Venetoclax (ABT-199) is a selective, oral bioavailable inhibitor of BCL-2 that has activity in a variety of hematologic malignancies.1–3 BCL-2 expression is high in leukemia stem cells of acute myeloid leukemia (AML),4 and BCL-2 inhibitors have the potential to sensitize AML cells to hypomethylating agents (HMA),5 thereby providing the rationale for combining venetoclax with HMA. In an ongoing phase II trial that enrolled treatment-naïve, older (≥65 years) AML patients deemed ineligible for intensive induction therapy, venetoclax, in combination with either decitabine or 5-azacitidine has shown high overall response rate (76%),6 and this response rate is significantly higher than what is observed with single agent HMA in this setting.7,8 The combination of venetoclax with HMA has not been tested systematically in relapsed/refractory (r/r) AML. In a phase II study (n=32), venetoclax was tested as a single agent in r/r AML and produced a modest complete remission (CR)/CR with incomplete recovery (CRi) rate of 19%.1 We present here “real world” evidence from our institution for the use of venetoclax in combination with HMA in patients with r/r AML treated outside of a clinical trial.
We retrospectively analyzed 33 consecutive adults with r/r AML (who failed at least one prior therapy for AML) and were treated with venetoclax in combination with decitabine or 5-azacitidine at the City of Hope Medical Center, Duarte, CA, USA, between October 2016 and October 2017. The study was approved by the Institutional Review Board of the City of Hope Medical Center. We restricted our analysis to evaluable patients who had completed at least one cycle of the combination therapy and had r/r disease based on the assessment of bone marrow biopsy or persistence of circulating leukemia blasts. Venetoclax was given at a dose of 400 mg daily (200 mg daily if the patient was concurrently on an azole). Decitabine dose was given at 20 mg/m2/day and either a 5-day (n=15) or 10-day (n=16) course was administered at the discretion of the treating physician. 5-azacitidine (n=2) was given at the dose of 75 mg/m2/day for seven days per cycle. Prior treatment with HMA was not an exclusion criterion.
We collected data regarding patients’ clinical characteristics, the number and types of prior therapy, cytogenetics and leukemia mutation profile, clinical characteristics and patients’ outcomes. Genetic risk stratification was based on European LeukemiaNet combined cytogenetic and molecular profile.9 We also collected data on serious adverse events that required hospitalization or prolonged hospitalization during treatment. Consensus criteria were used to define response.9,10 Descriptive and univariate analyses were performed for the baseline characteristics and clinical outcomes. Fisher’s exact test and log-rank test were used to explore the differences in tumor response and survival by characteristics. All tests were two-sided at a significance level of 0.05.
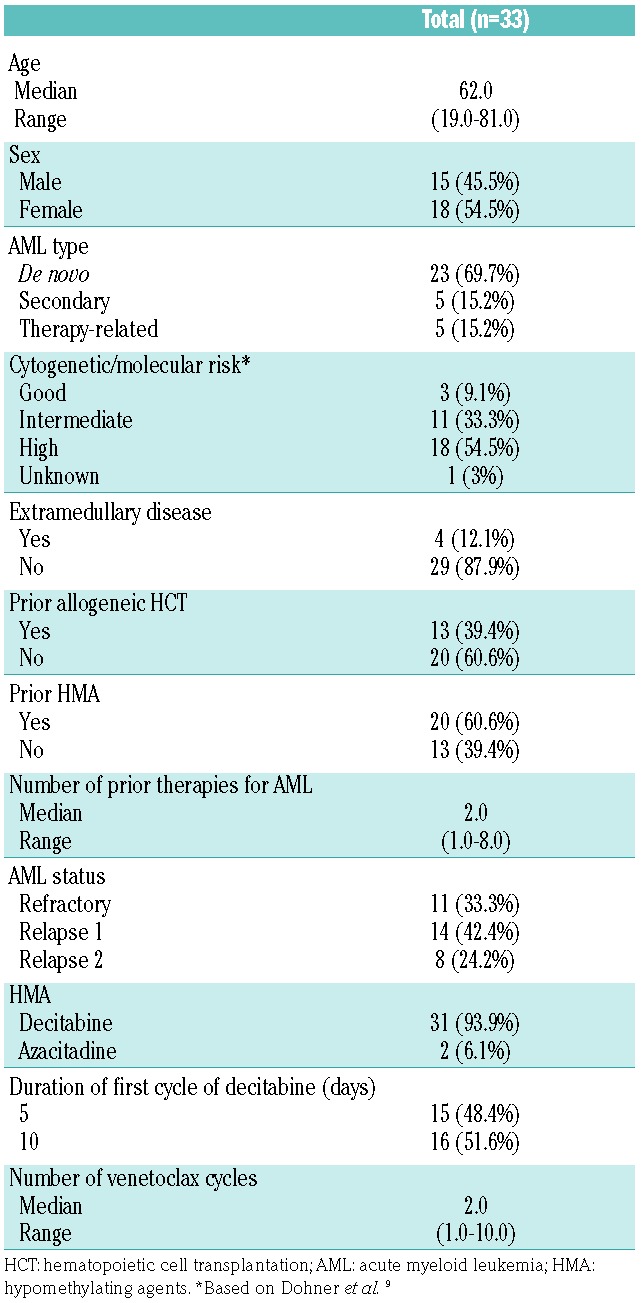
Patients’ and disease characteristics are shown in Table 1. Median age of patients was 62 years (range 19-81). Sixty-one percent (n=20) of patients had failed HMA therapy previously and 39% (n=13) had had prior allogeneic hematopoietic cell transplantation (HCT). Overall response rate (ORR) was 64% (n=21); 10 (30%) patients achieved CR, 7 (21%) CRi, and 4 (12%) morphological leukemia-free state (MLFS). The best response to therapy was seen after a median of 2 (range 1-3) cycles. Among 15 CR/CRi responders who had minimal residual disease (MRD) evaluated at the time of best response, 8 (53%) were MRD negative by multicolor flow cytometry. Three (14%) of the responders who achieved CR/CRi eventually underwent allogeneic HCT. One patient died in remission at day 96 post HCT due to sepsis, one patient relapsed at day 236, and one patient remains in remission at day 134 at the time of analysis.
Table 1.
Patients’ characteristics.
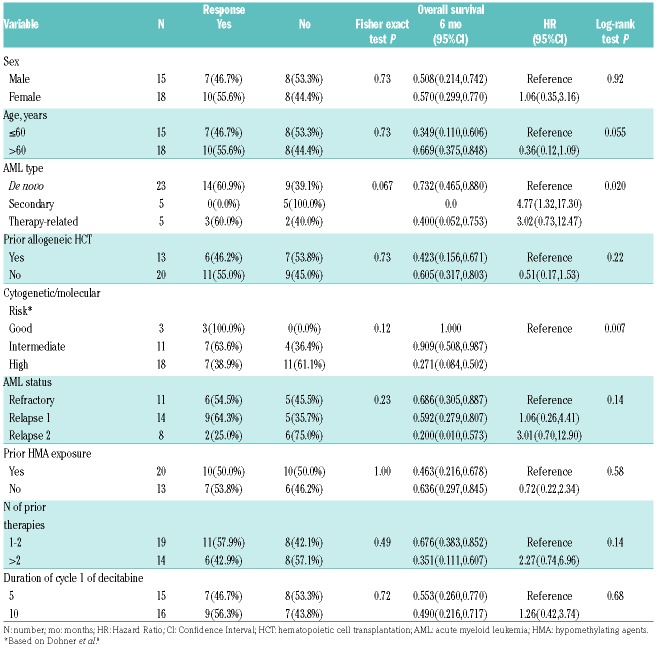
When analysis was restricted to patients who achieved CR/CRi, there was a trend towards higher response in de novo (61%) and therapy-related (60%) AML compared to secondary AML (0%) (P=0.067). None of the 5 patients with secondary AML who were treated with venetoclax and HMA combination responded, but one patient achieved MLFS. All 3 patients with good risk genetic risk [including 1 patient with mutant NPM1 and normal karyotype (NK), one with NK and CEBPA mutation, and 1 with inversion 16] achieved CR/CRi, while only 64% and 39% of patients, respectively, with intermediate- and poor-risk AML responded. There was no difference in response according to prior allogeneic HCT or HMA exposure (P=0.73) and (P=1.00). Nine patients had received HMA in the cycle immediately prior to HMA-venetoclax combination and 6 of them achieved CR/CRi. Response rate for specific molecular mutations were 67% for IDH 1/2 mutations, 44% for FLT3 mutations (ITD or TKD), and 67% in TP53 mutations. There was no difference in response rate between patients treated with a 10-day or a 5-day course of decitabine during the first cycle. The impact of leukemia-specific factors and treatment on response rates is shown in Table 2. The mutation profile and response of the 18 cases with available next generation sequencing (NGS) data are summarized in Online Supplementary Table S1.
Table 2.
Predictors of response and survival.
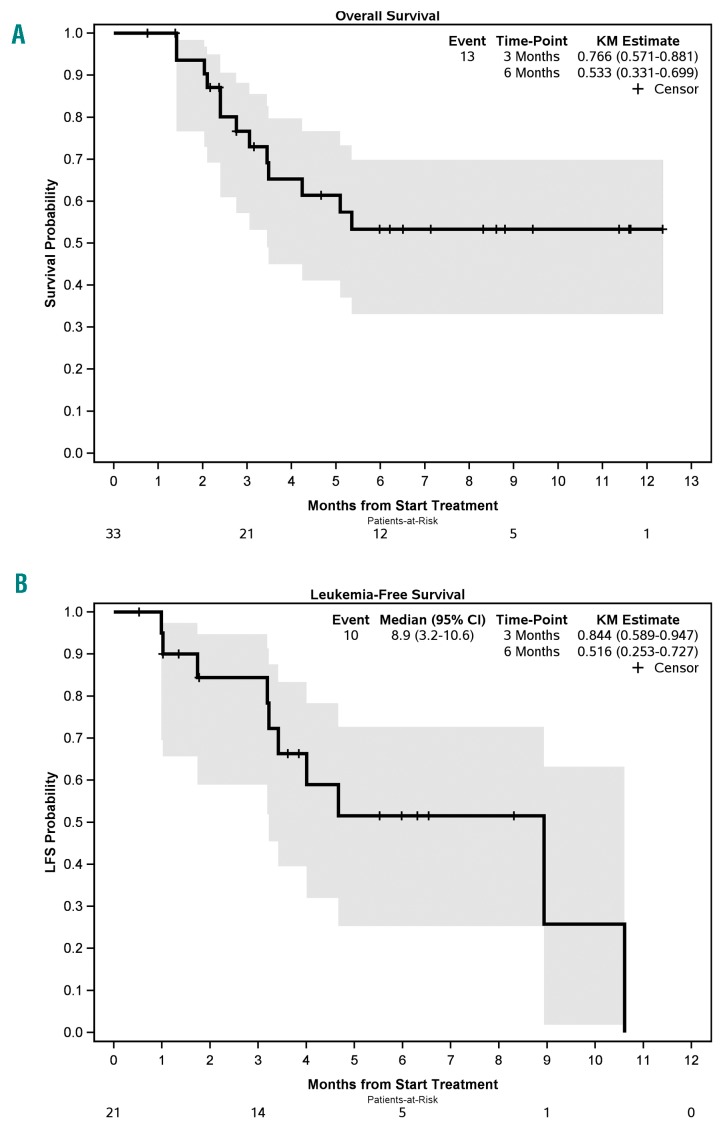
The median follow up for all patients was 6.5 months (range 0.8-12.4). The 1-year overall survival (OS) for all patients was 53%, and OS was longer for patients with de novo AML (1-year OS=73%, median not reached) compared to secondary or therapy-related AML (median OS=3.4 and 3.5 months, respectively) (P=0.02). The median LFS for responders (CR/CRi) was 8.9 months (95%CI: 3.2-10.6), and 6-month LFS was 52% (Figure 1). Among the 10 cases who achieved CR, neutrophil and platelet count recovery occurred during cycle 1 in 4 patients, during cycle 2 in another 4 patients, and during cycle 3 in the remaining 2 patients.
Figure 1.
Survival of refractory/relapsed acute myeloid leukemia (AML) after hypomethylating agent and venetoclax therapy. (A) Overall survival and (B) leukemia-free survival (LFS).
Among the 21 responders, 13 patients stopped venetoclax while 8 remained on therapy at the time of analysis. Reasons for stopping venetoclax were: disease progression in 5, receipt of HCT in 3, sepsis in 3, pregnancy in 1, and small bowel obstruction in 1 patient.
There were 19 serious adverse events (SAE) during the first cycle of venetoclax and HMA in 18 (54%) patients.
The majority of SAE were unrelated to therapy. Neutropenic infections were the most common and were observed in 14 cases. One patient each developed viral pneumonia, fungal pneumonia, hypotension and dehydration, atrial fibrillation, and one patient had prolonged cytopenia that lasted more than six weeks. For the 88 cycles beyond cycle 1, 17 cycles were complicated by SAEs including sepsis (n=11), pneumonia (n= 5), colitis and diarrhea (n=3), atrial fibrillation (n=2), and acute renal failure (n=2).
Although the early results of venetoclax-HMA combination are impressive in the front-line setting, the activity of this regimen in the relapsed r/r AML still has to be fully evaluated. Herein, we provide the first report of a “real world” experience in a relatively large cohort of patients with r/r AML uniformly treated with venetoclax and HMA outside of a clinical trial. We show that the ORR of 64% is higher than the 17-18% response rate expected for single agent HMA11,12 or the 19% response rate seen in a study of single agent venetoclax for r/r AML.1 In fact, the ORR in our series approaches that observed for the venetoclax and HMA combination in newly diagnosed AML,6 despite the fact that approximately 60% of our patients had failed to respond to HMA therapy previously administered for their AML or had relapsed after allogeneic HCT. Moreover, over 50% of responders who were evaluated for MRD achieved an MRD negative status.
While the relatively small number of patients in this series precludes making any definitive conclusions regarding response in particular AML subsets, the high rate of complete responses observed in the whole cohort, including patients with adverse molecular or clinical risk factors, suggest a good clinical activity for this combination even in those patients who would be expected to respond poorly to conventional combination chemotherapy (i.e. patients with TP53 and FLT3 mutations and who had relapsed after allogeneic HCT). This observation has been made in the ongoing clinical trial for de novo AML as well.6 Similar to previous reports, IDH-mutated AML showed a high response rate to venetoclax in this series (67%).1,6
Our data provide preliminary evidence for the excellent activity and safety of venetoclax and HMA in r/r AML among patients treated in the “real world” setting at a single institution. The CR rates with this combination are approximately double of those expected with conventional salvage chemotherapy, with a much better toxicity profile. Given the fact that allogeneic HCT is the only curative therapy for relapsed AML, the venetoclax and HMA regimen could enable a subset of responders to undergo HCT and provide meaningful survival benefit for the rest with acceptable toxicity.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6(10):1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):2401–2409. [DOI] [PubMed] [Google Scholar]
- 3.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogenberger JM, Kornblau SM, Pierceall WE, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28(8):1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiNardo CD, Pollyea DA, Jonas BA, et al. Updated safety and efficacy of venetoclax with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2017;130(Suppl 1):Abstract 2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–569. [DOI] [PubMed] [Google Scholar]
- 8.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 11.Stahl M, Podoltsev NA, DeVeaux M, et al. Hypomethylating agents (HMAs) in patients with relapsed and refractory acute myeloid leukemia (RR-AML): Clinical outcomes and their predictors in a large international patient cohort. Blood. 2016;128(22):1063.27283026 [Google Scholar]
- 12.Itzykson R, Thepot S, Berthon C, et al. Azacytidine for the treatment of relapsed and refractory AML in older patients. Leuk Res. 2015;39(2):124–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.