Abstract
Cardiovascular disease in patients with multiple myeloma may derive from factors unrelated to the disease (age, diabetes, dyslipidemia, obesity, prior cardiovascular diseases), related to the disease (cardiac AL-amyloidosis, hyperviscosity, high-output failure, arteriovenous shunting, anemia, renal dysfunction) and/or related to anti-myeloma treatment (anthracyclines, corticosteroids, alkylating agents, immunomodulatory drugs, proteasome inhibitors). Good knowledge of cardiovascular events, effective dose reductions, prevention and management of early and late cardiovascular side effects of chemotherapeutic agents are essential in current clinical practice. Myeloma experts are obliged to carefully balance the efficacy and toxicity of drugs for each individual patient. This review summarizes current data and novel insights into cardiovascular adverse events of today’s anti-myeloma treatment, focusing on carfilzomib, as a starting point for developing consensus recommendations on preventing and managing cardiovascular side effects in patients with multiple myeloma.
Introduction
Multiple myeloma (MM) is a plasma cell dyscrasia accounting for 1% of neoplastic diseases. It typically affects the elderly population, with the median age at diagnosis being 70 years.1,2 Cardiovascular disease is one of the most frequent comorbidities in MM patients,3 being the main cause of death in western countries.4 Since the global population is aging, the prevalence of both MM and cardiovascular disease is expected to increase in the near future.5,6
Cardiovascular disease in MM may derive from factors unrelated to the disease (age, diabetes, dyslipidemia, obesity, history of cardiovascular diseases), or those related to the myeloma (cardiac AL-amyloidosis, hyperviscosity, high-output failure, arteriovenous shunting, anemia, renal dysfunction) and/or be related to the treatment of the disease [anthracyclines, corticosteroids, alkylating agents, immunomodulatory drugs, proteasome inhibitors, high-dose conditioning and autologous/allogeneic stem cell transplantation (SCT)].7 Of interest, the incidence of cardiovascular disease has been described to be higher in patients with MM (60.1%) than in non-MM patients (54.5%)7 and a recent review and meta-analysis of patients enrolled in clinical trials with carfilzomib showed that the incidence of all grade and grades ≥3 cardiovascular adverse events (CVAEs) was 18.1% and 8.2%, respectively.8 Although carfilzomib is one of the most cardiotoxic and, at the same time, effective novel agents used in MM treatment, it is not the only anti-myeloma drug characterized by a high risk of CVAEs. Moreover, it is difficult to estimate the actual incidence of chemotherapy-induced cardiovascular diseases, because current data about drug-induced cardiotoxicity were generated in clinical trials, from which patients with severe cardiovascular comorbidities were excluded. However, in real-life clinical practice, MM patients may indeed suffer from cardiovascular disease, have cardiovascular risk factors, and/or may have already received several cardiotoxic drugs. Physicians managing patients with MM are, therefore, obliged to carefully balance expected drug efficacy with toxicity. This review is published as a consensus paper by the European Hematology Association (EHA), the European Myeloma Network (EMN) and the Italian Society of Arterial Hypertension (SIIA), and it aims to describe CVAEs related to anti-myeloma treatment, especially novel agents and the proteasome inhibitor carfilzomib. Furthermore, the paper intends to be a starting point for future debates on how to manage CVAEs appropriately. At present, studies to validate recommendations on prevention and management of CVAEs during MM treatment are lacking, and overcoming this lacuna is particularly relevant. Besides focusing on toxicity issues, this review also aims to provide a more complete overview of MM treatments by clarifying the efficacy and outcome advantages associated with such treatments. Of note, this paper is not only a review of the current literature, but it is also the result of a thorough discussion involving a panel of EMN clinical experts in MM and cardiology experts.
Cardiovascular toxicity of multiple myeloma treatment
Antineoplastic therapy is frequently complicated by the onset of CVAEs, which in turn may increase treatment-related morbidity and mortality.9 Cardiovascular disease or events are among the most frequent adverse events during chemotherapy, leading to the concept of cardiotoxicity, which may be due to the direct effects of antineoplastic treatment on the structure and function of the heart or may accelerate the onset of cardiovascular disease in high-risk patients with predisposing cardiovascular risks.10
Cardiovascular complications of antineoplastic therapy may be acute, subacute or chronic and can be classified depending on the type of damage,11 the organ structure or function targeted by the damage,10 or the drug responsible for the damage.12 Depending on the damage, two types of cardiotoxicity have been described: type I and type II. In type I cardiotoxicity, mostly associated with traditional anticancer therapies, there is irreversible destruction of myocytes, leading to congestive heart failure. Type II cardiotoxicity may involve a reversible loss of cardiac contractility and is mainly caused by new targeted therapies, such as vascular endothelial growth factor inhibitors and tyrosine kinase inhibitors.11
With regards to the organ structure or function that is the target of the damage, the classification of cardiovascular chemotherapy-induced complications recognizes myocardial dysfunction and heart failure, coronary artery disease and myocardial ischemia, arrhythmias, hypertension, thromboembolic disease, peripheral vascular disease and stroke, pulmonary hypertension, valvular diseases and pericardial complications.12 In MM, drug-related cardiotoxicity may be caused by chemotherapeutic agents or by new targeted therapies, such as immunomodulatory drugs (thalidomide, lenalidomide, pomalidomide) and proteasome inhibitors (bortezomib, ixazomib, carfilzomib).
Cardiovascular toxicity induced by chemotherapy
Myocardial dysfunction and heart failure are common side effects of chemotherapy and may appear early during antineoplastic treatment or years thereafter. The risk of heart failure is increased in elderly patients with cardiovascular risks. Anthracyclines and alkylating agents are associated with direct cytotoxic cardiac injury. Cardiac damage induced by anthracyclines involves myocyte cell membrane injury by oxygen free radicals and lipid peroxidation, leading to oxidative stress10 and reduced cardiac contractility. In <1% of patients the effects may be manifested acutely (as supraventricular arrhythmia, transient left ventricular dysfunction or electrocardiographic changes). More commonly, the manifestations occur early within the first year of treatment [decline in left ventricular ejection fraction (LVEF)] or late (chronic heart failure). Cardiotoxic effects are dose-dependent and increased in patients with pre-existing cardiovascular diseases and in elderly patients. Cardiotoxicity is amplified by combination treatment, such as with alkylating or antimicrotubule agents, immuno- and targeted drugs. Cyclophosphamide induces heart failure in 7-28% of cases,9 although mainly in relation to high doses before autologous SCT. The mechanism of damage may be direct endothelial injury, extravasation of toxic metabolites with interstitial hemorrhage, edema and structural damage to cardiomyocytes. Anthracyclines and alkylating agents may induce arrhythmias through direct cardiotoxicity, cardiac ischemia or metabolic changes caused by the chemotherapy.13 Moreover, all cancers lead to a prothrombotic state, release of proangiogenic cytokines and drug-induced hepatotoxicity, which may contribute further to thromboembolism. Thromboembolic risks are particularly high in patients with other predisposing factors, in those with disseminated tumors and in those with hematologic neoplasms, in particular MM.14 Arterial thromboembolism is a rare event and may occur in patients receiving anthracyclines. Venous thromboembolism is frequent, occurring in 15-20% of patients with MM.10 Anthracyclines and cyclophosphamide may also cause acute pericarditis,11 and high doses of alkylating agents have been associated with pulmonary veno-occlusive disease.10
Cardiovascular toxicity induced by new targeted therapies, such as immunomodulatory drugs
Immunomodulatory drugs may induce arrhythmias, such as bradycardia and atrioventricular block. The bradycardia and heart block may be caused by the antineoplastic drugs themselves, age-related fibrosis or AL-amyloidosis. Thalidomide is associated with sinus bradycardia in 5% of patients. Sinus bradycardia can lead to syncope and may require placement of a pacemaker.15 Immunomodulatory drugs are characterized by an increased risk of thromboembolic events. The mechanisms underlying this phenomenon are direct damage to endothelial cells, increased platelet aggregation and higher von Willebrand factor serum levels. In the absence of thromboprophylaxis, the risk of venous thromboembolism is 1.3 and 4.1 per 100 patient-cycles in newly diagnosed patients receiving thalidomide alone and in combination with dexamethasone, respectively. The corresponding risk at relapse is 0.4 and 0.8 per 100 patient-cycles and it increased to 6.7 in patients treated with thalidomide, dexamethasone and doxorubicin.16 In the absence of thromboprophylaxis, the risk of venous thromboembolism is 0.8 and 0.7 per 100 patient-cycles in newly diagnosed and relapsed patients, respectively, receiving lenalidomide and dexamethasone. Venous thromboembolism may be fatal if complicated by pulmonary embolism. Adequate prophylaxis, which is based on risk evaluation, is therefore mandatory. Risk factors can be related to characteristics of the patient (age, obesity, history of venous thromboembolism, central-venous catheter, comorbidities – such as diabetes, infections, cardiac diseases –, surgical procedures – including vertebroplasty and kyophoplasty –, and inherited thrombophilia); related to the myeloma (diagnosis per se, hyperviscosity) and related to therapy (high-dose dexamethasone, doxorubicin, or multi-agent chemotherapy). Prophylaxis in low-risk patients (≤1 patient/myeloma-related risk factor) consists of aspirin 100 mg, unless contraindicated. If more than one risk factor is present, low molecular weight heparin or full-dose warfarin should be used and continued for at least 4 months; subsequently, switching to aspirin may be an option.17,18 New oral anticoagulants are increasingly being considered, although these drugs have not been systematically studied in myeloma yet. Specific recommendations cannot, therefore, be made as yet.19 Recently, a pilot study with the novel oral anticoagulation agent apixaban was conducted to evaluate the feasibility of venous thrombo-prophylaxis in 104 patients with MM, during treatment with immunomodulatory drugs. Results were encouraging but further evaluation is needed.
Cardiovascular toxicity of proteasome inhibitors
The metabolism of cellular proteins has a pivotal role in regulating cell function and homeostasis. Intracellular protein degradation is mainly regulated by the ubiquitin-proteasome pathway, which acts on proteins involved in cell cycle functions, apoptosis, transcription and DNA repair.20 Proteins are tagged by ubiquitin and recognized by the 26S proteasome complex, which degrades proteins into small peptides.21
If proteasome activity is altered, cells stop growing and undergo apoptosis at an increased rate because of an increasingly aberrant proteome.22 In several cancers, neoplastic cells are more sensitive than untransformed cells to proteasome inhibition. Based on this evidence, proteasome inhibitors therefore represent a highly relevant therapeutic strategy in MM treatment.
The pathophysiology of the cardiotoxicity induced by proteasome inhibitors is not entirely clear. One mechanism could be the inhibition of sarcomeric protein turnover, resulting in myocyte death.23 Other hypotheses are increased apoptosis of endothelial progenitor cells, endothelial nitric oxide synthase dysfunction, functional/structural abnormalities of cardiomyocytes secondary to protein accumulation due to impaired protein degradation, inhibition of physiological NFκB activity, potentiation of the effects of other cardiotoxic agents, suppression of the adaptive/cytoprotective activity of the ubiquitin-proteasome pathway in an underlying cardiomyopathy, myocardial scarring and fibrosis, endoplasmic reticulum stress induction in cardiomyoblasts, dysfunction of cardiac mitochondria, contractile left ventricular dysfunction and/or increased smooth muscle cell apoptosis causing atherosclerotic plaque instability.24–29
Bortezomib
Bortezomib is a dipeptide boronic acid that inhibits the proteolytic activity of the proteasome chymotrypsin-like site, via the formation of a reversible interaction at the 26S proteasome. With regards to bortezomib-related cardiotoxicity, the information leaflet for patients contains a warning concerning the possibility of acute development or exacerbation of chronic heart failure and a new onset of decreased LVEF. There have also been isolated cases of QT-interval prolongation, but causality has not been established.30
The incidence of all grades of cardiac dysfunction in patients taking bortezomib shows marked variability from 2% to 17.9%, depending on the clinical trial.9,29 In the meta-analysis conducted by Xiao et al., the incidence of all grades and high-grade bortezomib-related cardiotoxicity was 3.8% and 2.3%, respectively.29 Of interest, bortezomib does not seem to significantly increase the risk of all grades or high-grade cardiotoxicity, if compared with control medications. Nevertheless, the overall incidence of bortezomib-related cardiotoxicity of all grades was higher in MM patients (4.3%) than in non-MM patients.29 The main limitation of this meta-analysis was, however, that many cardiovascular comorbidities, as well as concomitant therapies, were unknown or unrecorded. Moreover, cardiotoxicity was misreported in many studies and the trials’ treatment designs were very different, giving rises to heterogeneity of data regarding cardiovascular effects of bortezomib and a high probability of confounding effects secondary to other prior treatments or clinical conditions.
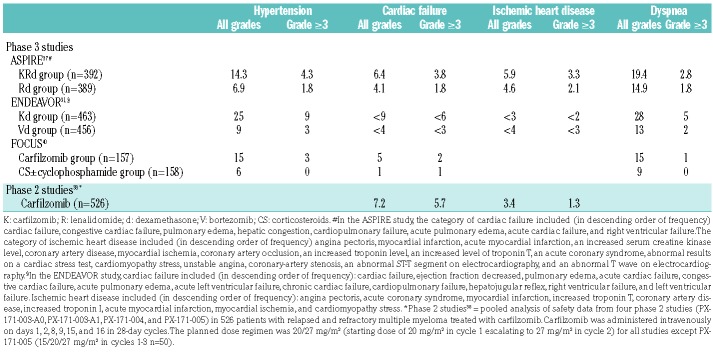
In the ENDEAVOR study, patients were randomized to receive carfilzomib and dexamethasone (Kd) or bortezomib and dexamethasone (Vd).31 The patients’ median age was fairly low (65 years), with only about 15% of patients aged ≥75 years. The incidence of CVAEs with Vd treatment is summarized in Table 1. During treatment or within 30 days of receiving the last study-dose, 5% of patients in the Vd group died and cardiovascular events were the second cause of death. Interestingly, dyspnea was reported as a CVAE, but its etiology (cardiac, pulmonary versus other causes) remained undetermined, so it could be classified as either a cardiovascular or pulmonary adverse event.
Table 1.
Incidence (in %) of cardiovascular events in patients with relapsed/refractory multiple myeloma treated with carfilzomib in phase 2 and 3 studies
Ixazomib
ixazomib is an oral analog of bortezomib that reversibly inhibits the chymotrypsin-like site of the 20S proteasome, and is responsible for caspase-dependent induction of apoptosis and inhibition of cell cycle in MM cells. Moreover, ixazomib inhibits the NFκB pathways in MM supporting cells, thus influencing cytokines important for MM cell growth and survival.20 Kumar et al. reported an incidence of grade ≥3 hypertension of 5% in treatment-naïve patients given ixazomib in combination with lenalidomide and dexamethasone (Rd).32 The TOURMALINE MM1 study group analyzed the safety and efficacy profile of ixazomib in a phase 3 trial in patients with relapsed, refractory or relapsed and refractory MM.33 Patients received ixazomib-Rd or placebo-Rd. The rates of serious adverse events were similar in both groups (47% and 49%), as were death rates during the study (4% and 6%, respectively); grade 3 or higher adverse events occurred in 74% and 69% of cases, respectively. There were no differences in the incidence of heart failure (4% in each group), arrhythmias (16% and 15%), hypertension (6% and 5%) and myocardial ischemia (1% and 2%, respectively). At present, there is only one case report in which ixazomib was described as possibly responsible for cardiotoxicity with a mechanism similar to that observed with bortezomib (type I chemotherapy-induced cardiotoxicity).34
Carfilzomib
Relapsed or refractory multiple myeloma
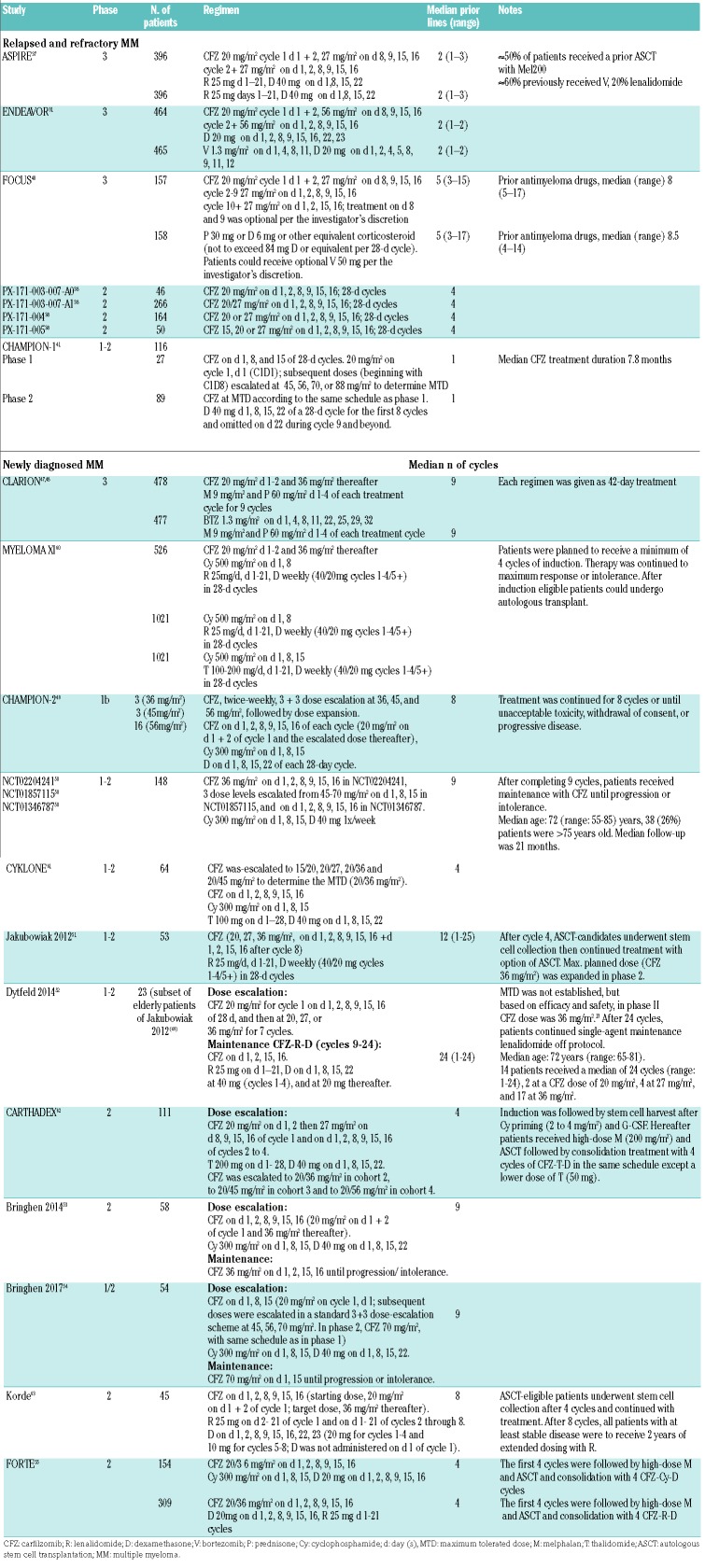
This second-generation proteasome inhibitor is an irreversible cell-permeable tetrapeptide epoxyketone, an analog of epoxomicin.35 The irreversible binding leads to the activity of carfilzomib being more sustained than that of bortezomib, as new proteasome subunit synthesis and assembly are required for restoration of proteasome activity.21 Carfilzomib has been demonstrated to be more specific than bortezomib, and the irreversible carfilzomib binding may provide better inhibition of proteasome activity, which may overcome bortezomib-resistance.36 In a head to head study of carfilzomib versus standard treatments, bortezomib or lenalidomide, carfilzomib demonstrated superior efficacy and was associated with improved overall survival. Data regarding cardiac events in MM patients receiving carfilzomib are available from various phase 1, 2 and 3 studies, as well as retrospective and observational analyses, in both relapsed/refractory MM and newly diagnosed MM. Data regarding the dosing and treatment schedules of relevant studies are reported in Table 2. The most frequent adverse event was hypertension. In randomized clinical trials, the relative risks of all-grade and grade ≥3 CVAEs were 1.8 and 2.2, respectively, for patients receiving carfilzomib compared with control patients. Carfilzomib doses of 45 mg/m2 or higher were associated with high-grade CVAEs.8
Table 2.
Data from clinical trials of carfilzomib in relapsed/refractory multiple myeloma and newly diagnosed multiple myeloma.
The incidence of hypertension in patients with relapsed/refractory MM being treated with carfilzomib was 14.3-25% for all grades and 3-9% for grade ≥3, the cumulative incidence of cardiac failure of any grade was 5-9% and that of grade ≥3 was 2-6%. Corresponding cumulative values for ischemic heart disease were 3-5.9% and 1.3-3.3%. The incidence of dyspnea of any grade was 15-19.4% while that of grade ≥3 was 1-5% (Table 1). The most frequent cardiovascular events were dyspnea and hypertension.31,37–43
Carfilzomib has been approved in Europe for the treatment of relapsed or refractory MM, in combination with Rd or dexamethasone, based on the randomized trials ASPIRE37 and ENDEAVOR,31 and after analysis of safety data derived from 526 patients enrolled in four phase 2 carfilzomib studies.38
In the ASPIRE study, the incidence of dyspnea was 19.4% considering all grades and 2.8% considering only grade ≥3 events, although the origin (cardiac, infectious, pulmonary) remained unspecified.37 There was also increased hypertension, which was almost double in the carfilzomib-Rd (KRd) group as compared to the Rd group (14.3% versus 6.9% for all grades and 4% versus 2% for grade ≥3 in the KRd and Rd groups, respectively). However, cardiac death rates were similar in the two arms: four deaths with KRd (3 myocardial ischemia, 1 cardiac failure) and four with Rd (1 myocardial ischemia, 3 cardiac failures).
The findings of the ENDEAVOR study31 were similar to those of ASPIRE, with a 28% incidence of dyspnea of unspecified origin and 25% of hypertension in patients treated with Kd. In this study, there were five cardiac deaths with Kd and six with Vd. Patients in the Kd group showed a higher incidence of grade ≥3 CVAEs, including hypertension, cardiac failure and dyspnea, while the incidence of ischemic heart disease was similar in the two groups. Venous thromboembolism was more common with Kd than with Vd (deep vein thrombosis: 3.7% for all grades and 0.9% for grade ≥3 in Kd recipients versus 0.9% for all grades and 0.4% for grade ≥3 in Vd recipients; pulmonary embolism: 2.6% for all grades and 1.7% for grade ≥3 in Kd recipients versus 0.9% for all grades and 0.9% for grade ≥3 in Vd recipients). Of interest, a preplanned prospective ENDEAVOR substudy (ECHO study) was performed to evaluate changes from baseline in LVEF, right ventricular function and pulmonary artery systolic pressure via echocardiography.39 Patients with a LVEF ≥40% and no evidence of New York Heart Association class III or IV heart failure, symptomatic ischemia, uncontrolled arrhythmias or recent myocardial ischemia were enrolled. All patients (75 treated with Kd, 76 treated with Vd) were assessed using two-dimensional transthoracic echocardiograms at baseline, every 12 weeks and at the end of treatment. Notable differences between the two treatment groups were that more patients in the Kd group were older than 75 years: 21.3% versus 14.5% in the Vd group) and more had a prior cardiac-related history (26.7% versus 14.5%, respectively). Patients in the Kd arm had a higher incidence of heart failure reported by the treating physician (10.8% versus 4.1%, respectively). A history of cardiac disorders was associated with an elevated but not statistically significant different risk of heart failure. Patients receiving carfilzomib had a higher incidence of hypertension compared to those receiving bortezomib (20.3% versus 8.1%, respectively). Twenty-three patients (15.2%) discontinued treatment because of adverse events; eight due to non-fatal cardiac-related adverse events (Kd: n=6 versus Vd: n=2). The primary endpoint was a reduction in LVEF by 24 weeks. The ECHO data were analyzed in a blinded fashion. Among all patients in both arms, only one (in the Vd group) had a significant reduction in LVEF within the first 24 weeks. Six patients (3 in each group) had significant LVEF reductions at some time during the study, with normal LVEF being recovered in all but one. Fourteen patients (Kd: n=8; Vd: n=6) had clinically meaningful changes in echocardiograms; 79% did not meet the echocardiographic criteria for decreased LVEF. More Kd than Vd recipients had clinical evidence of heart failure (n=4 versus n=0, respectively) or pulmonary hypertension (n=4 versus n=1, respectively) based on investigator assessment. Thus, heart failure and pulmonary hypertension occurred more frequently with Kd than with Vd, although a echocardiographically detectable significant decline in LVEF was low in both treatment arms and occurred with similar frequencies. The substudy found limited utility for serial screening with echocardiograms to mitigate cardiac risks for unselected patients receiving carfilzomib. Another subgroup analysis of the ENDEAVOR study, conducted in 109 Asian patients, showed a toxicity profile similar to that in the general population. In the carfilzomib arm, hypertension was reported in 26.4% of patients, dyspnea in 17.0%.44
The different incidence of cardiotoxicity in ENDEAVOR and ASPIRE can be explained by comparing both study designs. The first factor is the carfilzomib dose: there was a higher incidence of cardiotoxicity in the ENDEAVOR study, but carfilzomib doses were substantially higher (56 mg/m2 versus 27 mg/m2). The second issue is that nearly 20% of the patients enrolled in the ENDEAVOR trial had a creatinine clearance <50 mL/min (and 6% of them had a creatinine clearance <30 mL/min) and, therefore, had a higher cardiovascular risk, while in ASPIRE only 6.3% of patients had a creatinine clearance between 30 and 50 mL/min.45 Consequently, a side-by-side comparison of both trials is hampered by different patient eligibility criteria.
An integrated analysis of patients with advanced MM enrolled in four phase 2 studies showed that 73.6% of patients had a history of cardiovascular events, and 70% had baseline cardiac risks.38 Cardiac failure (including chronic heart failure, pulmonary edema, and decreased LVEF) occurred in 7.2% of patients. The overall mortality rate was the same in patients with or without cardiovascular risks at baseline. CVAEs occurred in 22.1% and 14.3% of patients had hypertension (mainly grade 1–2). Cardiac events leading to treatment discontinuation occurred in 4.4% of patients. Cardiac adverse events did not increase in later treatment cycles, suggesting that there was no cumulative toxicity. Among CVAEs, arrhythmias of any grade were observed in 13.3% of patients (grade ≥3 in 2.3%). Dyspnea was included in respiratory adverse events and appeared in 42.2% of patients (all grades) and was grade ≥3 in 4.9%.
Newly diagnosed multiple myeloma
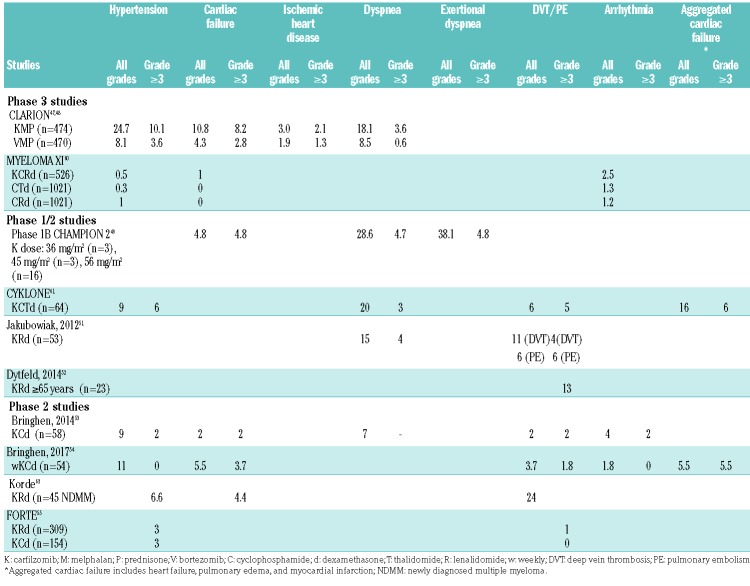
Carfilzomib, alone or in combination with other drugs, has also been investigated in newly diagnosed MM patients in several ongoing and completed studies.46 Data derived from these trials are very useful, because they are not biased by possible cardiotoxic effects of previous lines of therapy. The incidence of any grade hypertension was 9-24.7% while that of grade ≥3 was 2-10%. The incidence of any grade dyspnea was 18.1-28.6% while that of grade ≥3 was 3.6-4.8%. The most frequent cardiovascular events were dyspnea and hypertension (Table 3) with no apparent correlation with carfilzomib doses, schedules or other drug associations.
Table 3.
Incidence of cardiovascular events in patients with newly diagnosed multiple myeloma treated with carfilzomib in phase 1, 2 and 3 studies.
The CLARION trial compared carfilzomib, melphalan, and prednisone (KMP) versus bortezomib, melphalan, and prednisone (VMP) in newly diagnosed MM patients ineligible for transplant.47,48 The incidence of grade ≥3 adverse events was 74.7% versus 76.2%, respectively. Adverse events of interest included cardiac failure (all grades: 10.8% versus 4.3%; grade ≥3: 8.2% versus 2.8%), dyspnea (all grades: 18.1% versus 8.5%; grade ≥3: 3.6% versus 0.6%), and hypertension (all grades: 24.7% versus 8.1%; grade ≥3: 10.1% versus 3.6%, respectively).
The CHAMPION-2 study evaluated carfilzomib with cyclophosphamide and dexamethasone (KCd).49 One patient in the 45 mg/m2 cohort died due to sudden cardiac arrest, whereas no deaths occurred in the 36 or 56 mg/m2 cohorts. The authors concluded that carfilzomib administered at a dose of 56 mg/m2 twice weekly in combination with cyclophosphamide and dexamethasone showed acceptable toxicity. Likewise, an integrated analysis of newly diagnosed MM patients enrolled in three phase 1/2 studies evaluated CVAEs in patients treated with KCd.50 Any-grade cardiovascular events were reported in 42% of patients. The risk of CVAEs during treatment with carfilzomib was significantly higher in patients ≥75 years old and the most important risk factor, regardless of age, was hypertension. Grade ≥3 adverse events occurred in 30% of patients. In patients <75 years, the most frequent grade ≥ 3 adverse events were hypertension (9%) and dyspnea (10%). In patients ≥75 years, the most frequent grade ≥3 adverse events were hypertension and pulmonary edema (both: 13%). Thirty-four percent of patients who developed CVAEs had hypertension at baseline or developed it during treatment compared with 14% of patients who did not have CVAEs. The risk of cardiotoxicity was lower during maintenance, with 22% of patients developing a cardiovascular event of any grade. The most frequent CVAE during maintenance was again hypertension (12%), regardless of age. Five cardiovascular carfilzomib-related deaths were reported. No difference was observed in relation to different carfilzomib doses and schedules.
A phase 1/2 study in newly diagnosed MM patients assessed carfilzomib, lenalidomide and weekly dexamethasone. During induction, grade 3/4 CVAEs included deep venous thrombosis/pulmonary embolism in 9% of cases. Grade 3/4 dyspnea was observed only during phase 1 and within the first three cycles.51 An updated follow-up in a subset of 23 elderly patients (≥65 years) was also performed:52 during induction, non-hematologic grade 3/4 adverse events (>10%) included thromboembolic events (13%). During maintenance, most adverse events were grade 1/2 and did not include CVAEs. This could be partially because patients reaching the maintenance phase have deeper and longer responses to therapy, and tolerate the treatment better.
In a multicenter, open-label phase 2 trial of KCd in elderly newly diagnosed MM patients, ineligible for autologous SCT53, cardiac adverse events of any grade occurred during induction in 16% of patients and of grade ≥3 in 7% and included four cardiac events (heart failure, arrhythmia, myocardial ischemia and hypertension in one patient each) and stroke, acute pulmonary edema and pulmonary thromboembolism (also in one patient each). The safety profile was similar in the 15 patients >75 years who received one or more doses of KCd. As expected, no CVAEs occurred during maintenance. Seven patients died while on the study, one due to hypertension (related to carfilzomib) and another due to atrial fibrillation (deemed unrelated to carfilzomib).
A multicenter, open-label phase 1/2 trial determined the safety and efficacy of KCd in a weekly schedule in newly diagnosed patients aged ≥65 years who were ineligible for autologous SCT.54 Cardiopulmonary adverse events occurred in 9%. During induction, the incidence of CVAEs of any grade was 24%, while the incidence of CVAEs of grade 3–5 was 9%. Treatment-emergent serious adverse events occurred during induction in 26% of patients and included eight cardiac events (heart failure, pulmonary edema, sudden death and hypertension) and one pulmonary thromboembolism. During maintenance, any grade hypertension occurred in 15% of cases. Grade 3-5 non-hematologic adverse events were rare except for hypertension (10% of cases) and CVAEs (5%: 1 heart failure and 1 myocardial ischemia). Treatment-emergent serious adverse events occurred during maintenance in three patients and included heart failure and myocardial ischemia.
The FORTE trial compared KRd versus KCd in transplant-eligible patients with newly diagnosed MM.55 No significant differences were seen in rates of CVAEs (hypertension: 7% versus 6%, cardiac adverse events: 3% versus 5%), with the exception of venous thromboembolism/pulmonary embolism (8% versus 2%). The slightly lower incidence of CVAEs, as compared to other studies, could be partially explained by the fact that newly diagnosed MM patients eligible for transplantation are fitter than those with relapsed or refractory MM and/or patients ineligible for autologous SCT.
Real-life experience
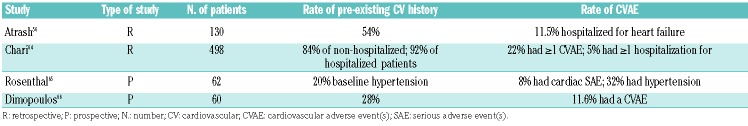
Data derived from “real-life” experience with carfilzomib unite more unselected patients than those treated within clinical trials, although they do not add much to currently available knowledge (Table 4). The most robust data are derived from the study by Atrash et al., in 130 relapsed/refractory patients. In that study, 20% of patients had CVAEs. A history of cardiac events was present in 54% of patients.56 Fifteen (11.5%) were hospitalized for chronic heart failure, serious arrhythmias or pulmonary edema. Of four patients hospitalized for arrhythmia, two had cardiac arrests.
Table 4.
Main studies conducted on real-life patients treated with carfilzomib-based regimens.
Conclusions
Melphalan, cyclophosphamide, and doxorubicin are frequently used in the treatment of MM, especially in the context of high-dose chemotherapy. Alkylating agents and anthracyclines are both characterized by the potential for cardiotoxicity. Cardiac function must be monitored carefully in patients taking these drugs, particularly in the long-term, because of late onset toxicity. Electrocardiography and transthoracic echocardiography are useful for assessing cardiac function before high-dose melphalan and/or anthracycline-based treatment.
Immunomodulatory drugs, especially in combination with corticosteroids and chemotherapy, are associated with a high frequency of thromboembolic complications. Prophylaxis with cardio-aspirin, low molecular weight heparin or warfarin is mandatory. The choice of the most appropriate drug depends on the thrombotic risk. If the probability of venous thromboembolism/pulmonary embolism is high, low molecular weight heparin or warfarin should be preferred.
Cardiac adverse events are thought to be a class effect of proteasome inhibitors because they have been reported with bortezomib, carfilzomib and ixazomib; however, they appear to be more frequent with carfilzomib. A recent meta-analysis showed that the most frequent CVAEs during treatment with carfilzomib are hypertension (all grades: 12.2%; grade ≥3: 4.3%), heart failure (all grades: 4.1%; grade ≥3: 2.5%) and ischemic heart disease (all grades: 1.8%; grade ≥3: 0.8%).8 The incidence of heart failure increases up to 20-25% in patients ≥75 years of age. Although true carfilzomib-induced cardiac failure is infrequent and usually reversible, MM patients are typically elderly individuals and may frequently have cardiovascular comorbidities, thereby increasing their risk of drug-related CVAEs.57 As found in the clinical trials described above, age is not the only risk factor for cardiovascular toxicity. Comorbidities, especially pre-existing hypertension or cardiovascular diseases, carfilzomib dose, infusion time, and volume of hydration may increase the risk of CVAEs during carfilzomib treatment.43
However, carfilzomib has been shown to prolong both progression-free and overall survival in MM patients, and maximizing the benefit while reducing cardiovascular risks has become a priority in the management of these patients. At present, there are no strategies to prevent CVAEs that have been validated in prospective studies. The EMN expert panel suggests, based on clinical experience, that cardiovascular risks should be assessed before starting carfilzomib and that measures to correct modifiable risk factors, such as hypertension, high cholesterol levels, hyperglycemia, tobacco use, incorrect diet, should be started. Patients with cardiac risks may benefit from a cardiology review prior to receiving treatment and should be closely monitored for fluid overload. Patients receiving antihypertensive medications may need drug adjustments to reduce their blood pressure while receiving carfilzomib. Regular clinical surveillance with blood pressure control is recommended before and during treatment. In a sub-study of the ENDEAVOR trial, the rate of hypertension was 25% in the entire cohort and 26.4% in an Asian cohort. These rates are higher than in other studies and suggest that particular attention is needed in patients treated with carfilzomib 56 mg/m2 and in subjects of Asian ethnicity. Serial monitoring of cardiac function via echocardiography39 or cardiac biomarkers such as NT-proBNP are considered of limited value in mitigating the risk of carfilzomib-associated cardiac failure.58 In the event of grade 3 or 4 cardiac events, carfilzomib should be withheld until recovery.59 Carfilzomib may be resumed at the physician’s discretion based on a benefit/risk assessment, although preferably at a reduced dose.
In general, the risk-benefit ratio for a drug must be considered in the context of the nature and severity of the disease for which it is used. Dynamic, interdisciplinary cooperation between hematologists and cardiologists is the key to the future studies that are needed to assess and manage cardiovascular safety in patients treated with carfilzomib.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/9/1422
References
- 1.Moreau P, San Miguel J, Ludwig H, et al. , on behalf of the ESMO Guidelines Working Group. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(6): vi133–vi137. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER Stat Fact Sheets: Myeloma. Available at: www.seer.cancer.gov/statfacts/html/mulmy.html. Accessed March 2016.
- 3.Kistler KD, Rajangam K, Faich G, Lanes S. Cardiac event rates in patients with newly diagnosed and relapsed multiple myeloma in US clinical practice. Blood. 2012;102(21):2916. [Google Scholar]
- 4.WHO 2016 Fact sheets-media centre. http://www.who.int/mediacentre/factsheets/fs317/en/.
- 5.WHO 2016 fact files. http://www.who.int/features/factfiles/ageing/ageing_facts/en/.
- 6.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kistler KD, Kalman J, Sahni G, et al. Incidence and risk of cardiac events in patients with previously treated multiple myeloma versus matched patients without multiple myeloma: an observational, retrospective, cohort study. Clin Lymphoma Myeloma Leuk. 2017;17(2):89–96. [DOI] [PubMed] [Google Scholar]
- 8.Waxman AJ, Clasen S, Hwang WT, et al. Carfilzomib-associated cardiovascular adverse events. A systematic review and meta-analysis. JAMA Oncol. 2018;4(3): e174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009; 53(24):2231–2247. [DOI] [PubMed] [Google Scholar]
- 10.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–2801. [DOI] [PubMed] [Google Scholar]
- 11.Bhave M, Akhter N, Rosen ST. Cardiovascular toxicity of biologic agents for cancer therapy. Cancer Network 2014. Available at http://www.cancernetwork.com. [PubMed]
- 12.Sheppard RJ, Berger J, Sebag IA. Cardiotoxicity of cancer therapeutics: current issues in screening, prevention and therapy. Front Pharmacol. 2013;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong RA, Limura T, Sumida KN, Eager RM. Cardio-oncology/onco-cardiology. Clin Cardiol. 2010;33(12):733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. ; International Myeloma Working Group. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414–423. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Eleutherakis-Papaiakovou V. Adverse effects of thalidomide administration in patients with neoplastic diseases. Am J Med. 2004;117(7):508–515. [DOI] [PubMed] [Google Scholar]
- 16.Carrier M, Le Gal G, Tay J, Wu C, Lee AY. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost. 2011;9(4):653–663. [DOI] [PubMed] [Google Scholar]
- 17.Terpos E, Kleber M, Engelhardt M, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica. 2015;100(10):1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Leleu X, Palumbo A, et al. Expert panel consensus statement on the optimal use of pomalidomide in relapsed and refractory multiple myeloma. Leukemia. 2014;28(8):1573–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pegourie B, Pernod G, Karlin L, et al. Evaluation of an oral direct anti-Xa anticoagulant, apixaban, for the prevention of venous thromboembolism in patients with myeloma treated with IMiD* compounds: a pilot study (MYELAXAT). J Clin Oncol. 2017;35(Suppl15):8019. [Google Scholar]
- 20.Kubiczkova L, Pour L, Sedlarikova L, et al. Proteasome inhibitors-molecular basis and current perspectives in multiple myeloma. J Cell Mol Med. 2014;18(6):947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dou PQ, Zonder JA. Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-protea-some system. Curr Cancer Drug Targets. 2014;14(6):517–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreau P, Richardson PG, Cavo M, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120(5):947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasinoff BB, Patel D, Wu X. Molecular mechanism of the cardiotoxicity of the proteasomal targeted drugs bortezomib and carfilzomib. Cardiovasc Toxicol. 2017;17(3): 237–250. [DOI] [PubMed] [Google Scholar]
- 24.Takamatsu H, Yamashita T, Kotani T, et al. Ischemic heart disease associated with bortezomib treatment combined with dexamethasone in a patient with multiple myeloma. Int J Hematol. 2010;91(5):903–906. [DOI] [PubMed] [Google Scholar]
- 25.Nowis D, Mczewski M, Mackiewicz U, et al. Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am J Pathol. 2010;176(6):2658–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hacihanefioglu A, Tarkun P, Gonullu E. Acute severe cardiac failure in a myeloma patient due to proteasome inhibitor bortezomib. Int J Hematol. 2008;88(2):219–222. [DOI] [PubMed] [Google Scholar]
- 27.Foley P, Hamilton MS, Leyva F. Myocardial scarring following chemotherapy for multiple myeloma detected using late gadolinium hyperenhancement cardiovascular magnetic resonance. J Cardiovasc Med. 2010;11(5): 386–388. [DOI] [PubMed] [Google Scholar]
- 28.Voortman J, Giaccone G. Severe reversible cardiac failure after bortezomib treatment combined with chemotherapy in a non-small cell lung cancer patient: a case report. BMC Cancer. 2006;6:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Y, Yin J, Shang Z. Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: a systematic review and meta-analysis. Plos One. 2014;9(1):e87671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000539/WC500048136.pdf
- 31.Dimopoulos M, Moreau P, Palumbo A, et al. ; ENDEAVOR Investigators. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomized, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. [DOI] [PubMed] [Google Scholar]
- 32.Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15(13):1503–1512. [DOI] [PubMed] [Google Scholar]
- 33.Moreau P, Masszi T, Grzasko N, et al. ; TOURMALINE-MM1 Study Group. Oral Ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634. [DOI] [PubMed] [Google Scholar]
- 34.Jouni H, Aubry MC, Lacy MQ, et al. Ixazomib cardiotoxicity: a possible class effect of proteasome inhibitors. Am J Hematol. 2017;92(2):220–221. [DOI] [PubMed] [Google Scholar]
- 35.Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121(6):893–897. [DOI] [PubMed] [Google Scholar]
- 36.Schlafer D, Shah KS, Hall Panjic E, Lonial S. Safety of proteasome inhibitors for treatment of multiple myeloma. Expert Opin Drug Saf. 2017;16(2):167–183. [DOI] [PubMed] [Google Scholar]
- 37.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. ; ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. [DOI] [PubMed] [Google Scholar]
- 38.Siegel D, Martin T, Nooka A, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98(11):1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hájek R, Russell SD, Lyon A, et al. A sub-study of the phase 3 ENDEAVOR study: serial echocardiographic assessment of patients with relapsed multiple myeloma (RMM) receiving carfilzomib plus dexamethasone or bortezomib plus dexamethasone. Haematologica. 2016;101(S1):263.26928246 [Google Scholar]
- 40.Hájek R, Masszi T, Petrucci MT, et al. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia. 2017;31(1):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berenson JR, Cartmell A, Bessudo A, et al. CHAMPION-1: a phase 1/2 study of once weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood. 2016;127(26):3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delforge M, Ludwig H. How I manage the toxicities of myeloma drugs. Blood. 2017;129(17):2359–2367. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig H, Delforge M, Facon T, et al. Prevention and management of adverse events of novel agents in multiple myeloma: a consensus of the european myeloma network. Leukemia. 2018. May 2 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Huang SY, Liu JH, et al. Outcomes for Asian patients with relapsed multiple myeloma treated with carfilzomib and dexamethasone Vs bortezomib and dexamethasone: a subgroup analysis of the phase 3 ENDEAVOR Study (NCT01568866). Haematologica. 2016;101(S1):549–550. [Google Scholar]
- 45.Dimopoulos MA, Siegel D, White DJ, et al. Superior efficacy of carfilzomib and dexamethasone (Kd56) vs bortezomib and dexamethasone (Vd) in multiple myeloma (MM) patients with moderate or serious renal failure: a sobgroup analysis of the phase 3 ENDEAVOR study. Blood. 2017;130(Suppl 1):1845.28716860 [Google Scholar]
- 46.Sugumar D, Keller J, Vij R. Targeted treatments for multiple myeloma: specific role of carfilzomib. Pharmgenomics Pers Med. 2015;20;8:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Facon T, Lee JH, Moreau P, et al. CLARION: Phase 3 study of carfilzomib, melphalan, prednisone vs bortezomib, melphalan, prednisone in newly diagnosed multiple myeloma. Clin Lymphoma Myeloma Leuk. 2017;17(Suppl):e26–e27. [Google Scholar]
- 48.Data available at https://www.amgen.com/media/news-releases/2016/09/amgenannounces-topline-results-from-phase-3-kyprolis-carfilzomib-clarion-study-innewly-diagnosed-multiple-myelomapatients/
- 49.Boccia RV, Bessudo A, Agajanian R, et al. A multicenter, open-label, phase 1b study of carfilzomib, cyclophosphamide, and dexamethasone in newly diagnosed multiple myeloma patients (CHAMPION-2). Clin Lymphoma Myeloma Leuk. 2017;17(7):433–437. [DOI] [PubMed] [Google Scholar]
- 50.Bringhen S, Petrucci MT, Giuliani N, et al. An integrated analysis of cardio-vascular adverse events of carfilzomib, cyclophosphamide and dexamethasone in elderly newly diagnosed myeloma patients enrolled in 3 phase I/II trials. Blood. 2016;128(22): 3336. [Google Scholar]
- 51.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dytfeld D, Jasielec J, Griffith KA, et al. Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124(1):63–69. [DOI] [PubMed] [Google Scholar]
- 54.Bringhen S, D’Agostino M, De Paoli L, et al. Phase 1/2 study of weekly carfilzomib, cyclophosphamide, dexamethasone in newly diagnosed transplant-ineligible myeloma. Leukemia. 2018;32(4):979–985. [DOI] [PubMed] [Google Scholar]
- 55.Gay F, Rota Scalabrini D, Belotti A, et al. Carfilzomib-lenalidomide-dexamethasone vs carfilzomib-cyclophosphamide-dexamethasone induction: planned interim analysis of the randomized FORTE trial in newly diagnosed multiple myeloma. J Clin Oncol. 2017;35(Suppl 15):8003. [Google Scholar]
- 56.Atrash S, Tullos A, Panozzo S, et al. Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J. 2015;5:e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenihan DJ, Potluri R, Bhandari H, Ranjan S, Chen C. Evaluation of cardiovascular comorbidities among patients with multiple myeloma in the United States. Blood. 2016;128(4):479–487.27207789 [Google Scholar]
- 58.Rosenthal A, Luthi J, Belohlavek M, et al. Carfilzomib and the cardiorenal system in myeloma: an endothelial effect¿ Blood Cancer J. 2016;6(1):e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atrash S, Tullos A, Panozzo S, et al. Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J. 2015;5(1):e272–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pawlyn C, Davies F, Cairns D, et al. Quadruplet vs sequential triplet induction Therapy for myeloma patients: results of the MYELOMA XI study. Haematologica. 2017; 102(S2):142. [Google Scholar]
- 61.Mikhael JR, Reeder CB, Libby EN, et al. Phase Ib/II trial of CYKLONE (cyclophosphamide, carfilzomib, thalidomide and dexamethasone) for newly diagnosed myeloma. Br J Haematol. 2015;169(2):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wester R, van der Holt B, Asselbergs E, et al. Phase 2 study of carfilzomib, thalidomide, and low-dose dexamethasone as induction/consolidation in newly diagnosed, transplant eligible patients with multiple myeloma, the carthadex trial. Blood. 2016;128(22):1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1(6):746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chari A, Aggarwal S, Mezzi K, et al. Cardiac events in real-world multiple myeloma patients treated with carfilzomib: a retrospective claims database analysis. Blood. 2016;128(22):3319. [Google Scholar]
- 65.Rosenthal AC, Luthi J, Belohlavek M, et al. The cardiovascular impact of carfilzomib in multiple myeloma. Blood. 2014;124(21): 4748. [Google Scholar]
- 66.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.