Abstract
Background
The combination chemotherapy regimens of nab-paclitaxel plus gemcitabine (nab-p + G) and FOLFIRINOX (FFX) have each demonstrated improved survival compared with gemcitabine monotherapy in clinical trials for metastatic pancreatic cancer; however, limited comparative data exist.
Objective
The objective of this study was to compare patient characteristics and clinical outcomes including time to treatment failure and overall survival in patients with metastatic pancreatic cancer receiving first-line chemotherapy in the community.
Methods
We conducted a retrospective, multi-site, observational cohort study of patients with metastatic pancreatic cancer receiving first-line nab-p + G, FFX, or gemcitabine monotherapy between April 2013 and October 2015, using data from the iKnowMed electronic health record database. Patients on clinical trials or with other cancer diagnoses were excluded. Time to treatment failure and overall survival were assessed by Kaplan–Meier methods.
Results
Four hundred and eighty-six patients met selection criteria, 255 nab-p + G, 159 FFX, and 72 gemcitabine patients. Median age was 61, 68, and 73 years for FFX, nab-p + G, and gemcitabine patients, respectively (p < 0.01 for nab-p + G vs. FFX). Eastern Cooperative Oncology Group performance status of 0–1 was 91% for FFX, 77% for nab-p + G, and 68% for gemcitabine patients (p < 0.01 for nab-p + G vs. FFX). For the nab-p + G vs. FFX cohorts, respectively, time to treatment failure was 3.7 vs. 4.3 months (log-rank p = 0.25); and OS was 9.8 vs. 11.4 months (log-rank p = 0.38). Among patients with Eastern Cooperative Oncology Group performance status 0–1, time to treatment failure was 4.2 vs. 4.3 months (log-rank p = 0.47); and overall survival was 12.1 vs 11.4 months (log-rank p = 0.68).
Conclusions
The nab-p + G patients were older and had worse performance status than FFX patients. Time to treatment failure and overall survival were not observed to be significantly different in first-line nab-p + G and FFX patients. Results were similar after stratifying by performance status.
Key Points
| Nab-paclitaxel plus gemcitabine and FOLFIRINOX are both first-line combination chemotherapy options for metastatic pancreatic cancer with demonstrated improved survival compared with single-agent gemcitabine chemotherapy. No direct randomized comparative studies of these two treatments exist. |
| This study found significant differences in age, performance status, and use of supportive care for toxicities among patients who received nab-paclitaxel plus gemcitabine and FOLFIRINOX. |
| No significant differences in clinical outcomes including time to treatment failure or overall survival were observed in patients who received nab-paclitaxel plus gemcitabine and FOLFIRINOX in this real-world observational study, overall, or when stratified by performance status. |
Introduction
Pancreatic cancer accounts for approximately 3% of all new cancer cases in USA. While the incidence is low, survival is poor, in part because it is difficult to detect early. Pancreatic cancer is the third leading cause of cancer death in USA, with 53,670 new cases estimated in 2017, and 43,090 deaths [1]. About 85% of all cases are pancreatic adenocarcinomas. The majority of patients present with stage IV metastatic disease, with a 5-year overall survival (OS) rate of 2% in those with metastasis [2]. Therefore, treatment is largely palliative.
Treatment of metastatic pancreatic cancer has evolved over the past decade with the emergence of new first-line (1L) treatment options for metastatic disease. Both single-agent chemotherapy and combination chemotherapy regimens are available treatment options. Gemcitabine monotherapy had long been the standard-of-care chemotherapy [3]. However, the combination regimens of nab-paclitaxel plus gemcitabine and FOLFIRINOX (fluorouracil, oxaliplatin, leucovorin, irinotecan) have emerged over the past few years as 1L treatment options for metastatic pancreatic adenocarcinoma based on phase III trials demonstrating significant improvement in OS when compared with gemcitabine monotherapy. Yet to date, no direct randomized comparative studies of nab-paclitaxel plus gemcitabine vs. FOLFIRINOX exist. Therefore, treatment selection often depends on patient performance status (PS) and toxicities [4].
In this study, we describe the clinical and demographic patient profiles for patients with metastatic pancreatic cancer receiving 1L treatment with either nab-paclitaxel plus gemcitabine, gemcitabine monotherapy, or FOLFIRINOX. We aimed to compare the real-world clinical outcomes observed including time to treatment failure (TTF) and OS by these treatment cohorts.
Methods
Data Source
This was a retrospective observational cohort study of patients with metastatic pancreatic cancer. Data were obtained via programmatic queries of The US Oncology Network iKnowMed™ electronic health record (EHR) system to collect the structured documented information available from the records. McKesson Specialty Health maintains iKnowMed™, an integrated web-based database and oncology-specific EHR system that captures outpatient practice encounter histories from network community oncology practices affiliated with over 1000 physicians in more than 25 practices across 400 sites of care in 19 states (Midwest, Northeast, South, and West US Census regions). Vital status data were supplemented with information obtained from the US Department of Social Security Death Index. Institutional review board approval was obtained. Supportive care utilization data were analyzed through use of matched outpatient claims data.
Study Design and Patients
The study population included patients with a diagnosis of pancreatic cancer, with evidence of metastatic disease defined as having stage IV at diagnosis. Patients were required to have initiated treatment with either FOLFIRINOX, nab-paclitaxel plus gemcitabine, or gemcitabine monotherapy as 1L treatment between 1 April 2013 and 31 October 2015. Patients must have received the qualifying number of doses of treatments [three doses of nab-paclitaxel (in nab-paclitaxel plus gemcitabine), three doses of gemcitabine (in gemcitabine monotherapy), and two doses of fluorouracil (in FOLFIRINOX) or one cycle of each]. The follow-up period was through 31 January 2016 or until the date of the last record, whichever occurred first, to allow for a minimum of 3 months of potential follow-up for each patient. The study time period was selected to capture utilization in the time frame following an initial report of improved survival findings from the phase III MPACT trial in 2013 [5].
Patients were required to have two or more visits within The US Oncology Network during the study period. Patients were excluded if they were < 18 years of age at diagnosis, had another documented primary cancer diagnosis, or were enrolled in a clinical trial during the 12 months prior to the initiation of 1L chemotherapy up through the end of the study. Patients with histologies other than ‘Adenocarcinoma’ or ‘Adenosquamous carcinoma’, or those diagnosed with earlier stages (I–III) or missing or unknown, with no information of metastasis documented were also excluded.
Endpoints
Time to treatment failure was defined as the time from the start of 1L chemotherapy treatment to the date of discontinuation for any reason. Overall survival was defined as the time from the start of 1L chemotherapy until death from any cause. Patients who survived to the end of the follow-up period or who were lost to follow-up by the end of the follow-up were censored for OS when they were last known to be alive. Non-hematologic toxicities that emerged during the course of 1L chemotherapy and up to 15 days after the last dose of 1L chemotherapy or until the day before the start of second-line treatment were captured from the structured data from the EHR. Hematologic toxicities were captured through laboratory values. Healthcare resource utilization was categorized through use of Current Procedural Terminology codes for submitted claims that occurred from the start of 1L chemotherapy throughout the 1L period.
Statistical Analysis
Descriptive analyses were conducted to assess demographic, clinical, and treatment characteristics among the cohorts. For comparisons of significance, nab-paclitaxel plus gemcitabine was the reference cohort. Analysis of variance was used for continuous variables, and chi-square or Fisher’s exact test tests were used for categorical variables. Missing data were identified and reported as percentages. The Kaplan–Meier method was used to estimate TTF and OS. Multivariable Cox regression analyses were performed using age, sex, PS, and treatment regimen as covariates. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
A total of 2901 patients with pancreatic cancer receiving chemotherapy during the study period were initially identified, of which 486 total patients met all eligibility criteria (Fig. 1). The most common reasons for exclusion were no evidence of metastases, did not receive one of the assigned regimens, and other histology. The final analysis included 255 nab-paclitaxel plus gemcitabine, 159 FOLFIRINOX, and 72 gemcitabine monotherapy patients.
Fig. 1.
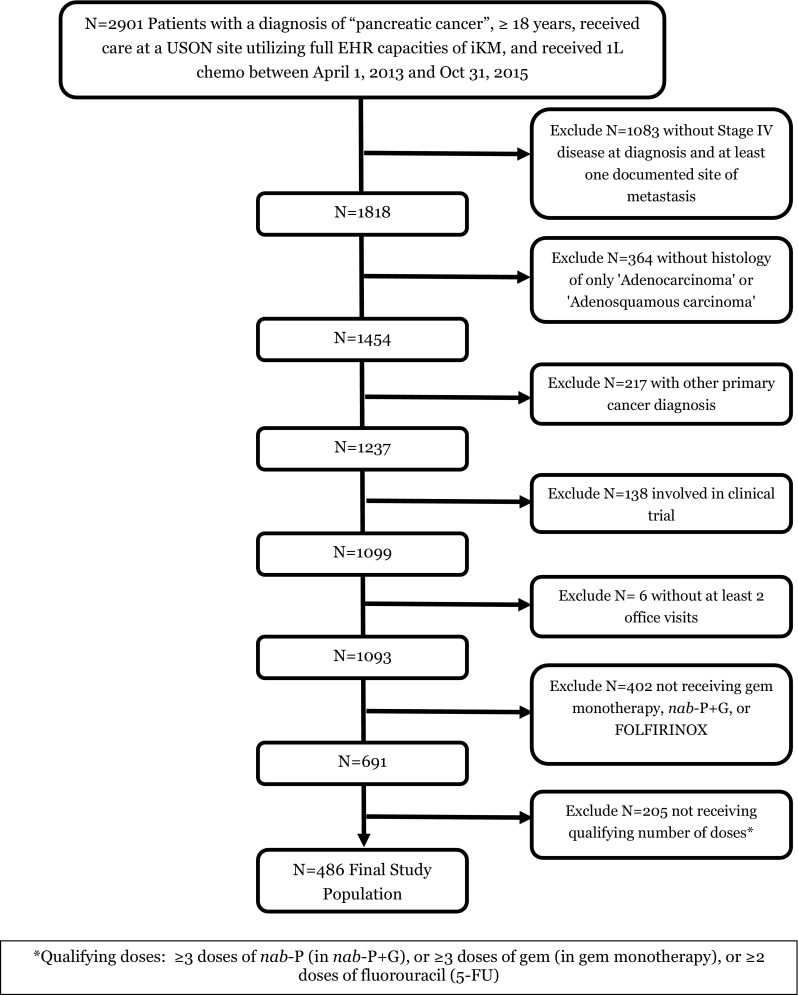
CONSORT diagram. 1L first-line, chemo chemotherapy, EHR electronic health record, gem gemcitabine, iKM iKnowMed™, nab-P + G nab-paclitaxel plus gemcitabine, USON US Oncology Network
The overall cohort was predominantly male (55%), Caucasian, (83%), and treated in the southern region of USA (60%) (Tables 1, 2). Patients in the FOLFIRINOX cohort were significantly younger than patients in the nab-paclitaxel plus gemcitabine cohort (p < 0.01) and patients in the gemcitabine mono cohort were significantly older than patients in the nab-paclitaxel plus gemcitabine cohort (p < 0.01), with median ages of 61, 68, and 73 years for FOLFIRINOX, nab-paclitaxel plus gemcitabine, and gemcitabine monotherapy, respectively. Eastern Cooperative Oncology Group (ECOG) PS was 0–1 in 77% of nab-paclitaxel plus gemcitabine patients, 91% of FOLFIRINOX patients, and 68% of gemcitabine monotherapy patients (p < 0.01 for nab-paclitaxel plus gemcitabine vs. FOLFIRINOX; p = 0.22 for nab-paclitaxel plus gemcitabine vs. gemcitabine monotherapy). Among the known metastatic sites of disease, the liver (34%), lung/pleura (11%), and peritoneum (10%) were the most common. Seventy-six percent of patients had one or no known documented co-morbidity within 12 months prior to 1L treatment, and 24% had two or more co-morbidities, consisting of hypertension (16%) and cardiovascular disease (14%), which were not statistically different between groups.
Table 1.
Patient demographics
| Demographics | Overall cohort N = 486 n (%) |
1L FOLFIRINOX (FFX) N = 159 n (%) |
1L nab-paclitaxel + gemcitabine (nab-P + G) N = 255 n (%) |
1L gemcitabine (GEM) monotherapy N = 72 n (%) |
p value (nab-P + G vs. FFX) | p value (nab-P + G vs. GEM) |
|---|---|---|---|---|---|---|
| Age (years), at 1L chemotherapy | ||||||
| Mean (SD) | 65.47 (10.52) | 59.13 (9.27) | 67.66 (9.09) | 71.71 (11.05) | < 0.0001 | 0.0016 |
| Median (min–max) | 66 (28–90 +) | 61 (28–84) | 68 (37–86) | 73 (41–90 +) | < 0.0001 | 0.0013 |
| Age group (years) | ||||||
| < 60 | 131 (27.0) | 73 (45.9) | 48 (18.8) | 10 (13.9) | < 0.0001 | 0.0127 |
| ≥ 60 to < 70 | 189 (38.9) | 70 (44.0) | 101 (39.6) | 18 (25.0) | ||
| ≥ 70 | 166 (34.2) | 16 (10.1) | 106 (41.6) | 44 (61.1) | ||
| Sex | ||||||
| Female | 219 (45.1) | 66 (41.5) | 118 (46.3) | 35 (48.6) | 0.3426 | 0.7257 |
| Male | 267 (54.9) | 93 (58.5) | 137 (53.7) | 37 (51.4) | ||
| Race | ||||||
| Caucasian | 401 (82.5) | 134 (84.3) | 204 (80.0) | 63 (87.5) | 0.4208 | 0.1068 |
| African American | 35 (7.2) | 7 (4.4) | 21 (8.2) | 7 (9.7) | ||
| All other | 7 (1.4) | 3 (1.9) | 3 (1.2) | 1 (1.4) | ||
| Not documented | 43 (8.8) | 15 (9.4) | 27 (10.6) | 1 (1.4) | ||
| Ethnicity | ||||||
| Hispanic/Latino | 46 (9.5) | 18 (11.3) | 24 (9.4) | 4 (5.6) | 0.694 | 0.029 |
| Not Hispanic/Latino | 391 (80.5) | 125 (78.6) | 200 (78.4) | 66 (91.7) | ||
| Not documented | 49 (10.1) | 16 (10.1) | 31 (12.2) | 2 (2.8) | ||
| US Census region of treating practice | ||||||
| Midwest | 72 (14.8) | 31 (19.5) | 28 (11.0) | 13 (18.1) | 0.003 | 0.3788 |
| Northeast | 21 (4.3) | 10 (6.3) | 8 (3.1) | 3 (4.2) | ||
| South | 290 (59.7) | 96 (60.4) | 153 (60.0) | 41 (56.9) | ||
| West | 103 (21.2) | 22 (13.8) | 66 (25.9) | 15 (20.8) | ||
| Payer type | ||||||
| Medicare/Medicaid | 187 (38.5) | 38 (23.9) | 114 (44.7) | 35 (48.6) | < 0.0001 | 0.1542 |
| Commercial | 152 (31.3) | 76 (47.8) | 66 (25.9) | 10 (13.9) | ||
| Other | 1 (0.2) | 0 | 1 (0.4) | 0 | ||
| Unknown | 146 (30.0) | 45 (28.3) | 74 (29.0) | 27 (37.5) | ||
1L first-line, max maximum, min minimum, SD standard deviation
Table 2.
Patient clinical characteristics
| Clinical characteristics | Overall cohort N = 486 n (%) |
1L FOLFIRINOX (FFX) N = 159 n (%) |
1L nab-paclitaxel + gemcitabine (nab-P + G) N = 255 n (%) |
1L gemcitabine (GEM) monotherapy N = 72 n (%) |
p value (nab-P + G vs. FFX) | p value (nab-P + G vs. GEM) |
|---|---|---|---|---|---|---|
| Histology | ||||||
| Adenocarcinoma | 484 (99.6) | 159 (100) | 254 (99.6) | 71 (98.6) | 0.6159 | 0.3445 |
| Other | 2 (0.4) | 0 | 1 (0.4) | 1 (1.4) | ||
| Sites of initial metastatic disease at 1L chemotherapy | ||||||
| Brain | 2 (0.2) | 0 | 2 (0.3) | 2 (1.2) | 0.4419 | 0.5055 |
| Bone/bone marrow | 40 (3.5) | 16 (4.3) | 22 (3.7) | 2 (1.2) | ||
| Lung/pleural | 119 (10.5) | 36 (9.6) | 67 (11.4) | 16 (9.6) | ||
| Liver | 382 (33.7) | 126 (33.5) | 195 (33.1) | 61 (36.7) | ||
| Mediastinal or other distant lymph nodes | 10 (0.9) | 1 (0.3) | 6 (1.0) | 3 (1.8) | ||
| Peritoneum | 114 (10.1) | 40 (10.6) | 59 (10.0) | 16 (9.6) | ||
| Other sites (GI, adrenal, kidney, ovary, skin, spleen, other) | 64 (95.7) | 25 (6.6) | 29 (4.9) | 7 (4.2) | ||
| Not documented | 401 (35.4) | 132 (35.1) | 210 (35.6) | 59 (35.5) | ||
| No. of sites of initial metastatic disease at 1L chemotherapy | ||||||
| Single | 308 (63.4) | 96 (60.4) | 164 (64.3) | 48 (66.7) | 0.3569 | 0.9293 |
| Multiple (≥ 2) | 178 (36.6) | 63 (39.6) | 91 (35.7) | 24 (33.3) | ||
| 2–3 | 167 (34.4) | 61 (38.4) | 84 (32.9) | 22 (30.6) | ||
| ≥4 | 11 (2.3) | 2 (1.3) | 7 (2.7) | 2 (2.8) | ||
| Co-morbidities < 12 mo before 1L chemotherapy | ||||||
| 0 | 172 (35.4) | 56 (35.2) | 86 (33.7) | 30 (41.7) | 0.9526 | 0.4474 |
| 1 | 196 (40.3) | 64 (40.3) | 105 (41.2) | 27 (37.5) | ||
| ≥2 | 118 (24.3) | 39 (24.5) | 64 (25.1) | 15 (20.8) | ||
| Performance status at 1L chemotherapy | ||||||
| ECOG0 | 48 (9.9) | 19 (11.9) | 20 (7.8) | 9 (12.5) | 0.0049 | 0.2244 |
| ECOG1 | 341 (70.2) | 125 (78.6) | 176 (69.0) | 40 (55.6) | ||
| ECOG2 | 71 (14.60 | 12 (7.5) | 41 (16.1) | 18 (25.0) | ||
| ECOG3–4 | 2 (0.4) | 1 (0.6) | 1 (0.4) | 0 | ||
| Unknown | 24 (4.9) | 2 (1.3) | 17 (6.7) | 5 (6.9) | ||
1L first-line, ECOG Eastern Cooperative Oncology Group, GI gastrointestinal
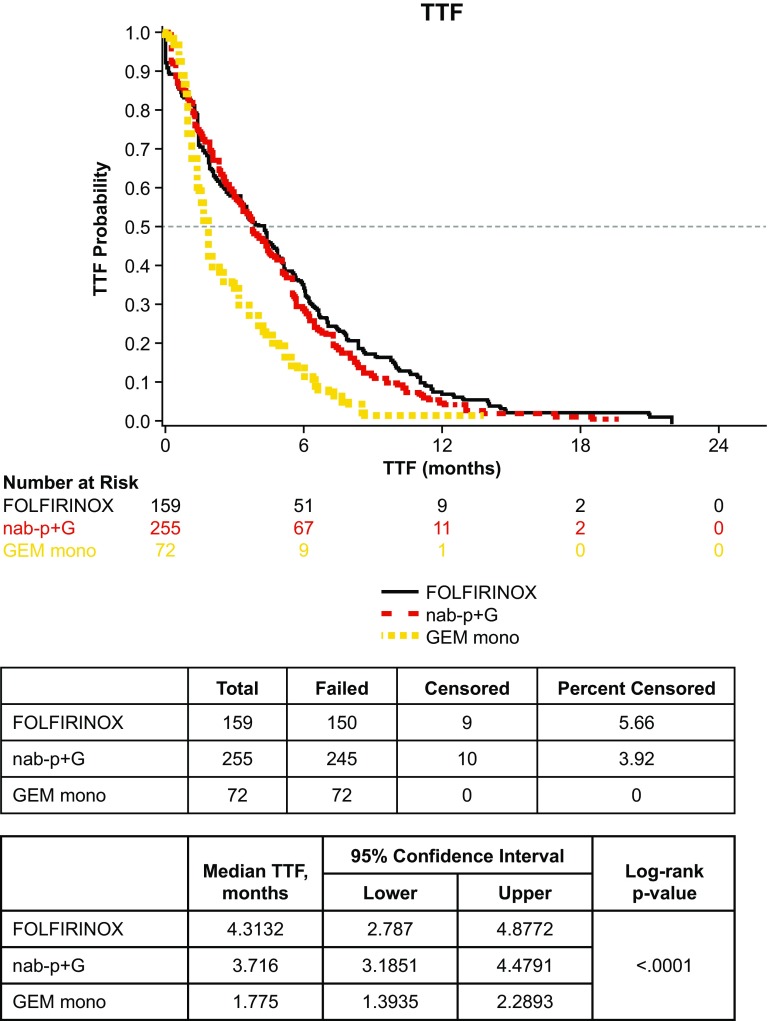
Time to Treatment Failure
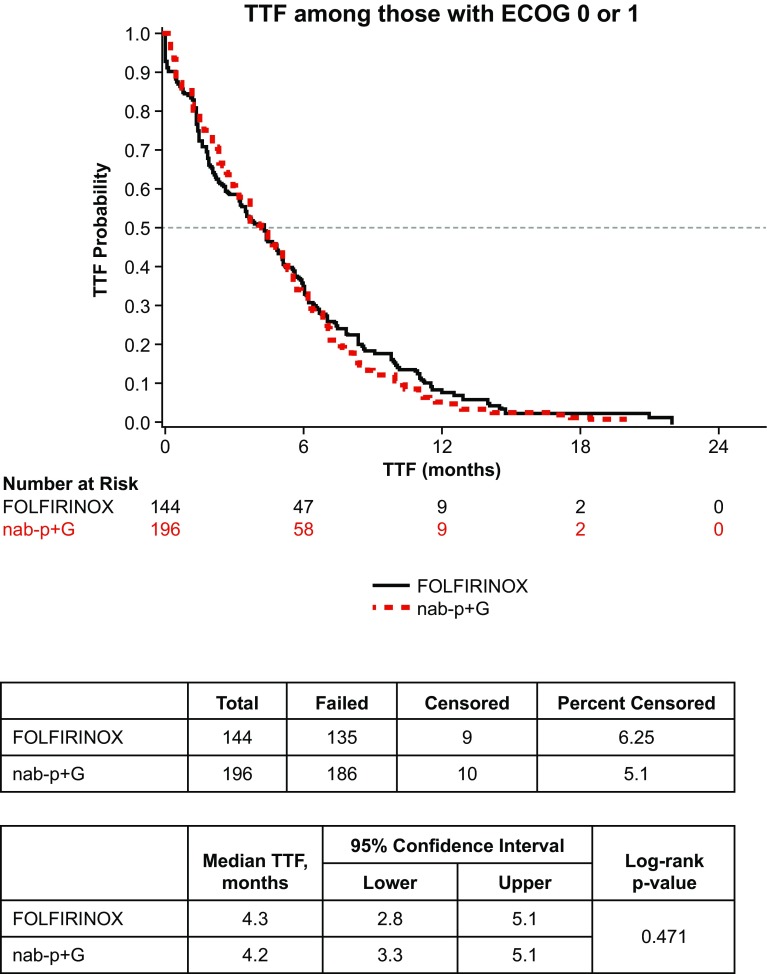
Median TTF in all patients was 3.5 months (95% confidence interval 3.0–3.9). In the three cohort analyses, a statistically significant difference in TTF was observed (log-rank p < 0.01) (Fig. 2). There was no statistical difference in median TTF for the nab-paclitaxel plus gemcitabine vs. FOLFIRINOX cohorts (3.7 vs. 4.3 months, log-rank p = 0.25). However, a statistically significant difference in median TTF was seen between the nab-paclitaxel plus gemcitabine vs. gemcitabine monotherapy cohorts (3.7 vs. 1.8 months, log-rank p < 0.01). Among the subgroup of patients with ECOG PS 0–1, TTF for nab-paclitaxel plus gemcitabine vs. FOLFIRINOX was also not different at 4.2 vs. 4.3 months, respectively (log-rank p = 0.47) (Fig. 3).
Fig. 2.
Time to treatment failure (TTF) by cohort (n = 486). GEM mono gemcitabine monotherapy, nab-P + G nab-paclitaxel plus gemcitabine
Fig. 3.
Time to treatment failure (TTF) for nab-paclitaxel plus gemcitabine (nab-P + G) vs. FOLFIRINOX; Eastern Cooperative Oncology Group (ECOG) performance status 0–1
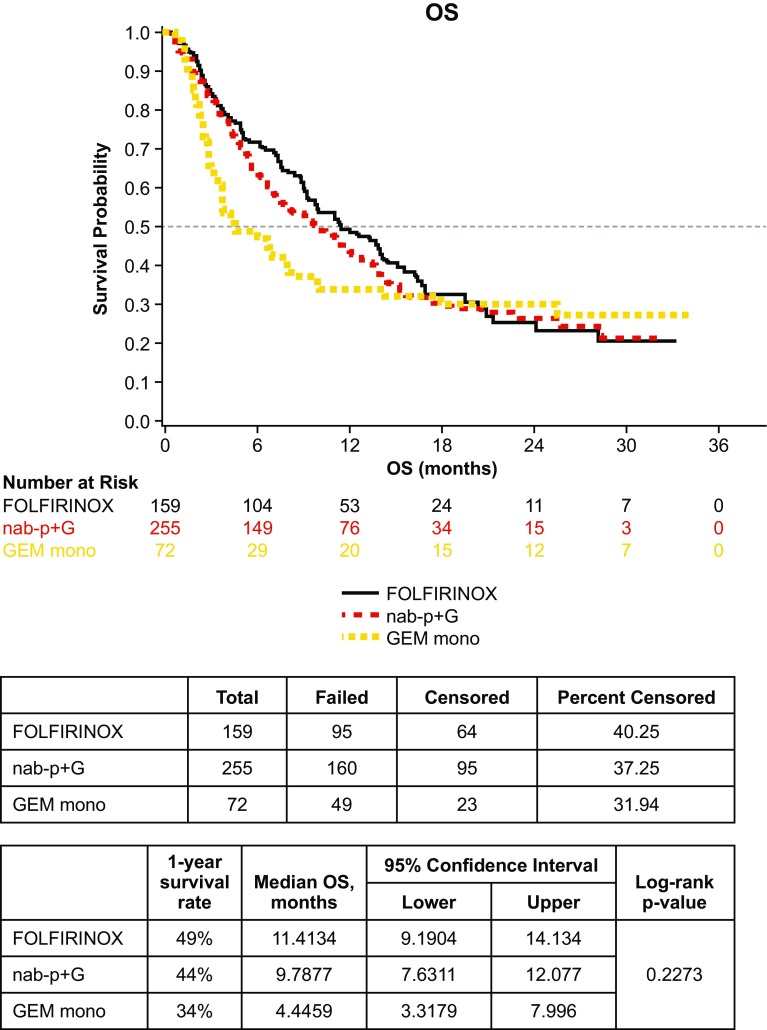
Overall Survival
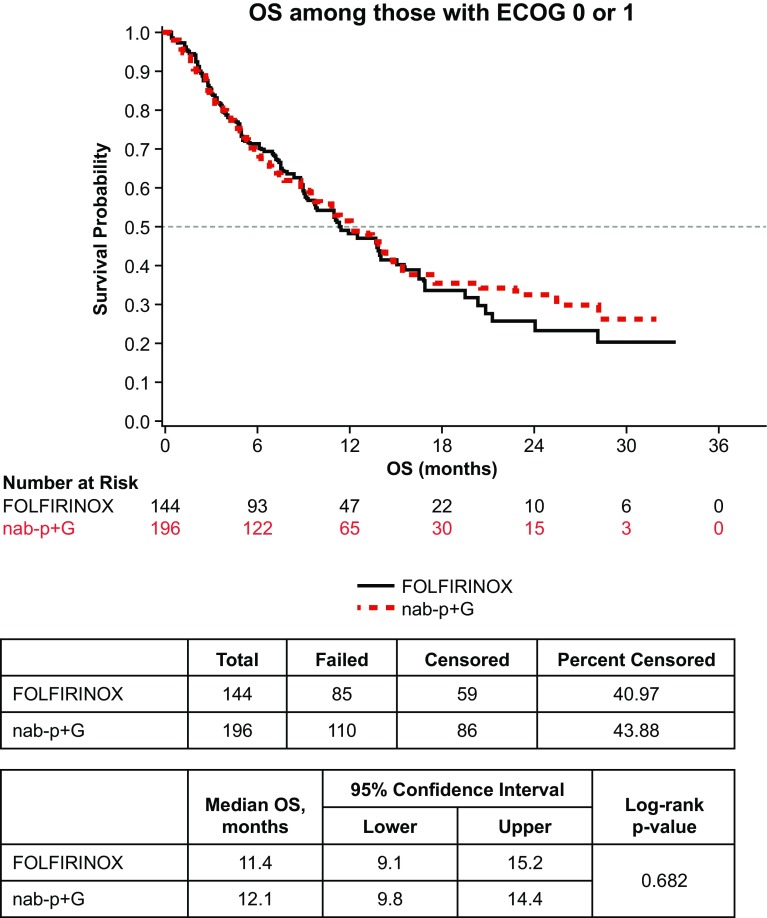
Median OS for all patients was 9.8 months (95% confidence interval 8.2–11.5). In the three cohort analyses of OS, there was no statistical difference across all three cohorts (log-rank p = 0.23) (Fig. 4). There was no statistical difference in median OS for the nab-paclitaxel plus gemcitabine vs. FOLFIRINOX cohorts (9.8 vs. 11.4 months, log-rank p = 0.38). While the median OS of the nab-paclitaxel plus gemcitabine cohort and FOLFIRINOX cohort was more than twice that of the gemcitabine monotherapy cohort, no significant difference was observed between the nab-paclitaxel plus gemcitabine vs. gemcitabine monotherapy cohorts (9.8 vs. 4.4 months, log-rank p = 0.19), or the FOLFIRINOX vs. gemcitabine monotherapy cohorts (11.4 vs. 4.4 months, log-rank p = 0.09). In the subgroup of patients with ECOG PS 0–1, OS for nab-paclitaxel plus gemcitabine vs. FOLFIRINOX was again not significantly different at 12.1 vs. 11.4 months (log-rank p = 0.68) (Fig. 5).
Fig. 4.
Overall survival (OS) by cohort (n = 486). GEM mono gemcitabine monotherapy, nab-P + G nab-paclitaxel plus gemcitabine
Fig. 5.
Overall survival (OS) for nab-paclitaxel plus gemcitabine (nab-P + G) vs. FOLFIRINOX; Eastern Cooperative Oncology Group (ECOG) performance status 0–1
Cox multivariable analyses revealed no significant difference in survival by regimen, age, or sex. A poor ECOG PS of 2 + vs. 0 significantly increased the risk of death (hazard ratio 2.4, p < 0.05).
Toxicity and Supportive Care Use
The rates of non-hematologic toxicity were similar between groups. The frequency of supportive care medication use is shown in Table 3. No significant difference was observed in the use of antibiotics, nausea, or pain medications in patients receiving FOLFIRINOX vs. nab-paclitaxel plus gemcitabine. Antibiotic utilization was higher in the nab-paclitaxel plus gemcitabine cohort than the gemcitabine monotherapy cohort (p < 0.01).
Table 3.
Supportive care utilization
| Overall cohort N = 486 n (%) |
1L FOLFIRINOX (FFX) N = 159 n (%) |
1L nab-paclitaxel + gemcitabine (nab-P + G) N = 255 n (%) |
1L gemcitabine (GEM) monotherapy N = 72 n (%) |
p value (nab-P + G vs. FFX) | p value (nab-P + G vs. GEM) | |
|---|---|---|---|---|---|---|
| Supportive care medications administered during 1L chemotherapya | ||||||
| Pegfilgrastimb | 109 (22.4) | 69 (43.4) | 33 (12.9) | 7 (9.7) | < 0.0001 | 0.4617 |
| Darbepoetinb | 68 (14.0) | 21 (13.2) | 44 (17.3) | 3 (4.2) | 0.2709 | 0.0039c |
| Common antibiotics (≥ 1 agent)a | 217 (44.7) | 67 (42.1) | 127 (49.8) | 23 (31.9) | 0.1285 | 0.0072 |
| Common medications for prophylaxis and treatment of CINV (≥ 1 agent)a | 466 (95.9) | 150 (94.3) | 244 (95.7) | 72 (100) | 0.5343 | 0.073 |
| Pain medicinesa | 369 (75.9) | 120 (75.5) | 194 (76.1) | 55 (76.4) | 0.8884 | 0.9565 |
1L first-line, CINV chemotherapy-induced nausea and vomiting
aSupportive care medications administered in the US Oncology Network clinic between the patient’s start and end of their first-line chemotherapy
bWhite blood cell and red blood cell growth factor use as captured from Current Procedural Terminology codes from a claims data analysis
cFisher’s exact test
Neutropenia (absolute neutrophil count < 1000/mm3) occurred in 11.3% of FOLFIRINOX patients, 9.8% of nab-paclitaxel plus gemcitabine patients, and 1.4% in the gemcitabine monotherapy cohort (p = 0.91 for FOLFIRINOX vs. nab-paclitaxel plus gemcitabine; p = 0.83 for nab-paclitaxel plus gemcitabine vs. gemcitabine monotherapy). White blood cell growth factor use with pegfilgrastim was used in 43% of FOLFIRINOX patients, 13% of nab-paclitaxel plus gemcitabine patients, and 10% of gemcitabine monotherapy patients (p < 0.01 for FOLFIRINOX vs. nab-paclitaxel plus gemcitabine; p = 0.46 for nab-paclitaxel plus gemcitabine vs. gemcitabine monotherapy).
Anemia (hemoglobin < 8 g/dL) occurred in 0, 0.8, and 2.8% of FOLFIRINOX, nab-paclitaxel plus gemcitabine, and gemcitabine monotherapy patients, respectively (p = 0.96 for FOLFIRINOX vs. nab-paclitaxel plus gemcitabine; p = 0.96 for nab-paclitaxel plus gemcitabine vs. gemcitabine monotherapy). Red blood cell growth factor use with darbepoetin was 13, 17, and 4% for FOLFIRINOX, nab-paclitaxel plus gemcitabine, and gemcitabine monotherapy patients, respectively (p = 0.27 for FOLFIRINOX vs. nab-paclitaxel plus gemcitabine; p < 0.01 for nab-paclitaxel plus gemcitabine vs. gemcitabine monotherapy).
Discussion
This study used a large EHR database population to examine real-world patient characteristics and clinical outcomes with the use of 1L therapy for metastatic pancreatic cancer. Outcomes for patients with metastatic pancreatic cancer have historically been poor. Gemcitabine monotherapy was considered a standard-of-care option for several years, until the emergence of combination chemotherapy regimens such as FOLFIRINOX and nab-paclitaxel plus gemcitabine, which reported significantly improved survival of approximately 2–5 months over gemcitabine alone in separate phase III clinical trials, providing new treatment options [6, 7]. However, currently no direct comparative data exist on nab-paclitaxel plus gemcitabine vs. FOLFIRINOX. Thus, real-world data may assist in helping optimize treatment selection and understanding treatment trends.
In this study, the majority of patients received combination chemotherapy with nab-paclitaxel plus gemcitabine (52%) or FOLFIRINOX (33%), vs. gemcitabine monotherapy (15%) as 1L therapy. This is consistent with other database studies showing temporal changes in utilization over time with declines in 1L gemcitabine monotherapy use following the published reports of survival advantages with the combination regimens [8, 9]. A recent US-based study that included academic, private, and community-based oncology practices reported similar rates of utilization, with 38, 29, and 16% use of nab-paclitaxel plus gemcitabine, FOLFIRINOX, and gemcitabine monotherapy as 1L therapy in 2015, respectively [9]. Similarly, a questionnaire study of physicians in 19 European countries reported high utilization of nab-paclitaxel plus gemcitabine (47%) and FOLFIRINOX (42%), when using intensified regimens as 1L therapy for metastatic pancreatic cancer [10].
Baseline patient characteristics in our study were also similar to other reported 1L studies. Patients receiving FOLFIRINOX were youngest in age, followed by nab-paclitaxel plus gemcitabine, then gemcitabine monotherapy in older patients [11–13]. The proportion of patients with good PS (ECOG 0–1) was also highest in patients receiving FOLFIRINOX, followed by nab-paclitaxel plus gemcitabine, then gemcitabine. Gemcitabine monotherapy is currently considered a 1L option for patients with poor PS, while both FOLFIRINOX and nab-paclitaxel plus gemcitabine are options in patients with good PS [4].
Significant differences were observed in age and PS between the treatment cohorts in this study. Although patients in the nab-paclitaxel plus gemcitabine cohort were older and had worse PS than patients who received FOLFIRINOX, median TTF and median OS were not statistically different between these groups. These findings are consistent with other recent real-world studies, reporting comparable progression-free survival and OS with either nab-paclitaxel plus gemcitabine or FOLFIRINOX as 1L therapy, but improved efficacy in comparison to gemcitabine monotherapy [12, 13]. The real-world study by Kang et al. reported comparable progression-free survival (6.8 vs. 5.1 months) and OS (11.4 vs. 9.6 months) in patients receiving nab-paclitaxel plus gemcitabine or FOLFIRINOX as 1L therapy, respectively [12]. The OS estimates from the present study were in the ranges reported from the phase III trials. Median OS from this study was 11.4 months for FOLFIRINOX and 9.8 months for nab-paclitaxel plus gemcitabine, compared to 11.1 months for FOLFIRINOX in the ACCORD trial and 8.5 months for nab-paclitaxel plus gemcitabine in the MPACT trial [6, 7]. Furthermore, our stratified analysis on patients with PS 0–1 and multivariate regression analysis examining the impact of PS on OS demonstrates the significance of PS on outcomes. Among patients with PS 0–1, OS was higher than the overall population, specifically in the nab-paclitaxel plus gemcitabine cohort (12.1 months for nab-paclitaxel plus gemcitabine vs. 11.4 months for FOLFIRINOX patients; p = 0.68).
Differences in outcomes were observed between the nab-paclitaxel plus gemcitabine and gemcitabine monotherapy groups. There was a statistically significant difference in median TTF favoring nab-paclitaxel plus gemcitabine. Median OS time was also numerically more than doubled in the nab-paclitaxel plus gemcitabine cohort; however, this was not statistically different. This may potentially be owing to the low number of patients in the gemcitabine monotherapy group (n = 72) and potentially because some patients received nab-paclitaxel subsequently.
Despite the different tolerability profiles for these treatment regimens, overall non-hematologic toxicities appeared similar between groups. Toxicities may have been underreported in this study of EHR data, as toxicities may not be graded objectively outside of clinical trials. Rates of neutropenia were also not significantly different. This may have been related to higher utilization of the white blood cell growth factor pegfilgrastim in patients receiving FOLFIRINOX, as upfront prophylactic use may have reduced the occurrence of observed neutropenia. In a previously published retrospective claims study utilizing inpatient- and hospital-based outpatient data, there was a similar pattern of use of pegfilgrastim observed, and supportive care utilization and cost was significantly lower with 1L nab-paclitaxel plus gemcitabine vs. FOLFIRINOX [14]. In the real-world study by Wang et al., a significantly higher proportion of patients required dose modifications with FOLFIRINOX (40%) compared with nab-paclitaxel plus gemcitabine (14%) and gemcitabine monotherapy (9%; p < 0.001) [13]. The European questionnaire study reported a higher likelihood of protocol deviation with FOLFIRINOX than nab-paclitaxel plus gemcitabine (p < 0.01) [10].
The strength of this study lies in the large multi-site population of patients with metastatic pancreatic cancer treated in the community oncology setting across USA. Data collected from the EHR database represent actual treatment information documented as part of routine clinical care, and therefore demonstrate real-world clinical treatment patterns and outcomes as opposed to data obtained or collected as part of a controlled clinical trial or protocol. Limitations of this study include the retrospective nature of the evaluation, the short follow-up time in more recently selected patients, and the potential for bias if there were blank fields or errors in the EHR database. Missing data cannot confirm the absence of a condition or value in patients’ medical histories, only that it was not documented. In addition, this study was limited to clinics that are part of The US Oncology Network only and could represent a specific trend of practice in an individual oncology group/network.
Conclusion
In this retrospective observational study, TTF and OS were not observed to be significantly different in 1L nab-paclitaxel plus gemcitabine and FOLFIRINOX patients. This represents real-world outcomes where randomized comparative analysis data do not exist. In a metastatic disease setting where the majority of patients are treated with palliative intent, many factors are considered when selecting treatment. These findings suggest that in the absence of differences in efficacy or known predictors of response to therapy, treatment selection may depend on patient PS and toxicities.
Funding
This study was funded by Celgene Corporation. The authors were fully responsible for all content and editorial decisions for this manuscript.
Conflict of interest
Thomas H. Cartwright has received consulting fees and financial compensation for speaker bureau activities for Celgene. Monika Parisi, Manish Patel, and Corey Pelletier are employees of and own stock in Celgene. Janet L. Espirito is an employee of and owns stocks in McKesson Specialty Health. Thomas W. Wilson has received consulting fees from McKesson Specialty Health. Hani M. Babiker has no conflict of interest directly relevant to the content of this article.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors. This study was approved by an institutional review board. For this type of study, formal consent is not required.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program. SEER stat facts: pancreas cancer. http://www.seer.cancer.gov. Accessed 9 Aug 2017.
- 3.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patient with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.NCCN. Clinical practice guidelines in oncology: pancreatic adenocarcinoma. Version 2. 2015. http://www.nccn.org. Accessed 3 Feb 2016.
- 5.Von Hoff DD, Ervin TJ, Arena FP, et al. Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT) [abstract] J Clin Oncol. 2013;31(Suppl. 4):LBA148. doi: 10.1200/jco.2013.31.4_suppl.lba148. [DOI] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Ervin T, Arena PF, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartwright TH, Ginsburg A, Wilfong L, et al. Use of first-line chemotherapy for advanced pancreatic cancer: FOLFIRINOX versus gemcitabine-based therapy [abstract] J Clin Oncol. 2014;32(5 Suppl.):4132. [Google Scholar]
- 9.Abrams TAA, Meyer G, Meyerhardt JA, et al. Patterns of chemotherapy use in a US-based cohort of patients with metastatic pancreatic cancer. Oncologist. 2017;22(8):925–933. doi: 10.1634/theoncologist.2016-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le N, Vinci A, Schober M, et al. Real-world clinical practice of intensified chemotherapies for metastatic pancreatic cancer: results from a Pan-European questionnaire study. Digestion. 2016;94(4):222–229. doi: 10.1159/000453257. [DOI] [PubMed] [Google Scholar]
- 11.Braiteh F, Patel MB, Parisi M, et al. Comparative effectiveness and resource utilization of nab-paclitaxel plus gemcitabine vs. FOLFIRINOX or gemcitabine for first-line treatment of metastatic pancreatic adenocarcinoma in a US community setting. Cancer Manag Res. 2017;9:141–148. doi: 10.2147/CMAR.S126073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang J, Hwang I, Yoo C, et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs. 2018 doi: 10.1007/s10637-018-0598-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Camateros P, Cheung WY. A real-world comparison of FOLFIRINOX, gemcitabine plus nab-paclitaxel, and gemcitabine in advanced pancreatic cancers. J Gastrointest Cancer. 2017 doi: 10.1007/s12029-017-0028-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim GP, Parisi MF, Patel MB, et al. Comparison of treatment patterns, resource utilization, and cost of care in patients with metastatic pancreatic cancer treated with first-line nab-paclitaxel plus gemcitabine or FOLFIRINOX. Expert Rev Clin Pharmacol. 2017;10(5):559–565. doi: 10.1080/17512433.2017.1302330. [DOI] [PubMed] [Google Scholar]