Abstract
Populations of neural stem cells (NSCs) reside in a number of defined niches in the adult central nervous system (CNS) where they continually give rise to mature cell types throughout life, including newly born neurons. In addition to the prototypical niches of the subventricular zone (SVZ) and subgranular zone (SGZ) of the hippocampal dentate gyrus, novel stem cell niches that are also neurogenic have recently been identified in multiple midline structures, including circumventricular organs (CVOs) of the brain. These resident NSCs serve as a homeostatic source of new neurons and glial cells under intact physiological conditions. Importantly, they may also have the potential for reparative processes in pathological states such as traumatic spinal cord injury (SCI) and traumatic brain injury (TBI). As the response in these novel CVO stem cell niches has been characterized after stroke but not following SCI or TBI, we quantitatively assessed cell proliferation and the neuronal and glial lineage fate of resident NSCs in three CVO nuclei—area postrema (AP), median eminence (ME), and subfornical organ (SFO) —in rat models of cervical contusion-type SCI and controlled cortical impact (CCI)-induced TBI. Using bromodeoxyuridine (BrdU) labeling of proliferating cells, we find that TBI significantly enhanced proliferation in AP, ME, and SFO, whereas cervical SCI had no effects at early or chronic time-points post-injury. In addition, SCI did not alter NSC differentiation profile into doublecortin-positive neuroblasts, GFAP-expressing astrocytes, or Olig2-labeled cells of the oligodendrocyte lineage within AP, ME, or SFO at both time-points. In contrast, CCI induced a pronounced increase in Sox2- and doublecortin-labeled cells in the AP and Iba1-labeled microglia in the SFO. Lastly, plasma derived from CCI animals significantly increased NSC expansion in an in vitro neurosphere assay, whereas plasma from SCI animals did not exert such an effect, suggesting that signaling factors present in blood may be relevant to stimulating CVO niches after CNS injury and may explain the differential in vivo effects of SCI and TBI on the novel stem cell niches.
Keywords: : circumventricular organs, controlled cortical impact, contusion, SCI, TBI
Introduction
Ongoing neurogenesis occurs in the adult mammalian central nervous system (CNS). In particular, the lateral subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampal dentate gyrus are widely accepted as multi-potent neural stem cell (NSC) niches within the adult brain. NSCs residing within these areas continually give rise to specific classes of newly born neurons (i.e., peri-glomerular and granule cells in the olfactory bulb and granule cells of the dentate gyrus) under intact physiological conditions.1–8 In addition, these stem cell niches can contribute to repair in response to perturbations such as neurodegenerative disease, traumatic injury, and stroke.9–17
Recent evidence suggests that in addition to these prototypical stem cell niches, there are other sites of adult neurogenesis along the third and fourth ventricles. The circumventricular organs (CVOs) constitute a midline series of novel stem cell niches in the adult brain,18,19 which include organum vasculosum of the lamina terminalis (OVLT), subfornical organ (SFO), median eminence (ME), pineal gland (PG), subcommissural organ (SCO), area postrema (AP), and the choroid plexus. These structures share many characteristics with SVZ and SGZ. NSCs within CVO niches show a similar expression profile as those in the traditional stem cell niches. For example, CVO cells express the NSC markers Sox2 and nestin, the class III intermediate filament protein vimentin, the marker for germinal astrocytes GFAP, as well as the cell-cycle regulation marker Ki67. Similar to SVZ and SGZ, CVO stem cells have the ability to proliferate and give rise to neurons and glial cells in vitro and in vivo and after transplantation into the adult brain.18,19 Additionally, similar to SVZ, CVOs have a network of permeable fenestrated capillaries and lack the intact blood–brain barrier that is present in the rest of the brain; therefore, CVOs are able to interact with extrinsic cues present in the blood and are often referred to as “windows of the brain.”20–22
In the quiescent state, cell proliferation and differentiation into mature neural lineages in SVZ, SGZ, and CVOs occur at relatively low levels. In response to ischemic injury (i.e., experimental stroke) or infusion of a NSC mitogen (bFGF), there are significant and long-lasting increases in proliferation and differentiation in the SVZ and in three CVOs (AP, ME, and SFO).23,24 Injury-related cues are possibly transmitted to these CVO niches via permeable fenestrated capillaries, and may serve as a mechanism for promoting the generation of new neurons and glia to facilitate brain repair.
Neurogenesis arising from NSCs that originate in the traditional stem cell niches has been studied in traumatic brain injury (TBI) models.25–27 Similarly, NSC changes within the SVZ and SGZ have been examined in animal paradigms of traumatic spinal cord injury (SCI).28,29 However, these analyses have not been extended to the novel CVO niches for either SCI or TBI.
Enhancing the response of NSCs and directing their differentiation fate at the site of trauma has become a long-standing therapeutic target for various types of CNS injury. SCI and TBI are debilitating conditions that exert their most devastating effects on proximal cellular structures located within the spinal cord and near the site of brain injury, respectively. This can include the degeneration of a variety of CNS cell types, as well as damage to axons traveling through and/or near the lesion site. Despite being traumatic events that occur focally within the spinal cord and brain, SCI and TBI also exert widespread changes throughout the CNS neuraxis. For example, damage to axons passing through a SCI site can induce significant retrograde atrophy of neuronal cell bodies or even overt death of neurons located in supraspinal structures.30 Stem cells residing in CVO niches near these vulnerable cells located distant to the injury represent a potentially powerful replacement source of newly born neurons. Similarly, changes in critical glial cell populations of the brain such as astrocytes and oligodendrocytes occur after SCI and TBI31; therefore, the importance of the stem cell response in these novel niches is not restricted solely to neurogenesis. To address these important issues relevant to repair following spinal cord and brain trauma, we examined the response of endogenous NSCs that reside in three CVO stem cell niches (AP, ME, and SFO) in rat models of both SCI and TBI.
Methods
Cervical contusion SCI
All procedures were approved by the Thomas Jefferson University IACUC. Adult female Sprague-Dawley rats weighing 275–300 g were anesthetized with a cocktail of ketamine (100 mg/kg), xylazine (5 mg/kg), and acepromazine (2 mg/kg). The skin and underlying muscle layers between the base of the skull and the top of the shoulder blades were incised and the cervical spinal column was exposed. Spinal cord was exposed by right-side unilateral laminectomy above spinal cord level C4. Unilateral C4 contusion was induced with the Infinite Horizon impactor (Precision Systems and Instrumentation, Lexington, KY) using a 1.5-mm tip at an impact force of 395 kdyn.32,33 The same surgical procedure, except for the administration of the contusion injury, was performed on laminectomy-only control animals. Following the completion of the injury procedure, the muscle and skin incisions were closed with sterile 4–0 silk suture and sterile wound clip, respectively. Animals were injected with lactated Ringers solution and 0.1 mg/kg of buprenorphine (buprenorphine HCl). Daily post-operative monitoring continued until sacrifice.
Controlled cortical impact (CCI)
While heavily anesthetized with isoflurane (3% induction and 2–2.5% maintenance), male Sprague-Dawley rats received a CCI injury using an electromagnetic impactor device (Leica Biosystems Inc.) at pre-determined parameters described previously by our laboratory.34,35 Briefly, CCI was induced on the right side at 1 mm to the right of the sagittal suture and 1 mm posterior of bregma (at a depth of 1 mm; impact velocity of 3 m/sec; 100 msec dwell time) using a 5-mm impactor tip. Craniotomy-only controls underwent all procedures (including the same exposure to isoflurane), with the exception of the impact injury.
Bromodeoxyuridine injections
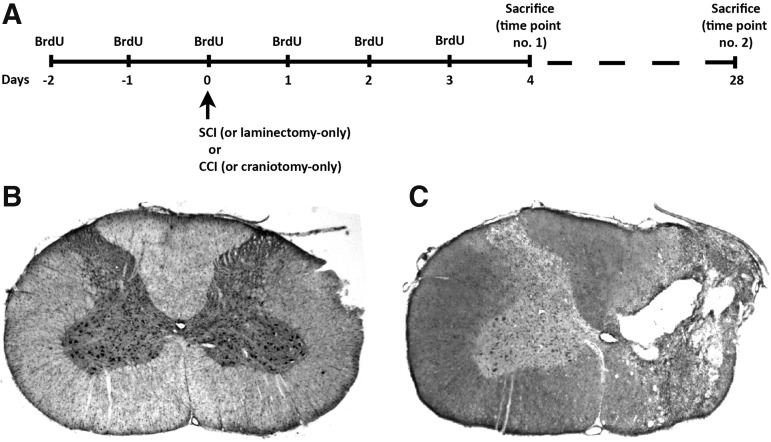
For both SCI and TBI, rats were injected with bromodeoxyuridine (BrdU) solution (Fisher Scientific; 100 mg/kg body weight) prepared in sterile saline. Intraperitoneal injections were performed in a pulse-chase manner twice per day, starting 2 days prior to cervical contusion (or laminectomy-only) or cortical impact (or craniotomy-only) and continuing until 3 days post-injury (or laminectomy/craniotomy) for a total of 6 days of injections. The injections were given approximately 12 h apart on a particular day to space out the timing of BrdU pulsing. The animals were then sacrificed at 4 days post-injury, a time-point based in part on our previous data combined with findings for novel stem cell niches in a stroke model.23 Follow-up experiments were performed at 28 days post-injury or laminectomy only in the SCI model.
Tissue processing for histology
At 4 or 28 days post-surgery, animals were transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde. Brains were harvested and post-fixed in 4% paraformaldehyde at 4°C for 24–36 h, washed with 0.1 M phosphate buffer, and cryoprotected in 30% sucrose at 4°C for 3 days. Tissue was then embedded in optimal cutting temperature (OCT) freezing medium (Tissue-Tek, Sakura, Japan) and flash-frozen. Coronal brain sections were cut at 30 μm for SCI and 20 μm for TBI tissues on a cryostat and collected onto glass slides. Slides were then stored at −20°C.
Immunohistochemistry
Slides were thawed on a heating block at 37°C for 1 h and rehydrated with 0.1 M phosphate-buffered saline (1x PBS). Following an antigen-retrieval step, slides were incubated in blocking buffer containing 0.1% Triton X100 and 5% normal donkey serum (NDS) in 1x PBS (pH 7.4). Slides were then incubated in primary antibodies for 48 h at 4°C, washed, and incubated in secondary antibodies for 2 h at room temperature in blocking buffer. Images were acquired with a Zeiss Imager M2 upright fluorescence microscope. High-resolution confocal images were obtained using a FluoView FV1000 confocal microscope (Olympus, Center Valley, PA) for SCI tissues or using a Leica TCS SP8w with a Leica Hybrid Detector for TBI tissues. For BrdU immunostaining, sections were incubated in 2N HCl for 20 min at 37°C. Sections were then washed with 1x PBS, followed by incubation for 10 min at room temperature in 0.1 M borate buffer (pH 8.5). Sections were then washed with PBS and processed for immunohistochemistry as described above.
Antibodies and reagents
The following primary antibodies were used for immunohistochemistry: mouse anti-GFAP (Millipore, 1:300), mouse anti-nestin (Millipore, 1:200), rabbit anti-doublecortin (Cell Signaling, 1:390), mouse anti-Sox2 (R&D Systems, 1:1000), rat monoclonal anti-BrdU (Accurate Chemical, 1:200), rabbit anti-Olig2 (Millipore, 1:500), and rabbit anti-Iba-1 (WAKO, 1:600). All secondary antibodies were AlexaFluor-conjugated from Invitrogen and included donkey anti-rabbit 647, donkey anti-mouse 594, and donkey anti-rat 488.
Quantitative analysis
All BrdU-positive nuclei in each CVO region (i.e., SFO, ME, AP) were counted using unbiased stereological methods by an observer blinded to experimental conditions,36 using methods modified from those previously described by our laboratory.37,38 A blinded observer counted cells with a visible BrdU-labeled nucleus. Eight to 20 sections per CVO or SVZ region for the BrdU-labeled sections were counted; cell counts for the ME were restricted to sections randomly selected from −2.56 to −2.80 mm posterior to bregma. Three to four sections per animal were counted for double-labeling of BrdU with Sox2, DCX, GFAP, Olig2, and Iba1. Cell counts for all sections were averaged and reported as the mean and standard error of the mean (SEM).
Neurosphere assay
SVZ from embryonic rat brain (E15.5) was dissected out and dissociated into single cells with Papain (Roche, 1 mg/mL) and DNaseI (Roche, 1 mg/mL). Primary neurosphere cultures were cultured in NEP basal medium with 0.36 units/mL heparin sodium plus 20 ng/mL bFGF and 20 ng/mL EGF (PeproTech), as described previously.39,40 Plasma was obtained from anti-coagulated cardiac blood samples from TBI, SCI, and control group rats before perfusion on day 4. Plasma was aliquoted and snap-frozen with liquid nitrogen for later use. After the formation of primary neurospheres, spheres were dissociated by Accutase (Sigma) and total cell numbers were counted using a Countess Automated Cell Counter (Invitrogen). On 24-well plates, 10,000 cells were seeded per well for the secondary neurosphere assay.41 For secondary neurosphere assays, cultures were incubated in NEP basal medium with heparin and grown under the following experimental conditions: control groups: low EGF and bFGF (1 ng/mL) plus 0.5% normal plasma; experimental groups: low EGF and bFGF (1 ng/mL) plus 0.5% plasma from TBI or SCI animals. For quantification of the secondary neurospheres, images were taken and total spheres were counted from each well after 7 days when secondary neurospheres formed. After quantification, spheres from each group were dissociated and total cell numbers were obtained immediately following dissociation, and 10,000 cells/well were seeded onto 24-well plates for the tertiary neurosphere assay. Similar to the secondary neurosphere assay, the tertiary neurosphere assay cultures were treated with plasma from experimental (TBI or SCI) or control groups and quantified as described above. The plasma used in our study was collected by two authors (one using the CCI model and one using the SCI model) following the exact same published protocol. All batches of neurospheres, their treatment with plasma and growth factors, as well as all cell counting and data analysis, were performed for all experiments by two independent investigators.
Statistical analysis
All data are presented as the mean ± SEM. The statistical significance of the mean was calculated by Student's t test to compare injured and control groups by regions of interest at matched end-points. Statistics were computed with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). A p value <0.05 was considered to be significant: *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Cervical contusion SCI did not alter cell proliferation in CVO or SVZ stem cell niches
To assess the supraspinal stem cell response in three CVO niches of the adult brain (AP, ME, and SFO), we employed a cervical contusion rat model of SCI.42–44 SCI is not a single disease, but is instead a heterogeneous set of conditions that differ on a patient-to-patient basis depending on the neuroanatomical location, type and severity of trauma, as well as on the consequent functional deficits produced.45 We chose to use the cervical contusion model because the majority of SCI patients suffer contusion-type trauma to the cervical region.46 We subjected adult, female Sprague-Dawley rats to unilateral cervical contusion at the C4 spinal cord level using the Infinite Horizon spinal impactor. This SCI model resulted in degeneration of a significant portion of the hemi-cord at the level of the contusion, whereas the contralateral hemi-cord was completely intact (at least based on analysis of neuronal degeneration and overall spinal cord structure; Fig. 1B–C). Further, we previously showed that the degeneration on the side of the contusion SCI was restricted to approximately only one spinal cord segment in the rostral-caudal axis.47,48
FIG. 1.
Experimental paradigm. Time line of BrdU pulse-chase protocol for both the control animals that received laminectomy/craniotomy-only and experimental animals that received unilateral C4 contusion or CCI (A) Animals received two daily injections of BrdU for 6 consecutive days (the first injection starting 2 days prior to the surgery) and were sacrificed either 4 days or 28 days after the surgery. Cresyl-violet staining of transverse spinal cord section from laminectomy-only control (B) and C4 contusion SCI (C). BrdU, bromodeoxyuridine; CCI, controlled cortical impact; SCI, spinal cord injury.
To label populations of proliferating cells including multi-potent NSCs and lineage-restricted neural progenitors in ME, AP, SFO, and SVZ, we injected both laminectomy-only uninjured control rats and C4 contusion SCI rats with BrdU. BrdU is permanently incorporated into the DNA of any cell that is dividing at the time of injection and, importantly, into any progeny of these cells (even if they are no longer proliferating and have differentiated from their immature stem/progenitor cell state). The control animals received laminectomy only, meaning that they underwent the exact same surgical procedure as SCI animals, but were not subjected to the contusion injury. The inclusion of the laminectomy control group allowed us to determine the effects specifically of SCI on the supraspinal stem cell response.
We conducted the BrdU-labeling experiment in a “pulse-chase” fashion (Fig. 1A). We first pulsed rats twice daily starting 2 days prior to injury and continuing twice daily until 3 days post-injury. We stopped administering BrdU at 3 days post-injury and then allowed the animals to live for either 4 days post-injury (i.e., one day after the final day of BrdU administration) or 28 days post-injury. This design allowed us to selectively track the response of stem cells that were proliferating at and/or close to the time of SCI and then to follow their localization and differentiation out to either an early time-point post-injury (4 days) or a chronic time-point (28 days; n = 4–6 per group per time-point). Sacrificing the rats at these two distinct time-points allowed us to temporally follow the stem cell response and provided adequate time by 28 days for maturation of the stem/progenitor cells residing in these novel niches.
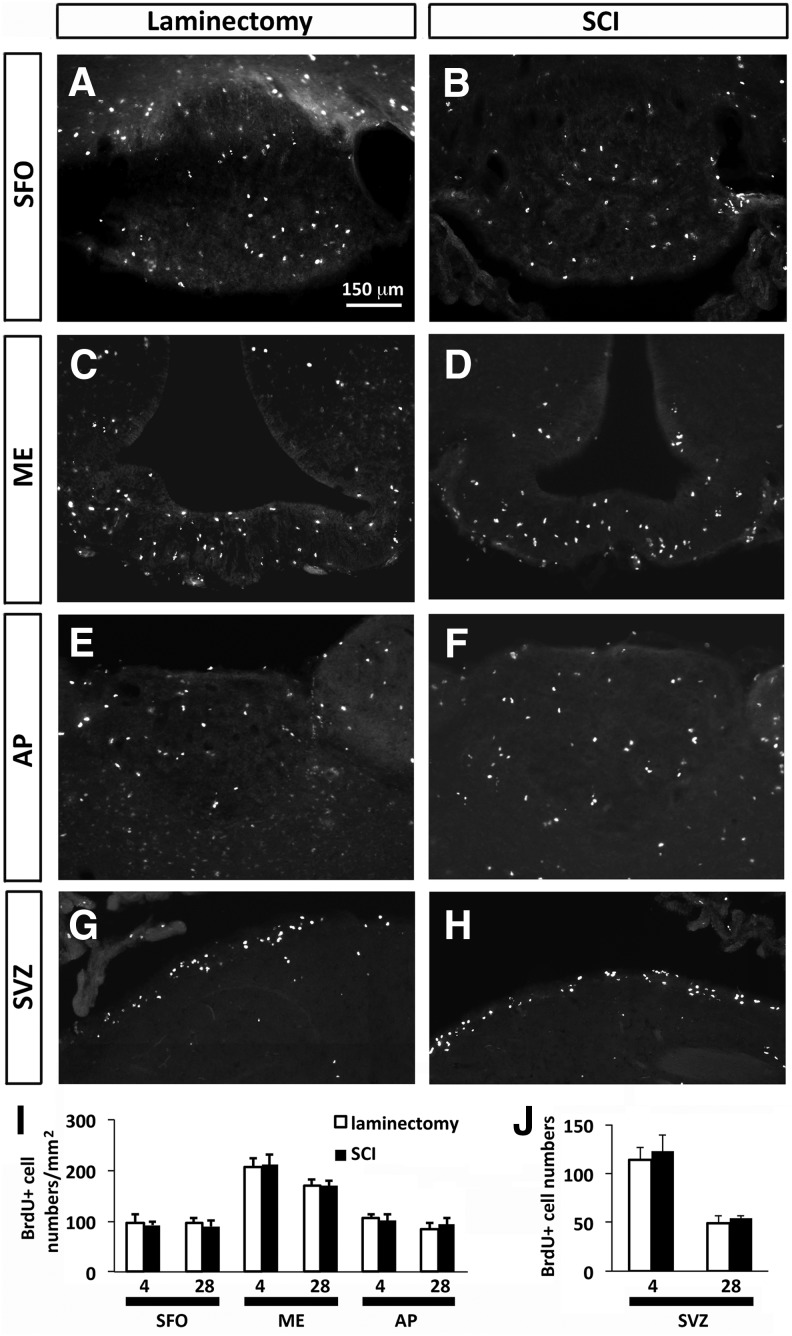
We quantitatively assessed the response of BrdU-labeled cells at 4 and 28 days post-injury or post-laminectomy by performing immunohistochemistry on brain sections using anti-BrdU antibody. We observed significant numbers of BrdU-positive cells in all three niches and at both time-points in both SCI and uninjured rats (Fig. 2A–F). The finding of cell proliferation in the uninjured condition was not unexpected as these niches contain populations of slowly dividing NSCs even in the intact situation. Interestingly, we did not find any statistically significant differences in the numbers of BrdU-labeled cells between uninjured and SCI groups in SFO, ME, or AP at either 4 or 28 days post-surgery (Fig. 2I).
FIG. 2.
Cervical contusion SCI did not alter cell proliferation in CVO and SVZ stem cell niches. Distribution of BrdU-labeled cells in the CVO regions: SFO (A, B), ME (C, D), and AP (E, F) at 4 days after laminectomy (A, C, E) and 4 days after SCI (B, D, F). Distribution of BrdU-labeled cells in the SVZ region at 28 days after laminectomy (G) and SCI (H). (I) Quantification of BrdU-labeled cells in the CVO regions at 4 and 28 days after laminectomy or SCI. (J) Quantification of BrdU-labeled cells in the SVZ at 4 and 28 days after laminectomy or SCI. Data are expressed as mean ± SEM. AP, area postrema; BrdU, bromodeoxyuridine; CVO, circumventricular organs; ME, median eminence; SCI, spinal cord injury; SEM, standard error of the mean; SFO, subfornical organ; SVZ, subventricular zone.
These data demonstrate that SCI does not alter the overall cell proliferation response in these three CVO niches, unlike the robust response elicited in a model of forebrain stroke.23 We also quantified numbers of BrdU-labeled cells in SVZ surrounding the lateral ventricles, a prototypical niche for endogenous NSCs in adult brain. Similar to results obtained in SFO, ME, and AP, C4 contusion did not induce any changes in BrdU cell numbers in SVZ compared with laminectomy-only control at 4 days or 28 days post-surgery (Fig. 2G–H, J).
Cervical contusion SCI did not alter NSC differentiation in CVO stem cell niches
Though we observed no differences in BrdU-positive cell numbers in the CVO niches in response to SCI, it is still possible that SCI altered the stem cell response in these areas. For example, without boosting overall NSC numbers (which was assayed with BrdU-labeled cell counts in all dividing cell populations), SCI may have instead altered NSCs only or the phenotypic trajectory of their differentiation response (i.e., proportion of neurons, astrocytes, and oligodendrocytes derived from BrdU-labeled neural stem/progenitor cells). Such an outcome would still have profound effects on the ability of the niche response to alter cellular makeup of important brain areas. To address this question, we performed double-immunohistochemistry for BrdU with a panel of lineage-specific antibodies to ascertain the phenotypic identity of these BrdU-positive cells that were proliferating around the time of injury/laminectomy. We performed these analyses on both laminectomy-only and C4 contusion animals at 4 and 28 days post-surgery.
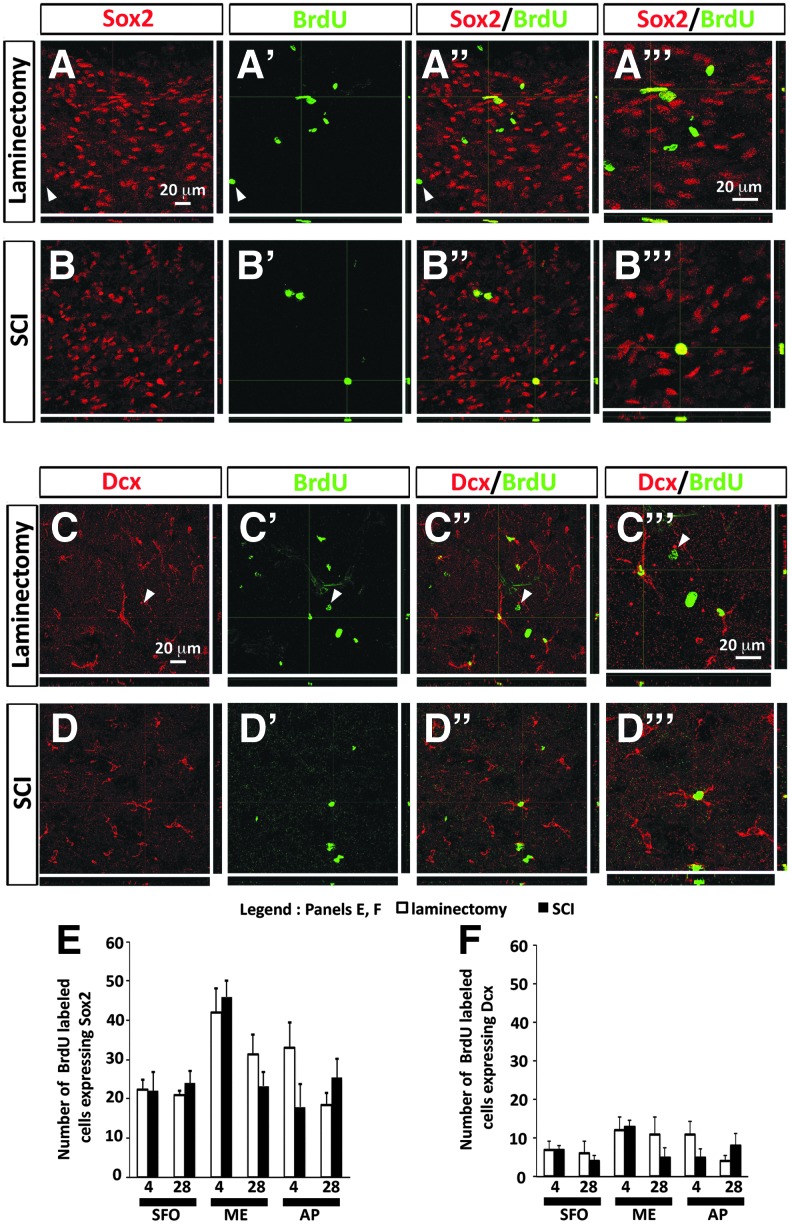
We first identified multi-potent NSCs with the marker Sox2 (Fig. 3A,B). Approximately 35–45% of BrdU-labeled cells in all three niches were Sox2-expressing at both time-points. SCI did not alter numbers of BrdU-Sox2 double-labeled cells in any region or at either time post-injury (Fig. 3E). We next assessed the neurogenic responses in these CVO niches after cervical SCI using doublecortin/Dcx, a marker of immature neurons/neuroblasts (Fig. 3C,D). Approximately 10% of BrdU-labeled cells in all three niches were Dcx-positive at both time-points. Similar to the Sox2 quantification, SCI did not alter numbers of BrdU-Dcx double-labeled cells in any region or at either time post-injury (Fig. 3F). These findings demonstrate that SCI did not alter the population of multi-potent NSCs residing in these CVO niches, nor did it modulate their neuronal differentiation.
FIG. 3.
Cervical contusion SCI did not alter NSC numbers or neuronal differentiation in CVO stem cell niches. (A, A’’) Expression of NSC marker Sox2 by BrdU-labeled cells in AP at 28 days after laminectomy. Arrowheads point to an example of BrdU-labeled cell not expressing Sox2. (A’’’) Higher magnification image showing co-localization of Sox2 and BrdU in laminectomy tissue. (B, B’’’) Expression of Sox2 by BrdU-labeled in AP at 28 days after SCI. (B’’’) Higher magnification image showing co-localization of Sox2 and BrdU in SCI tissue. (C, C’’) Expression of immature neuron/neuroblast marker Dcx by BrdU-labeled cells in AP at 28 days after laminectomy. Arrowheads point to an example of BrdU-labeled cell that is not expressing Dcx. (C’’’) Higher magnification image showing co-localization of Dcx and BrdU in laminectomy tissue. (D, D’’) Expression of Dcx by BrdU-expressing cells in AP at 28 days after SCI. (D’’’) Higher magnification image showing co-localization of Dcx and BrdU in SCI tissue. (E) Number of BrdU-labeled cells expressing Sox2 in CVO regions at 4 days and 28 days after laminectomy or SCI. (F) Number of BrdU-labeled cells expressing Dcx in CVO regions at 4 days and 28 days after laminectomy or SCI. AP, area postrema; BrdU, bromodeoxyuridine; CVO, circumventricular organs; Dcx, doublecortin; NSC, neural stem cell; SCI, spinal cord injury; SVZ, subventricular zone. Color image is available online at www.liebertpub.com/neu
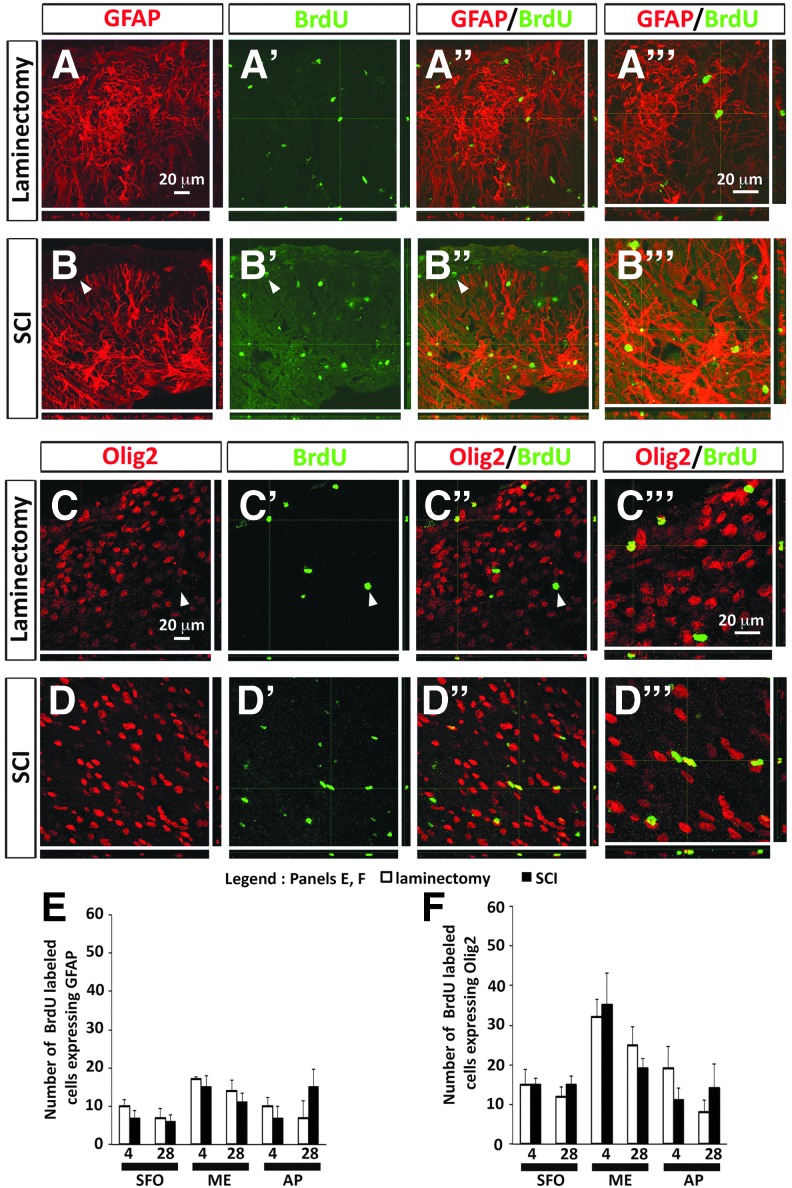
As the differentiation response in these CVO niches is not restricted to solely neurogenesis, we also examined differentiation of BrdU-labeled cells into astrocytes and along the oligodendrocyte lineage. Of BrdU-labeled cells, 10–15% in all three CVO regions and at both time-points were GFAP-positive astrocytes (Fig. 4A,B), whereas 20–30% of BrdU-labeled cells were lineage-restricted oliogendrocyte progenitor cells expressing Olig2 (Fig. 4C,D). Similar to the analyses with Sox2 and Dcx, SCI did not alter differentiation along either glial lineage in the SFO, ME, or AP at either time-point, as assessed by numbers of BrdU-GFAP (Fig. 4E) and BrdU-Olig2 (Fig. 4F) double-labeled cells.
FIG. 4.
Cervical contusion SCI did not alter astrocyte or oligodendrocyte lineage differentiation in CVO stem cell niches. (A, A”) Expression of astrocyte marker GFAP by BrdU-labeled cells in ME at 28 days after laminectomy. (A’’’) Higher magnification image showing co-localization of GFAP and BrdU in laminectomy tissue. (B’, B’’) Expression of GFAP by BrdU-labeled cells in ME at 28 days after SCI. Arrowheads point to an example of BrdU-labeled cell not expressing GFAP. (B’’’) Higher magnification image showing co-localization of GFAP and BrdU in SCI tissue. (C, C’’) Expression of oligodendrocyte lineage marker Olig2 by BrdU-labeled cells in AP at 28 days after laminectomy. Arrowheads point to an example of BrdU-labeled cell that is not expressing Olig2. (C’’’) Higher magnification image showing co-localization of Olig2 and BrdU in laminectomy tissue. (D, D’’) Expression of Olig2 by BrdU-labeled cells in AP at 28 days after SCI. (D’’’) Higher magnification image showing co-localization of Olig2 and BrdU in SCI tissue. (E) Number of BrdU-labeled cells expressing GFAP in CVO regions at 4 days and 28 days after laminectomy or SCI. (F) Number of BrdU-labeled cells expressing Olig2 in CVO regions at 4 days and 28 days after laminectomy or SCI. AP, area postrema; BrdU, bromodeoxyuridine; CVO, circumventricular organs; ME, median eminence; SCI, spinal cord injury. Color image is available online at www.liebertpub.com/neu
Cervical contusion SCI did not change the microglia/macrophage response in CVO niches
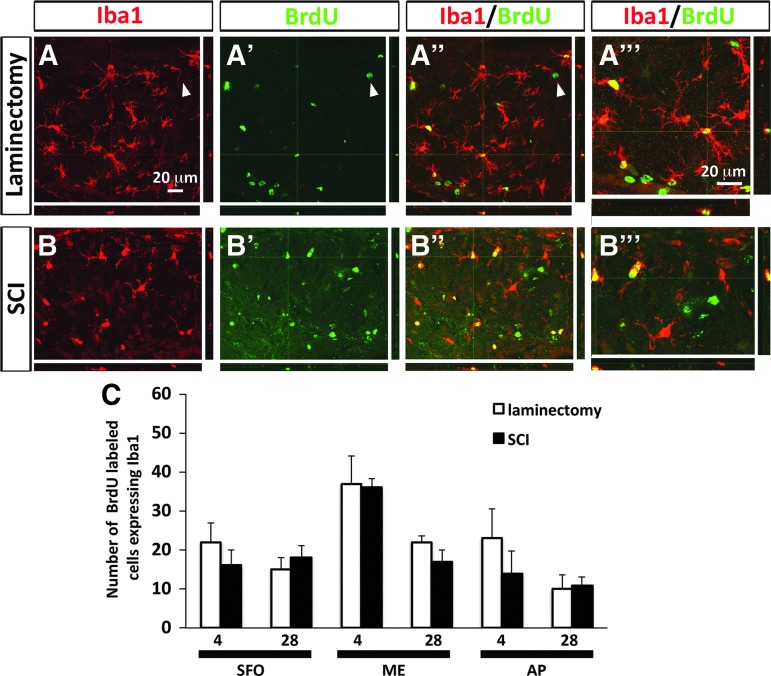
It is important to note that labeling of a cell with BrdU does not necessarily mean that it is still in a neural stem/progenitor cell state or that it was derived from a NSC that was proliferating at the time of SCI/BrdU pulsing. For example, already-mature cell types of the CNS such as microglia (that are of a non-ectodermal myeloid lineage) can revert back to a proliferative state in response to nervous system trauma.49 In addition, cells from outside the CNS such as activated, proliferating monocyte-derived macrophages can infiltrate CNS parenchyma after SCI.50 In such cases, the BrdU-labeled population in the SFO, ME, and AP would not necessarily be all NSCs or their progeny. To address this possibility, we performed double-immunohistochemistry for BrdU with the pan-microglia/macrophage marker Iba1 on our brain section samples (Fig. 5A,B). We found that a significant percentage of BrdU-labeled cells in all three niches were Iba1-positive; however, there were no differences in the number of BrdU+ cells co-expressing Iba1 between uninjured control and contusion groups at either time-point (Fig. 5C).
FIG. 5.
Cervical contusion SCI did not alter the microglia/macrophage response in CVO niches. (A, A’’) Expression of microglia/macrophage marker Iba-1 by BrdU-labeled cells in ME at 28 days after laminectomy. Arrowheads point to an example of a BrdU-labeled cell that is not expressing Iba-1. (A’’’) Higher magnification image showing co-localization of Iba-1 and BrdU in laminectomy tissue. (B, B’’) Expression of Iba-1 by BrdU-labeled cells in ME at 28 days after SCI. (B’’’) Higher magnification image showing co-localization of Iba-1 and BrdU in SCI tissue. (C) Number of BrdU-labeled cells expressing Iba-1 in CVO regions at 4 days and 28 days after laminectomy or SCI. BrdU, bromodeoxyuridine; CVO, circumventricular organs; ME, median eminence; SCI, spinal cord injury. Color image is available online at www.liebertpub.com/neu
Controlled cortical impact enhanced NSC proliferation in CVO stem cell niches
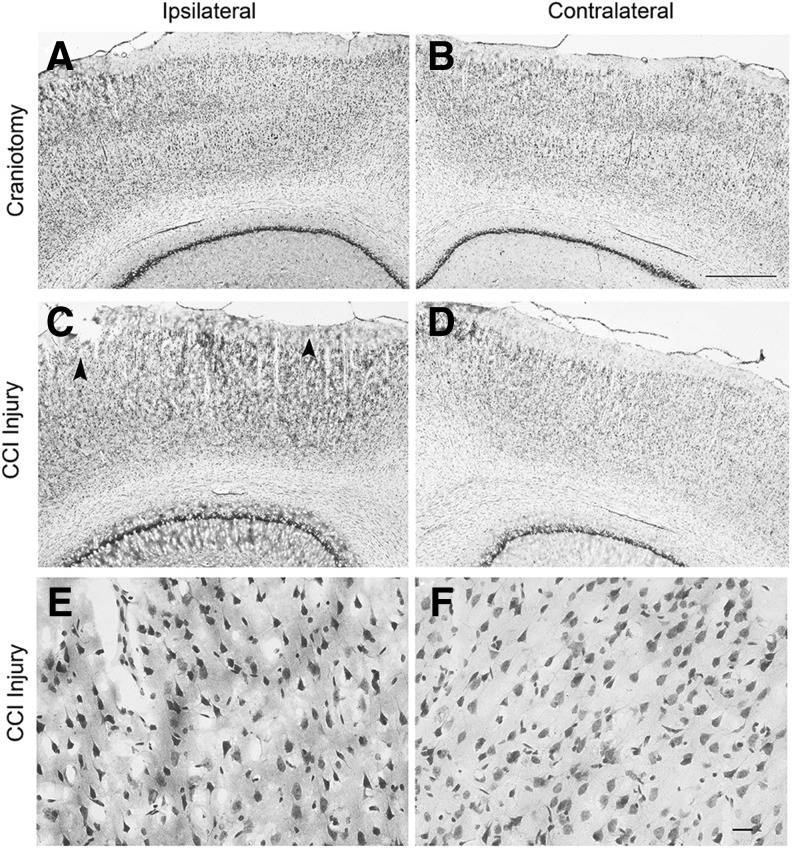
To examine the effects of TBI on CVO and traditional stem cell niches, we use a CCI model in adult rats, a paradigm of mild cortical injury. Compared with craniotomy-only control (Fig. 6A,B), CCI delivered at a 1-mm depth of impact using an electromagnetically controlled device induced slight tissue loss in the superficial cortical layers along with hyperchromic, shrunken neurons throughout the cortical layers on the side of injury at 4 days post-CCI (Fig. 6C–F). Our CCI model shows a similar mild tissue loss in the rat that has been reported for a similar CCI model using mice.51,52 After CCI, the lesion progressively develops, in which cells continue to degenerate up to 7–14 days post-injury, resulting in a necrotic injury core. Hyperchromatic neurons observed in the neocortex and hippocampus in models of TBI are indicative of pursuant cell death or present damage. Hyperchromic, shrunken neurons showed marked condensation of cytoplasm, mitochondrial swelling, and particularly aggregation of the nuclear chromatin.
FIG. 6.
Cresyl-violet stained sections showing craniotomy and controlled cortical impact (CCI) injury brains at 4 days post-injury. Ipsilateral and contralateral cortices are shown for craniotomy-only control (A, B) and CCI injured (C–F) brains. CCI brains at the center of the injury site at lower magnification show minimal necrotic tissue loss in the superficial cortical layers (arrowheads in C), whereas hyperchromic neurons are observed throughout all cortical layers on the side ipsilateral to CCI (C) when compared with few hyperchomic neurons appearing in the medial layers of the contralateral cortex (D). There is a lack of injured/dying hyperchomic neurons observed in cortex of the craniotomy-only rats (A, B). Higher magnification images show hyperchromic neurons in the ipsilateral cortex after CCI (E) and normal cresyl-violet stained neurons in the contralateral cortex of CCI animals (F). Scale bars: 500 μm (A–D); 20 μm (E–F). CCI, controlled cortical impact.
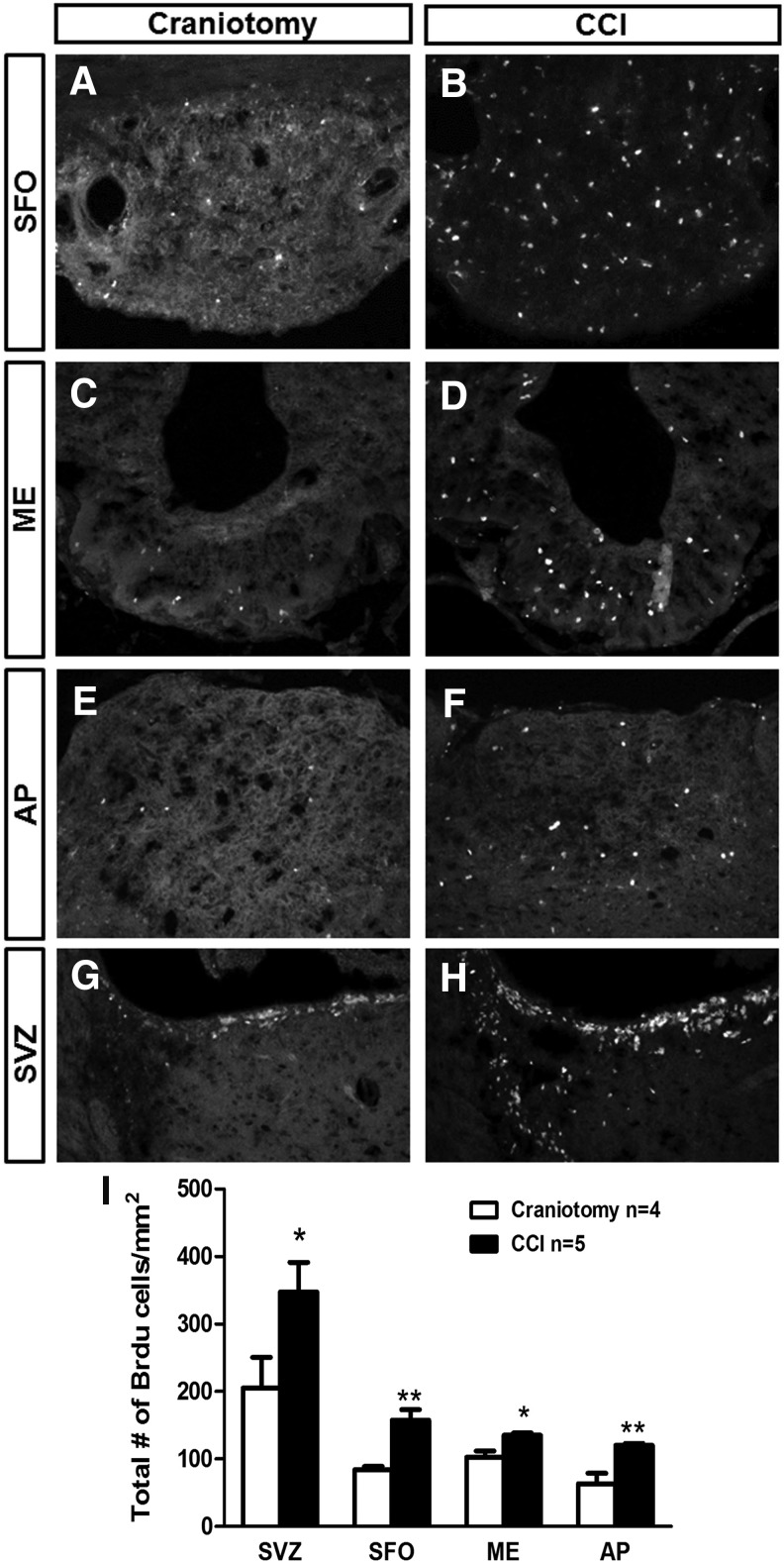
We quantitatively assessed the response of BrdU-labeled cells at 4 days post-CCI or after craniotomy-only control in AP, ME, SFO, and SVZ (n = 4–5 per group per time-point), as described for the SCI experiments. CCI increased numbers of BrdU-labeled cells compared with craniotomy-only in the SFO (Fig. 7A,B), ME (Fig. 7C,D), AP (Fig. 7E,F), and SVZ (Fig. 7G,H) at 4 days post-injury (p < 0.01 and p < 0.05, respectively; quantification in Fig. 7I). We performed histological analyses only at 4 days post-injury for the CCI model. Because we observed a robust response at 4 days in the CVO niches after CCI, we did not extend our analysis to the delayed 28-day time-point as we had after SCI (a paradigm in which we observed no changes at 4 days).
FIG. 7.
Controlled cortical impact (CCI) enhanced cell proliferation in CVO niches. Distribution of BrdU-labeled cells in the CVO regions: SFO (A, B), ME (C, D), and AP (E, F) at 4 days after craniotomy (A, C, E) or CCI (B, D, F). Distribution of BrdU-labeled cells in the SVZ at 4 days after craniotomy (G) or CCI (H). Quantification of BrdU-labeled cells in the CVO and SVZ regions at 4 days after craniotomy or CCI (I). Data are expressed as mean ± SEM. AP, area postrema; BrdU, bromodeoxyuridine; CVO, circumventricular organs; ME, median eminence; SEM, standard error of the mean; SFO, subfornical organ; SVZ, subventricular zone.
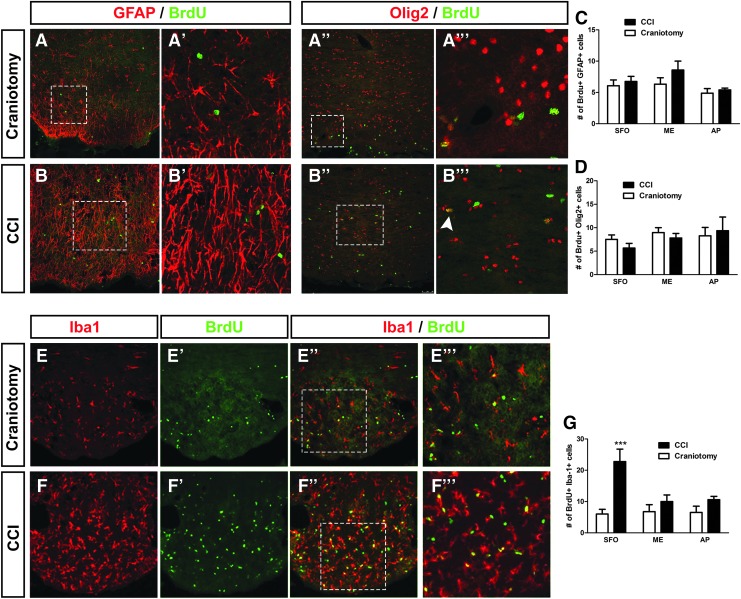
Controlled cortical impact altered lineage profiles in CVO stem cell niches
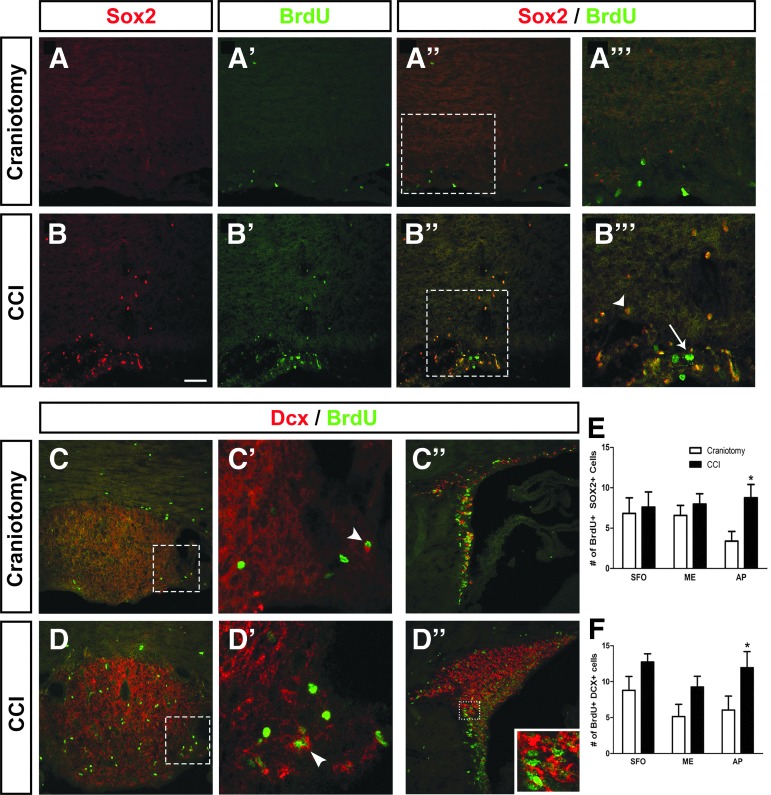
Unlike the results obtained in the cervical SCI model in which no effect of spinal cord trauma was noted in any CVO region, our BrdU quantification data demonstrate significant increases in cell proliferation in all three CVO stem cell niches following TBI. Given this response, we next sought to identify potential effects on the lineage fate of NSC-derived cells in the AP, ME, and SFO post-CCI. We used the same panel of lineage-specific markers as described above for the SCI model to identify Sox2+ NSCs (Fig. 8A,B), Dcx+ immature neurons/neuroblasts (Fig. 8C,D), GFAP+ astrocytes (Fig. 9A,B), Olig2+ cells of the oligodendrocyte lineage (Fig. 9A,B), and Iba1+ microglia/macrophages (Fig. 9E,F). At 4 days post-surgery, there was a significant increase in numbers of Brdu+/Sox2+ double-labeled cells only in the AP after CCI (p < 0.05; Fig. 8E), demonstrating that CCI increased the pool of NSCs in this CVO niche. There was a significant increase in BrdU/Dcx co-labeling in CCI compared with craniotomy-only in AP (p < 0.05) and a trend toward an increase in BrdU+/Dcx+ counts after CCI in both the ME (p < 0.06) and SFO (p < 0.053; Fig. 8F), demonstrating that CCI alters the neurogenic fate of NSCs in CVO niches, particularly in the AP. CCI did not affect the numbers of BrdU-labeled cells co-expressing GFAP compared with craniotomy-only at 4 days post-surgery in SFO, ME, or AP (p = 0.6, 0.1, and 0.5, respectively; Fig. 9C), although we noted a robust astrogliosis response characterized by changes in astrocyte morphology and intensity of GFAP expression in the SFO region (Fig. 8B’), but not in ME or AP (not shown). Similarly, CCI did not alter differentiation along the oligodendrocyte lineage in any CVO location, as assessed by quantification of co-labeling with BrdU and Olig2 (Fig. 9D). These Olig2 and GFAP results show that CCI did not alter glial differentiation in the CVO niches. Lastly, co-labeling of Iba1 and BrdU showed a robust increase in proliferating microglia/macrophages in the SFO following CCI, but not in the AP or ME (Fig. 9E–G).
FIG. 8.
Controlled cortical impact (CCI) increased NSC numbers and neurogenesis in AP. Expression of Sox2 by BrdU-labeled cells at 4 days after craniotomy (A, A’’) or CCI injury (B, B’’). Higher magnification image showing co-localization of Sox2 and BrdU in craniotomy (A’’’) or CCI tissue (B’’’). White arrow indicates BrdU-labeled cell that does not co-express Sox2, whereas arrowhead identifies BrdU+ cell double-labeled with Sox2 (A’’’). Expression of Dcx by BrdU-labeled cells in SFO at 4 days after craniotomy (C) or CCI (D), along with higher magnification images (C’, D’). BrdU-labeled cells expressing Dcx in the SVZ of craniotomy (C”) or CCI (D’’) animals. Higher magnification inset shows cells co-labeled with BrdU and Dcx (D’’). Number of BrdU-labeled cells expressing Sox2 (E) or Dcx (F) in CVO regions at 4 days after craniotomy or CCI. AP, area postrema; BrdU, bromodeoxyuridine; CVO, circumventricular organs; Dcx, doublecortin; ME, median eminence; SFO, subfornical organ; SVZ, subventricular zone. Color image is available online at www.liebertpub.com/neu
FIG. 9.
Controlled cortical impact (CCI) increased microlial proliferation in SFO, but did not alter astrocyte or oligodendrocyte lineage differentiation in any of the CVO stem cell niches. Expression of GFAP by BrdU-labeled cells in AP at 4 days after craniotomy (A) or in SFO after CCI (B). Higher magnification image showing co-localization of GFAP and BrdU in craniotomy (A’) or CCI (B’) tissue. Expression of Olig2 by BrdU-labeled cells in SFO at 4 days after craniotomy (A’’) or CCI (B”). Higher magnification image showing co-localization of Olig2 and BrdU after craniotomy (A’’’) or CCI (B’’’). White arrowhead indicates BrdU-labeled cell co-expressing Olig2. Number of BrdU-labeled cells expressing GFAP (C) or Olig2 (D) in CVO regions at 4 days after craniotomy or CCI. Expression of Iba1 by BrdU-labeled cells in SFO at 4 days after craniotomy (E, E’’) or CCI (F, F”). Higher magnification image showing co-localization of Iba1 and BrdU in craniotomy (E’’’) or CCI (F’’’) tissue. Number of BrdU-labeled cells expressing Iba1 in CVO regions at 4 days after craniotomy or CCI (G). AP, area postrema; BrdU, bromodeoxyuridine; CVO, circumventricular organs; SFO, subfornical organ; SVZ, subventricular zone. Color image is available online at www.liebertpub.com/neu
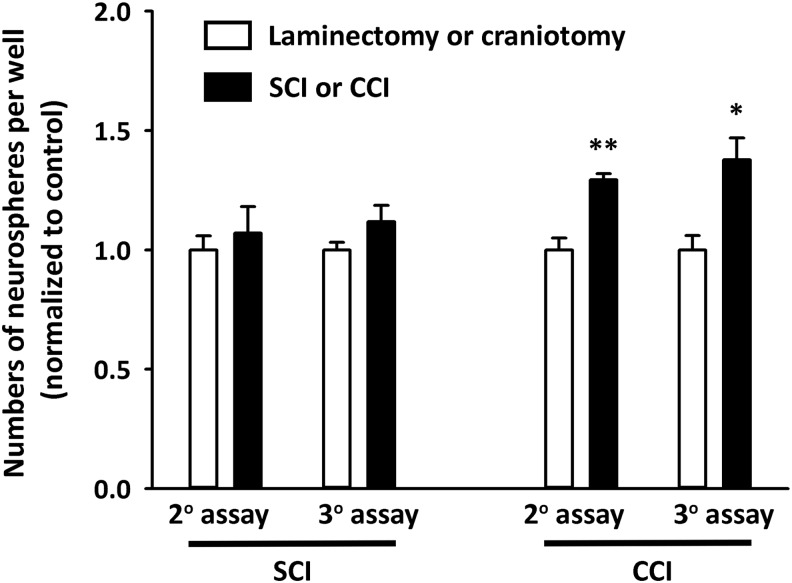
Diffusible signals from plasma derived from TBI rats—but not SCI—promoted neural stem cell proliferation
To begin to evaluate the ability of blood-derived factors to influence NSC properties in brain niches, we harvested plasma from laminectomy-only, C4 contusion, craniotomy-only and CCI rats at 4 days post-surgery for all groups. We then applied these plasma samples in vitro to cultures of neurospheres derived from rat SVZ to quantify effects on NSC proliferation. In preliminary studies, we determined proliferation efficiency with various concentrations of normal plasma. A final plasma concentration of 0.5% in the culture medium resulted in no obvious differentiation; therefore, we selected this concentration for our assay. We found a significant increase in the total number of neurospheres with TBI plasma added to the medium compared with craniotomy-only control, but we observed no effects with plasma derived from SCI animals compared with their laminectomy-only control (Fig. 10). As blood-derived signaling factors may be responsible for providing instructive cues to stem cell niches in the brain in response to various types of CNS trauma, the differential ability of TBI-derived plasma to stimulate NSC expansion in vitro may in part explain the robust in vivo changes observed in the CVO niches in the TBI model—but not in the SCI paradigm.
FIG. 10.
Diffusible signals from plasma derived from TBI rats—but not SCI—promote neural stem cell proliferation. No differences in secondary or tertiary neurosphere expansion were noted between cultures treated with plasma derived from SCI and laminectomy-only control animals. Significant increases in the total number of neurospheres per well were observed in cultures treated with TBI plasma compared with craniotomy-only control group. CCI, Controlled cortical impact; SCI, spinal cord injury; TBI, traumatic brain injury.
Discussion
We report that the proliferation and differentiation response of NSCs in three novel CVO niches of the adult brain were differentially affected in rodent models of SCI and TBI. TBI induced an early and robust activation of this response that included both increased NSC proliferation and an enhanced neurogenesis profile, whereas SCI exerted no effects on NSC mobilization or differentiation at either early or late time-points post-trauma.
Absence of response in CVO stem cells niches of the brain to SCI
Our results in the SCI model are in contrast to those obtained in a rodent model of forebrain stroke23 and in the current study with TBI. However, the CNS damage associated with stroke and TBI (including many forms of the human condition, as well as the matching animal models) likely induces more profound changes than SCI in the ME, AP, and SFO because of the irrelatively close proximity and possibly also due to greater severity. Whereas SCI can be incredibly devastating to patients on a functional level,45 the severity of the trauma itself (due to the relative small size of the spinal cord and the restricted nature of the lesion)—as well as its significant distance from the brain—likely reduce its impact on these supraspinal niches.
Given that SCI can exert profound supraspinal changes in the brain such as retrograde neuronal atrophy and even overt cell death,30 we originally hypothesized that we would observe an increase in the number of BrdU-positive cells (suggestive of an activation of stem cell niches) and an alteration in the differentiated fate of these stem cells. However, our results may not be surprising given the relatively large distance between the SCI site and these brain niches, especially if proximity is playing a key role in stem cell activation. This proximity issue may be relevant if, for example, the injury-induced stimulating factor(s) are traveling through CNS parenchyma and not via some other mode such as through the vasculature and/or cerebrospinal fluid. We calculated the approximate distances between each of the three CVO niches and both the SCI site (SCI-to-SFO: 27.7 mm; SCI-to-ME: 26.2 mm; SCI-to-AP: 15.0 mm) and the CCI site (CCI-to-SFO: 5.0 mm; CCI-to-ME: 10.1 mm; CCI-to-AP: 15.1 mm). In general, the SCI is significantly farther from the niches than the CCI. The AP is a similar distance from both injury locations, raising the possibility that contributions such as directionality of cerebrospinal fluid flow may play a role. It is most likely that the stimulus for inducing (or not) a stem cell response in these CVO niches is multi-factorial. At present, we do not understand the mechanism(s) responsible for conveying the injury stimulus into a supraspinal stem cell activation response. Interestingly, our neurosphere results demonstrate a differential ability of TBI-derived and SCI-derived plasma to promote NSC expansion, suggesting that signaling factors present in blood may be critical for stimulating these CVO niches after CNS injury and that the blood-transmitted stimulus is significantly weaker with spinal cord damage compared with TBI.
Our current results in the cervical contusion are in line with previous work in SCI animal models that demonstrated minimal-to-no NSC responses in SVZ and SGZ niches. Using similar BrdU and Dcx analysis as our study, Felix and colleagues showed that cervical hemi-section SCI at the C2 level transiently reduced BrdU counts in the dentate gyrus and olfactory bulb and persistently reduced neurogenesis in the dentate gyrus.28 In a model of thoracic contusion SCI, BrdU counts and neurogenesis were not altered in the SVZ or hippocampus out to 6 weeks post-injury.29 These findings show that SCI induces minimal effects on supraspinal stem cell niches (and even reduced neurogenesis in some conditions), including the prototypical SVZ and SGZ niches and the novel CVO niches examined in the current work.
Response in CVO stem cells niches to TBI
The mild cortical contusion model of TBI resulted in a significant increase in NSC proliferation in all examined niches. In addition, we observed changes in NSC numbers, neurogenesis, and microglial/macrophage response at some of these CVO locations. As we found no effects of cervical SCI at SFO, ME, AP, or SVZ, these data suggest that injury severity may be involved in the stem cell niche response. In our previous work, we showed that experimental stroke produces extensive damage with respect to neuroanatomical area of tissue degeneration and promotes robust changes in BrdU counts, along with Sox2 and Dcx responses in all three CVO niches.23 On the contrary, the mild CCI model results in significantly less tissue damage, as shown in previous studies,35 than the stroke paradigm, and in turn promoted changes in cell differentiation profiles at only a subset of the CVO locations and with only select lineage markers. To further address the impact of injury severity on CVO niches, we could test CCI models of different severity in future work.
As an alternative explanation to injury severity, our data support the notion that proximity of the CVO niche to the injury site may affect the stem cell response. Although BrdU counts were increased in all three CVO niches after CCI, we observed more pronounced increases in the SFO and AP compared with the ME. The differential effects of CCI on cell proliferation in the three niches may be associated with the dorsal nature of the CCI, considering that the ME lies more ventrally in the brain than SFO and AP. In addition, we found increased CCI-induced NSC numbers and neurogenesis only in the AP, which is the CVO location most distal to the CCI site. Conversely, we observed a robust neuroinflammatory microglia/macrophage response in the SFO, the most proximal niche.
It is possible that increases in proliferating microglia and/or infiltrating macrophages after cortical contusion may have suppressed neurogenesis in the more proximal stem cell niches (SFO, ME),53 whereas CCI induced a robust NSC and neurogenic response in the AP because of a minimized effect of CCI on inflammation at greater distances. Prolonged inflammation has been found to suppress Dcx-positive cells in the SVZ and SGZ in a rat CCI model.53 The inflammatory second messenger, inducible nitric oxide synthase, released from microglia cells was found to impair NSC proliferation.54 Previously, our laboratory reported microglia activation and increases in iNOS signaling proximal to the injury, which encroaches on regions such as the SFO and SVZ.55 Astrogliosis was also evident in the SFO near the site of impact, as has been reported in cortical and subcortical structures previously by our laboratory.56 Astrocytes represent an additional potential source of inflammation or other anti-neurogenic signaling mediators, possibly accounting for the lack of robust changes in the stem cell response in this niche.
Our results with the in vitro neurosphere assay demonstrate that plasma derived from CCI (but not SCI) animals significantly enhances NSC expansion. Therefore, all three CVO niches were subjected to plasma-derived signals in the CCI animals, but differential inflammatory responses at each niche location may have altered the response of NSCs to these plasma signals in vivo. One future approach to address the contribution of niche proximity would be to alter the CCI location such that the injury is placed closer to the AP than the SFO; we could hypothesize that this would result in opposite effects on Sox2, Dcx, and Iba1 changes in the CVO niches compared with the current study.
The potential of the traumatized brain to repair itself with respect to the traditional stem cell niches has been well studied in TBI research models, including NSC generation in the SVZ and hippocampal neurogenesis.25–27 One study demonstrated that a diminished stem cell pool compromised recovery in a model of TBI.26 Our findings support therapeutic strategies that combine enhancements in the NSC pool concomitant with anti-inflammatory or targeted immune cell therapies.
It is important to note that we used only females for the contusion SCI paradigm and only males for the CCI model. As sex-specific differences play a critical role across biological processes, it is possible that our results are missing the potential impact of sex on CVO stem cell niche responses to SCI and CCI, especially considering that previous work has revealed the effects of sex-related factors such as sex steroids on the properties of various stem cell populations.57,58
Interestingly, we noted differences in the control conditions of laminectomy versus craniotomy. Focusing on the total BrdU counts in the two models, we found that these baseline counts were similar between laminectomy-only and craniotomy-only conditions in both the SFO and AP, but were significantly different in the ME. This effect may be due to the inherent differences in these sham surgeries. For example, we and others have previously shown that in brain injury models craniotomy alone causes tissue injury, inflammation, and transient gliosis.59,60 It is possible that differences in the degree to which craniotomy and laminectomy induce these types of changes affect the stem cell response in CVO niches (in particular the ME).
We chose to BrdU-pulse animals in both injury models temporally at and close to the time of trauma given that this is the time frame contributing to the greatest degree of tissue degeneration. For example, we have quantified the time course of “secondary injury” in this C4 contusion SCI model; we find that, although some cellular loss and overall tissue degeneration does continue to occur after the first 3 days post-injury, the majority occurs during the first several days post-injury.47,48 Despite this continued process of ongoing degeneration, we did not want to pulse with BrdU for an extended time window because then we would not have much temporal resolution to understand when these CVO stem cell niches are being activated relative to the time of trauma. Therefore, we choose to deliver BrdU during the early time frame following SCI and CCI. Given that both the SCI and CCI models involve continued evolution over days, weeks and possibly even longer following the initial insult, it will be important in future work to examine the CVO stem cell niche responses at later time-points.
Conclusion
In summary, we report that SCI and TBI promote differential responses in CVO stem cell niches of the brain. Characterizing the nature of these changes is critical for designing interventions to boost the mobilization of NSCs within these important niches and, importantly, to instruct their differentiation along desired phenotypic lineages. Though we have not observed effects of SCI on stem cell responses in the AP, ME, or SFO, future studies could be geared toward attempts at therapeutically enhancing and/or altering the stem cell response in these novel niches after SCI. Such a strategy could have profound effects on pathological changes occurring in the brain after distant focal trauma to the spinal cord. Our findings suggest that location of the traumatic event (i.e., spinal cord versus brain, as well as dorsal-ventral and rostral-caudal position within the brain) may be involved in determining the CVO niche response. Further, our neurosphere expansion results support the notion that signaling factors transmitted in the blood play a role in regulating NSCs in CVO niches and that differences in this blood signal may in part underlie the differential responses we observed in our SCI and TBI models.
Acknowledgments
This work was supported by the Dean's Programmatic Science Grant (to L.I., M.B.E., and A.C.L.) and NS079702 (to A.C.L.). We thank Brittany Daiutolo for technical support in the early stages of these experiments.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Doetsch F., Caille I., Lim D.A., Garcia-Verdugo J.M., and Alvarez-Buylla A. (1999). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 [DOI] [PubMed] [Google Scholar]
- 2.Doetsch F., Garcia-Verdugo J.M., and Alvarez-Buylla A. (1999). Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. U S A 96, 11619–11624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doetsch F. (2003). A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 13, 543–550 [DOI] [PubMed] [Google Scholar]
- 4.Doetsch F. (2003). The glial identity of neural stem cells. Nat. Neurosci. 6, 1127–1134 [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A., and Lim D.A. (2004). For the long run: maintaining germinal niches in the adult brain. Neuron 41, 683–686 [DOI] [PubMed] [Google Scholar]
- 6.Lie D.C., Song H., Colamarino S.A., Ming G.L., and Gage F.H. (2004). Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu. Rev. Pharmacol. Toxicol. 44, 399–421 [DOI] [PubMed] [Google Scholar]
- 7.Mignone J.L., Kukekov V., Chiang A.S., Steindler D., and Enikolopov G. (2004). Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 469, 311–324 [DOI] [PubMed] [Google Scholar]
- 8.Ming G.L., and Song H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schouten J.W., Fulp C.T., Royo N.C., Saatman K.E., Watson D.J., Snyder E.Y., Trojanowski J.Q., Prockop D.J., Maas A.I., and McIntosh T.K. (2004). A review and rationale for the use of cellular transplantation as a therapeutic strategy for traumatic brain injury. J. Neurotrauma 21, 1501–1538 [DOI] [PubMed] [Google Scholar]
- 10.Sundholm-Peters N.L., Yang H.K., Goings G.E., Walker A.S., and Szele F.G. (2005). Subventricular zone neuroblasts emigrate toward cortical lesions. J. Neuropathol. Exp. Neurol. 64, 1089–1100 [DOI] [PubMed] [Google Scholar]
- 11.Park J.H., Cho H., Kim H., and Kim K. (2006). Repeated brief epileptic seizures by pentylenetetrazole cause neurodegeneration and promote neurogenesis in discrete brain regions of freely moving adult rats. Neuroscience 140, 673–684 [DOI] [PubMed] [Google Scholar]
- 12.Belluzzi O., Benedusi M., Ackman J., and LoTurco J.J. (2003). Electrophysiological differentiation of new neurons in the olfactory bulb. J. Neurosci. 23, 10411–10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ihrie R.A., and Alvarez-Buylla A. (2008). Cells in the astroglial lineage are neural stem cells. Cell. Tissue Res. 331, 179–191 [DOI] [PubMed] [Google Scholar]
- 14.Lois C., and Alvarez-Buylla A. (1994). Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145–1148 [DOI] [PubMed] [Google Scholar]
- 15.Zhao C., Deng W., and Gage F.H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 [DOI] [PubMed] [Google Scholar]
- 16.Ming G.L., and Song H. (2005). Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250 [DOI] [PubMed] [Google Scholar]
- 17.van Praag H., Schinder A.F., Christie B.R., Toni N., Palmer T.D., and Gage F.H. (2002). Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett L., Yang M., Enikolopov G., and Iacovitti L. (2009). Circumventricular organs: a novel site of neural stem cells in the adult brain. Mol. Cell. Neurosci. 41, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett L.B., Cai J., Enikolopov G., and Iacovitti L. (2010). Heterotopically transplanted CVO neural stem cells generate neurons and migrate with SVZ cells in the adult mouse brain. Neurosci. Lett. 475, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross P.M., and Weindl A. (1987). Peering through the windows of the brain. J. Cereb. Blood Flow. Metab. 7, 663–672 [DOI] [PubMed] [Google Scholar]
- 21.Moyse E., Bauer S., Charrier C., Coronas V., Krantic S., and Jean A. (2006). Neurogenesis and neural stem cells in the dorsal vagal complex of adult rat brain: new vistas about autonomic regulations–a review. Auton. Neurosci. 126–127, 50–58 [DOI] [PubMed] [Google Scholar]
- 22.Joly J.S., Osorio J., Alunni A., Auger H., Kano S., and Retaux S. (2007). Windows of the brain: towards a developmental biology of circumventricular and other neurohemal organs, Semin. Cell. Dev. Biol. 18, 512–524 [DOI] [PubMed] [Google Scholar]
- 23.Lin R., Cai J., Nathan C., Wei X., Schleidt S., Rosenwasser R., and Iacovitti L. (2015). Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol. Dis. 74, 229–239 [DOI] [PubMed] [Google Scholar]
- 24.Lin R., and Iacovitti L. (2015). Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 1628, 327–342 [DOI] [PubMed] [Google Scholar]
- 25.Acosta S.A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D.M., Sanberg P.R., Bickford P.C., Kaneko Y., and Borlongan C.V. (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One 8, e53376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaiss C.A., Yu T.S., Zhang G., Chen J., Dimchev G., Parada L.F., Powell C.M., and Kernie S.G. (2011). Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J. Neurosci. 31, 4906–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X., Enikolopov G., and Chen J. (2009). Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp. Neurol. 219, 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felix M.S., Popa N., Djelloul M., Boucraut J., Gauthier P., Bauer S., and Matarazzo V.A. (2012). Alteration of forebrain neurogenesis after cervical spinal cord injury in the adult rat, Front. Neurosci. 6, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franz S., Ciatipis M., Pfeifer K., Kierdorf B., Sandner B., Bogdahn U., Blesch A., Winner B., and Weidner N. (2014). Thoracic rat spinal cord contusion injury induces remote spinal gliogenesis but not neurogenesis or gliogenesis in the brain. PLoS One 9, e102896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Himes B.T., Goldberger M.E., and Tessler A. (1994). Grafts of fetal central nervous system tissue rescue axotomized Clarke's nucleus neurons in adult and neonatal operates. J. Comp. Neurol. 339, 117–131 [DOI] [PubMed] [Google Scholar]
- 31.O'Shea T.M., Burda J.E., and Sofroniew M.V. Cell biology of spinal cord injury and repair. (2017). J. Clin. Invest. 127, 3259–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicaise C., Hala T.J., Frank D.M., Parker J.L., Authelet M., Leroy K., Brion J.P., Wright M.C., and Lepore A.C. (2012). Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury, Exp. Neurol. 235, 539–552 [DOI] [PubMed] [Google Scholar]
- 33.Nicaise C., Frank D.M., Hala T.J., Authelet M., Pochet R., Adriaens D., Brion J.P., Wright M.C., and Lepore A.C. (2013). Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion. J. Neurotrauma 30, 1092–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macolino C.M., Daiutolo B.V., Albertson B.K., and Elliott M.B. (2014). Mechanical alloydnia induced by traumatic brain injury is independent of restraint stress. J. Neurosci. Methods 226, 139–146 [DOI] [PubMed] [Google Scholar]
- 35.Elliott M.B., Jallo J.J., and Tuma R.F. (2007). An investigation of cerebral edema and injury volume assessments for controlled cortical impact injury, J. Neurosci. Methods 168, 320–324 [DOI] [PubMed] [Google Scholar]
- 36.Mouton P.R. (2002). Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. The Johns Hopkins University Press: Baltimore [Google Scholar]
- 37.Daiutolo B.V., Tyburski A., Clark S.W., and Elliott M.B. (2016). Trigeminal pain molecules, allodynia, and photosensitivity are pharmacologically and genetically modulated in a model of traumatic brain injury. J. Neurotrauma 33, 748–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott M.B., Tuma R.F., Amenta P.S., Barbe M.F., and Jallo J.I. (2011). Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury, J. Neurotrauma 28, 973–981 [DOI] [PubMed] [Google Scholar]
- 39.Bennett L., Yang M., Enikolopov G., Iacovitti L. (2009). Circumventricular organs: a novel site of neural stem cells in the adult brain, Mol. Cell. Neurosci. 41, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutton S.R., Pevny L.H. (2008). Isolation, culture, and differentiation of progenitor cells from the central nervous system, CSH Protoc. 2008, pdb prot 5077. [DOI] [PubMed] [Google Scholar]
- 41.Ferron S.R., Andreu-Agullo C., Mira H., Sanchez P., Marques-Torrejon M.A., and Farinas I. (2007). A combined ex/in vivo assay to detect effects of exogenously added factors in neural stem cells. Nat. Protoc. 2, 849–859 [DOI] [PubMed] [Google Scholar]
- 42.Li K., Javed E., Hala T.J., Sannie D., Regan K.A., Maragakis N.J., Wright M.C., Poulsen D.J., Lepore A.C. (2015). Transplantation of glial progenitors that overexpress glutamate transporter GLT1 preserves diaphragm function following cervical SCI. Mol. Ther. 23, 533–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li K., Javed E., Scura D., Hala T.J., Seetharam S., Falnikar A., Richard J.P., Chorath A., Maragakis N.J., Wright M.C., and Lepore A.C. (2015). Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp. Neurol. 271, 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li K., Nicaise C., Sannie D., Hala T.J., Javed E., Parker J.L., Putatunda R., Regan K.A., Suain V., Brion J.P., Rhoderick F., Wright M.C., Poulsen D.J., and Lepore A.C. (2014). Overexpression of the astrocyte glutamate transporter GLT1 exacerbates phrenic motor neuron degeneration, diaphragm compromise, and forelimb motor dysfunction following cervical contusion spinal cord injury. J. Neurosci. 34, 7622–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., Fehlings M.G. (2017). Traumatic spinal cord injury. Nat. Rev. Dis. Primers 3, 17018. [DOI] [PubMed] [Google Scholar]
- 46.Warren P.M., and Alilain W.J. (2014). The challenges of respiratory motor system recovery following cervical spinal cord injury. Prog. Brain Res. 212, 173–220 [DOI] [PubMed] [Google Scholar]
- 47.Nicaise C., Frank D.M., Hala T.J., Authelet M., Pochet R., Adriaens D., Brion J.P., Wright M.C., and Lepore A.C. (2013). Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion, J. Neurotrauma 30, 1092–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicaise C., Hala T.J., Frank D.M., Parker J.L., Authelet M., Leroy K., Brion J.P., Wright M.C., and Lepore A.C. (2012). Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp. Neurol. 235, 539–552 [DOI] [PubMed] [Google Scholar]
- 49.Gu N., Peng J,. Murugan M., Wang X., Eyo U.B., Sun D., Ren Y., DiCicco-Bloom E., Young W., Dong H., and Wu L.J. (2016). Spinal microgliosis due to resident microglial proliferation is required for pain hypersensitivity after peripheral nerve injury. Cell Rep. 16, 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dennis A.M., Haselkorn M.L., Vagni V.A., Garman R.H., Janesko-Feldman K., Bayir H., Clark R.S., Jenkins L.W., Dixon C.E., and Kochanek P.M. (2009). Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J. Neurotrauma 26, 889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saatman K.E., Feeko K.J., Pape R.L., and Raghupathi R. (2006). Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma 23, 1241–1253 [DOI] [PubMed] [Google Scholar]
- 53.Acosta S.A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D., Sanberg P.R., Bickford P.C., Kaneko Y., and Borlongan C.V. (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One 8, e53376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carreira B.P., Morte M.I., Santos A.I., Lourenco A.S., Ambrosio A.F., Carvalho C.M., and Araujo I.M. (2014). Nitric oxide from inflammatory origin impairs neural stem cell proliferation by inhibiting epidermal growth factor receptor signaling. Front. Cell. Neurosci. 8, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amenta P.S., Jallo J.I,. Tuma R.F., Hooper D.C., and Elliott M.B. (2014). Cannabinoid receptor type-2 stimulation, blockade, and deletion alters the vascular inflammatory responses to traumatic brain injury. J. Neuroinflammation 11, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hazra A., Macolino C., Elliott M.B., and Chin J. (2014). Delayed thalamic astrocytosis and disrupted sleep-wake patterns in a preclinical model of traumatic brain injury. J. Neurosci. Res. 92, 1434–1492 [DOI] [PubMed] [Google Scholar]
- 57.Heo H.R., Chen L., An B., Kim K.S., Ji J., and Hong S.H. (2015). Hormonal regulation of hematopoietic stem cells and their niche: a focus on estrogen. Int. J. Stem Cells 8, 18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ray R., Novotny N.M., Crisostomo P.R., Lahm T., Abarbanell A., and Meldrum D.R. (2008). Sex steroids and stem cell function. Mol. Med. 14, 493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cole J.T., Yarnell A., Kean W.S., Gold E., Lewis B., Ren M., McMullen D.C., Jacobowitz D.M., Pollard H.B., O'Neill J.T., Grunberg N.E., Dalgard C.L., Frank J.A., and Watson W.D. (2011). Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma 28, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elliott M.B., Oshinsky M.L., Amenta P.S., Awe O.O., and Jallo J.I. (2012). Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache 52, 966–984 [DOI] [PMC free article] [PubMed] [Google Scholar]