Abstract
Purpose
Safety data of many drugs used during pregnancy remain scarce. This is especially true in developing countries characterised by the absence of a robust pharmacovigilance system, high prevalence of different tropical diseases affecting patients and potential for drug-drug interactions. This study aimed to assess the safety profile of drugs used in women at high risk of malaria during pregnancy and delivery in Burkina Faso’s health facilities. It also aimed to assess factors associated with the use of potentially risky drugs over the entire course of pregnancy.
Methods
We enrolled pregnant women from their first antenatal care visit and followed them up until delivery, and collected data on drug use. Based on United States Food and Drug Administration (FDA) or Australian Therapeutic Goods Administration (TGA) drug risk classification, drugs were classified into three groups: ‘probably safe’, ‘potentially risky’ or ‘unclassified’. A modified classification was built to take into account national malaria policy treatment guidelines and World Health Organization Malaria Treatment Guidelines recommending malaria chemoprophylaxis during pregnancy.
Results
Out of 2371 pregnant women enrolled, 56.7% used at least one medication during the entire course of the pregnancy (excluding sulphadoxine-pyrimethamine and iron-folic acid). A total of 101 different types of medications were used by study participants and 36.6, 49.5 and 13.9% were, respectively, classified as ‘probably safe’, ‘potentially risky’ and ‘unclassified’. Antimalarials and antibiotics were the most frequently used drugs. Around 39% of women used a least one medication classified as potentially risky. However, this proportion dropped to 26% with the modified classification. Living in urban areas and attending the first antenatal care within their first trimester of pregnancy (longer health surveillance) were associated with using ‘potentially risky’ medications.
Conclusion
This study provides rare and valuable information on the current use of drugs among pregnant women in Burkina Faso. Many pregnant women used medications classified as potentially risky. Our findings suggest the need for rational drug prescription and community education to reduce hazardous drug exposure during pregnancy.
Key Points
| To our knowledge, this study provides the first analysis on the current use of drugs in pregnant women in Burkina Faso. |
| About half of medications used to manage illness during pregnancy were classified as potentially risky. |
| Nearly one-seventh of medications lacked a safety classification (US-FDA and AU-TGA drug classification) and more than one-quarter of women used medications classified as potentially risky to use during pregnancy. |
Introduction
Through government and international efforts to improve maternal and child health in high mortality countries, there have been massive efforts to improve care during pregnancy, increase the number of antenatal consultations (ANC), deploy essential medicines in health facilities, and improve skilled attendance at birth [1, 2]. These efforts have increased patient access to medicines of different therapeutic classes, especially for tropical diseases. Improved access to essential drugs for the general population in many Sub-Saharan Africa countries [1, 3–5] and the introduction of an effective universal healthcare coverage in a few countries (Botswana, Namibia, Ghana and South Africa) [6] have made it clear that drug safety data are not available for many drugs that are commonly used during pregnancy. Regulatory requirements for drugs to be used during pregnancy are substantially stricter since the thalidomide disaster [7–9], but one consequence is that many medications commonly authorised for general use have no data on efficacy or safety for pregnant women. For legal and ethical reasons, the acquisition of safety data during pregnancy is normally collected during pharmacovigilance studies, post-registration [10, 11]. Since it takes years, or even decades, to acquire systematic safety data that can be used to extend the therapeutic indication to pregnancy, drug safety during pregnancy remains generally unknown, except for drugs used for obstetric conditions. For many important infections, including malaria and HIV where there are few safety data in pregnancy on effective medications, the World Health Organization (WHO) recommends treatment of pregnant women with severe malaria with artemisinin parenteral drugs and uncomplicated Plasmodium falciparum malaria during the first trimester with 7 days of quinine + clindamycin and artemisinin combination therapy (ACT) in the second and third trimester [12]. A high prevalence of HIV with poor safety evidence for treatment in pregnancy is equally important in many Sub-Saharan countries; at present, transition to efavirenz is sanctioned on condition that safety surveillance is carried out in implementing countries [13, 14].
Several studies have monitored drug use during pregnancy both in developed [15–18] and in developing countries [19–23]. Most studies found that pregnant women were prescribed and used a large number of drugs during pregnancy and that a number of potentially risky drugs are used, based on United States Food and Drug Administration (FDA), Australian Therapeutic Goods Administration (AU-TGA) or Farmaceutiska Spesialiteter i Sverige, Europe (FASS) drug-risk classification systems. In Sub-Saharan African countries, studies have reported that drug exposure to antibiotics, analgesics and antimalarials were the most common drugs used in pregnancy. A study conducted in Mozambique reported that antibiotic agents (41%) and antimalarial drugs (24%) were the most commonly used drugs [21]. Another study in Tanzania reported that analgesics were the most commonly used drugs (24%), followed by antibiotics (17%) and antimalarials (15%) [22].
We are unaware of any study carried out in Burkina Faso to assess the profile and the magnitude of medications exposure during pregnancy. Existing studies on the use of drugs during pregnancy have normally targeted a specific drug used often within a clinical trials framework [24, 25]. Therefore, there are no data available on the profiles, ranges and magnitude of drug exposure during pregnancy. This can be of concern. Indeed, the epidemiological profile of Burkina Faso, like that of most countries in Sub-Saharan Africa, is dominated by parasitical (such as malaria) and infectious diseases, where the pattern of medicine use would be a higher elevating risk for drug-drug interactions, in the presence of other risk factors affecting patient outcome (malnutrition, drug resistance and low quality or fake medicines) [26–28]. Furthermore, in many Sub-Saharan African countries [29], self-medication is common, potentially exposing the fetus to drugs that may have negative outcomes during gestation or at birth [30, 31]. Some studies have revealed that drug prescribing errors occur significantly more often in children and pregnant women [32]. Drug prescription (with/without errors) and self-medication may occur before a pregnancy is known [33]. For this reason, we conducted a study to assess the therapeutic classes and risk categories of drug use among pregnant women at high risk of malaria (during pregnancy and delivery, especially during labour) when they attended antenatal clinics (ANC) at peripheral health centres, in Burkina Faso. Furthermore, after categorizing the drugs according to their risk, we assessed the factors associated with use of potentially risky drugs during the entire course of pregnancy.
Methods
Study Settings
The study was conducted in two health districts located in the western region of Burkina Faso, Dafra and Do. In this region, malaria is the most common cause of outpatient attendance (45.0%) in peripheral health centres, followed by acute respiratory infection (21.8%) and diarrheal diseases (2.9%). In 2016, the health facilities of the region recorded 1,000,786 malaria cases in a population of 2,025,511, including 39,862 cases of severe malaria [34]. Dafra and Do are districts with mixed urban and rural health facilities. The sites are characterized by a high malaria transmission season from June to October [35]. In 2016, there were an estimated 799,951 inhabitants in both districts, 224,726 women of childbearing age and 48,163 pregnant women at risk of malaria. Data suggest that between 2012 and 2016 the frequency of one ANC visit during pregnancy was about 80.3% [34]. The literacy rate was 41.3%, with a gap of 18 points in favour of men [36]. Intermittent preventive treatment with sulphadoxine-pyrimethamine (IPTp/SP), provision of insecticide-treated nets and daily ferrous sulphate (200 mg) and folic acid (0.25 mg) are currently recommended as national policy for malaria and anaemia prevention during pregnancy [12, 37].
Study Design
This was a prospective observational cohort study in which we actively collected information on drug exposure for the entire course of the pregnancy using a pregnancy exposure registry. This study was nested within an intervention study (NCT01703884 [38]) entitled ‘ANC & Malaria Diagnostic in Pregnancy’ designed to assess the impact of a package of interventions aimed at reducing malaria-related mortality and morbidity in pregnant women and newborns.
Briefly, the main intervention study was a cluster-randomised study in which 16 health facilities were randomised to intervention (eight health facilities) and control (eight health facilities). At the community level women were encouraged by community-based health workers to access ANC early in the pregnancy, attend follow-up antenatal visits throughout the pregnancy and deliver at the health facility. In the control area, women were provided with standard care. In the intervention area, a pregnancy drug-exposure registry was set up in order to collect medication exposure and pregnancy outcomes. During the last trimester of the pregnancy, a malaria rapid diagnostic test was performed systematically, and those who had malaria were treated [38]. All pregnant women enrolled at their first ANC visit in the intervention group were included in this analysis.
Patient Recruitment and Follow-up
Study participants were recruited at antenatal clinics and were monitored by study staff (midwives, nurses and physicians) at their scheduled ANC visits until delivery. In Burkina Faso routine healthcare practice required a minimum of four ANC visits (first trimester, second trimester, third trimester and 2 weeks before delivery) [39]. During enrolment procedures, women were sensitized and encouraged to attend their health facility when they were sick and report any problems occurring with their health or pregnancy as early as possible. At enrolment, demographic data, vital signs and medical/pregnancy history were obtained, and a clinical/obstetric examination was performed. The woman’s medical card was used to record treatment and drug exposure history during or before pregnancy. All procedures were repeated at each scheduled or unscheduled ANC visit. A special effort was made by the study staff to record all medications, adverse events and any problems that occurred during the pregnancy.
Medication Use Reports
All information on medications was collected at every visit (scheduled or unscheduled). Pregnant women were asked for information regarding any new drug exposure, and in all cases evidence for the new medication was verified from antenatal cards and prescription sheets. Visual aids were used to facilitate the recognition (to enhance recall) of drug names, especially essential medicines available within the health facility drug stores and commonly used by the population: antimalarial drugs, antibiotics, analgesic/anti-inflammatory drugs and vitamins, etc. To facilitate data collection on medication use, the most common symptoms of acute illnesses (malaria, fever, sexual or urinary infection, vaginal discharge or bleeding) and the most prevalent chronic disorders (diabetes, high blood pressure, epilepsy, HIV) were itemised in the data collection tools, and women were asked if they had experienced these conditions during pregnancy or between the last visit and the interview. In the case of a positive answer, women were asked to report any medication used for the indication. The data collection tools also included traditional or herbal medicines, questions about use of over-the-counter medications and use of medications from illegal sources. All medications were coded into the corresponding Anatomical Therapeutic Chemical (ATC) codes at the fifth level in accordance with the WHO ATC index [40]. In addition, the drugs were also grouped into therapeutic classes. Drugs used for prevention of malaria and anaemia as recommended routinely by WHO and the national malaria control program during pregnancy, namely sulphadoxine-pyrimethamine and iron-folic acid [12, 37], were given to all women at ANC visits, and therefore excluded from the analysis.
Safety Classification of Medications According to Fetal Safety
Our purpose was to understand the clinical relevance and safety of drug exposure, and we categorised medicine exposure into ‘probably safe’, ‘potentially risky’ or ‘unclassified’. We classified drugs using two internationally recognised risk classification systems to attribute each drug in risk groups according to fetal safety. The FDA classification system, which uses five categories, A, B, C, D, and X [41], was used as the primary approach. In June 2016, the FDA updated their pregnancy risk categories. The A, B, C, D and X are no longer used, but were in use at the time of the present study [42]. As previously reported [17], when medications were part of a combination treatment, they were classified according to the dominant active ingredient. Likewise, for medications manufactured with several active pharmaceutical ingredients, the risk categorisation was performed according to the active substance with the highest risk. If, despite this method of classification, a specific medication was not covered by the FDA classification, the AU-TGA classification system [43] was used as a secondary method of classification. According to these two classifications, categories C, D, and X for FDA and categories B3, C, D and X for AU-TGA are classified as potentially causing fetal harm when administered to a pregnant woman (‘potentially risky’).
Secondly, findings of the classification from the FDA or AU-TGA were modified according to several considerations. Indeed, this modified classification was built by taking in consideration the epidemiological profile of the country, the WHO recommendations and Burkina Faso national policy guidelines for the management of malaria cases and its symptoms during pregnancy. Some drugs classified as ‘potentially risky’ by the FDA or AU-TGA are authorised for use during pregnancy, because post-marketing studies have increased the evidence base that depending upon the trimester of exposure, these drugs could be considered as safe for treatment during pregnancy [12, 17, 37, 44–47]. Consequently, quinine treatment during pregnancy and ACTs when used after the first trimester of pregnancy were re-classified as ‘probably safe’. Likewise, ‘unclassified medicines’, within the FDA or AU-TGA classification such as phloroglucinol, metopimazine and drotaverine were re-classified as ‘probably safe’. However, traditional or herbal medicines or drugs from illicit sources were grouped together as ‘potentially risky’ medications [48, 49].
Drugs that could not be classified were maintained as ‘unclassified’. In general, in assigning individual pregnancies to a particular risk group, we always classified participants who were exposed to multiple drugs in the highest risk group.
Data Management and Statistical Analysis
Data were collected and recorded on paper Case Report Forms (CRFs). All data were processed from the CRFs into a Clinical Data Management System using OpenClinica® software (OpenClinica LLC and collaborators, Waltham, MA, USA). Statistical analyses were performed using R software (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria).
The period or trimester of drug exposure was calculated as the difference between the date of drug prescription or self-medication and the date of the last menstrual period. The period was assigned to the first trimester if the time calculated was less than 15 weeks, otherwise it is assigned to the second (15–28 weeks) or third trimester (> 28 weeks) [50].
Descriptive statistics were used to summarize the prevalence of drug exposure according to the risk categories and trimester of pregnancy and at delivery. To explore the association between ‘potentially risky’ medications use and co-variables, a logistic regression model was applied. Based on our modified classification, the outcome variable is a binary, exposure to at least one ‘potentially risky’ medication (1) and not having been exposed to any ‘potentially risky’ medications (0) throughout the pregnancy. Variables were selected for inclusion in the multivariable model if associated with the respective outcome variable was p < 0.2. A complete case analysis was used and observations with missing values were omitted.
Odds ratios (OR) with 95% confidence interval were computed in univariate and multivariable analyses and two-tailed p values < 0.05 were considered significant.
Ethics
The study was approved by the ethics committee of Centre Muraz, Bobo-Dioulasso, Burkina Faso and the National ethics committee of Burkina Faso, and the WHO Ethics Review Committee, and was part of an intervention trial registered in the ClinicalTrial.gov registry (identifier: NCT01703884). The study purpose and all procedures were explained to the potential participants by the study clinical team. Women who fulfilled the inclusion criteria and consented to participate in the study provided written informed consent.
Results
Baseline Characteristics
From August 2012 to July 2014, 2371 women aged 14–50 years were enrolled. The median pregnant women age was 25.0 (20.0–30.0) years. Overall, we included 932 women (39.3%) from rural areas and 1439 women (60.7%) from urban areas. About one-quarter (25.8%) of women enrolled were nulliparous. More than half of women were enrolled during their second trimester of pregnancy. A few women did not know their exact date of last menstruation (n = 122; 5.1%). The baseline characteristics of study participants are summarized in Table 1.
Table 1.
Baseline characteristics of women at enrolment
| No drugs | Use drugs | Traditional medicinea | Drugs from illicit source | Row total | |
|---|---|---|---|---|---|
| Maternal age (years), n (%) | |||||
| < 18 | 69 (62.2) | 35 (31.5) | 7 (6.3) | 0 (0.0) | 111 (100) |
| 18–29 | 1078 (66.4) | 435 (26.8) | 102 (6.3) | 8 (0.5) | 1623 (100) |
| 30–39 | 416 (72.6) | 133 (23.2) | 21 (3.7) | 3 (0.5) | 573 (100) |
| ≥ 40 | 30 (88.2) | 3 (8.8) | 1 (2.9) | 0 (0.0) | 34 (100) |
| Place of residence, n (%) | |||||
| Rural area | 813 (87.2) | 90 (9.7) | 25 (2.7) | 4 (0.4) | 932 (100) |
| Urban area | 804 (55.9) | 521 (36.2) | 106 (7.4) | 8 (0.6) | 1439 (100) |
| Parity, n (%) | |||||
| 0 | 358 (58.8) | 210 (34.5) | 39 (6.4) | 2 (0.3) | 609 (100) |
| 1 | 329 (63.1) | 160 (30.7) | 30 (5.8) | 2 (0.4) | 521 (100) |
| ≥ 2 | 872 (70.8) | 288 (23.4) | 64 (5.2) | 8 (0.6) | 1232 (100) |
| Time of first visits of the HF, n (%) | |||||
| 1st trimester | 500 (64.7) | 233 (30.1) | 34 (4.4) | 6 (0.8) | 773 (100) |
| 2nd trimester | 925 (69.1) | 327 (24.4) | 82 (6.1) | 4 (0.3) | 1338 (100) |
| 3rd trimester | 99 (71.7) | 31 (22.5) | 7 (5.1) | 1 (0.7) | 138 (100) |
| Alcohol consumption, n (%) | |||||
| Yes | 273 (83.5) | 40 (12.2) | 11 (3.4) | 3 (0.9) | 327 (100) |
| No | 1333 (65.6) | 570 (28.1) | 120 (5.9) | 9 (0.4) | 2032 (100) |
Study sample, n = 2,371; numbers may not add up to total number due to missing values
HF health facilities
aTraditional medicines included, herbs/plants, herb/plant preparations and finished herb/plant products, containing parts of plants or other plant materials, animal components perceived to have therapeutic benefits as active ingredients. Traditional medicines can be often combined with traditional practices such as scarification, incisions etc.
Drug Exposure During the Study Period
Drug Risk Classification According to the FDA or AU-TGA
Twenty-six percent (611/2371) of women had used medications before their first ANC visit and some of them used drugs from illicit sources (n = 12; 0.51%). Overall, a total of 101 different medications were used by the study participants and classified as specified above. The most frequently used medications classified as ‘probably safe’, ‘potentially risky’, and ‘unclassified’, respectively, are summarized in Table 2.
Table 2.
Most commonly taken medications during pregnancy, classified as probably safe, potentially risky, or unclassified, according to the US FDA and AU-TGA classifications
| Probably safea | ||
|---|---|---|
| Therapeutic class and INN | ATC code | n (%) |
| Analgesic/antipyretics | ||
| Paracetamol | N02BE01 | 447 (18.9) |
| Antibacterial agents | ||
| Amoxicillin | J01CA04 | 169 (7.1) |
| Erythromycin | J01FA01 | 112 (4.7) |
| Ceftriaxone | J01DD04 | 41 (1.7) |
| Antiprotozoal agents | ||
| Metronidazole | A01AB17 | 83 (3.5) |
| Metronidazole | G01AF01 | 19 (0.8) |
| Antifungal agents | ||
| Miconazole | G01AF04 | 133(5.6) |
| Nystatin | G01AA01 | 63 (2.7) |
| Vitamins | ||
| Vitamin C | A11GA01 | 78 (3.3) |
| Multivitamins | A11AA05 | 75 (3.2) |
| Antispasmodics | ||
| Butylscopolamine | A03BB01 | 28 (1.2) |
| Antiemetics | ||
| Metoclopramide | A03BB01 | 32 (1.3) |
| Levosulpiride | N05AL07 | 20 (0.8) |
| Meclozine | R06AE55 | 11 (0.5) |
| Anti-allergic agents | ||
| Cyproheptadine | R06AX03 | 32 (1.3) |
| Chlorphenamine | R06AB04 | 16 (0.7) |
| Potentially riskyb | ||
|---|---|---|
| Therapeutic class and INN | ATC code | n (%) |
| Antimalarials | ||
| Quinine | P01BC01 | 536 (22.6) |
| Amodiaquine/Artesunate | P01BF03 | 249 (10.5) |
| Artemether/Lumefantrine | P01BF01 | 17 (0.7) |
| Bacterial vaccines | ||
| Tetanus vaccines | J07AM01 | 272 (11.5) |
| Antibacterial agents | ||
| Neomycin | D07CB01 | 9 (0.4) |
| Cotrimoxazole | J01EE01 | 4 (0.2) |
| Ciprofloxacin | J01MA02 | 137 (5.8) |
| Antiparasitic agents | ||
| Albendazole | P02CA03 | 13 (0.5) |
| Mebendazole | P02CA01 | 48 (2.0) |
| Analgesic/Antipyretics | ||
| Metamizole | N02BB02 | 22 (0.9) |
| Lysine acetylsalicylate | N02BA01 | 17 (0.7) |
| Sedatives | ||
| Diazepam | N05BA01 | 10 (0.4) |
| Anti-inflammatory | ||
| Diclofenac | M01AB05 | 12 (0.5) |
| Ibuprofen | M01AE51 | 5 (0.2) |
| Antiretrovirals | ||
| Combivir | J05AR01 | 1 (0) |
| Zidovudine | J05AF01 | 5 (0.2) |
| Unclassified | ||
|---|---|---|
| Therapeutic class and INN | ATC code | n (%) |
| Antispasmodics | ||
| Phloroglucinol | A03AX12 | 320 (13.5) |
| Drotaverine | A03AD02 | 15 (0.6) |
| Antiemetics | ||
| Metopimazine | A03FA01 | 44 (1.9) |
| Antimalarials | ||
| Dihydroartemisinin/piperaquine | P01BF05 | 23 (1) |
| Artesunate | P01BE03 | 2 (0.1) |
| Antibacterial agents | ||
| Spiramycin | J01FA02 | 3 (0.1) |
| Antiprotozoal agents | ||
| Tilbroquinol | P01AA05 | 3 (0.1) |
AU-TGA: Australian Therapeutic Goods Administration, US-FDA: United States Food and Drug Administration, ATC: Anatomical Therapeutic Chemical, INN: International Non-proprietary Name
Women may have used more than one medication
Study sample, n = 2371. These medicines have been used women of the study by 1345
aExcluding iron+folic acid for anaemia prevention
bExcluding sulphadoxine-pyrimethamine for intermittent preventive treatment in pregnancy
With FDA/AU-TGA classification, 36.6% (37/101) of medications were classified as ‘probably safe’ for use during pregnancy. With 49.5% (50/101), about half of exposures during pregnancy were classified as ‘potentially risky’. The most frequently used drugs in this group were antimalarials, and the most frequent vaccine was tetanus vaccine. It appeared that 13.9% of medications could not be classified and the medications that frequently fell into this group were phloroglucinol, drotaverine and metopimazine.
Therapeutic Class Use According to Gestational Age
During the study period, a total of 4279 drugs were used by pregnant women, including 1492 in the first trimester, 1526 in the second trimester and 539 in the third trimester. The median number (interquartile range) of drugs taken was estimated at 2 (1–3). Out of the 2371 women enrolled, 1345 (56.7%) used at least one medication during their pregnancy.
The proportion of women who had received a new drug prescription (used for the first time) was 25.7% (610/2371), 26.6% (630/2371) and 10.6% (251/2371), respectively, during the first, second and third trimester.
Antimalarials, analgesics/antipyretics, antispasmodics and antibiotics were the most commonly used medicines to which women were exposed during their first trimester. Moreover, it was noted that traditional or herbal medicine use was considerable, especially during the first trimester of pregnancy, with 24.4% (149/610) of women being exposed to traditional or herbal medicines. During the second trimester, antimalarials were the most common medicinal exposures, followed by antibiotics, analgesics/antipyretics, tetanus vaccines and antispasmodics. However, during the third trimester, antibiotics became the most commonly used drugs, followed by antimalarials, antifungals and antispasmodics (Table 3).
Table 3.
Therapeutic classes used by the study pregnant womena during the study period
| Trimester of drug consumptiond | Delivery | |||
|---|---|---|---|---|
| First | Second | Third | ||
| n = 610b | n = 630b | n = 251b | n = 400b | |
| Therapeutic class n (%)c | ||||
| Antimalarials | 384 (62.9) | 250 (39.7) | 68 (27.1) | 77 (19.3) |
| Analgesics/antipyretics | 278 (45.6) | 153 (24.3) | 44 (17.5) | 16 (4) |
| Traditional/herbal medicine | 149 (24.4) | 95 (15.1) | 22 (8.8) | 3 (0.8) |
| Antispasmodics | 147 (24.1) | 118 (18.7) | 49 (19.5) | 27 (6.8) |
| Antibiotics | 108 (17.7) | 223 (35.4) | 134 (53.4) | 33 (8.3) |
| Vitamins | 92 (15.1) | 69 (10.9) | 28 (11.2) | 5 (1.2) |
| Antiemetics | 75 (12.3) | 21 (3.3) | 6 (2.4) | 2 (0.5) |
| Antifungals | 42 (6.9) | 104 (16.5) | 52 (20.7) | 9 (2.3) |
| Antiparasitics | 40 (6.6) | 67 (10.6) | 17 (6.8) | 36 (9.0) |
| Tetanus vaccine | 37 (6.1) | 130 (20.6) | 11 (4.4) | 61 (15.3) |
| Antacids | 13 (2.1) | 11 (1.7) | 0 (0) | 0 (0) |
| Sedatives | 10 (1.6) | 2 (0.3) | 2 (0.8) | 3 (0.8) |
| Antihistaminics (H1) | 8 (1.3) | 8 (1.3) | 3(1.2) | 1 (0.3) |
| Mucolytics | 5 (0.8) | 14 (2.2) | 5 (2.0) | 0 (0) |
| Non-steroidal anti-inflammatory | 4 (0.7) | 7 (1.1) | 6 (2.4) | 2 (0.5) |
| Progesterones | 3 (0.5) | 0 (0) | 0 (0) | 0 (0) |
| Corticoids | 2 (0.3) | 2 (0.4) | 2 (0.8) | 0 (0) |
| Hemostatics | 2 (0.3) | 0 (0) | 0 (0) | 0 (0) |
| Antiulcer (proton pump inhibitors) | 2 (0.3) | 1 (0.2) | 0 (0) | 0 (0) |
| Mineral elements | 2 (0.3) | 3 (0.5) | 2 (0.8) | 1 (0.3) |
| Antiretrovirals | 1 (0.2) | 4 (0.6) | 0 (0) | 1 (0.3) |
| Contact laxatives | 1 (0.2) | 3 (0.5) | 2 (0.8) | 0 (0) |
| Antihypertensives | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.3) |
| Diuretics | 1 (0.2) | 0 (0) | 1 (0.4) | 0 (0) |
| Antihistaminics (H2) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Anxiolytics | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Nasal preparation combinations | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Nutraceutical agents | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Venotonic | 1 (0.2) | 1 (0.2) | 3 (1.2) | 0 (0) |
| Decongestants | 0 (0) | 3 (0.5) | 0 (0) | 0 (0) |
| Local antiseptics | 0 (0) | 4 (0.6) | 1 (0.4) | 0 (0) |
| Antidiarrheals | 0 (0) | 1 (0.2) | 1 (0.4) | 0 (0) |
| Local anesthetics | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Neuroprotective agents | 0 (0)) | 1 (0.2) | 0 (0) | 0 (0) |
| Tetanus serum | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) |
| Electrolytes | 0 (0) | 2 (0.3) | 1 (0.4) | 0 (0) |
| Concentrated red blood cells | 0 (0) | 1 (0.2) | 2 (0.8) | 0 (0) |
aIn whom the date of drug intake and the date of last period of menstruation was known
bNumber of pregnant women. The number of women in each period is not mutually exclusive
cPregnant women could have many episodes of disease and could have more than one episode of drug exposure
dExcluding sulphadoxine-pyrimethamine for intermittent preventive treatment in pregnancy and ferrous sulphate + folic acid for anaemia prevention
Therapeutic Class Use at Delivery
At delivery, the proportion of women who received a new drug prescription was 16.9% (400/2371). The median number of drugs taken per pregnant woman was 1. Antimalarials remained the most common class of drugs used, followed by tetanus vaccine, antiparasitics and antibiotics.
Some pregnant women had more than one episode of disease during the pregnancy, and consequently more than one drug was prescribed during their pregnancy. Altogether, at delivery, 46.8% and 43.3% of women were exposed to drugs classified as ‘probably safe’ and ‘potentially risky’, respectively.
At the individual level (individual risk assignment) and irrespective of trimester, 39.2% (930/2371) of the women used medications classified as ‘potentially risky’. Although antimalarials remained the most widely used drugs, the frequency of their use decreased as the pregnancy progressed.
Modified Drug Risk Classification According to WHO and National Malaria Policy Treatment Guidelines
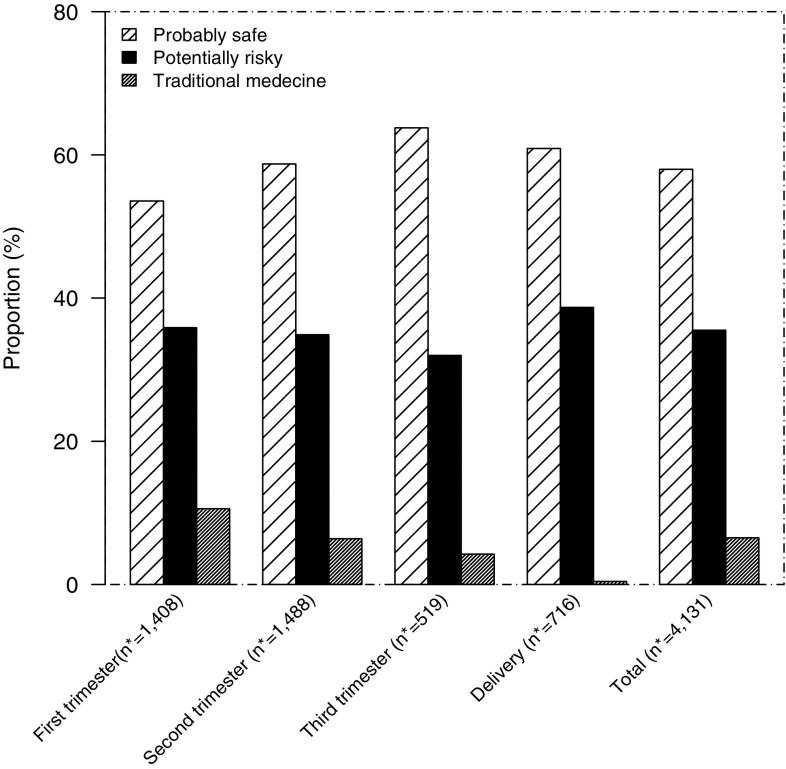
After pooling traditional or herbal medicines and drugs from illicit sources as ‘potentially risky’, and antimalarials (except first trimester of pregnancy for ACT), phloroglucinol, metopimazine and drotaverine as ‘probably safe’ (Table 4), more than half of pregnant women (56.9%) used medications classified as ‘probably safe’. The results from the analysis on medications used by place of residence are shown in Fig. 1. We found that medications classified as risky were most often prescribed in rural areas. According to the trimester of pregnancy, 53.6, 58.7, 63.8 and 60.9% of medications use were classified as ‘probably safe’, respectively, during the first, second, third trimester and delivery (Fig. 2). For individual pregnancies, regardless of trimester, 26.4% (626/2371) of the women used medications classified as ‘potentially risky’.
Table 4.
Most probably-safe and potentially-risky medications used during pregnancy: modified classification from US-FDA and AU-TGA
| Probably-safea | Potentially-riskyb | ||||
|---|---|---|---|---|---|
| Therapeutic class and INN | ATC code | n (%) | Therapeutic class and INN | ATC code | n (%) |
| Antimalarials | Antimalarials | ||||
| Quinine | P01BC01 | 536 (22.6) | Quinine | P01BC01 | – |
| Amodiaquine/Artesunate | P01BF03 | 161 (6.8) | Amodiaquine/Artesunate | P01BF03 | 85 (3.58) |
| Artemether/Lumefantrine | P01BF01 | 9 (0.4) | Artemether/Lumefantrine | P01BF01 | 8 (0.3) |
| Dihydroartemisinin/piperaquine | P01BF05 | 11 (0.5) | Dihydroartemisinin/piperaquine | P01BF05 | 12 (0.5) |
| Artesunate | P01BE03 | 1 (0) | Artesunate | P01BE03 | 2 (0.1) |
| Analgesic/antipyretics | Bacterial vaccines | ||||
| Paracetamol | N02BE01 | 447 (18.9) | Tetanus vaccines | J07AM01 | 272 (11.5) |
| Antibacterial agents | Antibacterial agents | 9 (0.4) | |||
| Amoxicillin | J01CA04 | 169 (7.1) | Neomycin | D07CB01 | 4 (0.2) |
| Erythromycin | J01FA01 | 112 (4.7) | Cotrimoxazole | J01EE01 | |
| Ceftriaxone | J01DD04 | 41 (1.7) | Ciprofloxacin | J01MA02 | 137 (5.8) |
| Antiprotozoal agents | Antiparasitic Agents | 13 (0.5) | |||
| Metronidazole | A01AB17 | 83 (3.5) | Albendazole | P02CA03 | |
| Metronidazole | G01AF01 | 19 (0.8) | Mebendazole | P02CA01 | 48 (2.0) |
| Antifungal agents | Analgesic/Antipyretics | 22 (0.9) | |||
| Miconazole | G01AF04 | 133 (5.6) | Metamizole | N02BB02 | |
| Nystatin | G01AA01 | 63 (2.7) | Lysine acetylsalicylate | N02BA01 | 17 (0.7) |
| Vitamins | Sedatives | ||||
| Vitamin C | A11GA01 | 78 (3.3) | Diazepam | N05BA01 | 10 (0.4) |
| Multivitamins | A11AA05 | 75 (3.2) | Anti-inflammatory | ||
| Antispasmodics | Diclofenac | M01AB05 | 12 (0.5) | ||
| Phloroglucinol | A03AX12 | 320 (13.5) | Ibuprofen | M01AE51 | 5 (0.2) |
| Butylscopolamine | A03BB01 | 28 (1.2) | Antiretrovirals | ||
| Drotaverine | A03AD02 | 15 (0.6) | Combivir | J05AR01 | 1 (0.0) |
| Antiemetics | Zidovudine | J05AF01 | 5 (0.2) | ||
| Metopimazine | A03FA01 | 44 (1.9) | |||
| Metoclopramide | A03BB01 | 32 (1.3) | |||
| Levosulpiride | N05AL07 | 20 (0.8) | |||
| Meclozine | R06AE55 | 11 (0.5) | |||
| Anti-allergic agents | |||||
| Cyproheptadine | R06AX03 | 32 (1.3) | |||
| Chlorphenamine | R06AB04 | 16 (0.7) | |||
AU-TGA Australian Therapeutic Goods Administration, US-FDA United States Food and Drug, ATC Anatomical Therapeutic Administration Chemical, INN International Non-proprietary Name
Women may have used more than one medication
Study sample, n = 2371. These medicines have been used by 1345 women of the study
aExcluding iron+folic acid for anaemia prevention
bExcluding sulphadoxine-pyrimethamine for intermittent preventive treatment in pregnancy
Fig. 1.
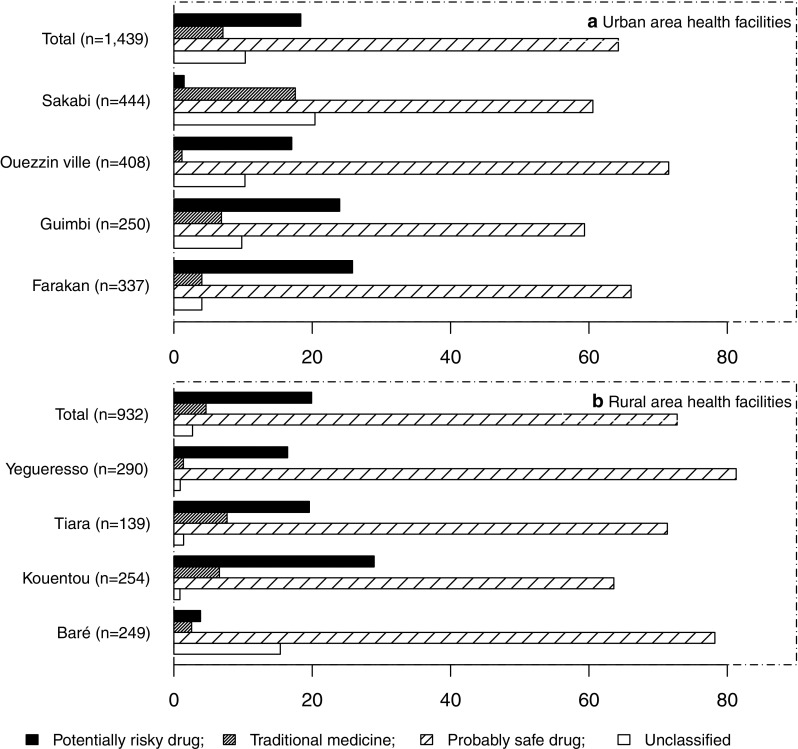
Proportion of women (%) using ‘potentially risky’, traditional or herbal medicines, ‘probably safe’ and unclassified medications during pregnancy according to health facilities and place of residence according to the modified classifications
Fig. 2.
Safety of medication used during the trimesters of pregnancy and at delivery according to the to the modified classifications. Asterisk indicates total number of drugs used by women according to pregnancy trimester and at delivery
Factors Associated with Potentially Risky Medications Use
Maternal factors that were associated with the use of ‘potentially risky’ medications during pregnancy are shown in Table 5. In univariate analysis, we found that maternal age had no significant effect on ‘potentially risky’ medication use. However, place of residence, alcohol consumption, parity (≥ 1) and time of first visit of the health facilities were negatively associated with ‘potentially risky’ drug use throughout the pregnancy. In multivariable analysis, women living in urban areas, attending the first ANC at their first trimester of pregnancy are more likely to use potentially risky drugs.
Table 5.
Risk factors associated with use of potentially-risky drugs among pregnant women attending antenatal consultation in Bobo-Dioulasso, Burkina Faso
| Maternal characteristics | Potentially-risky drugs usea | Crude ORb [95% CI] | P | Adjusted ORb [95% CI] | P |
|---|---|---|---|---|---|
| Maternal age (years) | 0.36 | ||||
| < 18 | 27.3 (30/110) | 1 | |||
| 18–29 | 27.4 (443/1619) | 1.00 [0.66–1.57] | |||
| 30–39 | 24.6 (140/569) | 0.87 [0.55–1.40] | |||
| ≥ 40 | 17.6 (6/34) | 0.57 [0.20–1.44] | |||
| Place of residence | < 0.001 | 0.019 | |||
| Urban | 29.6 (423/1431) | 1 | 1 | ||
| Rural | 21.8 (196/901) | 0.66 [0.54–0.80] | 0.77 [0.62–0.96] | ||
| Alcohol consumption | < 0.001 | 0.005 | |||
| No | 28.0 (563/2013) | 1 | 1 | ||
| Yes | 17.6 (56/319) | 0.55 [0.40–0.74] | 0.63 [0.45–0.87] | ||
| Parity | 0.007 | 0.07 | |||
| 0 | 30.8 (187/608) | 1 | 1 | ||
| ≥ 1 | 25.1 (432/1724) | 0.75 [0.61–0.92] | 0.83 [0.67–1.02] | ||
| Time of first visits of the health facilities | < 0.001 | < 0.001 | |||
| 1st trimester | 32.3 (246/762) | 1 | 1 | ||
| 2nd trimester | 25.7 (339/1318) | 0.73 [0.06–0.88] | 0.76 [0.62–0.92] | ||
| 3rd trimester | 21.5 (29/135) | 0.57 [0.36–0.88] | 0.57 [0.36–0.88] | ||
HF health facilities, CI confidence Interval
a Pregnant women who have been exposed to at least one medication classified a ‘potentially-risky’
b Odds Ratios [95% confidence interval]
Discussion
To our knowledge, this is the first study examining the safety profile and magnitude of medications during pregnancy in Burkina Faso. Our findings are important in understanding the range of drug exposures and their safety profiles during pregnancy. The modified classification system applied took advantage of classifying a larger number of drugs than would have been possible with only one classification system [17]. However, nearly one-seventh (13.9%) of drugs to which women were exposed could not be classified. This finding is consistent with a Danish study’s findings that 12% of prescriptions could not be classified [51], but is considerably less than a European multinational medication utilisation study that was unable to classify 23.2% of the prescriptions during pregnancy [17]. The reasons why some medications cannot be classified could probably be explained by the fact that some drugs do not have FDA approval. Thus, either no application for a marketing license has been made for the USA or the drug does not yet meet the required standards for safety, effectiveness, quality and labelling [52]. The Summary of Product Characteristics for most unclassified medications had limited or no information available in the pregnancy section. Our substantial rate of unclassified drugs highlights the fact that an important proportion of drugs used during pregnancy have poor pregnancy safety information.
Approximatively, half of medications available in the study setting and used to treat illness during pregnancy were classified as ‘potentially risky’. A substantial proportion of several classes of medicines used during pregnancy were antimalarials, which corresponds with the epidemiological risk of the study area [34]. To reduce the risk of malaria during pregnancy, women need to be protected by an effective long-acting antimalarial in order to both limit the malaria and anaemia risks for the mother and reduce risks of poor pregnancy outcomes (low birthweight, prematurity, miscarriage, etc.). Unfortunately, effective medicines for malaria case-management are classified by the FDA/AU-TGA as ‘potentially risky’ (category C) when used during pregnancy [41]. Because animal reproductive toxicology studies may have revealed adverse effects and there are limited safety data in humans, the WHO examines whether potential benefits may warrant use of the drug in pregnant women despite potential risks [12]. In our study, the majority of women were exposed to antimalarials for severe acute or life-threatening infections, and these antimalarial drugs to which they were exposed are authorised for use by the national drug regulatory authority and WHO. Although the FDA classified these antimalarials as ‘potentially risky’ drugs, worldwide experience with antimalarial drugs, especially quinine (all trimesters of pregnancy) and ACTs in second-third trimesters (over 4000 documented pregnancies), is reassuring. Risk–benefit assessments have shown no adverse effects on the mother or fetus [12] and WHO now recommends use of these medications for treatment during pregnancy, depending upon the trimester of exposure and the severity of illness [33]. For this reason, we modified the FDA/AU-TGA classification to the study context.
Excluding antimalarials, antibiotics were the second largest class of reported drug exposure. This finding is similar to reports in previous studies showing that these drugs are the most frequent medicines taken during pregnancy mainly for the treatment of infectious diseases [19, 21–23]. After antibiotics, analgesics/antipyretics and antispasmodics were the most frequently prescribed drugs, and this might be explained by the presence of concomitant symptoms (fever, headache, uterine contraction) of infectious diseases.
Prescription during pregnancy may vary according to the epidemiology of the area and this limits comparisons between developing countries and developed counties [17, 21, 53]. Despite this caveat, the average number of drugs taken (1.8) by study participants was slightly greater than findings reported in Ethiopia where the average number of medications prescribed per pregnant woman was 1.1 [23] but is lower than the number reported for Mozambique (3.9) and Nigeria (3.0) [19, 21]. The latter difference could be explained by the exclusion of the drugs recommended by the national policy to prevent malaria and anaemia during pregnancy in our study [12, 37]. Drug prescription and the proportion of prescriptions that fell into the ‘potentially risky’ category declined during the entire course of the pregnancy, similar to findings reported in Denmark [51].
Traditional or herbal medicines use was non-negligible, especially in the first trimester of pregnancy, probably for gastrointestinal disorders and malaria treatment [54]. According to WHO, about 80% of the population of developing countries relies on traditional or herbal medicines for their primary-care needs [54]. Traditional or herbal medicines use during pregnancy is of concern because of their limited safety profiles. There may well be culturally specific knowledge about the use of medicinal plants by traditional healers [54], but herbal medicines can put both mother and fetus at risk [48, 49]. Moreover, a non-negligible number of women practiced self-medication with drugs purchased from illicit sources (such as shops and markets), although studies have demonstrated that these medicines are of poor quality [27].
In our study area, pregnant women who consumed alcohol were less likely to be exposed to ‘potentially risky’ medications (aOR: 0.63; 95% CI 0.45–0.87); this finding could be partly attributed to the small sample size in this group. However, previous studies reported that poor/low-income pregnant women have higher vulnerability to alcohol consumption in both developed and developing country settings [55, 56]. Therefore, in our study alcohol consumption might be confounded by lower social status (poor pregnant women have less access to healthcare and medicines), explaining the fact that women who consumed alcohol were less likely to be exposed to ‘potentially risky’ drugs.
Women attending their first ANC after the first trimester of pregnancy were less likely to use ‘potentially risky’ medication. This could be explained by the fact that the study participation period of women who came to ANC after the first trimester was shorter than that of women attending their first ANC within the first trimester; the latter group were also more likely to have malaria infection episodes [57] and so more drugs might have been prescribed to this group.
A strength of our study is that the collection of drug exposure data was performed both retrospectively (history at first ANC visit) and prospectively after the first ANC visit. Although this research achieved its aims, there were some limitations.
First, the study population was pregnant women attending antenatal clinic for prenatal care and who were systematically screened for malaria throughout pregnancy, and therefore more likely to be treated with antimalarials drugs than the general population of pregnant women. Regular sensitisation of pregnant women in their community probably had the desirable effect of improving the routine rates of ANC attendance. Although this may have had the effect of increasing drug consumption (especially, antimalarial drugs) compared to routine practice (since any health problem requiring medical attention would have been monitored), the study population might not be representative of the national population, and therefore it may not be possible to generalize these results to the general population.
A second limitation of the study is the fact that our classification depended on the US and Australian classification system, which is relevant to drugs authorised for use in those countries. We partially resolved this limitation by adapting the classification system to incorporate WHO guidelines. The FDA have recently modified their pregnancy risk categories in order to make risk categorisation more meaningful for both patients and healthcare providers [42] and have replaced the former pregnancy risk letter categories by available information regarding the potential benefits and risks for the mother, fetus and breastfeeding child.
Third, although women aged below 17 years were enrolled in the study and technically would be considered minors and fall into the paediatric category, we did not use the FDA paediatric labelling in our classification of risk.
Conclusion
This study provides rare and valuable information on the current use of drugs among pregnant women in Burkina Faso. More than half of pregnant women used ‘probably safe’ medications. However, it is reassuring that the majority of ‘potentially risky’ medications used by pregnant women are essential medications for important diseases, which are harmful to both mother and developing fetus. Both living in urban areas and early attendance at antenatal care were associated with the use of ‘potentially risky’ medications during pregnancy; the latter finding is probably related to a longer period of antenatal care and increased health surveillance during the period. This challenges the health system to reinforce the rationale of drug prescriptions during pregnancy and the need for community education, especially in urban areas, to reduce hazardous drug exposure. Continuous training of health professionals in pharmacovigilance and teratovigilance will be important to ensure safe medication use for both mother and child.
Funding statement
This study is part of a main study entitled ‘ANC & Malaria Diagnostic in Pregnancy’ supported by the Alliance for Health Policy and Systems Research (AHPSR), World Health Organization Special Programme for Research and Training in Tropical Diseases (WHO/TDR).
Authors’ contributions
FKS, HT, MG, IV and TR designed the study protocol; JDB and KH coordinated the fieldwork and performed the data collection; TR performed statistical analysis with inputs from FKS and drafted the manuscript; HT, MG and FKS revised the article. All authors read and approved the final manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee (Centre Muraz Bobo-Dioulasso, Burkina Faso), National health research ethics committee and WHO Ethics Review Committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
Toussaint Rouamba, Innocent Valea, Joel D. Bognini, Herve Kpoda, Petra F. Mens, Melba F. Gomes, Halidou Tinto, and Fati Kirakoya-Samadoulougou declare that they have no competing interests.
Data availability
The datasets generated during and/or analysed during the current study are available from the World Health Organization data repository and from the Clinical Research Unit of Nanoro on reasonable request.
References
- 1.Ridde V, Nitièma AP, Dadjoari M. Improve the accessibility of essential drugs for the populations of one medical region in Burkina Faso. Cah Santé. 2005;15:175–82. http://www.ncbi.nlm.nih.gov/pubmed/16207580. Accessed 6 Sep 2017. [PubMed]
- 2.World Health Organization | Malaria control improves for vulnerable in Africa, but global progress off-track. WHO. 2016. http://www.who.int/mediacentre/news/releases/2016/malaria-control-africa/en/. Accessed 28 Sep 2017.
- 3.Mbwasi R, Mlaki W. Increasing access to medicines in Tanzania. Lancet. 2008;372:205–206. doi: 10.1016/S0140-6736(08)61070-3. [DOI] [PubMed] [Google Scholar]
- 4.Brazier E, Andrzejewski C, Perkins ME, Themmen EM, Knight RJ, Bassane B. Improving poor women’s access to maternity care: Findings from a primary care intervention in Burkina Faso. Soc Sci Med. 2009;69:682–690. doi: 10.1016/j.socscimed.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Ndyomugyenyi R, Neema S, Magnussen P. The use of formal and informal services for antenatal care and malaria treatment in rural Uganda. Health Policy Plan. 1998;13:94–102. http://www.ncbi.nlm.nih.gov/pubmed/10178189. Accessed 7 Sep 2017. [DOI] [PubMed]
- 6.Hogan DR, Stevens GA, Hosseinpoor AR, Boerma T. Monitoring universal health coverage within the Sustainable Development Goals: development and baseline data for an index of essential health services. Lancet Glob Heal. 2018;6:e152–e168. doi: 10.1016/S2214-109X(17)30472-2. [DOI] [PubMed] [Google Scholar]
- 7.McBride W. Thalidomide and congenital malformations. Lancet. 1961;2:1358. doi: 10.1016/S0140-6736(61)90927-8. [DOI] [Google Scholar]
- 8.Lenz W. Thalidomide and congenital abnormalities. Lancet. 1962;1:271–272. [Google Scholar]
- 9.WARD SP. Thalidomide and congenital abnormalities. Br Med J. 1962;2:646–7. http://www.ncbi.nlm.nih.gov/pubmed/14004945. Accessed 16 Nov 2017. [DOI] [PMC free article] [PubMed]
- 10.Shields KE, Wiholm B-E, Hostelley LS, Striano LF, Arena SR, Sharrar RG. Monitoring outcomes of pregnancy following drug exposure: a company-based pregnancy registry program. Drug Saf. 2004;27:353–67. http://www.ncbi.nlm.nih.gov/pubmed/15144230. Accessed 7 Sep 2017. [DOI] [PubMed]
- 11.Howard TB, Tassinari MS, Feibus KB, Mathis LL. Monitoring for teratogenic signals: pregnancy registries and surveillance methods. Am J Med Genet Part C Semin Med Genet. 2011;157:209–214. doi: 10.1002/ajmg.c.30304. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Guidelines For The Treatment of Malaria. Third. Geneva, Switzerland; 2015. http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf.
- 13.Vitoria M, Ford N, Clayden P, Pozniak AL, Hill AM. When could new antiretrovirals be recommended for national treatment programmes in low-income and middle-income countries: results of a WHO Think Tank. Curr Opin HIV AIDS. 2017;12:414–422. doi: 10.1097/COH.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zash R, Jacobson DL, Diseko M, Mayondi G, Mmalane M, Essex M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Heal. 2018;6:e804–e810. doi: 10.1016/S2214-109X(18)30218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakker M, Jentink J, Vroom F, Den Van, Berg P, De Jong-Van Den Walle H, Berg L. Maternal medicine: drug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. BJOG An Int J Obstet Gynaecol. 2006;113:559–568. doi: 10.1111/j.1471-0528.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 16.Lupattelli A, Spigset O, Twigg MJ, Zagorodnikova K, Mårdby AC, Moretti ME, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014;4:e004365. doi: 10.1136/bmjopen-2013-004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trønnes JN, Lupattelli A, Nordeng H. Safety profile of medication used during pregnancy: results of a multinational European study. Pharmacoepidemiol Drug Saf. 2017;26:802–811. doi: 10.1002/pds.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daw JR, Hanley GE, Greyson DL, Morgan SG. Prescription drug use during pregnancy in developed countries: a systematic review. Pharmacoepidemiol Drug Saf. 2011 doi: 10.1002/pds.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eze UI, Eferakeya AE, Oparah AC, Enato EF. Assessment of prescription profile of pregnant women visiting antenatal clinics. Pharm Pract (Granada). 2007; 5:135–9. http://www.ncbi.nlm.nih.gov/pubmed/25214930. Accessed 22 Sep 2017. [DOI] [PMC free article] [PubMed]
- 20.Kebede B, Gedif T, Getachew A. Assessment of drug use among pregnant women in Addis Ababa, Ethiopia. Pharmacoepidemiol Drug Saf. 2009;18:462–468. doi: 10.1002/pds.1732. [DOI] [PubMed] [Google Scholar]
- 21.Sevene E, Bardají A, Mariano A, Machevo S, Ayala E, Sigaúque B, et al. Drug exposure and pregnancy outcome in Mozambique. Pediatr Drugs. 2012;14:43–49. doi: 10.2165/11591270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Mosha D, Mazuguni F, Mrema S, Abdulla S, Genton B. Medication exposure during pregnancy: a pilot pharmacovigilance system using health and demographic surveillance platform. BMC Pregnancy Childbirth. 2014;14:1–10. doi: 10.1186/1471-2393-14-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molla F, Assen A, Abrha S, Masresha B, Gashaw A, Wondimu A, et al. Prescription drug use during pregnancy in Southern Tigray region, North Ethiopia. BMC Pregnancy Childbirth. 2017;17:170. doi: 10.1186/s12884-017-1359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellicour S, ter Kuile FO, Stergachis A. Pregnancy exposure registries for assessing antimalarial drug safety in pregnancy in malaria-endemic countries. PLoS Med. 2008;5:e187. doi: 10.1371/journal.pmed.0050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinto H, Sevene E, Dellicour S, Calip GS, d’Alessandro U, Macete E, et al. Assessment of the safety of antimalarial drug use during early pregnancy (ASAP): protocol for a multicenter prospective cohort study in Burkina Faso, Kenya and Mozambique. Reprod Health. 2015;12:112. doi: 10.1186/s12978-015-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zongo F, Nikiema JB. Contrôle national postmarketing de la qualité des produits pharmaceutiques. Annual report. Direction de l’Approvisionnement Pharmaceutique, Service Contrôle Qualité. Ministère de la Santé. Ouagadougou, Burkina Faso. 2010.
- 27.Tipke M, Diallo S, Coulibaly B, Störzinger D, Hoppe-Tichy T, Sie A, et al. Substandard anti-malarial drugs in Burkina Faso. Malar J. 2008;7:95. doi: 10.1186/1475-2875-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton PN, Green MD, Fernández FM. Impact of poor-quality medicines in the ‘developing’ world. Trends Pharmacol Sci. 2010;31:99–101. doi: 10.1016/j.tips.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mwita S, Jande M, Marwa K, Hamasaki K, Katabalo D, Burger J, et al. Medicines dispensers’ knowledge on the implementation of an artemisinin-based combination therapy policy for the treatment of uncomplicated malaria in Tanzania. J Pharm Heal Serv Res. 2017;8:227–233. doi: 10.1111/jphs.12187. [DOI] [Google Scholar]
- 30.Ouédraogo LT, Somé IT, Diarra M, Guissou IP. Self-medication in the treatment of acute malaria: study based on users of private health drug stores in Ouagadougou, Burkina Faso. Bull Soc Pathol Exot. 2008;101:124–7. http://www.ncbi.nlm.nih.gov/pubmed/18543706. Accessed 8 Jul 2018. [PubMed]
- 31.Müller O, Traoré C, Becher H, Kouyaté B. Malaria morbidity, treatment-seeking behaviour, and mortality in a cohort of young children in rural Burkina Faso. Trop Med Int Health. 2003;8:290–6. http://www.ncbi.nlm.nih.gov/pubmed/12667146. Accessed 6 Sep 2017. [DOI] [PubMed]
- 32.Krause G, Borchert M, Benzler J, Heinmüller R, Kaba I, Savadogo M, et al. Rationality of drug prescriptions in rural health centres in Burkina Faso. Health Policy Plan. 1999; 14:291–8. http://www.ncbi.nlm.nih.gov/pubmed/10621246. Accessed 6 Sep 2017. [DOI] [PubMed]
- 33.Lagoy CT, Joshi N, Cragan JD, Rasmussen SA. Medication use during pregnancy and lactation: an urgent call for public health action. J Womens Health (Larchmt). 2005;14:104–109. doi: 10.1089/jwh.2005.14.104. [DOI] [PubMed] [Google Scholar]
- 34.Ministère de la Santé : Direction Générale des Etudes et des Statistiques Sectorielles. Annuaire statistique. Burkina Faso. 2016. http://www.sante.gov.bf/index.php?option=com_edocman&task=document.viewdoc&id=363&Itemid=1123. Accessed 8 Sep 2017.
- 35.Coulibaly SO, Gies S, D’Alessandro U. Malaria burden among pregnant women living in the rural district of Boromo, Burkina Faso. Am J Trop Med Hyg. 2007; 77(6 Suppl):56–60. http://www.ncbi.nlm.nih.gov/pubmed/18165475. Accessed 8 Sep 2017. [PubMed]
- 36.Institut national de la statistique et de la démographie (INSD). Enquête multisectorielle continue : Alphabétisation et scolarisation. Ouagadougou, Burkina Faso; 2015. http://www.insd.bf/n/contenu/enquetes_recensements/Enq_EMC/Alphab%E9tisation_et_scolarisation.pdf. Accessed 8 Sep 2017.
- 37.Ministère de la Santé/Programme National de Lutte contre le Paludisme. Directives Nationales pour la prise en charge du paludisme au Burkina Faso. Ouagadougou, Burkina Faso; 2017.
- 38.ANC & Malaria Diagnostic in Pregnancy - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/study/NCT01703884. Accessed 11 Jul 2018.
- 39.Le suivi de la grossesse - Ministère de la Santé. http://www.sante.gov.bf/index.php?option=com_content&view=article&id=229:le-suivi-de-la-grossesse&catid=162&Itemid=1059. Accessed 11 Jul 2018.
- 40.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2017. Norway, Olso: Norwegian Institute of Public Health. 2017. https://www.whocc.no/filearchive/publications/2017_guidelines_web.pdf. Accessed 22 Sep 2017.
- 41.US Food and Drug Administration (FDA). Medicine use during Pregnancy or Breastfeeding drugs.com. https://www.drugs.com/pregnancy/. Accessed 22 Sep 2017.
- 42.US Food and Drug Administration (FDA). Drug Safety Label Changes—July 2016 Drugs.com. https://www.drugs.com/labeling-changes/. Accessed 14 Sep 2017.
- 43.Australian Government Department of Health. Australian categorisation system for prescribing medicines in pregnancy. https://www.tga.gov.au/australian-categorisation-system-prescribing-medicines-pregnancy. Accessed 22 Sep 2017.
- 44.Matok I, Gorodischer R, Koren G, Sheiner E, Wiznitzer A, Levy A. The Safety of Metoclopramide Use in the First Trimester of Pregnancy. N Engl J Med. 2009;360:2528–2535. doi: 10.1056/NEJMoa0807154. [DOI] [PubMed] [Google Scholar]
- 45.Pasternak B, Svanström H, Mølgaard-Nielsen D, Melbye M, Hviid A. Metoclopramide in pregnancy and risk of major congenital malformations and fetal death. JAMA. 2013;310:1601. doi: 10.1001/jama.2013.278343. [DOI] [PubMed] [Google Scholar]
- 46.Lacroix I, Hurault-Delarue C, Kessler S, Guitard C, Vidal S, Albouy-Cossard C, et al. First epidemiologic data about phloroglucinol exposure during first trimester of pregnancy. Gynécologie Obs Fertil. 2011;39:694–697. doi: 10.1016/j.gyobfe.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 47.PREGACT Study Group, Pekyi D, Ampromfi AA, Tinto H, Traoré-Coulibaly M, Tahita MC, et al. Four artemisinin-based treatments in African pregnant women with malaria. Malawi Med J. 2016; 28:139–49. http://www.ncbi.nlm.nih.gov/pubmed/27895848. Accessed 23 Oct 2017. [PMC free article] [PubMed]
- 48.Tiran Denise. The use of herbs by pregnant and childbearing women: a risk–benefit assessment. Compl Ther Nurs Midwifery. 2003;9:176–181. doi: 10.1016/S1353-6117(03)00045-3. [DOI] [PubMed] [Google Scholar]
- 49.Cuzzolin L, Benoni G. Safety issues of phytomedicines in pregnancy and paediatrics. In: Herbal drugs: ethnomedicine to modern medicine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. pp 381–96. 10.1007/978-3-540-79116-4_21.
- 50.Cunningham FG, MacDonald PC, Gant NF, Leveno KJ, Gilstrap III LC. Williams obstetrics. 19. United States, Norwalk, Conn.: Appleton & Lange; 1993. [Google Scholar]
- 51.Olesen C, Sørensen HT, de Jong-van den Berg L, Olsen J, Steffensen FH. Prescribing during pregnancy and lactation with reference to the Swedish classification system. A population-based study among Danish women. The Euromap Group. Acta Obstet Gynecol Scand. 1999;78:686–92. http://www.ncbi.nlm.nih.gov/pubmed/10468060. Accessed 24 Sep 2017. [DOI] [PubMed]
- 52.US Food and Drug Administration. Unapproved Prescription Drugs: Drugs Marketed in the United States That Do Not Have Required FDA Approval. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/SelectedEnforcementActionsonUnapprovedDrugs/default.htm. Accessed 12 Jul 2018.
- 53.Rohra DK, Das N, Azam SI, Solangi NA, Memon Z, Shaikh AM, et al. Drug-prescribing patterns during pregnancy in the tertiary care hospitals of Pakistan: a cross sectional study. BMC Pregnancy Childbirth. 2008;8:24. doi: 10.1186/1471-2393-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nadembega P, Boussim JI, Nikiema JB, Poli F, Antognoni F. Medicinal plants in Baskoure, Kourittenga Province, Burkina Faso: an ethnobotanical study. J Ethnopharmacol. 2011;133:378–395. doi: 10.1016/j.jep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Onah MN, Field S, van Heyningen T, Honikman S. Predictors of alcohol and other drug use among pregnant women in a peri-urban South African setting. Int J Ment Health Syst. 2016;10:38. doi: 10.1186/s13033-016-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muckle G, Laflamme D, Gagnon J, Boucher O, Jacobson JL, Jacobson SW. Alcohol, smoking, and drug use among Inuit women of childbearing age during pregnancy and the risk to children. Alcohol Clin Exp Res. 2011;35:1081–1091. doi: 10.1111/j.1530-0277.2011.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983; 61:1005–16 https://www.ncbi.nlm.nih.gov/pubmed/6370484. [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the World Health Organization data repository and from the Clinical Research Unit of Nanoro on reasonable request.