance treatment with an opioid agonist. Another limitation is the likelihood that participants did not report all stress events or drug uses. Although participants received incentives for reporting drug use, compliance was not 100%. We have no way of confirming stress events; however, there was substantial internal validity within the stress-event reports, as we have discussed elsewhere (Preston et al. 2017a).
Another limitation is that we had to infer within-person changes across time from a combination of within-person and across-person data. The only way to avoid the problem entirely would be to impose a very intense regimen of random prompts (at least one per hour, all day), so time courses of random-prompt reports would be directly assessed within each participant. Our analyses rely on the assumption that the random-prompt entries were not systematically biased such that, for example, the 1-hr bin was populated by people more prone to craving than the 5-hr, or greater than 5-hr bin. If that did happen by chance, then our multilevel models, by accounting for person-level effects, should have partitioned the covariance appropriately. The separate exploratory analyses of patients treated with methadone and buprenorphine are limited by their post-hoc nature and by the fact that participants were not randomized to the treatment groups
In the analyses reported here, we did not attempt to control for effects of drug-cue exposure or other situational variables. Because we were already dividing the data into one-hour bins, we did not want to slice them further, because this would both reduce statistical power and increase the likelihood of overfitted, nongeneralizable estimates. Related to this issue of model fitting, we note that the present analyses primarily address a descriptive question: “Given that an event occurs, what mental states typically surround that event, before and after, relative to mental states at other times?” This is different from the question: “Given a set of changes in ongoing mental state, how accurately can we predict the arrival of an event?” The latter question is better approached through machine learning than through descriptive or inferential statistics, and the answer is likely to be a model so complex that it is of little or no heuristic value (Yarkoni & Westfall, 2017). Our goal in these analyses was to highlight what is generally true about certain events (e.g., that lapses to drug use do not improve mood in the ensuing hours) rather than to maximize certainty about when events will or will not occur.
As we noted above, the nature of potential stressors could be quite variable, either something brief or something quite prolonged. As it turned out, more of the stressful events fell under the latter category (see Preston et al., 2017, for a more complete description). In asking our participants to make a stress event report whenever they felt stressed, overwhelmed, or anxious more than usual, we left it up to them to make the determination of what they found to be stressful, which likely led to variability in the results. Having gotten this experience and dataset of the stressors our participants experience, we may be able hone our EMA questions in future studies to more precisely define and separate types of stressors.
The results of this study highlight the complexity of relationships among stress, mood, craving, and drug use, which are clearly not independent of one another. We have shown in previous analyses, for example, that craving increases in the presence of both stress and drug cues (Preston et al., 2018). We used several sensitivity analyses in the present study to interrogate our data, but more work is needed to understand the time scales of changes in stress and craving and how they interact to influence (and are affected by) drug use. Further studies should collect data to adequately examine fluctuations in time scales of minutes, as well as days and weeks.
In summary, stress ratings systematically increased before stressful events, but were less evident before drug use. Craving was a sensitive predictor of both impending drug use and impending reports of stressful events. The results suggest a more direct link between drug use and craving than between drug use and stress.
Supplementary Material
Acknowledgments
Funding and Disclosure
This study was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. The authors do not have any competing financial interests related to the research presented.
References
- Abroms LC, Johnson PR, Leavitt LE, Cleary SD, Bushar J, Brandon TH, Chiang SC (2017) A randomized trial of text messaging for smoking cessation in pregnant women. Am J Prev Med 53: 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle MS, Ma P, Kendzor DE, Frank SG, Wetter DW, Vidrine DJ (2016) Using intensive longitudinal data collected via mobile phone to detect imminent lapse in smokers undergoing a scheduled quit attempt. J Med Internet Res 18: e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Jia H, Shankar A, Hanson D, Luo W, Masciotra S, Owen SM, Oster AM, Galang RR, Spiller MW, Blosser SJ, Chapman E, Roseberry JC, Gentry J, Pontones P, Duwve J, Peyrani P, Kagan RM, Whitcomb JM, Peters PJ, Heneine W, Brooks JT, Switzer WM (2017) Detailed transmission network analysis of a large opiate-driven outbreak of HIV infection in the United States. J Infect Dis 216: 1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Arias E, Kochanek K, Anderson R, Guy GP Jr., Losby JL, Baldwin G (2017) Contribution of opioid-involved poisoning to the change in life expectancy in the United States, 2000-2015. JAMA 318: 1065–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL (2010) Tobacco, cocaine, and heroin: Craving and use during daily life. Addict Behav 35: 318–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL (2009) Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry 66: 88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari M, Epstein DH, Phillips KA, Jobes ML, Kowalczyk WJ, Vahabzadeh M, Lin JL, Preston KL (2015) Some of the people, some of the time: Field evidence for associations and dissociations between stress and drug use. Psychopharmacology 232: 3529–37 [DOI] [PubMed] [Google Scholar]
- Gustafson DH, McTavish FM, Chih MY, Atwood AK, Johnson RA, Boyle MG, Levy MS, Driscoll H, Chisholm SM, Dillenburg L, Isham A, Shah D (2014) A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA Psychiatry 71: 566–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Mahmooth Z, Dedhia N, Frutchey R, Mercado CE, Epstein DH, Preston KL, Gibbons MC, Bowie JV, Labrique AB, Cheskin LJ (2015) Tailored, interactive text messages for enhancing weight loss among African American adults: The TRIMM randomized controlled trial. Am J Med 128: 896–904 [DOI] [PubMed] [Google Scholar]
- Mahoney JJ 3rd, Kalechstein AD, De La Garza R 2nd, Newton TF (2007) A qualitative and quantitative review of cocaine-induced craving: The phenomenon of priming. Prog Neuropsychopharmacol Biol Psychiatry 31: 593–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP (1985) New data from the Addiction Severity Index: Reliability and validity in three centers. J Nerv Ment Dis 173: 412–23 [DOI] [PubMed] [Google Scholar]
- Preston KL, Epstein DH (2011) Stress in the daily lives of cocaine and heroin users: Relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology 218: 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH (2017a) Context and craving during stressful events in the daily lives of drug-dependent patients. Psychopharmacology 234: 2631–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH (2018) Exacerbated craving in the presence of stress and drug cues in drug-dependent patients. Neuropsychopharmacology 43: 859–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH (2009) Cocaine craving and use during daily life. Psychopharmacology 207: 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM III (1995) The Diagnostic Interview Schedule, Version IV. St. Louis, MO, Washington University [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L (2016) Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep 65: 1445–1452 [DOI] [PubMed] [Google Scholar]
- Scott CK, Dennis ML, Gustafson DH (2017) Using smartphones to decrease substance use via self-monitoring and recovery support: study protocol for a randomized control trial. Trials 18: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LA, Lantini R, Jennings EG, Thind H, Rosen RK, Salmoirago-Blotcher E, Bock BC (2016) Text messaging-based interventions for smoking cessation: A systematic review and meta-analysis. JMIR Mhealth Uhealth 4: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR (2008) Ecological momentary assessment. Annu Rev Clin Psychol 4: 1–32 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ (2004) Negative affect and smoking lapses: A prospective analysis. J Consult Clin Psychol 72: 192–201 [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006) Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63: 324–31 [DOI] [PubMed] [Google Scholar]
- Treloar H, Piasecki TM, McCarthy DM, Sher KJ, Heath AC (2015) Ecological evidence that affect and perceptions of drink effects depend on alcohol expectancies. Addiction 110: 1432–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahabzadeh M, Epstein DH, Mezghanni M, Lin J-L, Preston KL (2004) An electronic diary software for ecological momentary assessment (EMA) in clinical trials. Proceedings of the 17th IEEE Symposium on Computer-Based Medical Systems (CBMS): 167–172 [Google Scholar]
- Vahabzadeh M, Mezghanni M, Lin J-L (2012) Context aware mobile device software for substance abuse interventions and behavioral modification. Federal Register 77: 48997–48998 [Google Scholar]
- Yarkoni T, Westfall J (2017) Choosing prediction over explanation in psychology: lessons from machine learning. Perspectives on Psychological Science 12: 1100–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, Holtzman D (2018) increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 108: 175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


