Abstract
Ixodes pacificus Cooley & Kohls (Acari: Ixodidae), the primary vector of Lyme disease spirochetes to humans in the far-western United States, is broadly distributed across Pacific Coast states, but its distribution is not uniform within this large, ecologically diverse region. To identify areas of suitable habitat, we assembled records of locations throughout California where two or more I. pacificus were collected from vegetation from 1980 to 2014. We then employed ensemble species distribution modeling to identify suitable climatic conditions for the tick and restricted the results to land cover classes where these ticks are typically encountered (i.e., forest, grass, scrub-shrub, riparian). Cold-season temperature and rainfall are particularly important abiotic drivers of suitability, explaining between 50 and 99% of the spatial variability across California among models. The likelihood of an area being classified as suitable increases steadily with increasing temperatures >0°C during the coldest quarter of the year, and further increases when precipitation amounts range from 400 to 800 mm during the coldest quarter, indicating that areas in California with relatively warm and wet winters typically are most suitable for I. pacificus. Other consistent predictors of suitability include increasing autumn humidity, temperatures in the warmest month between 23 and 33°C, and low-temperature variability throughout the year. The resultant climatic suitability maps indicate that coastal California, especially the northern coast, and the western Sierra Nevada foothills have the highest probability of I. pacificus presence.
Keywords: western blacklegged tick, Ixodes pacificus, Lyme disease, climate suitability
The western blacklegged tick, Ixodes pacificus Cooley & Kohls (Acari: Ixodidae), is a primary vector of Borrelia burgdorferi and Anaplasma phagocytophilum, the etiological agents of Lyme disease and human granulocytic anaplasmosis, respectively (Lane et al. 1994, Teglas and Foley 2006). I. pacificus also is suspected to be a vector of Borrelia miyamotoi based on demonstrated vector competence of the closely related blacklegged tick Ixodes scapularis Say (Acari: Ixodidae) (Scoles et al. 2001) and consistent detection of the spirochete in field-collected I. pacificus in California (Mun et al. 2006, Padgett et al. 2014).
Previous surveys demonstrated that I. pacificus occurs in all but two Californian counties (Dennis et al. 1998, Eisen et al. 2016a), and habitat suitability based on county-scale records indicate suitability for the tick broadly across most of the state (Hahn et al. 2016). However, most I. pacificus-borne diseases are reported from counties in northwestern or north-central California (Lane et al. 1992, Eisen et al. 2006b, Padgett et al. 2014, Yoshimizu et al. 2016). Nevertheless, county-scale distribution maps have limited value in targeting vector surveillance and prevention resources at the sub-county level, particularly in large and ecologically diverse counties in the western United States, generally, and California in particular (Eisen and Eisen 2007, 2008). In Mendocino County, for example, host-seeking nymphs, the life stage associated with most B. burgdorferi infections in humans (Clover and Lane 1995), are most commonly encountered in dense woodlands having leaf or fir-needle litter, and nymphal densities are elevated in woodlands having annual growing degree-days between 2,600 and 3,000 (10°C base) (Eisen et al. 2006b). These vegetation and climatic characteristics, which support elevated densities of host-seeking nymphs in a single north-coastal Californian county (Mendocino), were extrapolated across the state to identify potential areas of risk for exposure to I. pacificus nymphs. Overall, the sub-county distribution of acarological risk mirrored the distribution of reported human Lyme disease cases (Eisen et al. 2006b).
Since adult ticks also have the potential to transmit each of the three pathogens described above and larval ticks potentially could serve as vectors of B. miyamotoi (Rollend et al. 2013, Molloy et al. 2015), we endeavored to develop species distribution models for California that encompass all parasitic life stages. Species distribution models estimate the existence of a process (a detailed probability map describing the likely niche of a species) from a sample of the process (known locations of a species) through the development of statistical relationships between species presence and absence and key climatic variables (Graham et al. 2004, Guo et al. 2005, Keenan et al. 2011, Easterday et al. 2016). To capture the broad range in climatic conditions associated with I. pacificus presence, we developed species distribution models using more than 600 geocoded tick records from 51 of 58 Californian counties. To minimize extrapolation of our models to land-cover classes that were not sampled or included in model development, we applied a spatial land-cover mask to the resulting models that displays areas classified as forest, grass, scrub-shrub or riparian with suitable climatic conditions for I. pacificus. Our models reveal substantial variability in climate suitability for I. pacificus both within and among counties.
Methods
I. pacificus Records
We used published and unpublished data on locations where host-seeking I. pacificus larvae, nymphs, and/or adults were collected in California. These data were compiled from Eisen et al. (2006a) and the Vector-Borne Disease Section, California Department of Public Health (CDPH) tick database. The CDPH tick database maintains the following historic and contemporary information on California tick collection sites: number of ticks collected by species and life stage, location, date, and pathogen test-results, when available. While the majority of tick database records reflect environmental surveillance, primarily as part of human risk assessment, some records are from ticks recovered from people and animals and submitted to CDPH for identification. In this analysis, we only included I. pacificus records from those locations from which two or more I. pacificus of any life stage were collected from vegetation between 1980 and 2014. Although the CDPH tick database dates back to 1900, a contemporary time period, 1980–2014, was chosen and matched temporally with the climate data employed in our models. Tick locations that were within 1.415 km of each other (i.e., the length of the diagonal of a 1-km grid cell, which is the resolution of the environmental data discussed below) were excluded to remove potential oversampling bias and pseudo-replication. In total, 621 unique presence locations from 51 counties were included (Table 1). We used the random points tool in ESRI ArcMap (Environmental Systems Research Institute; Redlands, CA) to generate a set of background (pseudo-absence) data, at a rate of ~four background points per presence point; points were confined within the state borders, and no background points were located within 1.415 km of each other or of any location where I. pacificus was present.
Table 1.
Percentage of Californian counties classified as forest, grass, scrub-shrub or riparian and estimated to be climatically suitable for I. pacificus by three or more models having sensitivities of 90% or 99%
| County | No. tick locations | % area classified as suitable | |
|---|---|---|---|
|
| |||
| 90% sensitivity | 99% sensitivity | ||
| Alameda | 21 | 46.28 | 62.56 |
| Alpine | 0 | 0.00 | 2.00 |
| Amador | 8 | 63.62 | 76.65 |
| Butte | 6 | 51.29 | 63.42 |
| Calaveras | 14 | 73.06 | 89.55 |
| Colusa | 6 | 26.20 | 49.05 |
| Contra Costa | 17 | 39.40 | 53.77 |
| Del Norte | 4 | 11.30 | 75.10 |
| El Dorado | 25 | 46.60 | 68.07 |
| Fresno | 0 | 8.61 | 30.10 |
| Glenn | 2 | 11.32 | 57.86 |
| Humboldt | 18 | 33.32 | 85.31 |
| Imperial | 0 | 0.00 | 0.00 |
| Inyo | 1 | 0.00 | 0.00 |
| Kern | 2 | 0.00 | 18.57 |
| Kings | 1 | 0.00 | 9.51 |
| Lake | 28 | 66.37 | 83.89 |
| Lassen | 1 | 0.00 | 0.01 |
| Los Angeles | 30 | 23.18 | 47.31 |
| Madera | 8 | 19.02 | 47.21 |
| Marin | 14 | 71.03 | 71.04 |
| Mariposa | 14 | 59.32 | 79.93 |
| Mendocino | 62 | 75.34 | 90.74 |
| Merced | 0 | 0.00 | 33.16 |
| Modoc | 0 | 0.00 | 0.04 |
| Mono | 0 | 0.00 | 0.00 |
| Monterey | 12 | 18.45 | 83.71 |
| Napa | 11 | 74.94 | 74.94 |
| Nevada | 19 | 48.60 | 57.36 |
| Orange | 7 | 25.80 | 40.31 |
| Placer | 20 | 31.74 | 50.86 |
| Plumas | 3 | 3.19 | 33.21 |
| Riverside | 10 | 7.18 | 20.71 |
| Sacramento | 5 | 9.44 | 31.26 |
| San Benito | 4 | 4.12 | 82.97 |
| San Bernardino | 19 | 1.31 | 4.21 |
| San Diego | 10 | 16.66 | 59.40 |
| San Francisco | 0 | 5.26 | 5.26 |
| San Joaquin | 5 | 1.17 | 20.98 |
| San Luis Obispo | 6 | 18.21 | 81.90 |
| San Mateo | 15 | 66.89 | 66.89 |
| Santa Barbara | 18 | 32.12 | 87.12 |
| Santa Clara | 15 | 57.56 | 71.62 |
| Santa Cruz | 20 | 82.62 | 82.62 |
| Shasta | 36 | 41.77 | 75.01 |
| Sierra | 4 | 5.29 | 26.56 |
| Siskiyou | 7 | 6.05 | 45.62 |
| Solano | 5 | 19.87 | 31.14 |
| Sonoma | 13 | 75.49 | 75.52 |
| Stanislaus | 3 | 4.19 | 47.75 |
| Sutter | 2 | 1.44 | 15.88 |
| Tehama | 5 | 51.36 | 80.46 |
| Trinity | 21 | 22.37 | 64.66 |
| Tulare | 8 | 5.18 | 31.18 |
| Tuolumne | 9 | 41.50 | 54.83 |
| Ventura | 12 | 51.58 | 74.11 |
| Yolo | 4 | 27.39 | 35.90 |
| Yuba | 11 | 54.92 | 64.48 |
Climate Data Sources
Thirty-five candidate climatic predictor variables were selected based on our knowledge of the biological and ecological requirements of I. pacificus (Table 2). These variables have been described previously (Springer et al. 2015, Hahn et al. 2016). We computed monthly, seasonal, and annual averages of precipitation, maximum temperature, minimum temperature, vapor pressure, and day length using the daily 1 km spatial resolution Daymet (Version 2) dataset for each year of the 35-yr (1980–2014) dataset (Thornton et al. 2012). We also computed monthly, monthly cumulative (from 1 January), seasonal, and annual growing degree-days with respect to 10°C from Daymet monthly maximum and minimum temperature data. The 35-yr annual average number of days with a snow-water equivalent depth > 1 mm was computed from the daily Daymet snow-water equivalent field. Nineteen ‘bioclimatic’ variables (Hijmans et al. 2005) were computed from the 35-yr monthly averages of Daymet maximum temperature, minimum temperature, and precipitation using the ‘bioclim’ function in the ‘dismo’ species distribution modeling package (Hijmans et al. 2005) in the R statistical computing language.
Table 2.
Candidate and selected variables used to model climatic suitability of I. pacificus in California
| Candidate variables | Selected variables |
|---|---|
| Annual mean temperature (BIO1) | |
| Mean diurnal temperature range (BIO2) | |
| Isothermality (BIO3) | x |
| Temperature seasonality (BIO4) | |
| Max. temperature of warmest month (BIO5) | x |
| Min. temperature of coldest month (BIO6) | |
| Temperature annual range (BIO7) | |
| Mean temperature of wettest quarter (BIO8) | |
| Mean temperature of driest quarter (BIO9) | |
| Mean temperature of warmest quarter (BIO10) | |
| Mean temperature of coldest quarter (BIO11) | x |
| Annual precipitation (BIO12) | |
| Precipitation of wettest month (BIO13) | |
| Precipitation of driest month (BIO14) | |
| Precipitation seasonality (BIO15) | x |
| Precipitation of wettest quarter (BIO16) | |
| Precipitation of driest quarter (BIO17) | |
| Precipitation of warmest quarter (BIO18) | x |
| Precipitation of coldest quarter (BIO19) | x |
| Average day length annual | |
| Average day length winter (Dec., Jan., Feb.) | |
| Average day length summer (Jun., Jul., Aug.) | x |
| Average day length spring (Mar., Apr., May) | |
| Average day length fall (Sep., Oct., Nov.) | |
| Average growing degree days annual | |
| Average growing degree days winter (Dec., Jan., Feb.) | |
| Average growing degree days summer (Jun., Jul., Aug.) | |
| Average growing degree days spring (Mar., Apr., May) | |
| Average growing degree days fall (Sep., Oct., Nov.) | |
| Annual average number of days with snow depth > 1 mm | |
| Average vapor pressure annual | |
| Average vapor pressure winter (Dec., Jan., Feb.) | |
| Average vapor pressure summer (Jun., Jul., Aug.) | |
| Average vapor pressure spring (Mar., Apr., May) | |
| Average vapor pressure fall (Sep., Oct., Nov.) | x |
Variable Selection
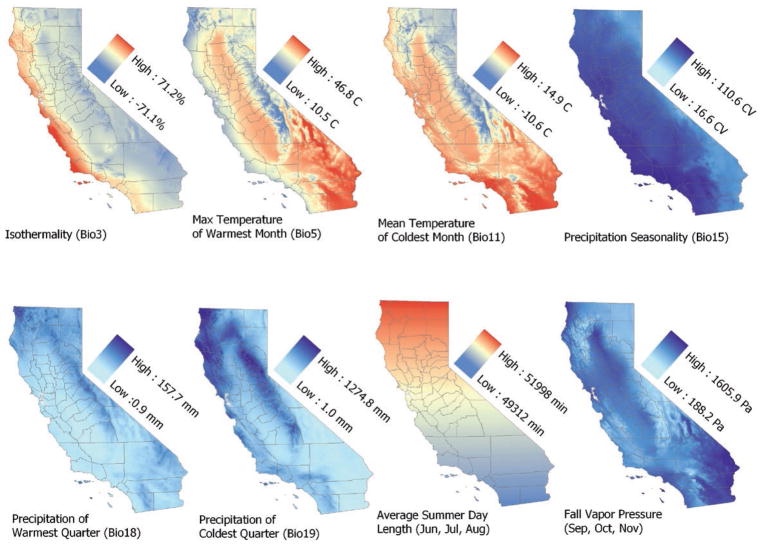
We used VisTrails Software for Assisted Habitat Modeling (SAHM; version 2.0) (Morisette et al. 2013) to manage data, perform species distribution modeling, and create ensemble models. Using SAHM, we prepared our data for the analysis (e.g., by clipping the environmental variables data to the extent and shape of California) and tested the environmental variable data for collinearity by comparing each pair of variables. We performed three different correlation tests (Pearson, Spearman, and Kendall) to evaluate collinearity and limited inclusion of variables into models to those with pairwise correlations <0.80. When variables were strongly correlated, we retained the variable with the highest percent deviance explained (e.g., a measure of how well a single variable predicts tick presence/absence) and/or deemed to be most biologically meaningful. The selected variables included isothermality (a measure of consistency in temperature), maximum temperature of warmest month, mean temperature of coldest quarter, precipitation seasonality, precipitation of warmest quarter, precipitation of coldest quarter, average day length in summer, and average vapor pressure in fall (Table 2). The spatial distributions of these variables are shown in Fig. 1.
Fig. 1.
Geographic distribution of the eight explanatory variables used to model the distribution of I. pacificus in California. This figure is available in colour at Journal of Medical Entomology online.
Modeling the Distribution of I. pacificus
The species distribution models for I. pacificus were fit with five algorithms using SAHM software: 1) boosted regression trees (BRT), 2) generalized linear model (GLM), 3) multivariate adaptive regression spline (MARS), 4) maximum entropy (Maxent), and 5) random forest (RF) (Talbert and Talbert 2001). We modeled the distribution of I. pacificus using these five algorithms, rather than just one of them, to identify potential biases of the individual models. Each model run produced a continuous relative probability surface of suitable climatic conditions for I. pacificus presence. We used the locations of I. pacificus, pseudo-absence locations, and the raster climatic layers as inputs to the models. For each algorithm, we used the SAHM default parameters, with the exception of the BRT model where we used a tree complexity of two, a learning rate of 0.005, and 5,000 trees (Elith et al. 2008, Springer et al. 2015, Hahn et al. 2016).
We converted each continuous surface for each model run into a binary representation of suitable habitat using a probability threshold that represented 90% or 99% model sensitivity. That is, at the selected threshold, 90 or 99% of tick presence points were correctly classified as suitable by the model. The resulting binary raster classified each 1 km cell as either suitable or unsuitable, with a score of 1 or 0, respectively (Fielding and Bell 1997, Guisan et al. 2007). Next, we evaluated model agreement by combining the five binary models for each sensitivity threshold into a single layer showing the number of models predicting presence for each 1 km raster cell based on fixed suitability thresholds.
Evaluation and Visualization of Model Results
We used a 10-fold cross-validation method to test the performance of our models (Elith et al. 2006). The location data were partitioned into 10 equal samples—1 test sample and 9 training samples—and the model was run 10 times with a different test-sample used each time. This type of validation reduced the overall variance by averaging the 10 different samples (Rodriguez et al. 2010). We evaluated each model using standard accuracy metrics including Area Under the Receiver-Operator Curve(AUC), and specificity at fixed sensitivity thresholds (Phillips et al. 2006). AUC is a threshold independent measure of model performance and ranges from 0 to 1 with a value of 0.5 representing a model with prediction probabilities close to random, and values greater than 0.5 signifying a model with a greater power to predict areas of high suitability in locations of known species presence. We evaluated specificity of models at 90 and 99% sensitivity thresholds (Hahn et al. 2017). Next, we calculated the relative contribution of each climatic variable to each modeling algorithm. We also plotted the response curves for each environmental variable by algorithm to aid in comparing the relationship between an environmental variable and I. pacificus climatic suitability.
Finally, because I. pacificus is primarily a woodland-associated species that has been collected from forest, grass, and scrub-shrub vegetation (Furman and Loomis 1984, Lane and Stubbs 1990, Kramer and Beesley 1993, Eisen et al. 2004), we applied a spatial mask representing these categories, as well as those categorized as ‘riparian’ in order to detect fragmented woodlands following rivers and streams, to our final models predicting suitability for the tick. We recognize that tick abundance differs among these land cover classes; however, our model assesses only the likelihood of presence, not abundance. We chose not to include land cover class as a potential predictor in the model for two reasons. First, the categories included in the mask are those where sampling was focused; these included land cover types where ticks were perceived to be most common (e.g., wooded, grassy, or scrubby vegetation). Second, if the models are projected under different climate change scenarios, our certainty in how land-cover will change over time is low, and therefore inclusion of land cover class in the models would increase uncertainty in model projections. To create the mask, we used a California vegetation layer developed by the California Department of Forestry and Fire Protection (http://frap.fire.ca.gov/data/frapgisdata-sw-fveg_download). This layer is a compilation of the ‘best available’ land cover data for California into a single comprehensive statewide raster data set at 30 m resolution. We reclassified the layer to show only the following classes: forest, grass, scrub-shrub, and riparian. Then, we reprojected and resampled the resulting dataset to have the same cell size (e.g., from 30 m to 1 km) as the input rasters used in the modeling effort. Functionally, this mask allowed us to exclude areas that are climatically suitable, but where land-cover types are unfavorable to the survival of I. pacificus (e.g., water, wetlands, agriculture, and impervious surface land cover classes).
Results
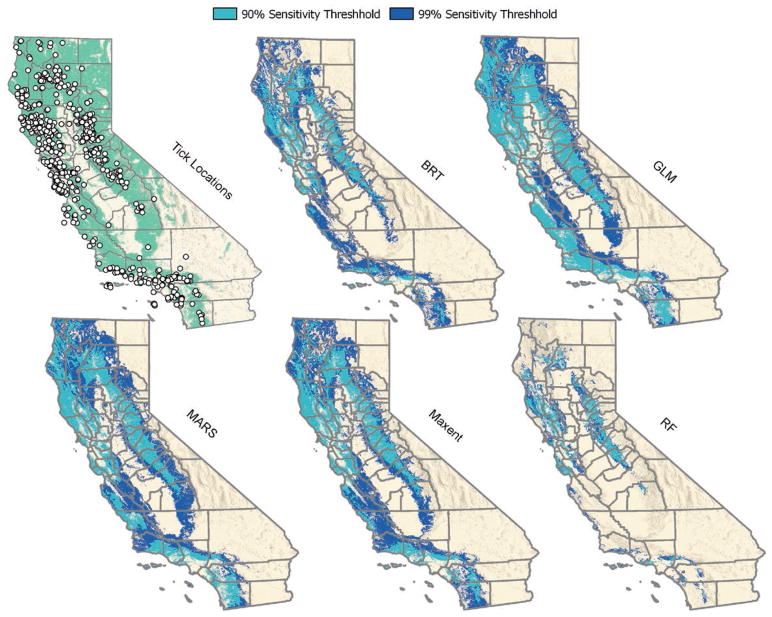
In total, 4,546 I. pacificus presence locations were considered for inclusion as presence points in our model development. The full database included ticks collected from 55 of 58 (95%) California counties and the majority (3,489 of 4,546; 76.8%) were collected from either forest, grass, scrub-shrub or riparian land cover classes. To yield a more contemporary dataset, we included only samples collected in 1980 or later and to avoid over-representing areas that were sampled intensively, we excluded locations in close proximity to one another (e.g., within 1.415 km). As a result, our final presence dataset included 621 I. pacificus presence locations distributed across 51 counties; most (499 or 80.3%) were collected from forest, grass, scrub-shrub or riparian land cover class categories (Table 1, Fig. 2).
Fig. 2.
Predicted distribution of forest, grass, scrub-shrub, and riparian land classes with suitable climatic conditions for I. pacificus provided by five different environmental niche models based on 90 and 99% sensitivities. Locations of I. pacificus in California also are overlaid on the distribution of the four land cover classes for which the results are masked. This figure is available in colour at Journal of Medical Entomology online.
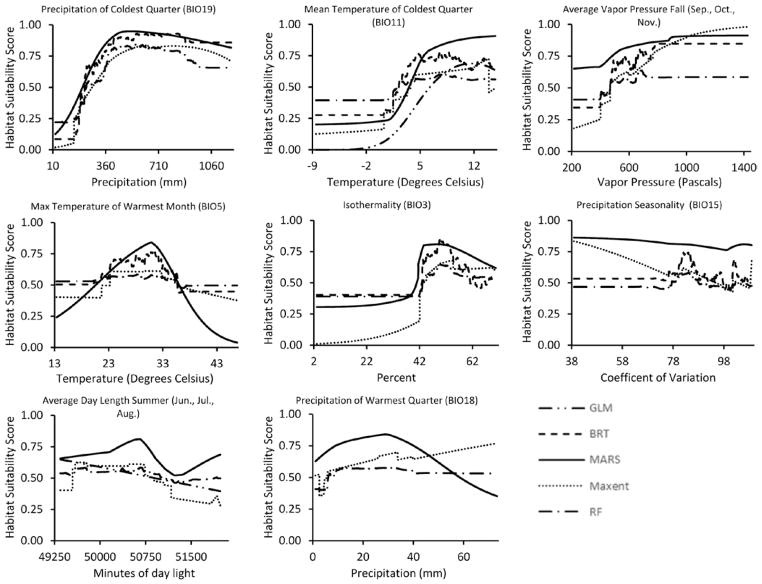
We reduced the original set of 35 climate variables to eight uncorrelated variables (Table 2). Among these variables, one model (GLM) retained only three variables, two (BRT and MARS) retained six variables and two (Maxent and RF) retained all eight (Table 3). All five models retained precipitation of the coldest quarter (Bio 19) and mean temperature of the coldest quarter (Bio 11) with normalized contributions ranging from 37.8 to 70.3% for the former and from 10.4 to 32.5% for the latter (Table 3). Suitability for I. pacificus increases between 0 and 400 mm of cold-season precipitation, levels off between 400 and 800 mm, and declines somewhat for precipitation amounts >800 mm (Fig. 2). Thus, the highest suitability is in areas that receive a moderate amount of cold-season precipitation, and is lower in drier regions in southern California as well as in the high precipitation regions in the Sierra Nevada and Klamath mountains and in coastal northern California (Fig. 1). Conditions are unsuitable for I. pacificus when cold-season average temperatures are below freezing (0°C), but suitability gradually increases for conditions above freezing, leveling off at beyond ~5°C. Therefore, Bio11 excludes areas in the Sierra Nevada and Klamath mountains that have cold season temperatures below freezing. Collectively, Bio19 and Bio11 contribute between 49.7 and 98.8% to the five I. pacificus models (Table 3). Other explanatory variables in order of summed normalized contributions across models are autumn vapor pressure (positively associated with suitability indicating a preference for higher humidity); maximum temperature of the warmest month (Bio5; peaking around 30°C and decreasing for lower or higher temperatures indicating an optimal range for survival between ~23 and 33°C); isothermality (Bio3; positively associated with suitability indicating a preference for small temperature fluctuations); precipitation seasonality (Bio15; no consistent response among models); average summer day length (generally negatively associated with suitability indicating a preference for shorter days); and precipitation of the warmest quarter (Bio18; no consistent association).
Table 3.
Normalized contributions of the climate variables used in each of the modeling algorithms for I. pacificus
| Description | Normalized contribution values (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| BRT | GLM | MARS | Maxent | RF | |
| Precipitation of coldest quarter (Bio 19) | 37.8 | 70.3 | 48.9 | 50.7 | 37.9 |
| Mean temperature of coldest quarter (Bio 11) | 19.0 | 28.5 | 32.5 | 10.4 | 11.8 |
| Autumn vapor pressure (Sep, Oct, Nov) | 12.3 | - | - | 10.9 | 11.7 |
| Max. temperature of warmest month (Bio 5) | 10.0 | - | 9.4 | 6.6 | 8.2 |
| Isothermality (Bio 3) | 10.2 | - | 2.2 | 8.2 | 8.2 |
| Precipitation seasonality (Bio 15) | 10.7 | - | - | 2.6 | 8.9 |
| Average summer day length (Jun., Jul., Aug.) | - | 1.2 | 3.6 | 7.9 | 7.6 |
| Precipitation of warmest quarter (Bio 18) | - | - | 3.4 | 2.8 | 5.7 |
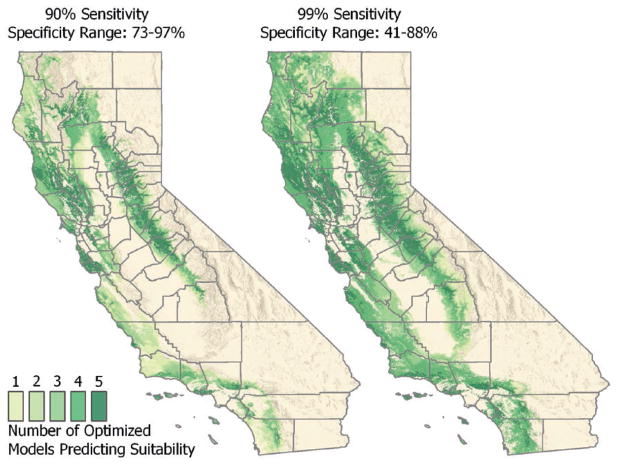
Among models, AUC values based on training datasets ranged from 0.85 (GLM) to 0.95 (BRT) and AUC values based on test sets ranged from 0.86 (GLM) to 0.90 (RF), indicating very good predictive capability for each model. The difference between test and training set AUC values ranged from 0.00 (RF) to 0.06 (BRT), showing good consistency in model predictions between training and testing datasets. We dichotomized continuous probabilities of suitability based on 90 and 99% sensitivity thresholds, which resulted in specificity ranges from 73 to 97% and 41 to 88%, respectively (Table 4, Fig. 4). As expected, as sensitivity increases, the area predicted to be suitable expands, resulting in lower specificity.
Table 4.
Threshold independent (AUC) and dependent (90 and 99% sensitivity thresholds) performance metrics for BRT, GLM, MARS, Maxent, and RF models
| Model | |||||
|---|---|---|---|---|---|
|
| |||||
| BRT | GLM | MARS | Maxent | RF | |
| AUC (test/train) | 0.89/0.95 | 0.86/0.85 | 0.89/0.88 | 0.88/0.91 | 0.90/0.90 |
| Specificity (%) | |||||
| 90% sensitivity | 87 | 67 | 73 | 77 | 97 |
| 99% sensitivity | 64 | 43 | 41 | 49 | 88 |
Fig. 4.
Maps indicating the number of models predicting climatic suitability for the presence of I. pacificus in forests, grasslands, scrub-shrub, and riparian areas of California. Each map displays a different threshold used to dichotomize continuous probabilities into a binary outcome of suitable or not. This figure is available in colour at Journal of Medical Entomology online.
All models predict suitable habitat (i.e., forest, grass, scrub-shrub or riparian land cover classes with suitable climatic conditions) for I. pacificus in the coastal regions from Humboldt County southward and include suitable habitat in the western foothills of the Sierra Nevada (Figs. 2 and 4). In general, excluded areas are dry, hot, or high elevation. Based on models with 90 or 99% sensitivity, 17.5% (71,636 km2) and 37.9% (155,243 km2) of California land surface were considered suitable by three or more models, respectively (Fig. 4). Using the most inclusive threshold to dichotomize the model (99% sensitivity) and counting only counties where three or more models predicted suitability, 55 of 58 counties were classified as suitable; however, the percentage of land within counties considered suitable based on these criteria ranged from 0.01% in Lassen County to as high as 90.74% in Mendocino County (Table 1, Fig. 4).
Discussion
Our models indicate consistently that precipitation and average temperatures during the coldest quarter of the year play a key role in defining the ecological niche of I. pacificus in California. The likelihood of an area being classified as suitable increases steadily with increasing temperatures above 0°C during the coldest quarter of the year, and further increases when precipitation amounts range from 400 to 800 mm during the coldest quarter, indicating that areas in California with relatively warm and wet winters typically are most suitable for the tick. Other consistent predictors of suitability include increasing autumn humidity, maximum temperatures in the warmest month ranging from 23 to 33°C, and low temperature variability throughout the year. Consistent with previously published distribution records (Bishopp and Trembley 1945, Furman and Loomis 1984), our suitability maps indicate that coastal California, especially the northern coast, and the western Sierra Nevada foothills have the highest probability of I. pacificus presence.
Compared with a recently published habitat suitability model for I. pacificus in the western United States (Hahn et al. 2016) that was based on county records showing where the tick was reportedly established (Dennis et al. 1998, Eisen et al. 2016a), our models identify a similar geographical range in the distribution of suitable habitat, but reveal substantial variability in suitability at the sub-county scale. Specifically, although 55 of 58 counties were classified by our most inclusive ensemble model as suitable (i.e., agreement among at least three models based on 99% sensitivity), within these counties, the percentage of the county’s total area that was classified as suitable ranged from 0.01% in Lassen County, located in the northeastern portion of the state to 90.74% in Mendocino County, located along the north-coast.
The variables included in our models are biologically plausible. The two leading variables, precipitation and mean temperature both measured in the coldest quarter, correspond to the host-seeking periods of the adult ticks (Lane and Stubbs 1990, Kramer and Beesley 1993, Clover and Lane 1995, Padgett and Lane 2001, Eisen et al. 2004, Salkeld et al. 2014, MacDonald and Briggs 2016), but these variables likely impact other life stages as well. Ixodes spp. ticks generally can tolerate short periods of very cold conditions, though prolonged exposure to sub-zero temperatures for long durations is almost universally lethal to the tick (Eisen et al. 2016b). On the other hand, snow cover (associated with increased winter precipitation) may provide some protective insulation (Eisen et al. 2016b). Further, survival and host-seeking rates in ixodid ticks increase with increasing humidity (Needham and Teel 1991, Eisen et al. 2002, 2003), which explains the significance of precipitation and autumn vapor pressure as predictive variables. Areas that are generally warmer and wetter during the adult host-seeking season likely promote successful host-seeking conditions for adult ticks that are vulnerable to desiccation and require a minimal level of warmth and humidity to effectively find hosts, mate, and reproduce (Salkeld et al. 2014, MacDonald and Briggs 2016).
Maximum temperature of the warmest month was retained by four of the five models and indicated an optimal range for survival between approximately 23–33°C. This range might appear slightly higher than expected based on previous studies that measured host-seeking of nymphs relative to air temperature (Eisen et al. 2002), but such ticks typically do not actively host-seek during the warmest months of the year and may be experiencing much lower temperatures in certain refugia (e.g., in leaf litter). This variable is strongly correlated with maximum temperatures during other warm months that are more apt to correspond with the peak activity periods of immature ticks, but those were excluded here as candidate predictors. A previous study from Mendocino County demonstrated that in oak woodlands the abundance of host-seeking nymphs typically begins to decline when the mean maximum daily air temperatures exceed 23°C (Eisen et al. 2002). Overall, excessive heat (generally between 32 and 40°C) increases ixodid tick mortality and reduces oviposition success (Needham and Teel 1991, Peavey and Lane 1996, Ogden et al. 2004, Eisen et al. 2016b).
Recent technological improvements, such as digitization of records, cloud-based processing, freeware and open source analytical tools, have facilitated the integration of increasing amounts of geo-referenced biological data with high-resolution global climate data and made species distribution models more available to researchers (Graham et al. 2004, Jimenez-Valverde et al. 2008, Elith and Leathwick 2009). Species distribution models are used often to examine potential climate change impacts on biodiversity and to aid in conservation (Loarie et al. 2008), and they are increasingly being used in public health contexts to prioritize vector management or eradication (Dicko et al. 2014, Fuller et al. 2016).
Despite their utility, species distribution models have been criticized for theoretical and practical reasons. First, these models assume that the niche being modeled is based solely on physical, noninteractive requirements for a population to exist, and can be ascertainable from a species’ current distribution (Austin 2007, Easterday et al. 2016). These can be problematic assumptions, but the benefits of mapping potential niche for medically important species to target vector surveillance and prevention resources outweigh these concerns. Additionally, these models can be biased by the choice of the temporal and spatial scale of the input data (e.g., data are not detailed enough to reveal important niches). This is especially problematic when downscaled climate data are not available for a given region. Finally, recent studies have revealed that projections by alternative models can be variable (Araujo and New 2007), and reliance on a single model is not useful. Our use of an ensemble approach helps shield against biases imposed by reliance on a single model.
Although our model yielded a good fit to the available tick-collection records, there are several remaining limitations to address. We restricted our model extrapolation only to forest, grass, scrubshrub, and riparian areas because these were the main land cover classes where tick sampling was conducted and therefore believed to be the most significant classes for I. pacificus survival (Furman and Loomis 1984, Lane and Stubbs 1990, Kramer and Beesley 1993, Eisen et al. 2004). Applying the mask avoided classifying intensive agricultural lands, impervious surfaces, and aquatic environments as suitable for the tick. However, given the spatial resolution and quality of our land cover mask, small isolated woodlands may have been excluded. These kinds of small or isolated habitats are difficult to map using remote sensing, and might have been missed in the original land cover dataset (Nagendra 2001), and in the resampled mask. For example, in parts of the northern Central Valley where woodlands are extremely fragmented but climatic conditions are consistent with classification as suitable, these areas may not be coded as suitable on our maps. As a result, field sampling may reveal that the tick is present in woodlands in this region that are not represented in our maps.
Our models should be evaluated further by means of ground-truthing field studies and to assess geographic variation in the density of host-seeking ticks and in prevalence of infection in those ticks. Also, they may prove useful for guiding vector surveillance efforts. Although those models that were based on a 99% sensitivity threshold should portray the most inclusive distribution of suitable habitat for the tick, many such sites might yield low tick abundance either because certain regions manifest lower probabilities of suitability, or because of model error. Indeed, specificity of the models based on this threshold ranged from 44 to 88%. By contrast, when surveillance resources are scarce, focusing tick collection efforts on areas classified as suitable based on a 90% sensitivity threshold are more likely to yield ticks compared with the models based on a 99% sensitivity.
Although presence of a vector tick is a prerequisite for risk of tickborne diseases, our models are not intended to predict the likelihood of I. pacificus-borne disease occurrence. Among counties reporting an incidence of ≥3.0 confirmed Lyme diseases cases per 100,000 person-years from 2007 to 2016 (Mendocino, Humboldt, Trinity and Sierra) (Yoshimizu et al. 2016), our models predicted between 26.56 and 90.74% of those counties to be suitable for I. pacificus and reveal considerable spatial variability within counties. For example, suitable habitat within Sierra County appears to be restricted to lower elevations in the western portion of the county, whereas it is more widespread in Mendocino County. Within these suitable areas, risk of encountering infected ticks may differ based on spatial variation in the abundance of infected ticks, presence or absence of primary reservoir hosts capable of infecting ticks with zoonotic agents, how much time people spend in various vegetation types infested with ticks, or regional differences in human behaviors that put people at risk for exposure to tick bites (Lane et al. 2004). In general, the density of host-seeking nymphs or the density of host-seeking nymphs infected with B. burgdorferi are better indicators of Lyme disease incidence than simple measures of the tick’s presence (Mather et al. 1996, Stafford et al. 1998, Eisen et al. 2006b, Pepin et al. 2012). Moreover, the density of host-seeking I. pacificus nymphs differs among land cover classes such that nymphs are typically more common in dense woodlands compared with grass, chaparral or woodland-grass, but there also are notable differences in the densities of host-seeking nymphs among woodland types and between northern and southern regions of the state (Lane and Stubbs 1990; Kramer and Beesley 1993; Clover and Lane 1995; Eisen et al. 2003, 2004, 2006a,b, 2010; Salkeld et al. 2014; MacDonald and Briggs 2016; MacDonald et al. 2017). Thus, within areas classified as suitable for I. pacificus, a tremendous amount of variability is expected in the abundance of host-seeking ticks and in their infection rates with zoonotic agents, which are often associated with host community composition (Mather et al. 1989, LoGiudice et al. 2003, Salkeld and Lane 2010), a variable not included in our models.
In conclusion, our study updates maps of suitable habitat for I. pacificus in California and reveals substantial variability within and among counties. Moreover, we identified climatic variables associated with suitability, which provides a basis for predicting future changes in the tick’s distribution in response to climate variability.
Fig. 3.
Response curves of environmental variables selected by the I. pacificus models. The x-axis represents the range of each environmental variable in the training dataset; the y-axis represents the probability of suitable and non-suitable habitat, 1 and 0, respectively.
References Cited
- Araujo MB, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2007;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Austin M. Species distribution models and ecological theory: a critical assessment of some possible new approaches. Ecol Modell. 2007;200:1–19. [Google Scholar]
- Bishopp FC, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol. 1945;31:1–54. [Google Scholar]
- Clover JR, Lane RS. Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of Lyme disease in California. Am J Trop Med Hyg. 1995;53:237–240. doi: 10.4269/ajtmh.1995.53.237. [DOI] [PubMed] [Google Scholar]
- Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- Dicko AH, Lancelot R, Seck MT, Guerrini L, Sall B, Lo M, Vreysen MJ, Lefrancois T, Fonta WM, Peck SL, et al. Using species distribution models to optimize vector control in the framework of the tsetse eradication campaign in Senegal. Proc Natl Acad Sci USA. 2014;111:10149–10154. doi: 10.1073/pnas.1407773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterday KJ, McIntyre PJ, Thorne JH, Santos MJ, Kelly M. Assessing threats and conservation status of historical centers of oak richness in California. Urban Plan. 2016;1:65–78. [Google Scholar]
- Eisen L, Eisen RJ. Need for improved methods to collect and present spatial epidemiologic data for vectorborne diseases. Emerg Infect Dis. 2007;13:1816–1820. doi: 10.3201/eid1312.070211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L. Spatial modeling of human risk of exposure to vector-borne pathogens based on epidemiological versus arthropod vector data. J Med Entomol. 2008;45:181–192. doi: 10.1603/0022-2585(2008)45[181:smohro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Lane RS. Seasonal activity patterns of Ixodes pacificus nymphs in relation to climatic conditions. Med Vet Entomol. 2002;16:235–244. doi: 10.1046/j.1365-2915.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Castro MB, Lane RS. Environmentally related variability in risk of exposure to Lyme disease spirochetes in northern California: effect of climatic conditions and habitat type. Environ Entomol. 2003;32:1010–1018. [Google Scholar]
- Eisen RJ, Mun J, Eisen L, Lane RS. Life stage-related differences in density of questing ticks and infection with Borrelia burgdorferi sensu lato within a single cohort of Ixodes pacificus (Acari: Ixodidae) J Med Entomol. 2004;41:768–773. doi: 10.1603/0022-2585-41.4.768. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Lane RS. Predicting density of Ixodes pacificus nymphs in dense woodlands in Mendocino County, California, based on geographic information systems and remote sensing versus fieldderived data. Am J Trop Med Hyg. 2006a;74:632–640. [PubMed] [Google Scholar]
- Eisen RJ, Lane RS, Fritz CL, Eisen L. Spatial patterns of Lyme disease risk in California based on disease incidence data and modeling of vector-tick exposure. Am J Trop Med Hyg. 2006b;75:669–676. [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Girard YA, Fedorova N, Mun J, Slikas B, Leonhard S, Kitron U, Lane RS. A spatially-explicit model of acarological risk of exposure to Borrelia burgdorferi-infected Ixodes pacificus nymphs in northwestern California based on woodland type, temperature, and water vapor. Ticks Tick Borne Dis. 2010;1:35–43. doi: 10.1016/j.ttbdis.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol. 2016a;53:349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Ogden NH, Beard CB. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J Med Entomol. 2016b;53:250–261. doi: 10.1093/jme/tjv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elith J, Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Ann Rev Ecol Evol Syst. 2009;40:677–697. [Google Scholar]
- Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, Guisan A, Hijmans R, Huettmann F, Leathwick JR, Lehmann A, et al. Novel methods improve prediction of species’ distributions from occurence data. Ecography. 2006;29:129–151. [Google Scholar]
- Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- Fielding A, Bell J. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24:38–49. [Google Scholar]
- Fuller DO, Alimi T, Herrera S, Beier JC, Quinones ML. Spatial association between malaria vector species richness and malaria in Colombia. Acta Trop. 2016;158:197–200. doi: 10.1016/j.actatropica.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Furman DP, Loomis EC. The ticks of California (Acari: Ixodida) Bull Calif Insect Surv. 1984;25:354. [Google Scholar]
- Graham CH, Ferrier S, Huettman F, Moritz C, Peterson AT. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol Evol. 2004;19:497–503. doi: 10.1016/j.tree.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Guisan A, Graham CH, Elith J, Huettmann F. Sensitivity of predictive species distribution models to change in grain size. Drivers Distrib. 2007;13:332–340. [Google Scholar]
- Guo Q, Kelly M, Graham CH. Support vector machines for predicting distribution of sudden oak death in California. Ecol Modell. 2005;182:75–90. [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, Eisen RJ. Modeling the geographic distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J Med Entomol. 2016;53:1176–1191. doi: 10.1093/jme/tjw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, Eisen RJ. Response: the geographic distribution of Ixodes scapularis (Acari: Ixodidae) revisited: the importance of assumptions about error balance. J Med Entomol. 2017;54:1104–1106. doi: 10.1093/jme/tjx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra L, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- Jimenez-Valverde A, Lobo JM, Hortal J. Not as good as they seem: the importance of concepts in species distribution modelling. Divers Distrib. 2008;14:885–890. [Google Scholar]
- Keenan TF, Carbone MS, Reichstein M, Richardson AD. The model-data fusion pitfall: assuming certainty in an uncertain world. Oecologia. 2011;167:587. doi: 10.1007/s00442-011-2106-x. [DOI] [PubMed] [Google Scholar]
- Kramer VL, Beesley C. Temporal and spatial distribution of Ixodes pacificus and Dermacentor occidentalis (Acari: Ixodidae) and prevalence of Borrelia burgdorferi in Contra Costa County, California. J Med Entomol. 1993;30:549–554. doi: 10.1093/jmedent/30.3.549. [DOI] [PubMed] [Google Scholar]
- Lane RS, Stubbs HA. Host-seeking behavior of adult Ixodes pacificus (Acari: Ixodidae) as determined by flagging vegetation. J Med Entomol. 1990;27:282–287. doi: 10.1093/jmedent/27.3.282. [DOI] [PubMed] [Google Scholar]
- Lane RS, Manweiler SA, Stubbs HA, Lennette ET, Madigan JE, Lavoie PE. Risk factors for Lyme disease in a small rural community in northern California. Am J Epidemiol. 1992;136:1358–1368. doi: 10.1093/oxfordjournals.aje.a116448. [DOI] [PubMed] [Google Scholar]
- Lane RS, Brown RN, Piesman J, Peavey CA. Vector competence of Ixodes pacificus and Dermacentor occidentalis (Acari: Ixodidae) for various isolates of Lyme disease spirochetes. J Med Entomol. 1994;31:417–424. doi: 10.1093/jmedent/31.3.417. [DOI] [PubMed] [Google Scholar]
- Lane RS, Steinlein DB, Mun J. Human behaviors elevating exposure to Ixodes pacificus (Acari: Ixodidae) nymphs and their associated bacterial zoonotic agents in a hardwood forest. J Med Entomol. 2004;41:239–248. doi: 10.1603/0022-2585-41.2.239. [DOI] [PubMed] [Google Scholar]
- Loarie SR, Carter BE, Hayhoe K, McMahon S, Moe R, Knight CA, Ackerly DD. Climate change and the future of California’s endemic flora. Plos One. 2008;3:e2502. doi: 10.1371/journal.pone.0002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AJ, Briggs CJ. Truncated seasonal activity patterns of the western blacklegged tick (Ixodes pacificus) in central and southern California. Ticks Tick Borne Dis. 2016;7:234–242. doi: 10.1016/j.ttbdis.2015.10.016. [DOI] [PubMed] [Google Scholar]
- MacDonald AJ, Hyon DW, Brewington JB, III, O’Connor KE, Swei A, Briggs CJ. Lyme disease risk in southern California: abiotic and environmental drivers of Ixodes pacificus (Acari: Ixodidae) density and infection prevalence with Borrelia burgdorferi. Parasit Vectors. 2017;10:7. doi: 10.1186/s13071-016-1938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather TN, Wilson ML, Moore SI, Ribeiro JM, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT. Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- Molloy PJ, Telford SR, III, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. Borrelia miyamotoi disease in the Northeastern United States: a case series. Ann Intern Med. 2015;163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- Morisette J, Jarnevich C, Holcombe T, Talbert C, Ignizio D, Talbert M, Silva C, Koop D, Swanson A, Young N. VisTrails SAHM: visualization and workflow management for species habitat modeling. Ecography. 2013;36:129–135. [Google Scholar]
- Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43:120–123. doi: 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- Nagendra H. Using remote sensing to assess biodiversity. Int J Remote Sens. 2001;22:2377–2400. [Google Scholar]
- Needham GR, Teel PD. Off-host physiological ecology of ixodid ticks. Annu Rev Entomol. 1991;36:659–681. doi: 10.1146/annurev.en.36.010191.003303. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Beauchamp G, Charron D, Maarouf A, O’Callaghan CJ, Waltner-Toews D, Barker IK. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J Med Entomol. 2004;41:622–633. doi: 10.1603/0022-2585-41.4.622. [DOI] [PubMed] [Google Scholar]
- Padgett KA, Lane RS. Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J Med Entomol. 2001;38:684–693. doi: 10.1603/0022-2585-38.5.684. [DOI] [PubMed] [Google Scholar]
- Padgett K, Bonilla D, Kjemtrup A, Vilcins IM, Yoshimizu MH, Hui L, Sola M, Quintana M, Kramer V. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. Plos One. 2014;9:e110853. doi: 10.1371/journal.pone.0110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavey CA, Lane RS. Field and laboratory studies on the timing of oviposition and hatching of the western black-legged tick, Ixodes pacificus (Acari: Ixodidae) Exp Appl Acarol. 1996;20:695–711. doi: 10.1007/BF00051555. [DOI] [PubMed] [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, Diuk-Wasser MA. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am J Trop Med Hyg. 2012;86:1062–1071. doi: 10.4269/ajtmh.2012.11-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributons. Ecol Modell. 2006;190:231–259. [Google Scholar]
- Rodriguez JD, Perez A, Lozano JA. Sensitivity analysis of kappa-fold cross validation in prediction error estimation. IEEE Trans Pattern Anal Mach Intell. 2010;32:569–575. doi: 10.1109/TPAMI.2009.187. [DOI] [PubMed] [Google Scholar]
- Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Lane RS. Community ecology and disease risk: lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology. 2010;91:293–298. doi: 10.1890/08-2106.1. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Castro MB, Bonilla D, Kjemtrup A, Kramer VL, Lane RS, Padgett KA. Seasonal activity patterns of the western black-legged tick, Ixodes pacificus, in relation to onset of human Lyme disease in northwestern California. Ticks Tick Borne Dis. 2014;5:790–796. doi: 10.1016/j.ttbdis.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Springer YP, Jarnevich CS, Barnett DT, Monaghan AJ, Eisen RJ. Modeling the present and future geographic distribution of the lone star tick, Amblyomma americanum (Ixodida: Ixodidae), in the Continental United States. Am J Trop Med Hyg. 2015;93:875–890. doi: 10.4269/ajtmh.15-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford KC, III, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–1244. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert C, Talbert M. U. S. G. Survey, editor. U.S. Geological Survey; Fort Collins, CO: 2001. User documentation for the Software for Assisted Habitat Modeling (SAHM) package in VisTrails. https://www.fort.usgs.gov/pub-types/software-documentation. [Google Scholar]
- Teglas MB, Foley J. Differences in the transmissibility of two Anaplasma phagocytophilum strains by the North American tick vector species, Ixodes pacificus and Ixodes scapularis (Acari: Ixodidae) Exp Appl Acarol. 2006;38:47–58. doi: 10.1007/s10493-005-5293-5. [DOI] [PubMed] [Google Scholar]
- Thornton P, Thornton M, Mayer B, Wilhelmi N, Wei Y, Devarakonda R, Cook R. DAACfBD Oak Ridge National Laboratory, editor. Daymet: daily surface weather data on a 1-km grid for North America, 1980–2008. Oak Ridge, TN: 2012. [Google Scholar]
- Yoshimizu M, Kjemtrup A, Porse CC. Chapter 3: tick-borne diseases. In: Kjemtrup A, Kramer V, editors. California department of public health vector-borne disease section annual report. California Department of Public Health; Sacramento, CA: 2016. pp. 8–13. [Google Scholar]