Abstract
Despite extensive studies, defining culture conditions in which hematopoietic stem cells can be expanded ex vivo has been challenging. Here we show that chemical inhibition of the NF-κB signaling pathway leads to a significant improvement of hematopoietic stem cell function from ex vivo cultured human umbilical cord blood derived CD34+ cells. We found a distinct peak of activation of the NF-κB pathway shortly after cells were put in culture, and consequently inhibition of the pathway was both necessary and sufficient during the first 24 hours of culture where it reduced the levels of several pro-inflammatory cytokines. Taken together, NF-κB pathway inhibition facilitates propagation of hematopoietic stem cells in culture and may complement other strategies for hematopoietic stem cell expansion by relieving stress signals that are induced as an immediate response to culture initiation.
Introduction
Umbilical cord blood (CB) has emerged as a promising source of hematopoietic stem and progenitor cells (HSPCs) for transplantation. Unfortunately, the use of CB grafts is mainly restricted to pediatric transplantation as the number of HSPCs per unit is usually too low to allow the infusion of the minimal cell dose required for successful transplantation in adults.1–3 Potentially, the ex vivo expansion of CB-derived HSPCs prior to transplantation could extend the use of CB transplantation to adult patients.4 Successful HSPC expansion would further facilitate the development of more advanced cell therapies for hematologic diseases, including gene therapy applications.5
Hematopoietic stem cell self-renewal is regulated by a combination of positive-negative feedback signaling.6 An incomplete understanding of this complex regulatory mechanism and how it would fit in a culture system has limited successful HSC ex vivo expansion. Despite the well-studied role of positive signals such as growth factors on HSC self-renewal, several studies highlighted the importance of inhibitory signals in restricting HSC self-renewal and function ex vivo. It has been suggested that, when under culture, HSCs undergo an immediate and transient “culture shock” that compromises their maintenance and function. This is due to upregulation of negative regulators of HSC expansion such as tumor necrosis factor (TNF signaling) and members of the aryl hydrocarbon receptor (AhR) signaling pathway.7,8 Furthermore, the secretion of inhibitory signaling factors, mainly from more differentiated cells, constitutes a major restriction to all HSC culture systems.8 To further explore this notion, our laboratory has performed high-throughput forward RNAi screens and identified negative regulators of ex vivo expansion of human HSPCs, including the cohesin family of genes, and p38 (MAPK14).9,10 Through these studies, we also identified shRNAs which display a remarkable ability to expand phenotypically-defined HSPCs in culture but whose gene target has not been validated. We reasoned that, although it would be very difficult to identify the gene targets responsible for the phenotype, the molecular characterization of cells targeted by such off-target shRNAs could provide general clues about the context under which HSPCs can be propagated ex vivo. Here, we report that the expression signature of cells showing enhanced expansion from one specific off-target shRNA (sh758) involved the downmodulation of genes involved in NF-κB signaling pathway. We found that pharmacological inhibition of the NF-κB signaling leads to a significant improvement in HSC function from ex vivo cultured CB-derived CD34+ cells, as assessed by transplantation to NSG mice. The effect of NF-κB pathway inhibition was most critical early during the culture where it reduced the levels of several pro-inflammatory cytokines induced as an immediate response to culture initiation.
Methods
shRNA experiments
The RNAi screening strategy has been thoroughly described previously.9,10 The target sequence for the candidate shRNA sh758 is GATATGCAAGTCTGTGAATTT. CD34+ cells were transduced with a pLKO1-GFP lentiviral vector harboring either sh758 or control (scrambled) shRNA and subsequently cultured for several weeks according to previously described protocol.9,10
Cord blood CD34+ isolation and culture
Umbilical CB samples were collected from full-term deliveries at maternity wards of Lund, Malmö and Helsingborg Hospitals. CB unit collection, mononuclear cell isolation, and CD34+ cell enrichment and culture were carried out as previously described.10 IKKβ inhibitors, PF184 and TPCA1 (Tocris Bioscience), kept in DMSO, were added at a final concentration of 400nM. Control wells were supplemented with DMSO at a matching concentration. Cultures were kept at 37°C and 5% CO2 and the medium (including inhibitors) was refreshed after four days.
Flow cytometry and cell sorting
For cell surface marker staining, cells were collected, washed once with PBS supplemented with 2% FCS (FACS buffer). Cells were incubated with anti CD34 (#343516581), CD90 (#3281145E10) and EPCR (#351906) (BioLegend) antibodies for 30 minutes (min) at 4°C, and washed once with cold FACS buffer. For cell sorting, CD34+ cells were quickly thawed and stained for CD34, CD38 (#345806), CD45RA (#560362) (BD Bioscience) and CD90 following the same procedure as above. When specified, cells were stained with the Annexin V Apoptosis Detection Kit, according to the manufacturer’s protocol (BD Bioscience). All data were collected on FACS Canto II or LSRII analyzer (Becton Dickinson), and analyzed with FlowJo software. Cells were sorted on a FACS Aria II or III (Becton Dickinson).
Human engraftment assay
All experiments with mice were reviewed and conducted under approved protocol from the Lund/Malmö Local Ethical Committee. NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice (NSG; Jackson Laboratory) were sublethally irradiated (300 cGy) before transplantation. Fresh cells or the cultured equivalent of 30,000 input CD34+ cells were injected intravenously into 10-12-week old NSG mice. Human cell contribution in peripheral blood (PB) and bone marrow (BM) of NSG was assessed 16 weeks post transplantation.
Cytokine secretion and Bioplex assay
Supernatants were collected from duplicate samples after six hours treatment of CB CD34+ cells with TPCA1 or STF. The secreted cytokines in the supernatants were measured by using human 27-plex panel (M500KCAF0Y, Bio-Rad) in the Bio-Rad Luminex instrument. Samples were prepared and analyzed as per the manufacturer’s protocol.
Statistical analysis
Statistical significance was calculated using a two-tailed Student t-test with GraphPad Prism software unless otherwise stated. Statistical significance in the figures are indicated: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Error bars indicate Standard Error of the Mean (SEM) unless otherwise stated and n represents the number of independent experiments.
Methods describing microarray analysis, CFC assays, western blot and cell cycle analysis can be found in the Online Supplementary Appendix.
Results
The NF-κB pathway modulates ex vivo cultured human HSPCs
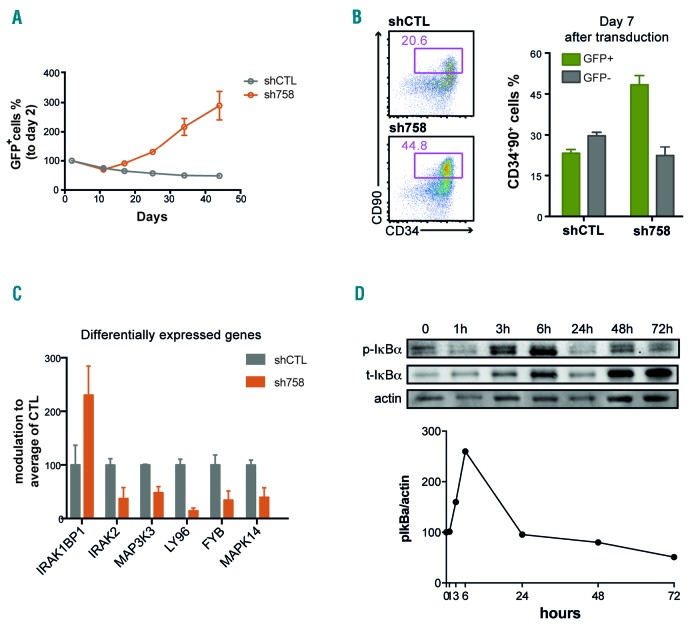
From RNAi-based screens conducted in our laboratory aimed at identifying novel modifiers of HSPC expansion,10,11 we have identified several off-target hits: shRNAs that display profound effects on HSPC expansion but do not affect the expression of their predicted target. One such shRNA, TRC00000758 (sh758), predicted to specifically knock-down (CLK1), induced an especially strong proliferative advantage of CB CD34+ cells, as the transduced, GFP+ population eventually dominated the culture (Figure 1A). Moreover, when cultured for seven days, CD34+CD38−CD90+CD45RA− cells expressing sh758 maintained a substantially higher percentage of immature CD34hiCD90+ cells, which is known to contain the engraftable HSCs (Figure 1B). Given the dramatic increase of CD34hiCD90+ cells induced by sh758, we reasoned that the molecular signature of cells expressing sh758 could provide valuable clues about the genes and pathways that modulate HSC expansion ex vivo. We performed global transcriptional profiling of CD34hiCD90+ cells expressing either sh758 or a control shRNA (shCTL) after seven days of culture. The gene expression profiles revealed a downmodulation of cellular stress-related genes and pathways, such as MAPK14 and genes involved in NF-κB signaling, in sh758 transduced cells (Figure 1C). Of note, the expression levels of CLK1, as well as other predicted targets of sh758 (based on sequence homology), were not repressed by the shRNA. We have previously reported on the role of MAPK14 (p38) in HSPC expansion10 and therefore decided to further investigate the role of NF-κB signaling in this context. We first addressed whether the NF-κB pathway is activated upon ex vivo culture of CB CD34+ cells and found that the key regulator of NF-κB signaling, IkBa,12 was readily detected and also showed an increased expression during culture (Figure 1D). We further measured the phosphorylated form of the IκBα protein (p-IκBa), as an indicator of NF-κB activity, at different time points during culture of CB CD34+ cells. We observed that the p-IκBα signal was detected throughout the culture and with particularly high levels during the first 24 hours (Figure 1D). Collectively, this suggests that the NF-κB pathway is activated in cultured HSPCs and may negatively influence their expansion.
Figure 1.
The sh758-dependent ex vivo expansion effect is associated with a downmodulation of the NF-κB pathway. (A) Growth of GFP+ cells was assessed by FACS and normalized to day 2-transduction efficiency. (B) The percentage of CD34+ 90+ cells in the GFP+ fraction was quantified by FACS seven days after transduction of CD34+CD38− CD90+CD45RA− cells (n=2). (C) Gene expression data mining showed modulation of members of NF-κB signaling in sh758 expressing CD34+ CD90+ cells. (D) The expression of total and phosphorylated ΙκΒα was quantified by western blot in CD34+ cells at indicated culture time points. Actin expression is shown as loading control.
NF-κB pathway inhibition mediated by targeting IKKβ increases expansion of CD34+CD90+ cells ex vivo
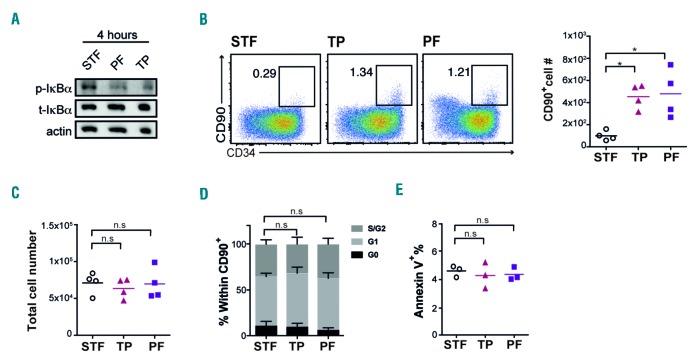
To further explore the role of NF-κB signaling in cultured HSPCs, we next blocked the pathway pharmacologically. As the down-regulated genes detected in the gene expression profiling (Figure 1C) are acting through canonical NF-κB signaling, we decided to target IKKβ which is a central molecule in integrating upstream signals in the canonical pathway.13 Thus, we treated CD34+ cells with two specific inhibitors of the IKKβ subunit: PF184 and TPCA1.14,15 IκBα is the canonical target of IKKβ and both inhibitors induced a strong reduction of the phosphorylated-to-total IκBα ratio after four hours treatment (Figure 2A). After seven days of culture, we observed a marked increase in both the frequency and number of CD34+CD90+ cells (Figure 2B).
Figure 2.
Targeting of the NF-κB regulator IKKβ increases the number of human hematopoietic stem and progenitor cells (HSPCs) in vitro without affecting cell proliferation and survival. (A) The protein level of total and phosphorylated IκBα were quantified in CD34+ cells after 4-hour treatment with STF/PF/TP in culture. Actin expression is shown as loading control (n=2). (B) FACS plots and graph show cell surface expression of CD34 and CD90 and number of CD34+CD90+ cells respectively at day 7 (n=4). (C) Graph displays total live (7AAD−) cell number at day 7 (n=4). (D) Cell cycle distribution, and (E) apoptosis (7AAD−/AnnexinV+) of CD34+CD90+ cells were assessed by FACS at days 4 and 7, respectively (n=3). p-IκBa: phosphorylated IκBa; t-IκBa: total IκBa; PF: PF184; TP: TPCA1.
However, the total cell number remained unaffected (Figure 2C), suggesting that the increase in CD34+CD90+ cells number is not due to a generally higher proliferation rate of cells treated with NF-κB inhibitors. Since NF-κB has well described effects on apoptosis16 and proliferation,17 we assayed cell cycle and apoptosis parameters in CD34+ cells treated with PF184 and TPCA1 in an attempt to understand the basis for the increased numbers of CD34+CD90+ cells. However, we could not detect any significant difference in cell cycle status (Figure 2D) or apoptotic cell levels (Figure 2E) of CD34+90+ cells.
NF-κB pathway inhibition enhances the ex vivo propagation of transplantable HSCs
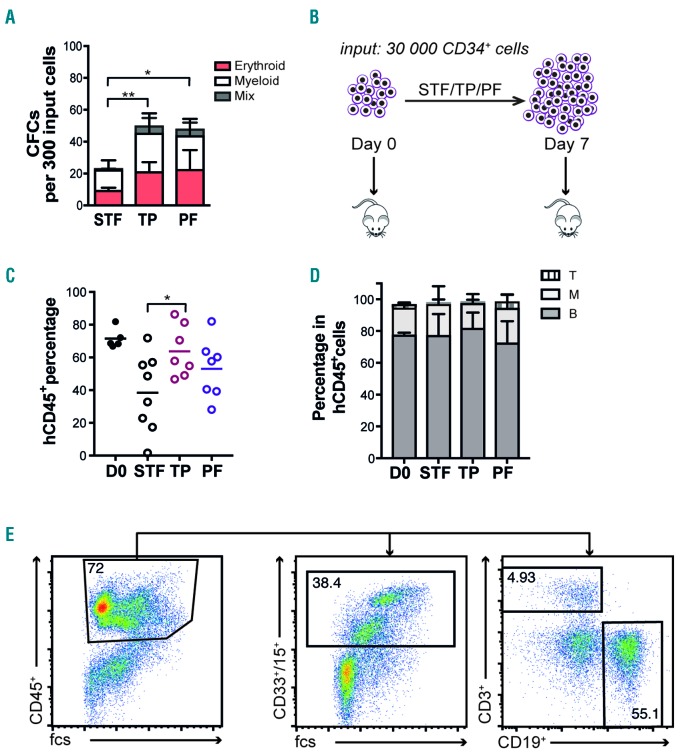
To assess in more detail the functional consequences of growing HSPCs in the presence of NF-κB inhibition, we cultured CD34+ cells for seven days with or without IKKβ inhibitors, and then assayed the cells for colony forming ability in methylcellulose medium. Cells cultured in the presence of inhibitors produced a significantly higher number of colonies compared to the control, and also generated a higher proportion of primitive colonies with a mixed phenotype (Figure 3A), indicating improved functional integrity of the cultured progenitor cells. Next, to test the in vivo regenerative capacity of HSCs cultured with IKKβ inhibitors, we performed transplantation experiments. Fresh CB CD34+ (30,000) cells or 7-day cultured equivalents of 30,000 CB CD34+ were transplanted into sub-lethally irradiated NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice (Figure 3B). Cells treated with PF184 and TPCA1 yielded 53% and 64% human engraftment, respectively, while control cells generated lower (38%) human engraftment in bone marrow 16 weeks post transplantation (Figure 3C). The engrafted cells contributed to both myeloid and lymphoid lineages (Figure 3D and E). Finally, the frequency of the HSC enriched CD34+CD38-population among the engrafted human cells was similar to that of control cells (Online Supplementary Figure S1), demonstrating an overall higher long-term engraftment of also the most immature cells from cultures with PF184 and TPCA1. Although IKKβ inhibitor treated cells showed slightly lower long-term engraftment compared to non-cultured cells, our data demonstrate a strong benefit of NF-κB inhibition in preserving functional HSPCs during ex vivo expansion cultures.
Figure 3.
Targeting of the NF-κB regulator IKKβ improves the in vitro and in vivo function of cultured cord blood (CB)-derived hematopoietic stem and progenitor cells (HSPCs). (A) Colonies per 300 CD34+ input cells were scored after 14 days (n=3). (B) Schematic representation of in vivo transplantation experiment. (C) Human engraftment was assessed by quantifying the percentage of human CD45+ cells in the bone marrow (BM) of NSG recipients four months post transplantation (data from 2 independent experiments). (D) Lineage distribution was quantified by FACS analysis of human B cells (CD19), T cells (CD3), and myeloid (CD33/CD15) in CD45+ cells of the BM of recipients. (E) Representative FACS plots for gating strategy of lineage reconstitution. PF: PF184; TP: TPCA1.
NF-κB inhibition is most beneficial during an early time window where it down-regulates several pro-inflammatory cytokines
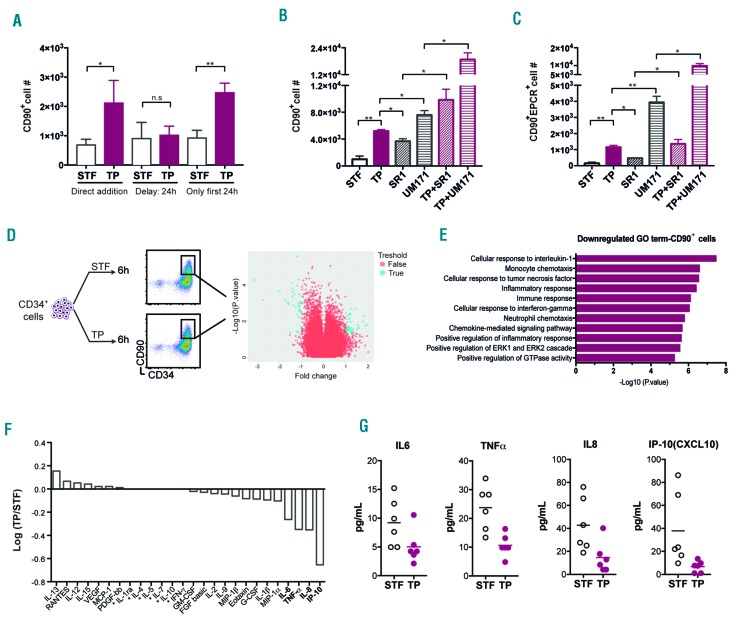
We had previously observed a peak in p-IκBα levels during the first 24 hours of culture (Figure 1D), and therefore next assessed if there is a critical time window for addition of IKKβ inhibitors. We found that delaying IKKβ inhibition for 24 hours abolished the beneficial effects and resulted in lower numbers of CD34+CD90+ cells after five days compared to cultures where the inhibitor was added directly. Similarly, treating cells for only the first 24 hours with IKKβ inhibitor resulted in similarly elevated numbers of CD34+CD90+ cells compared to 5-day treatment (Figure 4A). Collectively, these findings suggest that NF-κB activation is most detrimental during the first 24 hours of culture and define a time window when NF-κB inhibition is both necessary and sufficient to execute protective effects on HSPCs.
Figure 4.
The benefit of NF-κB pathway inhibition is limited to a short time window, and results in downregulation of inflammatory signaling. (A) Number of CD34+CD90+ cells was assessed after five days by FACS, when TP/STF was added directly or 24 hours after culture start or removed after 24 hours (n=3). (B) Graph shows number of CD34+CD90+ cells after treatment with STF, TP, SR1, UM171 and combination of TP with either SR1 or UM171 at day 6 (representative data from 2 experiments). (C) Graph shows number of CD34+CD90+EPCR+ cells after treatment with STF, TP, SR1, UM171 and combination of TP with either SR1 or UM171 at day 6 (representative data from 2 experiments). (D and E) CD34+ cells were treated with or without TP for six hours and subjected to transcriptome analysis by microarrays after sorting for CD34+90+ population. Volcano plot and GO classification of 2-fold modulated (down-regulated) genes are shown for each condition. See also Online Supplementary Figure S3 and Online Supplementary Table S1. (F) The log ratio (TP/STF) of factors present in supernatant after six hours of culture of CB-derived CD34+ cells is shown (n=3). (G) Each graph displays individual data point for significantly modulated cytokines (IL6, TNFa, IL8, and IP10), extracted from the experiments presented in (F). The stars in (F) indicate that those factors were not detectable. PF: PF184; TP: TPCA1.
In order to further characterize the potential of NF-κB inhibition in improving expansion protocols, we compared TPCA1 to two other known expansion molecules for human HSCs: SR1 and UM171.18,19 We evaluated the output of CD34+90+ cells as well as CD34+CD90+EPCR+ cells, as EPCR expression recently has been associated with the regenerative activity of ex vivo cultured HSPCs in the context of UM171 mediated expansion.20 All three compounds showed a clear increase in both CD34+CD90+ and CD34+CD90+EPCR+ cell numbers compared to control cultures (Figure 4B and C, and Online Supplementary Figure S2). However, TPCA1 treatment resulted in larger numbers of these cell populations compared to SR1, while UM171 showed a significant increase compared to both TPCA1 and SR1 (Figure 4B and C). Interestingly, the combination of the IKKβ inhibitor with either SR1 and UM171 led to a marked increase in CD34+90+ and CD34+CD90+EPCR+ cell numbers, compared to each compound alone (Figure 4B and C), indicating that targeting NF-κB signaling may enhance HSPC expansion protocols driven by other compounds.
NF-κB proteins are considered key players in inflammation and immunity. However, they also play an important role in other processes such as cell growth, survival and development.13 To further identify which NF-κB associated functions were targeted in our cultured HSPCs, we subjected bulk CD34+ and sorted CD34+CD90+ cells to global gene expression profiling using microarrays (Figure 4D and E, Online Supplementary Table S1 and Online Supplementary Figure S3). We used 6-hour culture time for the transcriptome analysis as this time point showed a clear increase in NF-κB activity (Figure 1D). Although relatively few transcriptional changes were detected within the more heterogeneous bulk of CD34+ cells (Online Supplementary Figure S3), 51 annotated genes were modulated in the CD34+CD90+ population upon exposure to the IKKβ inhibitor (Figure 4D and E, and Online Supplementary Table S1). Out of these, all coding genes (23 genes) were down-regulated and mainly related to inflammatory processes (15 genes), for example, IL1a, TNF-α, GM-CSF (CSF2), MCP-1 (CCL2), TCA3 (CCL1), and MIP-1β (CCL4) (Online Supplementary Table S1). Using Bioplex bead arrays, we confirmed the reduction of several pro-inflammatory factors, including TNF-α, IL-6, IP-10 (CXCL10) and IL-8 in supernatants collected from cultures of CB CD34+ cell after a 6-hour exposure to the IKKβ inhibitor (Figure 4F and G). Taken together, these findings indicate that the beneficial effect of IKKβ inhibitors on cultured human HSPCs is associated with the downregulation of NF-κB-dependent pro-inflammatory molecules.
Discussion
In this study, we demonstrate that the NF-κB signaling has a negative impact on ex vivo cultured human CB HSPCs, and that pharmacological inhibition of the pathway improves their propagation and regenerative potential after culture. Our findings further showed that this is associated with inhibition of pro-inflammatory signals in CD34+CD90+ cells during the early phases of the culture.
NF-κB signaling can be activated in both immune and non-immune tissues by various extracellular signals, such as reactive oxygen species,21 pro-inflammatory cytokines such as interleukin-1,22 and members of the TNF superfamily.23 Cellular responses largely depend on the cell type, include positive or negative modulation of proliferation and apoptosis,16 differentiation,24 development,13,25 and are mediated by secretion of a large variety of signals21 and direct expression control of cell cycle/apoptosis mediators.16 In hematopoiesis, knockout mouse models of different NF-κB subunits have revealed defects in HSPCs function, as well as impaired immune response, lymphopoiesis, granulocytosis, and splenomegaly.26,27 In human settings, overexpression of p65 or a constitutively active form of IKKβ did not influence growth and differentiation of CB-derived CD34+ cells.28 However, the actual role of NF-κB signaling and the consequences of inhibiting the pathway in normal HSPCs had not previously been addressed.
We initially identified the NF-κB pathway as part of a molecular signature associated with sh758, an shRNA that dramatically expands phenotypically defined HSPCs. However, even if chemical inhibition of IKKβ, the integrating kinase of NF-κB signaling, did lead to a robust and consistent increase in CD34+CD90+ cells, it could not reproduce the full extent of the sh758 phenotype. Specifically, the effect of IKKβ inhibition was limited to a short time window during the first 24 hours of culture. This suggests that the profound and persistent expansion of CD34+CD90+ cells induced by sh758 is only partly mediated by reduced activity of the NF-κB pathway, and that other genes and pathways must be involved as well. One strong candidate is MAPK14 (p38), which was also down-regulated in sh758-transduced cells. We have previously shown that p38 inhibition enhances the stem cell output from cultured HSPCs.
Despite the well described function of NF-κB in regulating proliferation and apoptosis,29 we did not observe any significant changes in cell cycle, or apoptotic status of CD34+CD90+ cells upon NF-κB pathway inhibition. Additionally, NF-κB signaling has been linked to ROS production,21 which must be tightly regulated to maintain HSPC function.30 However, intracellular ROS levels were not significantly altered during the early phase of the culture (data not shown), when the effects of NF-κB pathway inhibition were seen to enhance HSPC activity. This suggests that the preserved stem cell output of cultured HSPCs upon NF-κB pathway inhibition is not related to ROS modulation.
Based on the IκBα phosphorylation pattern, we showed that there is a critical 6-24-hour time frame necessary and sufficient for NF-κB pathway inhibition to benefit the propagation of the CD34+CD90+ population. In agreement with this, previous studies also reported on an immediate variation in gene expression profile of inhibitory HSPC regulators as the cultured cells experience an initial culture shock.7,8 In fact, we showed downregulation of several pro-inflammatory genes such as MCP-1 (CCL2), TCA3 (CCL1), IL1a, TNF-α and MIP-1β (CCL4) upon NF-κB inhibition, which are known to inhibit HSPC function.31 Although these pro-inflammatory cytokines are essential for a proper defense mechanism,24 they have been shown to negatively influence self-renewal and lineage commitment of HSCs.32,33 Therefore, considering the lack of effect on cell proliferation, the increased HSC output after NF-κB pathway inhibition may rather be associated with preserved stem cell integrity by restricting differentiation. It is likely that the most immature HSPC are particularly sensitive to the early culture shock as they showed the most profound transcriptional changes upon NF-κB pathway inhibition.
It could be argued that pro-inflammatory stress could be relieved by simply exchanging the media similar to the fed-batch system.8 However, during the critical first 24 hours, the cell density is very low and a media exchange system is not likely to have an impact during this short time frame. It is possible that restricting inhibitory signals in expansion cultures have a bi-phasic pattern; one pulse that is induced immediately upon culture initiation and one that accumulates over time and that is dependent on cell density and media changes. The latter can be relieved by fed-batch systems, but the acute effect may rather be intrinsically triggered and require pharmacological targeting, in this case NF-κB pathway inhibition, to be reduced. Our finding that targeting NF-κB signaling further enhances HSPC expansion driven by other compounds suggests that this should be further exploited in strategies aimed at HSC expansion for clinical benefit.
Supplementary Material
Acknowledgments
The authors are thankful to the technical staff and management at the Division of Molecular Medicine and Gene Therapy, The Lund Stem Cell Center FACS and Vector cores, as well as the Animal facility at the Biomedical Center. This work was funded by grants from the Swedish Research Council, the Swedish Cancer Foundation, the Swedish Pediatric Cancer Foundation and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement n. 648894) to JL. The work was further supported by the HematoLinné and StemTherapy programs at Lund University.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/9/xxx
References
- 1.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner JE, Gluckman E. Umbilical cord blood transplantation: the first 20 years. Semin Hematol. 2010;47(1):3–12. [DOI] [PubMed] [Google Scholar]
- 3.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broxmeyer HE. Enhancing the efficacy of engraftment of cord blood for hematopoietic cell transplantation. Transfus Apher Sci. 2016;54(3):364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farahbakhshian E, Verstegen MM, Visser TP, et al. Angiopoietin-like protein 3 promotes preservation of stemness during ex vivo expansion of murine hematopoietic stem cells. PLoS One. 2014;9(8):e105642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirouac DC, Ito C, Csaszar E, et al. Dynamic interaction networks in a hierarchically organized tissue. Mol Syst Biol. 2010;6:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnusson M, Sierra MI, Sasidharan R, et al. Expansion on stromal cells preserves the undifferentiated state of human hematopoietic stem cells despite compromised reconstitution ability. PLoS One. 2013;8(1): e53912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csaszar E, Kirouac DC, Yu M, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10(2):218–229. [DOI] [PubMed] [Google Scholar]
- 9.Galeev R, Baudet A, Kumar P, et al. Genome-wide RNAi Screen Identifies Cohesin Genes as Modifiers of Renewal and Differentiation in Human HSCs. Cell Rep. 2016;14(12):2988–3000. [DOI] [PubMed] [Google Scholar]
- 10.Baudet A, Karlsson C, Safaee Talkhoncheh M, et al. RNAi screen identifies MAPK14 as a druggable suppressor of human hematopoietic stem cell expansion. Blood. 2012;119(26):6255–6258. [DOI] [PubMed] [Google Scholar]
- 11.Ali N, Karlsson C, Aspling M, et al. Forward RNAi screens in primary human hematopoietic stem/progenitor cells. Blood. 2009;113(16): 3690–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. [DOI] [PubMed] [Google Scholar]
- 13.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12(8):695–708. [DOI] [PubMed] [Google Scholar]
- 14.Mora E, Guglielmotti A, Biondi G, Sassone-Corsi P. Bindarit: an anti-inflammatory small molecule that modulates the NFkappaB pathway. Cell Cycle. 2012;11(1):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podolin PL, Callahan JF, Bolognese BJ, et al. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell Proliferation. J Pharmacol Exp Ther. 2005;312(1):373–381. [DOI] [PubMed] [Google Scholar]
- 16.Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6910–6924. [DOI] [PubMed] [Google Scholar]
- 17.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fares I, Chagraoui J, Gareau Y, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345(6203):1509–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fares I, Chagraoui J, Lehnertz B, et al. EPCR expression marks UM171-expanded CD34(+) cord blood stem cells. Blood. 2017;129(25):3344–3351. [DOI] [PubMed] [Google Scholar]
- 21.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stylianou E, O’Neill LA, Rawlinson L, et al. Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J Biol Chem. 1992;267(22):15836–15841. [PubMed] [Google Scholar]
- 23.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11(9):372–377. [DOI] [PubMed] [Google Scholar]
- 24.Espin-Palazon R, Stachura DL, Campbell CA, et al. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159(5):1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein SJ, Baldwin AS. Deletion of the NF-kappaB subunit p65/RelA in the hematopoietic compartment leads to defects in hematopoietic stem cell function. Blood. 2013;121(25):5015–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakata S, Matsumura I, Tanaka H, et al. NF-kappaB family proteins participate in multiple steps of hematopoiesis through elimination of reactive oxygen species. J Biol Chem. 2004;279(53):55578–55586. [DOI] [PubMed] [Google Scholar]
- 28.Schepers H, Eggen BJ, Schuringa JJ, Vellenga E. Constitutive activation of NF-kappa B is not sufficient to disturb normal steady-state hematopoiesis. Haematologica. 2006;91(12): 1710–1711. [PubMed] [Google Scholar]
- 29.Radhakrishnan SK, Kamalakaran S. Proapoptotic role of NF-kappaB: implications for cancer therapy. Biochim Biophys Acta. 2006;1766(1):53–62. [DOI] [PubMed] [Google Scholar]
- 30.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431(7011):997–1002. [DOI] [PubMed] [Google Scholar]
- 31.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32(2): 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dybedal I, Bryder D, Fossum A, Rusten LS, Jacobsen SE. Tumor necrosis factor (TNF)-mediated activation of the p55 TNF receptor negatively regulates maintenance of cycling reconstituting human hematopoietic stem cells. Blood. 2001;98(6):1782–1791. [DOI] [PubMed] [Google Scholar]
- 33.Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130(15):1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.