Abstract
Neuroinflammation is a fundamental mechanism in Alzheimer’s disease (AD) progression. The stress-induced activation of the p38α mitogen-activated protein kinase (MAPK) leads to increased production of proinflammatory cytokines and neurodegeneration. We investigated the effects of an isoform selective p38α MAPK inhibitor, MW01-18-150SRM (MW150), administered at 2.5 mg/kg/day (i.p.; 14 days) on early entorhinal cortex (EC) alterations in an AD mouse model carrying human mutations of the amyloid precursor protein (mhAPP). We used electrophysiological analyses with long-term potentiation (LTP) induction in EC-containing brain slices and EC-relevant associative memory tasks. We found that MW150 was capable of rescuing LTP in 2-month old mhAPP mice. Acute delivery of MW150 to brain slices was similarly effective in rescuing LTP, with a comparable efficacy to that of the widely used multi-kinase inhibitor SB203580. MW150-treated mhAPP mice demonstrated improved ability to discriminate novel associations between objects and their position/context. Our findings suggest that the selective inhibition of the stress-activated p38α MAPK with MW150 can attenuate the EC dysfunctions associated with neuroinflammation in an early stage of AD progression.
Keywords: β-amyloid, entorhinal cortex, neuroinflammation, p38αMAPK inhibitor, long-term potentiation, novel object recognition
1. Introduction
Early stage neuroinflammation has emerged to crucially contribute to neurodegeneration from genetic studies (Benedet et al., 2015; C, 2015; Saykin et al., 2015), preclinical animal models and clinical pathology (Heppner et al., 2015; Van Eldik et al., 2016; Wyss-Coray and Rogers, 2012). Specific to the pathophysiology of Alzheimer’s disease (AD), the p38 MAPK cascade is activated in both glia and neurons in response to an array of central nervous system (CNS) disease-relevant stressors (Schnoder et al., 2016; Xing et al., 2011, 2015). In particular, Aβ peptide binding to the microglial receptor for advanced glycation end products (RAGE) induces p38 MAPK signaling and cytokine overproduction, which then leads to the activation of p38 MAPK in neurons, and ultimately to intraneuronal translocation of Aβ, tau phosphorylation in AD-relevant residues and cortical synaptic dysfunction (Anderton et al., 2001; Criscuolo et al., 2017; Ferrer et al., 2005; Li et al., 2003; Origlia et al., 2008; Peel et al., 2004; Takuma et al., 2009; Zhu et al., 2005). Approaches based on drug-resistant knock-in mice (O’Keefe et al., 2007), tissue-selective conditional knock-out mice (Kang et al., 2008), and isoform specific p38αMAPK inhibitors (Roy et al., 2015; Watterson et al., 2013; Zhou et al., 2017) demonstrated that the p38α isoform is critically involved in the increased cytokine production after an inflammatory challenge. Therefore, the p38α MAPK appears as an ideally located target for pharmacological strategies aimed at attenuation of disease progression or neuroprotection. Recently, a highly selective p38α MAPK inhibitor named MW01-18-150SRM (referred to as MW150) has been made available, which proved effective in ameliorating hippocampus-dependent associative and spatial memory deficits in two distinct AD-relevant animal models (Roy et al., 2015). MW150 was shown to reduce brain interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFα) release and to increase the microglia in close proximity to amyloid plaques, with no significant effects on normal microglia protective functions or plaque load (Zhou et al., 2017). Moreover, pharmacokinetic assays demonstrated that MW150 has good oral bioavailability, high cell permeability, and attractive distribution across the blood-brain barrier (Roy et al., 2015). Finally, MW150 screening against a critical set of cytochrome P450 (CYP) isoforms indicated a low potential for pharmacological interaction and adverse events (Roy et al., 2015), making it a good candidate for a clinical trial. Recent evidence points to the entorhinal cortex (EC) as a crucial site for associative memory formation (Moser et al., 2008) and as the brain region primarily affected during neurodegeneration (Stranahan and Mattson, 2010). In the context of the EC, layer II neurons are exquisitely vulnerable to age-related alterations (Braak and Braak, 1991; Gomez-Isla et al., 1996). In our previous work, we reported that transgenic mice (mhAPP) with progressive Aβ accumulation (Mucke et al., 2000) present synaptic dysfunctions in the EC layer II as early as at 2 months of age (Criscuolo et al., 2017). These, and the related associative memory deficits, can be reverted by inhibition of microglial RAGE which reduces the activation of p38 MAPK (Criscuolo et al., 2017). We therefore wanted to investigate the impact of p38αMAPK inhibition on EC impairment in the mhAPP mouse model of AD using MW150 systemic administration.
2. Materials and Methods
2.1. Animals
We used either 2- or 6-month old male mhAPP transgenic mice (APPsweInd, lineJ20) and their littermate controls (C57BL/6J background). mhAPP transgenic mice overexpress an alternatively spliced human APP (hAPP) minigene that encodes hAPP695, hAPP751, and hAPP770 bearing mutations linked to familial AD (V717F, KM670/671NL) (Mucke et al., 2000). All experiments were conducted in accordance with the principles of animal care and experimentation in the guidelines of the Italian Ministry of Health (Legislative Decree n. 116/92) and the European Community (European Directive 86/609/EEC). The Ministry of Health approved the use of animals in this protocol (n. 192/2000-A and n.74/2017). Every effort was made to minimize the number of animals used and their suffering.
2.2. Drugs and treatment
MW01-18-150SRM (MW150) was provided by Dr. M. Watterson. MW150 is an isoform-selective p38αMAPK inhibitor, which was synthesized and characterized as previously reported (Roy et al., 2015). The MW150 compound was in hydrochloride hydrate form (MW = 490.43) and was dissolved in sterile 0.9% saline. We treated 2-month and 6-month old mhAPP and WT mice with a daily dose of either MW150 (2.5 mg/kg/day) or saline vehicle by intraperitoneal injection (i.p.) for 14 days. 4-(4-Fluorophenyl)-2-(4-metylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580), an isoform unspecific inhibitor of p38 MAPK (Tocris Bioscience) was prepared in dimethyl sulfoxide (DMSO) as stock solutions. For the in vitro administration, drugs were diluted to the final concentration in artificial CSF (aCSF) solution (mM: NaCl, 119; KCl, 2.5; CaCl2, 2; MgSO4, 1.2; NaH2PO4, 1; NaHCO3, 6.2; glucose, 10; HEPES, 10) and continuously perfused over slices starting at least 15 min before HFS, as in Origlia et al. (2008; 2014).
2.3. EC slice preparation and electrophysiology
For field recordings, we prepared EC-containing slices from mhAPP or WT mice as described previously (Origlia et al., 2008; Criscuolo et al., 2017). To induce long-term potentiation (LTP) we used a high frequency stimulation (HFS, 3 trains of 100 pulses at 100 Hz, at 10 s interval). The magnitude of LTP was calculated as the average of the field excitatory post-synaptic potentials (fEPSPs) amplitudes recorded in the last 10 min and expressed as percentage change relative to the baseline period. The application of solutions containing different compounds and vehicles were performed in different slices during the same experimental session. Data collection and analysis were performed in blind by two different operators.
2.4. Behavioral testing for EC-dependent associative memory tasks
The behavioral testing was performed as described in (Wilson et al., 2013). The Novel object-place recognition task (OPRT) and the Novel object-place-context recognition task (OPCRT) took place the day after and the second day after the 14th day of treatment, respectively. Testing took place in a 60-cm square box with 40-cm high walls that could be shaped with two sets of contextual features (hereby 1 and 2). The objects used were easily cleanable 3D household objects approximately the same size as a mouse in at least one dimension and made from plastic, metal or glass. Exploration of the objects was monitored via an overhead camera. After one week of extensive handling to habituate the mice to the experimenter, mice were individually habituated to the white context for one hour for 3 consecutive days. Behavioral testing proceeded in the following stages:
OPRT. Following 5 min of exploration in context 1, mice were given a sample trial where they were allowed to explore two different novel objects freely for 3 min. They were then removed from the box and placed in a holding cage for 1 min inter trial interval while the box was cleaned and shaped for the test trial. In the test trial, mice were presented with the same context as used in the sample trial, except there were two copies of one of the previously presented objects. Therefore, in the test trial one of the copies held the same location as in the sample trial (familiar OP association), while the other copy was presented in a novel location (novel OP association).
-
OPCRT. The first two sessions were repeated as above. For sample trial 2 (3 min), mice were exposed to context 2 and left to explore the same two objects but in opposite position from where they were in context 1. In the test trial, two copies of one of the previously presented objects were presented within one of the contexts, so that one of the copies had been seen in that location within that context before (familiar OPC association), while the other copy had been presented in that location and context before, but not in that location within that particular context (novel OPC association).
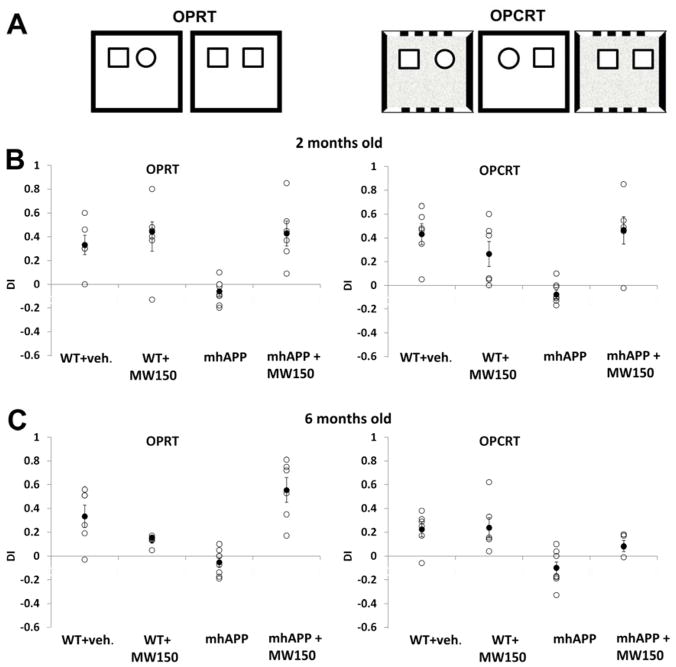
The object used in novel/familiar association, the side of presentation of the novel association, and the context used were counterbalanced as much as possible among experimental groups. In all the experiments, exploration time was counted when the mice were in the object close proximity (within 2 cm) with the nose directed towards it, sniffing or touching the object with the nose. Exploration time was not counted when the nose was pointing away from the object even if the mice were beside the object, running around it, sitting or climbing on it. To check for reliability the same separate observer re-scored a subset of videos in a blind fashion for each task and these scores were found to be consistent within 10% of the experimenter’s. The reliability among the two raters, (G.R. and M.S) who performed and scored behavioral tests independently was evaluated for a subset of videos (22%) that were scored in a blind fashion by both the experimenters. We calculated intra-class coefficient (ICC) estimates and their 95% confident intervals using SPSS statistical package version 22 (SPSS Inc, Chicago, IL) based on a mean-rating (k = 2), absolute-agreement, 2-way mixed-effects model. We obtained an ICC (2, 2) value of 0.958 (95% CI 0.874–0.984), indicating excellent inter-rater reliability (Portney and Watkins, 2000). For each task we converted observation scores into discrimination indices [DI = (time at novel − time at familiar)/(time at novel + time at familiar)] to determine the rates of exploration of novel relative to familiar OPC associations. Figure 2.A reports a schematic representation of the tasks. The Open Field Test was used to control for changes in locomotion and anxious behavior. Testing took place in the same box described above. Each animal was placed in the box for five minutes. We used the open-source toolbox developed by Patel, et al. 2014 to automatically compute the total ambulatory distance as well as the amount of time spent in outer zones versus inner zones (40×40), presented as a function of total time in the maze (Patel et al., 2014).
2.5 Statistical analysis
All data are reported as mean ± SEM. For electrophysiology statistical comparisons between experimental groups or between fEPSP amplitudes measured during baseline and after HFS were performed by applying a two-way repeated-measures ANOVA with pair wise multiple comparison procedures (Holm–Sidak method, Sigmaplot 12.0). For behavioral experiments, a one-way ANOVA was applied to determine the differences in average DI, exploration rates, total ambulatory distance, and thigmotaxis. One-sample t-tests were also used to determine whether the average discrimination index for each group was different from chance (hypothesized mean = 0). A p value < 0.05 was considered significant. For tests that did not reveal a statistical difference, we have reported the observed (post-hoc) power analyses, which were performed using G*Power 3.0.10.
3. Results
3.1. MW150 rescues LTP in EC-containing slices from early stage AD mice
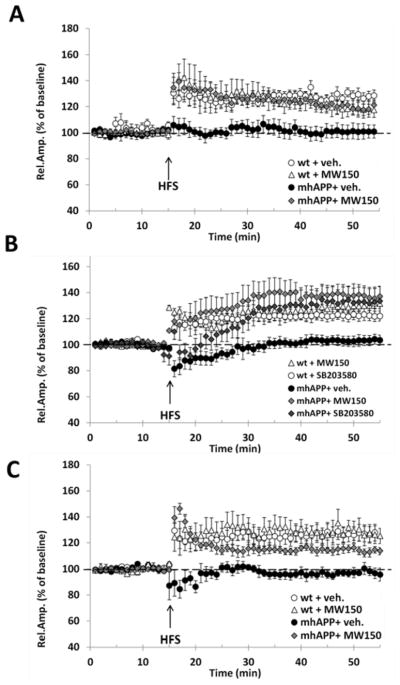
Young (2-month old) mhAPP mice present no alterations in basic synaptic transmission but a selective LTP impairment in the EC intrinsic circuitry (Criscuolo et al., 2017). We tested whether MW150 could prevent synaptic plasticity impairment at this age. We found a statistically significant difference between groups (F (3, 27) = 5.436, p = 0.005). Treating mhAPP mice with MW150 for 14 days was sufficient to restore a robust LTP upon HFS of the EC superficial layer. The mean LTP was in fact significantly increased with respect to mhAPP vehicle-treated mice (120 ± 6% of baseline, mice n = 4, slices n = 7, vs. 100 ± 4% of baseline, mice n = 4, slices n = 5; p = 0.024; Fig. 1.A) and was comparable to that recorded in WT vehicle-treated slices (128 ± 4% of baseline, mice n = 5, slices n = 11, p = 0.208; Fig. 1A). Notably, MW150 at the concentration tested did not affect synaptic plasticity in WT mice, as mean LTP was not significantly different from that in vehicle-injected WT mice (118 ± 4%, mice n = 4, slices n = 8; p = 0.235, with a power of 0.829; Fig. 1.A). Screening assay demonstrate that MW150 has a high permeability to CNS (Roy et al., 2015), however we further confirmed the efficacy of the compound after acute application on EC slices using a concentration that was shown to inhibit the phosphorylation activity of p38αMAPK. Perfusion with 10μM MW150 was capable of rescuing LTP expression in EC slices from mhAPP mice (F(4,25) = 4.561, p = 0.007; 137 ± 7% of baseline, mice n = 4, slices n = 6 vs. 104 ± 4% of baseline, mice n = 4, slices n = 6 in vehicle mhAPP slices; p = 0.007; figure 1.B). A comparable rescue was obtained with perfusion with 1μM SB203580 (133 ± 10% of baseline, mice n = 4, slices = 6, p = 0.014; figure 1.B) even though the effect was limited to the late-phase of LTP, consistently with a previous finding in slices treated with Aβ (Origlia et al., 2008). This first part of results demonstrated that MW150 has a protective effect on synaptic function at an early stage of neurodegeneration in mhAPP mice by acting on MAPK. In order to verify whether this effect could be retained as the AD phenotype progresses, we investigated 6-month old mhAPP mice. At this stage of neurodegeneration, evidence exists of diffuse amyloid immunoreactivity in the molecular layer of the dentate gyrus, and in the neocortex (Mucke et al., 2000). As reported in figure 1C, there was a statistically significant difference between groups (F(3, 21) = 8.005, p < 0.001), as HFS induced a stable LTP in vehicle-injected WT, but not in mhAPP EC slices (128 ± 7% of baseline, mice n = 4, slices n = 6 vs 97 ± 3% of baseline, mice n = 4, slices n = 6; p = 0.002). A statistically significant LTP was observed, however, after 14-day treatment with MW150 (114 ± 3% of baseline, mice n = 4, slices n = 7; vs. vehicle-treated mhAPP, p = 0.022). The LTP in mhAPP mice treated with MW150 was not significantly different as compared to WT slices (p = 0.128 vs vehicle-treated WT; p = 0.095 vs MW150-treated WT, with an acceptable power of 0.959; figure 1.C).
Figure 1. Impairment of LTP in mhAPP slices is prevented by MW150.
A) In 2-month old animals, LTP induction and maintenance was not affected by 14-day treatment with MW150 (2.5 mg/kg i.p.) in WT (open triangles) but rescued a normal LTP in slices from mhAPP mice (grey diamonds). Controls received 14-day i.p. injection of the vehicle (saline). B) Acute inhibition of p38MAPK by slices perfusion with either SB203580 (1μM) or MW150 (10μM) did not affect LTP in control WT (open circles and triangles) but was capable of rescuing a robust LTP in mhAPP slices with a comparable efficacy (light and dark grey diamonds). C) In vivo treatment with MW150 (2.5 mg/kg i.p.) confirmed its efficacy in 6-month old mhAPP mice. The mean LTP in the EC of mhAPP + MW150 mice (grey triangles) was comparable to controls (open circles and triangles) and significantly increased respect to mhAPP + vehicle group. Error bars indicate SEM
3.2. MW150 improves EC-dependent associative memory in mhAPP mice
We have previously reported that synaptic dysfunction is associated with impairment in associative memory, as the lateral EC superficial layers are involved in the combined elaboration of spatial (referred to context and objects position) and non-spatial (referred to objects) information (Criscuolo et al., 2017). In particular, the lateral EC is required for recognition of objects that have been experienced in a specific context and its lesion causes a selective impairment in in the execution of the OPRT and OPCRT memory tasks in the rat (Tsao et al., 2013; Wilson et al., 2013). We have confirmed that WT mice with selective lesions of the lateral EC show a similar impairment without affecting the recognition of a novel object in the execution of the ORT task (Criscuolo et al., 2017). Moreover, we demonstrated that synaptic deficit and activation of p38MAPK observed in the EC intrinsic circuitry of mhAPP mice are associated with a selective impairment in EC-dependent tasks as associative (OPRTand OPCRT) but not non-associative memories (ORT) were affected. Therefore, the next step was to investigate behaviorally the effect of MW150 in mhAPP mice. At 2 months of age, vehicle-treated (14 days of i.p. saline) mhAPP mice displayed impaired ability to discriminate novel associations of objects with their position and surrounding context, as compared to age-matched vehicle-treated WT mice (OPRT, F (3, 22) = 7.954, p < 0.001; OPCRT, F (3, 22) = 9.509, p < 0.001). The mean DI for mhAPP mice was significantly lower than the DI of WT mice for both OPRT (−0.06 ± 0.03, n = 8 vs. 0.33 ± 0.08, n = 6; p = 0.003) and OPCRT (−0.08 ± 0.03, n = 8 vs. 0.43 ± 0.09, n = 6; p = 0.001). According to the above findings from electrophysiology, a 14-day MW150 treatment had no effect on memory function in control WT mice (0.40 ± 0.12, n = 6, p = 0.584 with a power of 0.960 in OPRT and 0.26 ± 0.10, n = 6, p =0.195 with a power of 0.986 in OPCRT vs. vehicle treated WT); however, it was capable of ameliorating the cognitive performances of mhAPP mice as revealed by DI, which resulted in significant increase with respect to vehicle-injected mhAPP mice, and comparable to controls, in both tests (OPRT: 0.43 ± 0.10, n = 6; p = 0.002 vs. vehicle-treated mhAPP; p = 0.733 vs. vehicle-treated WT; OPCRT: 0.46 ± 0.11, n = 6; p = 0.001 vs. vehicle-treated mhAPP; p = 0.797 vs. vehicle-treated WT; Fig. 2B). No significant differences were found in locomotor and anxiety-related behavior. At 2 months of age, mice in different groups traveled overlapping total ambulatory distance in the box (F (3, 8) = 1.240, p = 0.36) and spent similar amount of time in outer zones (F (3, 8) = 0.363, p = 0.78) versus inner zones (F (3, 8) = 0.934, p = 0.47).
Figure 2.
MW150 treatment improves associative memory based on object recognition in mhAPP mice. In (A) left and right panel report a schematic depiction of the sample and test trials within the novel object place recognition task (OPRT) and novel object place/contest recognition task (OPCRT), respectively; In (B–C) plots represent individual discrimination indices (DI) for each mouse (white circles) and the average DI (black circle) calculated for each group of either 2-month old or 6-month old mice. Average DI are presented as mean ± SEM.
At a later stage of neurodegeneration (6 months of age), MW150 confirmed its protective effect on EC-dependent associative memory functions (OPRT, F (3, 22) = 14.980, p < 0.001; OPCRT, F (3, 22) = 7.407, p = 0.001). Novel association-discriminating performances were significantly improved in mhAPP mice treated with MW150 for 14 days (OPRT: 0.56 ± 0.13, n = 6; p < 0.001 vs. −0.05 ± 0.04, n=8 in vehicle treated mhAPP mice; OPCRT: 0.09 ± 0.04, n = 6; p = 0.028 vs. −0.10 ± 0.05, n= 8 in vehicle treated mhAPP mice), with no significant differences in DI as compared to vehicle-treated WT mice (OPRT: p = 0.218 with a power of 1.0; OPCRT: p = 0.161 with a power of 0.942). No significant differences were observed between groups either in terms of total locomotor activity (F (3, 8) = 4.846, p = 0.33) or time spent in outer zones (F (3, 8) = 0.383, p = 0.77) versus inner zones (F (3, 8) = 0,515, p = 0.68).
4. Discussion
The EC intrinsic circuitry is selectively vulnerable to synaptic plasticity alterations and corresponding associative memory impairment in a mouse model of progressive amyloid-dependent neurodegeneration (mhAPP) (Criscuolo et al., 2017). These deficits are associated with increased activation of RAGE and downstream phosphorylation of the p38MAPK, key events in amyloid-dependent neuroinflammation (Anderton et al., 2001; Criscuolo et al., 2017; Dai et al., 2016; Ferrer et al., 2005; Li et al., 2003; Origlia et al., 2008; Peel et al., 2004; Zhu et al., 2005). Therefore, p38MAPK are particularly suited as targets to retard or even halt neurodegeneration. Aβ-dependent synaptic failure driven by microglia is prevented by the p38MAPK inhibitor SB203580 in hippocampal slices (Wang et al., 2004) and EC (Origlia et al., 2014; Origlia et al., 2008). The α isoform of the p38MAPK has emerged as an interesting druggable site, as its inhibitors showed attenuation of neuroinflammation, synaptic alterations, and cognitive impairments (Bachstetter and Van Eldik, 2010; Watterson et al., 2013). In the present study we found that the selective inhibition of the p38α MAPK with an established efficacy dose of MW150 is capable of rescuing LTP in EC-containing slices and ameliorating EC-dependent associative memory functions in 2-month mhAPP mice. MW150 efficacy profile in the EC, one of the earliest affected brain regions in amyloid-induced neurodegeneration (Stranahan and Mattson, 2010), supports a potential role for MW150 as a disease-modifying drug in an early disease stage, before the appearance of AD-like histopathological findings. Our results were confirmed in a later stage of the disease progression, that is in mhAPP mice aged 6 months. The same drug could therefore act in different time windows, with increased potential for efficacy.
Our findings support MW150 as a drug candidate aimed at modifying the neuroinflammation-synaptic dysfunction cycle via the selective inhibition of the stress-activated p38α MAPK in the interacting microglia and neurons (Rask-Andersen et al., 2014; Watterson et al., 2013). A potential limitation of our study, which could confound the interpretation of results, is the variability in the early phase post-HFS for up to 15 min, emerging from the visual inspection of scatterplots in figure 1. We used fEPSP recordings in the EC, which has been rarely investigated with respect to hippocampal slices. For consistency with our previous work, as the main parameter we used the changes in the amplitude of fEPSP, which mirror the changes in the slope of the negative potentials and correlate with changes in the magnitude of a monosynaptic current sink when using cortical slices preparation (Mitzdorf, 1985). This may generate more variability in the early phase post-HFS with the presence of either post-tetanic potentiation or depression (Origlia et al., 2009; Origlia et al., 2008). This may be due also to the more complex circuitry that is present in the cortical layering with respect to the classical CA1 recording in the hippocampus, where the slope is the commonly used parameter.
MW150 treatment has already proved efficacious in the suppression of hippocampal-dependent associative and spatial memory deficits either in a preventative or in treatment paradigm (Roy et al., 2015). These effects are mediated by the modulation of stress-induced proinflammatory cytokine release driven by overactivated p38α MAPK, without changes in the microglia protective responses (Zhou et al., 2017). Specifically, the effects of MW150 were mediated by the increased release of IL-1β and TNFα but not IL-6. Together with efficacy, what makes MW150 particularly promising in the pursuit of drug candidates for AD are its pharmacological features of high target selectivity, oral bioavailability, brain penetrance and safety (Roy et al., 2015). These issues have indeed limited the development of p38α MAPK inhibitors so far, in the context of complex illnesses that require repeated administration, long-lasting exposure, and compatible use with other approved molecules in vulnerable subjects. In conclusion, MW150 and similar p38α MAPK inhibitors are worth exploring as therapeutic interventions to improve clinical outcomes in the early stages of neurodegeneration.
Highlights.
The p38αMAPK inhibitor MW150 rescues Entorhinal cortex -synaptic plasticity in AD mice
MW150 treatment ameliorates EC-dependent associative memory in early stage AD mice
The p38α MAPK inhibitors are worth exploring as therapeutics in neurodegeneration
Acknowledgments
Funding sources
This work was supported by the CNR Research Project on Aging (N.O.) and by the U01 AG043415 grant (M.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderton BH, Betts J, Blackstock WP, Brion JP, Chapman S, Connell J, Dayanandan R, Gallo JM, Gibb G, Hanger DP, Hutton M, Kardalinou E, Leroy K, Lovestone S, Mack T, Reynolds CH, Van Slegtenhorst M. Sites of phosphorylation in tau and factors affecting their regulation. Biochemical Society symposium. 2001;(67):73–80. doi: 10.1042/bss0670073. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Van Eldik LJ. The p38 MAP Kinase Family as Regulators of Proinflammatory Cytokine Production in Degenerative Diseases of the CNS. Aging and disease. 2010;1(3):199–211. [PMC free article] [PubMed] [Google Scholar]
- Benedet AL, Labbe A, Lemay P, Zimmer ER, Pascoal TA, Leuzy A, Mathotaarachchi S, Mohades S, Shin M, Dionne-Laporte A, Beaudry T, Picard C, Gauthier S, Poirier J, Rouleau G, Rosa-Neto P Alzheimer’s Disease Neuroimaging I. Epistasis analysis links immune cascades and cerebral amyloidosis. Journal of neuroinflammation. 2015;12:227. doi: 10.1186/s12974-015-0436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- C.I.G.o.A.s.D. Convergent genetic and expression data implicate immunity in Alzheimer’s. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2015;11(6):658–671. doi: 10.1016/j.jalz.2014.05.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscuolo C, Fontebasso V, Middei S, Stazi M, Ammassari-Teule M, Yan SS, Origlia N. Entorhinal Cortex dysfunction can be rescued by inhibition of microglial RAGE in an Alzheimer’s disease mouse model. Scientific reports. 2017;7:42370. doi: 10.1038/srep42370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai HL, Hu WY, Jiang LH, Li L, Gaung XF, Xiao ZC. p38 MAPK Inhibition Improves Synaptic Plasticity and Memory in Angiotensin II-dependent Hypertensive Mice. Scientific reports. 2016;6:27600. doi: 10.1038/srep27600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Current Alzheimer research. 2005;2(1):3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature reviews. Neuroscience. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y, Han J. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. Journal of immunology. 2008;180(7):5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23(5):1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiological reviews. 1985;65(1):37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Ann Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe SJ, Mudgett JS, Cupo S, Parsons JN, Chartrain NA, Fitzgerald C, Chen SL, Lowitz K, Rasa C, Visco D, Luell S, Carballo-Jane E, Owens K, Zaller DM. Chemical genetics define the roles of p38alpha and p38beta in acute and chronic inflammation. The Journal of biological chemistry. 2007;282(48):34663–34671. doi: 10.1074/jbc.M704236200. [DOI] [PubMed] [Google Scholar]
- Origlia N, Capsoni S, Cattaneo A, Fang F, Arancio O, Yan SD, Domenici L. Abeta-dependent Inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE. Journal of Alzheimer’s disease: JAD. 2009;17(1):59–68. doi: 10.3233/JAD-2009-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N, Criscuolo C, Arancio O, Yan SS, Domenici L. RAGE inhibition in microglia prevents ischemia-dependent synaptic dysfunction in an amyloid-enriched environment. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(26):8749–8760. doi: 10.1523/JNEUROSCI.0141-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N, Righi M, Capsoni S, Cattaneo A, Fang F, Stern DM, Chen JX, Schmidt AM, Arancio O, Yan SD, Domenici L. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(13):3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TP, Gullotti DM, Hernandez P, O’Brien WT, Capehart BP, Morrison B, 3rd, Bass C, Eberwine JE, Abel T, Meaney DF. An open-source toolbox for automated phenotyping of mice in behavioral tasks. Frontiers in behavioral neuroscience. 2014;8:349. doi: 10.3389/fnbeh.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel AL, Sorscher N, Kim JY, Galvan V, Chen S, Bredesen DE. Tau phosphorylation in Alzheimer’s disease: potential involvement of an APP-MAP kinase complex. Neuromolecular medicine. 2004;5(3):205–218. doi: 10.1385/NMM:5:3:205. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Prentice Hall; New Jersey: 2000. [Google Scholar]
- Rask-Andersen M, Zhang J, Fabbro D, Schioth HB. Advances in kinase targeting: current clinical use and clinical trials. Trends in pharmacological sciences. 2014;35(11):604–620. doi: 10.1016/j.tips.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Roy SM, Grum-Tokars VL, Schavocky JP, Saeed F, Staniszewski A, Teich AF, Arancio O, Bachstetter AD, Webster SJ, Van Eldik LJ, Minasov G, Anderson WF, Pelletier JC, Watterson DM. Targeting human central nervous system protein kinases: An isoform selective p38alphaMAPK inhibitor that attenuates disease progression in Alzheimer’s disease mouse models. ACS chemical neuroscience. 2015;6(4):666–680. doi: 10.1021/acschemneuro.5b00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Shen L, Yao X, Kim S, Nho K, Risacher SL, Ramanan VK, Foroud TM, Faber KM, Sarwar N, Munsie LM, Hu X, Soares HD, Potkin SG, Thompson PM, Kauwe JS, Kaddurah-Daouk R, Green RC, Toga AW, Weiner MW Alzheimer’s Disease Neuroimaging I. Genetic studies of quantitative MCI and AD phenotypes in ADNI: Progress, opportunities, and plans. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2015;11(7):792–814. doi: 10.1016/j.jalz.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoder L, Hao W, Qin Y, Liu S, Tomic I, Liu X, Fassbender K, Liu Y. Deficiency of Neuronal p38alpha MAPK Attenuates Amyloid Pathology in Alzheimer Disease Mouse and Cell Models through Facilitating Lysosomal Degradation of BACE1. The Journal of biological chemistry. 2016;291(5):2067–2079. doi: 10.1074/jbc.M115.695916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s disease. Neural plasticity. 2010;2010:108190. doi: 10.1155/2010/108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Moser MB, Moser EI. Traces of experience in the lateral entorhinal cortex. Current biology: CB. 2013;23(5):399–405. doi: 10.1016/j.cub.2013.01.036. [DOI] [PubMed] [Google Scholar]
- Van Eldik LJ, Carrillo MC, Cole PE, Feuerbach D, Greenberg BD, Hendrix JA, Kennedy M, Kozauer N, Margolin RA, Molinuevo JL, Mueller R, Ransohoff RM, Wilcock DM, Bain L, Bales K. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimer’s & dementia. 2016;2(2):99–109. doi: 10.1016/j.trci.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(13):3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson DM, Grum-Tokars VL, Roy SM, Schavocky JP, Bradaric BD, Bachstetter AD, Xing B, Dimayuga E, Saeed F, Zhang H, Staniszewski A, Pelletier JC, Minasov G, Anderson WF, Arancio O, Van Eldik LJ. Development of Novel In Vivo Chemical Probes to Address CNS Protein Kinase Involvement in Synaptic Dysfunction. PloS one. 2013;8(6):e66226. doi: 10.1371/journal.pone.0066226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DI, Watanabe S, Milner H, Ainge JA. Lateral entorhinal cortex is necessary for associative but not nonassociative recognition memory. Hippocampus. 2013;23(12):1280–1290. doi: 10.1002/hipo.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor perspectives in medicine. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Bachstetter AD, Van Eldik LJ. Microglial p38alpha MAPK is critical for LPS-induced neuron degeneration, through a mechanism involving TNFalpha. Molecular neurodegeneration. 2011;6:84. doi: 10.1186/1750-1326-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Bachstetter AD, Van Eldik LJ. Inhibition of neuronal p38alpha, but not p38beta MAPK, provides neuroprotection against three different neurotoxic insults. Journal of molecular neuroscience: MN. 2015;55(2):509–518. doi: 10.1007/s12031-014-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Bachstetter AD, Spani CB, Roy SM, Watterson DM, Van Eldik LJ. Retention of normal glia function by an isoform-selective protein kinase inhibitor drug candidate that modulates cytokine production and cognitive outcomes. Journal of neuroinflammation. 2017;14(1):75. doi: 10.1186/s12974-017-0845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Mei M, Lee HG, Wang Y, Han J, Perry G, Smith MA. P38 activation mediates amyloid-beta cytotoxicity. Neurochemical research. 2005;30(6–7):791–796. doi: 10.1007/s11064-005-6872-x. [DOI] [PubMed] [Google Scholar]