Abstract
A large proportion of patients suffering from spinal cord injury (SCI) develop chronic central neuropathic pain. Previously, we and others have shown that sensorimotor training early after SCI can prevent the development of mechanical allodynia. To determine whether training initiated in the subchronic/chronic phase remains effective, correlates of below-level neuropathic pain were analyzed in the hindpaws 5–10 weeks after a moderate T11 contusion SCI (50 kDyn) in adult female C57BL/6 mice. In a comparison of SCI and sham mice 5 weeks post-injury, about 80% of injured animals developed mechanical hypersensitivity to light mechanical stimuli, whereas testing of noxious stimuli revealed hypo-responsiveness. Thermal sensitivity testing showed a decreased response latency after injury. Without intervention, mechanical and thermal hyper-responsiveness were evident until the end of the experiment (10 weeks). In contrast, treadmill training (2 × 15 min/day; 5 × /week) initiated 6 weeks post-injury resulted in partial amelioration of pain behavior and this effect remained stable. Analysis of calcitonin gene-related peptide (CGRP)–labeled fibers in lamina III-IV of the lumbar dorsal horn revealed an increase in labeling density after SCI. This was not due to changes in the number or size distribution of CGRP-labeled lumbar dorsal root ganglion neurons. Treadmill training reduced the CGRP-labeling density in the spinal cord of injured mice, whereas the density of non-peptidergic isolectin-B4 (IB4)+ fibers showed no changes in lamina IIi and a slight reduction of sparse IB4 labeling in laminae III-IV. Thus, sensorimotor activity initiated in the subchronic/chronic phase of SCI remains effective in ameliorating pain behavior and influencing structural changes of the nociceptive system.
Keywords: : CGRP, contusion, sensory, spinal cord injury, sprouting, training
Introduction
Spinal cord injury (SCI) results not only in motor, sensory, and autonomic dysfunction, but a large proportion of SCI patients also develop spontaneous or evoked chronic central neuropathic pain (CNP), which is often severe and disabling.1–3 Although studies in rodents have uncovered potential mechanisms contributing to neuropathic pain after SCI, pharmacologic treatment options remain inadequate and have limited effects on neuropathic pain.4,5 Therefore, alternative options preventing the development of CNP and treating it are urgently needed.
From animal models of neuropathic pain, it is known that structural and electrophysiological changes in peripheral, spinal, subcortical, and cortical structures are associated with the development of CNP after SCI. Loss of descending modulatory projections and disruption of ascending sensory tracts clearly contribute to altered sensory perception after SCI. Studies in the context of pain following SCI focusing on spinal mechanisms have suggested that reduced inhibitory control6 due to loss of GABAergic interneurons,7 reduced glutamic acid decarboxylase-65 expression,8 downregulation of KCC2,9 and upregulation of NKCC1,10 as well as an excessive ionotropic glutamate receptor activation in the spinal cord,11 contribute to CNP. In sensory neurons, upregulation of voltage-gated calcium channels,12 increased spontaneous activity of sensory neurons13 by upregulation of TRPV1,14 and NaV1.815 also appear to play a role in CNP. Neuroinflammation, including the activation of microglia and astrocytes,16–18 contribute to central and peripheral sensitization and the development of CNP after SCI. As a result, sprouting and robust arborization of primary nociceptive afferents into deeper laminae of the dorsal horn, even several segments away from the injury, might occur.19–24 As the dorsal horn acts as an integration center between afferent information and sensory perception, the distribution, termination, and connection pattern of sensory fibers within the dorsal horn are critical.
A better understanding of the dynamic changes of nociceptive afferents after SCI may lead to new therapies that target aberrant structural rearrangements and misconnectivity in the dorsal horn. Several studies have shown that sensorimotor activity is able to modulate neural plasticity after SCI.19,25–29 Previously, we, along with others, have shown that sensorimotor training early after SCI in rodents can ameliorate mechanical allodynia.19,24,27 However, most SCI patients have chronic injuries, training is not feasible for all patients in the acute phase, and pathophysiology and structural changes differ substantially between the acute and chronic phase. It is therefore critical to characterize pain-associated changes in chronic SCI to investigate whether treatments with benefits early post-injury such as sensorimotor training can also reverse pain in chronic SCI.
Thus, we examined effects of delayed sensorimotor training on the reversal of pain-like behavior and structural changes after chronic contusion SCI in mice. Our data indicate that treadmill training remains effective even after chronic pain is established and this decrease in pain behavior is accompanied by a reduction in nociceptive fiber density in the spinal dorsal horn.
Methods
Animal subjects and experimental groups
All experiments were conducted in accordance with the European Communities Council Directive (European Union and institutional guidelines) and approved by the local governing body (Regierungsprasidium Karlsruhe). A total of 53 adult female C57BL/6 mice (8–12 weeks old; Janvier) weighing between 20–25 g were used and animals were housed in groups of 4–5 mice per cage on a 12/12-h light/dark cycle and had access to water and food ad libitum. Surgeries and behavioral tests were done in three cohorts of animals to ensure that training and behavioral testing can be done by the same investigator. In each cohort, animals were assigned to one of four experimental groups (numbers in parentheses indicate the group size in each cohort): spinal cord injury (SCI; n = 6; n = 5; n = 4), spinal cord injury with training (SCI+T; n = 7; n = 4; n = 4), sham-operated (Sham; n = 4; n = 4; n = 3) and sham operated with training (Sham+T; n = 4; n = 5; n = 3). Individual animals were assigned to training or no training groups based on paw withdrawal responses to the 0.4 g von Frey hair filament at 35 dpi to match trained and untrained animals. Data from the three cohorts were combined for statistical analysis. Spinal cord injured mice were subdivided post hoc into animals with pain (SCI-Pain) or without pain (SCI-No Pain) following completion of all behavioral tests to maintain blinding in behavioral tests (see below).
Spinal cord injury
Mice were deeply anesthetized using a cocktail (2.5 mL/kg) containing ketamine (31.25 mg/kg; Bremer Pharma, Warburg, Germany), xylazine (1.58 mg/kg; Ecuphar, Oostkamp, Belgium), and acepromazine (0.31 mg/kg; Sanofi-Ceva, Düsseldorf, Germany). After exposing the vertebral column and localization of the target region, mice underwent a T9 laminectomy (corresponding to T11 spinal cord) followed by a moderate contusion with a force of 50 kDyn using the Infinite Horizon Impact Device (IH-0400 Impactor; Precision Systems & Instrumentation, Lexington, KY) equipped with a standard mouse steel-tipped impactor with a diameter of 1.3 mm as previously described.24,30 Briefly, the spinal processes of the adjacent vertebral bodies rostrally (T8) and caudally (T10) from the site of laminectomy were used to stabilize the spinal column using extra fine forceps (Extra Fine Graefe Forceps; Fine Science Tools, Foster City, CA) attached to the impactor device. The exposed spinal cord was aligned perpendicular to the impounder tip. The position of the impactor tip was adjusted if necessary and positioned 3–4 mm above the exposed spinal cord. After contusion, the paravertebral muscle layers were sutured and the skin incision was stapled. In sham mice, skin incisions and separation of paravertebral muscles to isolate the T9 vertebra was performed followed by suturing and stapling of the skin. To prevent and compensate dehydration during surgery, animals received a subcutaneous (s.c.) injection of lactated Ringer's solution (100 μL) immediately after surgery. Post-surgical care included close observation, manual bladder voiding twice per day, and treatment with antibiotics to prevent bladder infection (100 μL ampicillin s.c., 33 mg/kg, twice per day; Ratiopharm, Ulm, Germany) for about 10–14 days after surgery until reflexive bladder function was restored. Analgesics (0.01 mg/kg buprenorphine-HCL, 100 μL s.c., Temgesic; Reckitt Benckiser, Heidelberg, Germany) were administered to injured and sham-operated animals immediately after recovery from anesthesia and twice daily during the first 2 post-operative days. All surgeries were performed by the same experienced investigator.
Behavioral testing
Mice were acclimated to the individual testing set-up for 2–3 days for at least 1.5 h per day prior to behavioral testing and for 30–60 min in each set-up on testing days. All behavioral testing was performed in awake, unrestrained mice by the same investigator blinded to group identity.
Mechanical sensitivity testing: von Frey
To evaluate changes in mechanical sensitivity after injury and sensorimotor training, von Frey hair filaments with ascending diameter and force (0.16 g, 0.4 g, 0.6 g, 1.4 g; Touch Test Sensory Evaluators; North Coast Medical, Gilroy, CA) were applied to the hindpaws of mice. The filaments were chosen based on results from a previous study evaluating effects of early-onset treadmill training in mice with the same SCI.24 Mice were placed in plexiglass chambers (modular animal-enclosure; Ugo Basile Srl, Gemonio, Italy) on a 90 × 39 cm perforated metal platform (framed testing surface; Ugo Basile). Starting with the smallest filament (0.16 g) each hindpaw was stimulated five times resulting in 10 stimuli per filament per mouse. Up to 12 animals were tested in parallel and only after testing one hindpaw with a single stimulus in all mice and testing the other paw once in all mice, the paw was re-tested with the same filament. Thereby a stimulus-induced sensitization of the paw was avoided. The response rate defined as paw withdrawal frequency (number of positive responses divided by the number of stimuli) was used to express mechanical sensitivity. The sensitivity towards each filament per animal represents the average response rate of the trials for the left and right paw. To define the baseline sensitivity for all filaments in each animal, mechanical sensitivity testing was performed on 2 consecutive days before surgery. The mean of the two pre-injury values per animal and filament was taken as pre-operative baseline response rate. After surgery, mechanical sensitivity was assessed 35 days post-injury, as well as 2 and 4 weeks (56 dpi and 70 dpi) after training was started (Fig. 1).
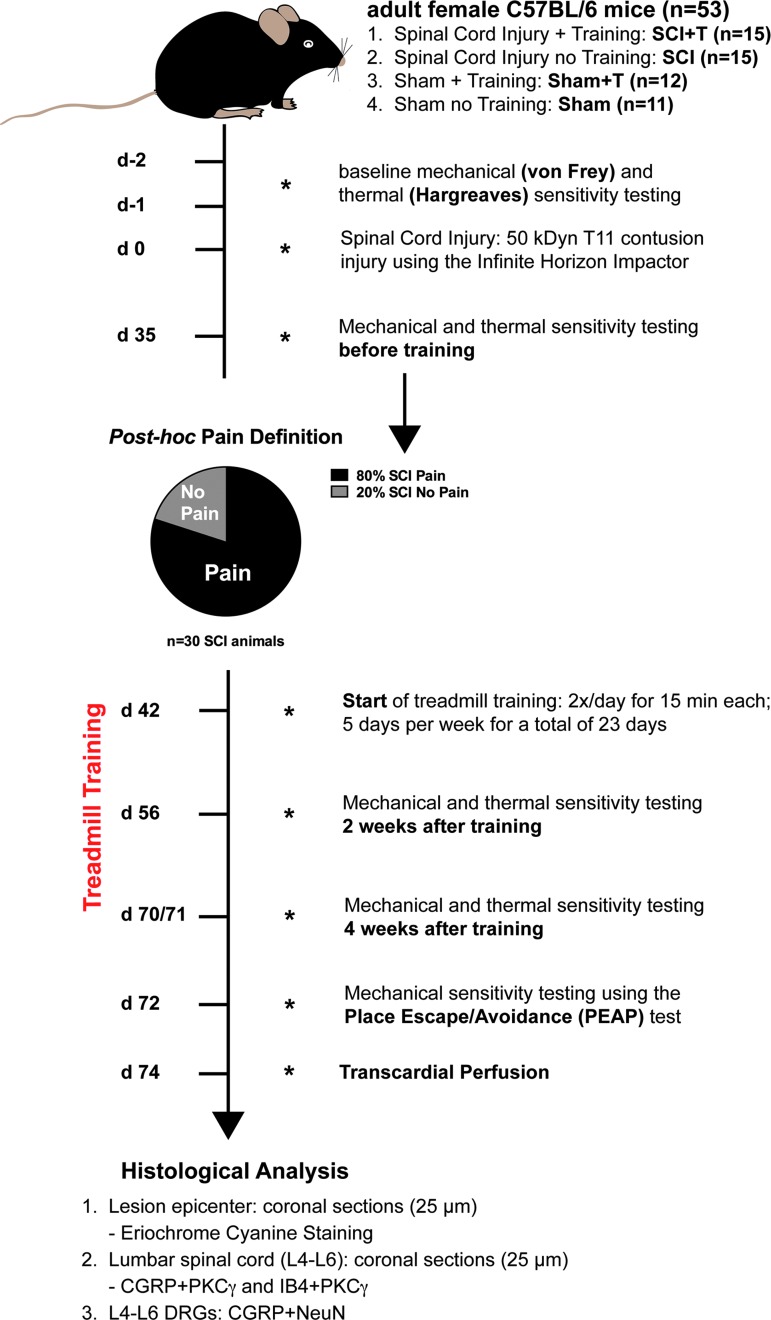
FIG. 1.
Experimental design. Adult female C57BL/6 mice (n = 53) were assigned to four groups (spinal cord injury [SCI], n = 15; SCI with training [SCI+T], n = 15; sham-operated [Sham], n = 11; Sham with training [Sham+T], n = 12) after baseline testing and 35 dpi before treadmill training was initiated. Mechanical sensitivity data 35 dpi were used to define “Pain” and “No Pain” animals. Starting from 42 dpi, mice in the training groups (SCI+T; Sham+T) were trained 2 × /day for 15 min each, 5 days per week for a total of 23 days. To investigate the effect of moderate treadmill training on nociceptive behavior, mechanical and thermal sensitivity testing was performed 2 and 4 weeks after training start. Supraspinal pain processing was tested using a place escape/avoidance paradigm (PEAP). Coronal sections (25 μm) of the lesion epicenter and lumbar spinal cord were analyzed by eriochrome cyanine staining, calcitonin gene-related peptide (CGRP)-/protein kinase Cγ (PKC)- and isolectin-B4 (IB4)-/PKCγ-labeling. Sections of L4-L6 dorsal root ganglia (DRGs; bilaterally; n = 3 per group of the last cohort; see Methods) were labeled for CGRP and NeuN.
Mechanical sensitivity testing: place escape/avoidance paradigm
Mechanical sensitivity testing using von Frey hair filaments is a stimulus-evoked behavioral measurement. Since changes in these measures might only be the result of evoked hyperreflexia, responses in a place escape/avoidance paradigm (PEAP) addressing the aversive quality of pain and thereby supraspinal processing of nociception were evaluated. The PEAP is based on the assumption that animals escape and/or avoid an aversive mechanical stimulus. Animals have the active choice between a naturally preferred dark environment and pain relief in a more aversive light environment.31 The testing environment, modified from LaBuda and Fuchs,32,33 consists of the same plexiglass enclosure used in the von Frey testing modified to create a box consisting of a dark and a light side. One half of the box was darkened using mat black self-adhesive tape, whereas the other half was coated with mat white self-adhesive tape creating a dark and a light side equal in size (10 cm × 10 cm × 14 cm).
To further enhance the contrast between both sides, a removable partition was positioned in the middle of the chamber separating the dark from the white side creating a passage to either side. The height of the border was adjusted to allow for normal crossing and the partition was also laminated with black and white adhesive foil. Additionally, the lid of the dark side was covered with black foil while the other lid was left transparent. The enclosure was placed on a perforated metal platform and it was ensured that the light side was uniformly illuminated by the ceiling light. Animals were placed in the middle of the chamber and the time spent on the dark side of the chamber was measured. An initial 10 min exploration phase (baseline) without any stimulus was followed by a 15 min testing phase (3 × 5 min blocks) in which the hindpaws were stimulated using a 0.16 g von Frey filament every 15 sec when the mouse was present on the dark side of the chamber. The left and right hindpaw were stimulated in an alternating manner. The PEAP was used to assess mechanical sensitivity changes after injury at the end of the experiment, 72 dpi.
Thermal sensitivity testing: Hargreaves' method
Thermal sensitivity was assessed according to Hargreaves' method using the plantar test (Hargreaves Apparatus; Ugo Basile). Mice were enclosed in plexiglass chambers (modular animal-enclosure; Ugo Basile) placed on a 90 × 39 cm metal platform with a glass floor (Ugo Basile). An infrared laser beam was presented to the plantar surface of the hindpaws and the time (sec) until withdrawal was recorded resulting in the response latency to the heat stimulus. The laser intensity was adjusted to give a latency of approximately 8–9 sec in naïve animals (190 ± 1 mW/cm2). To avoid tissue damage, a 15-sec cut-off time, as well as a delay of at least 1 min between two trials for the same paw and mice, was introduced. The paw testing order was chosen randomly to minimize an order effect. In each animal, the mean of four trials for the left and right hindpaw was used to express the thermal sensitivity. Similar to the von Frey testing, the mean latency of 2 consecutive testing days before surgery determined the baseline of the animals (pre-operatively). After surgery, thermal sensitivity was assessed 35 days post-injury, as well as 2 and 4 weeks (56 and 70 days post-injury) after training was started. The response latencies for the left and right hindpaw were averaged.
Motor recovery: Basso Mouse Scale
Recovery of motor function was assessed using the Basso Mouse Scale (BMS) for locomotion.34 Single mice were placed randomly in an open field and observed by two experienced investigators blinded to group identity for at least 5 min. The BMS is a 9-point scoring system developed and validated for spinal cord contusion injuries in mice. A score of 0 reflects complete hindlimb paralysis without ankle movement, whereas a score of 9 indicates normal locomotion. Lower scores (0–3) express changes in ankle movement and plantar placement of the hindpaw, continuing with stepping, coordination, and paw position in intermediate scores (4–6), and assessment of trunk stability and tail position in higher scores (7–9). Dorsal and/or plantar stepping starts at a score of 3, becomes frequent or consistent plantar with a score of 5 with some coordination, whereas scores of 6 and 7 indicate mostly coordinated stepping with parallel or rotated paw placement at initial contact and lift off. The motor function was scored 1 day post-injury to confirm an adequate injury and weekly starting 35 days post-injury. Each hindpaw was scored individually and averaged to get a single score for each animal per testing day.
Treadmill training
To test the impact of delayed sensorimotor activity on neuropathic pain, treadmill training was used as previously described.24 The custom-made motorized treadmill consists of three individual lanes (7 × 10 cm) separated by walls of red acryl glass, allowing for parallel training of up to three animals. A motor-driven rotor (RollerDrive EC310; Interroll Automation, Sinsheim, Germany) controlled by a proprietary software developed under LabVIEW 2012 (version 12.0; National Instruments Corporation, Austin, TX) enabled spinning of the belts with adjustable velocity. A load-independent constant velocity was achieved by implementation of a proportional-integral-derivative controller. Training was initiated in the subchronic/chronic phase after injury (42 days post-injury). The paradigm consisted of training twice a day for 15 min each, 5 days per week, over a total of 23 training days (Fig. 1). By increasing the speed, training intensity was adjusted on a daily basis according to the motor performance of injured mice. To expose trained sham animals (Sham+T) to the same training load as trained SCI animals (SCI+T), similar treadmill velocities were used and adjustments were also done on a daily basis. Animals of the untrained groups (SCI, Sham) were exposed to the same environment by placing them into the treadmill without turning on the treadmill. The criteria for moderate treadmill training were defined as intensity or velocity in which animals run most of the time in the center of the lane without any signs of increased stress (e.g., increased defecation or respiration rate). Animals were closely observed during the exercise period to adapt the velocity when the criteria for moderate training were not met.
Tissue processing
Animals were euthanized 74 days post-injury with an overdose of anesthesia cocktail and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PFA). The spinal column was dissected, post-fixed in 4% PFA for 1 h at room temperature, and briefly rinsed with ddH2O followed by a 5 min washing in 0.1 M phosphate buffer before the tissue was cryoprotected in 30% sucrose in 0.1 M phosphate buffer at 4°C. The lesion site (T11), the L4-L6 spinal cord, as well as dorsal root ganglia (DRGs) were identified using anatomical landmarks, embedded in Tissue-Tek® O.C.T compound (Sakura Finetek, Germany) followed by serial sectioning (spinal cord, 25 μm coronal; DRG, 10 μm) and mounting in seven or 14 series onto slides (Microscope Slides Superfrost® Plus; Thermo Fisher). Animals from all groups were processed, sectioned and mounted at the same time. Slides were stored at −80°C until used for immunolabeling or staining.
Eriochrome-cyanine staining
The lesion size of injured animals was analyzed by quantifying the spared tissue at the lesion epicenter. Two consecutive series were stained for myelin using eriochrome-cyanine. Slides were dried for 2 h at 37°C and placed in fresh acetone for 5 min, removed, and air dried for 20 min at room temperature. Next, the sections were stained in eriochrome cyanine solution (0.4 g eriochrome cyanine R, 1 mL concentrated H2SO4, 192 mL ddH2O, and 8 mL of 10% ferric ammonium sulfate solution) for 30 min at room temperature followed by a rinsing step for 5 min in ddH2O. Sections were differentiated in 5% aqueous ferric ammonium solution for 10–20 min until the gray matter started to appear. After the slides were rinsed in ddH2O for 10 min, the differentiation was completed in a borax-ferricyanide solution for 10 min (2 g disodium tetraborate decahydrate, 2.5 g potassium ferricyanide, and 200 mL ddH2O). Differentiation was followed by a further rinsing step in ddH2O for 5 min, the tissue was dehydrated by graded ethanol solutions (two changes each: 70%, 95% and 100%), cleared through three changes of Neo-clear (Merck, Darmstadt, Germany) for 2 min each and cover-slipped (Neo-Mount; Merck). Sections of the lesion epicenter (smallest area of spared white matter) were photographed using a XC30 camera mounted on an Olympus BX53 microscope (Olympus, Hamburg, Germany). Tissue sparing was quantified by outlining areas with light and dark blue staining and expressed as the percent of cross-sectional area calculated by dividing the white matter area by the total cross-sectional area. Imaging and analysis was performed by an investigator blinded to group identity using ImageJ.
Immunohistochemistry
Immunohistochemical labeling was performed on slides with serial sections of the lumbar spinal cord (L4-L6) and L4-L6 DRGs. Slides were dried for 30 min at room temperature, encircled with a liquid blocker and rinsed in 0.1 M Tris-buffered saline (TBS) 3 × 5 min each. Sections were blocked and permeabilized by incubating in TBS/0.25% Triton TX-100/5% donkey serum for 1 h at room temperature. All primary antibodies were diluted in TBS/0.25% Triton TX-100/1% donkey serum and the samples were incubated overnight in a humid box at 4°C. After primary antibody incubation, sections were rinsed 3 × in TBS/1% donkey serum at room temperature for 10 min each. Secondary antibodies and 4',6-diamidino-2-phenylindole (DAPI) were diluted in TBS/1% donkey serum and the sections were incubated for 2.5 h at room temperature in the dark, rinsed 3 × in TBS for 10 min, briefly dried and cover-slipped with Fluoromount G (Southern Biotechnology Associates, Birmingham, AL).
To evaluate SCI- and sensorimotor activity-induced changes in primary nociceptive afferents terminating in the dorsal horn, lumbar spinal cord sections were labeled for calcitonin gene-related peptide (CGRP; rabbit anti-CGRP, 1:200; Immunostar, Hudson, WI) and isolectin-B4 (IB4; biotinylated Isolectin B4 from Bandeiraea simplicifolia, 1:250; Sigma-Aldrich, St. Louis, MO) identifying peptidergic and non-peptidergic C/Aδ fibers, respectively. To identify lamina IIi, rabbit anti-protein kinase Cγ (PKCγ [C19], 1:200; Santa Cruz Biotechnology, Dallas, TX) was used. Two consecutive series were labeled for CGRP and PKCγ, respectively, or double immunofluorescence labeling using IB4 and PKCγ was performed. For the analysis of CGRP+ DRG neurons, L4-L6 DRGs were labeled for CGRP and NeuN (guinea pig anti-NeuN, 1:2000; Millipore ABN90, Billerica, MA). The following secondary antibodies were used: donkey anti-rabbit conjugated to Alexa Fluor 488 and Alexa Fluor 594 for CGRP and PKCγ labeling, respectively (1:300; Life Technologies, Carlsbad, CA), Alexa Fluor 594-conjugated streptavidin for IB4 (1:300; Jackson ImmunoResearch, Suffolk, UK), Rhodamine Red-X donkey anti-guinea pig for NeuN (1:500; Jackson ImmunoResearch).
For each animal, images of the left and right dorsal horn of three labeled sections with an intersection distance ranging from 700–1400 μm were taken at 200 × magnification with the same resolution, lens aperture, and exposure time using a XC30 camera mounted on an Olympus BX53 microscope with epifluorescence illumination and appropriate filter cubes. Lumbar DRGs (L4-L6) were imaged bilaterally from each animal at 100 × magnification using the above-described set-up, and images from three sections per DRGs (140 μm apart) were analyzed.
Quantification of labeling density in the spinal cord
Neurons in lamina III-IV usually receive little input from nociceptive C-fibers but rather respond to light mechanical stimuli. Therefore, the termination pattern of peptidergic-and non-peptidergic fiber was analyzed in this region of the dorsal horn as previously described,24 with the region of interest set using the mouse spinal cord atlas and PKCγ-labeling of lamina IIi. The dorsoventral extent of lamina IIi defined by PKCγ-labeling was defined as distance “x.” The region of interest analysis box was placed in the center of the dorsal horn by taking the midpoint of the medio-lateral border of lamina IIi followed by outlining a squared box with the dimension 2 × in the dorso-ventral and 4 × in the mediolateral direction adjoining the ventral border of lamina IIi. For the analysis of CGRP-labeling density, the analysis box was outlined in the PKCγ-labeled section and overlaid on the consecutive CGRP-labeled section, whereas IB4-labeling was evaluated in sections double-labeled for PKCγ and IB4. Quantification of labeling density was performed by an investigator blinded to group identify using ImageJ. Images were processed by setting a labeling threshold minimizing background and accurately reflecting CGRP-and IB4 positive fiber labeling, respectively. Labeling density was expressed as percentage of positive labeling within the analysis box and the values for three sections per animal were averaged. Only SCI-Pain animals were included into the analysis.
Due to tissue damage following dissection, three animals (SCI+T, n = 3) had to be excluded from histological analysis. Upon examination of CGRP- and IB4-immunolabeled sections, two animals (CGRP: SCI, n = 1; SCI+T, n = 1; IB4: Sham+T, n = 1) showed only poor labeling in the superficial dorsal horn and no labeling in deeper laminae. These animals were excluded from immunohistochemical analysis leaving a total of n = 42 for quantification of CGRP and n = 43 for quantification of IB4-labeling density.
Quantification of DRG neurons
In addition to spinal CGRP-labeling, the number and size of CGRP-labeled L4-L6 DRG neurons were quantified by an investigator blinded to treatment conditions using ImageJ. For each animal, L4-L6 DRG (bilaterally) neurons were analyzed in three sections spaced 140 μm apart. First the total number of NeuN+ neurons displaying a visible DAPI-stained nucleus was counted, followed by the number of neurons labeled for CGRP and NeuN (CGRP+/NeuN+). For size distribution, the diameter of CGRP-/NeuN+ and CGRP+/NeuN+ neurons was measured by drawing a straight line between two lateral borders covering the entire cell (longest distance between the two borders). Size distribution of CGRP+ neurons was evaluated by computing the frequency distribution using a bin size of 10 μm and expressed as the percentage of CGRP+ neurons within a certain size range (number of CGRP+/NeuN+ cells in a certain size range divided by the total number of CGRP+/NeuN+ cells). Values collected for DRGs of the same animals were averaged and group means compared. DRGs were only collected and quantified from sham animals and SCI animals that developed pain of the third cohort of this study (n = 3/group).
Statistical analysis
Behavioral results were analyzed by repeated measures analysis of variance (ANOVA) to reveal overall group difference and significant changes over time, followed by Fisher's least significant difference (LSD) post hoc testing. One-way ANOVA with post hoc Fisher's LSD was used to analyze lesion size and labeling density. The size distribution of CGRP-labeled DRG neurons was analyzed by two-way ANOVA (group × diameter) followed by post hoc Fisher's LSD, and one-way ANOVA with post hoc Fisher's LSD was used to compare the percentage of CGRP-labeled DRG neurons. Due to unequal variance and large differences in group size, Kruskal Wallis and Dunn's test was used to compare injured animals without pain and with pain with sham animals. Data are presented as mean ± standard error of the mean unless otherwise noted and statistical analysis was done using Prism 6 software (GraphPad Software Inc., La Jolla, CA) and an alpha level of 0.05 for significance.
Results
Sensorimotor recovery
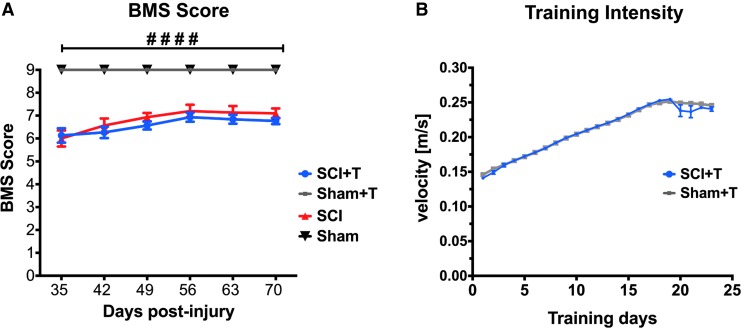
Motor recovery was examined weekly starting 5 weeks post-injury using the BMS. SCI mice showed a significant deficit in hindlimb motor function at all time-points compared with sham animals independent of training (two-way ANOVA p < 0.0001 for group differences, protected least significant difference (PLSD) p < 0.0001). Injured animals showed a slight recovery of motor function over time (two-way ANOVA p < 0.0001 for time, PLSD p < 0.001 comparing 35 dpi with 70 dpi). However, treadmill training did not influence motor recovery (Fig. 2A), which also was reflected in similar tissue sparing at the lesion site in trained and untrained injured animals (data presented below). As previously described,24 moderate 50 kDyn spinal cord contusion injury results in complete hindlimb paralysis immediately after injury and an average BMS score of ∼6 at 35 days post-injury (weight support in both hindlimbs, at least frequent plantar stepping, some coordination, and parallel or rotated paw placement), allowing for sensory testing and sensorimotor training. Based on the motor performance, the intensity of treadmill training was adjusted on a daily basis with continuing training to meet the criteria for a moderate training paradigm (Fig. 2B). Starting with velocities of about 0.14 m/sec, training reached a maximum velocity of 0.25 m/sec. As an appropriate control, trained sham mice were exposed to the same training intensity as SCI mice, whereas untrained mice were placed into the same environment without turning the treadmill on.
FIG. 2.
Treadmill training does not influence motor recovery. (A) Animals were tested weekly starting from 35 dpi in an open field and their motor recovery was scored according to the Basso Mouse Scale (BMS). Spinal cord injury (SCI) mice in the trained and untrained group showed a significant motor deficit (analysis of variance, p < 0.0001), which is not influenced by treadmill training. (B) As animals became accustomed to the treadmill training, the training intensity was adjusted on a daily basis by increasing the treadmill velocity. Training was initiated 42 dpi, which is represented as Day 1 in the graph. Both Sham and SCI mice were trained using similar velocities. Error bars in (B) are mostly too small to be visible.
Post hoc definition of pain
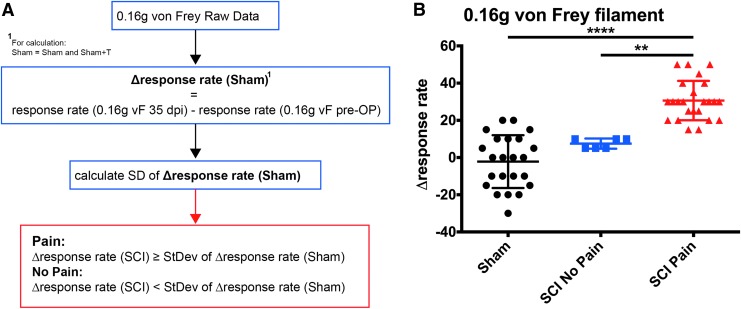
Previously, it has been reported that similar to SCI patients, only a subpopulation of injured animals develops chronic signs of mechanical allodynia.35 As animals underwent baseline testing prior to injury and 5 weeks post-injury prior to the start of training, we were able to evaluate whether all animals with SCI developed mechanical allodynia. Response rates to a 0.16 g von Frey filament were used to define SCI animals with and without pain as this light mechanical stimulus previously showed the largest difference between sham and injured animals.24 To exclude potential influences of age and repeated testing on exclusion criteria, response rates of all sham animals at baseline testing and 5 weeks later were taken into account by calculating for each sham animal the change in the response rate over time. Animals with contusion injuries that showed a difference in response rates that fell within one standard deviation of the changes in sham animals were not considered to have developed pain (SCI-No Pain). Only animals that showed a change exceeding one standard deviation of the changes observed in sham animals were regarded as animals with pain (SCI-Pain; Fig. 3A). Using this definition, 80% (24 out of 30) of injured animals developed significant mechanical allodynia when comparing response rates to a 0.16 g von Frey filament before and 35 days post-injury (Fig. 3B).
FIG. 3.
Post hoc pain definition. (A) Injured animals were subdivided based on whether they developed mechanical allodynia (spinal cord injury [SCI]-Pain) or not (SCI-No Pain). All animals were tested at baseline prior to injury and 5 weeks post-injury before initiation of training. Using the response rates to the 0.16 g von Frey filament at these time-points, the mean change in the response rate over time was calculated for sham animals (Δresponse rate Sham). Injured animals were only considered to show signs of mechanical allodynia (SCI-Pain) if the change in response rate from baseline to post-injury exceeded one standard deviation (SD) of the change observed in sham animals. (B) Changes in response rates to the 0.16 g von Frey filament between baseline and 35 dpi before training was initiated in SCI-Pain, SCI-No Pain, and sham animals shows no significant difference between sham animals and SCI-No Pain animals, but significant differences of SCI-Pain animals to the other groups (Kruskal Wallis test p < 0.0001; Dunn's post hoc **p < 0.01; ****p < 0.0001, mean ± standard deviation).
Statistical analysis of changes in response rates pre-injury and 35 dpi indicated an overall group difference (Kruskall Wallis p < 0.0001), no difference between SCI-No Pain and sham animals, but a significant difference between SCI-Pain animals and sham or SCI-No Pain animals (Dunn's test). While SCI-No Pain animals showed only a moderate increased response rate of 7.5%, the mean increase for SCI-Pain animals was about 30%. Based on these results, all training and pain responses including histochemical analyses described below are only reported for animals that developed pain according to this definition.
Treadmill training initiated in the subchronic phase after injury ameliorates mechanical allodynia
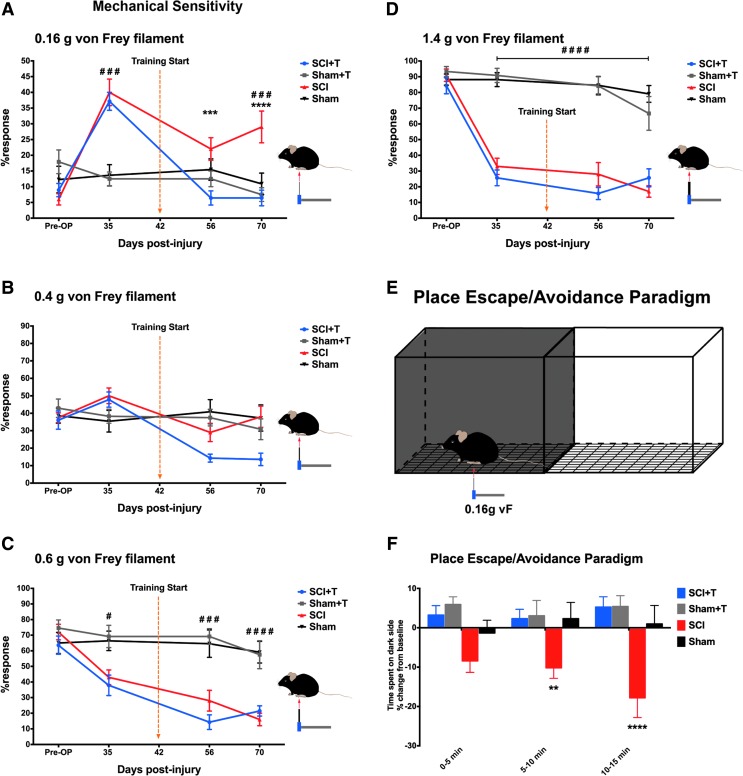
To examine whether SCI-induced nociceptive behavior is stable in the subchronic/chronic phase after injury and to evaluate if treadmill training initiated in this phase is beneficial, response rates before injury, 35 days post-injury (before training), and 2 as well as 4 weeks after training was started were compared between control (untrained and trained sham animals) and SCI animals that developed pain (Fig. 1). All animals had weight support when training was initiated. Determining the hindpaw withdrawal frequency to small-diameter filaments (0.16 g), statistical analysis (two-way ANOVA) indicated overall group differences (p < 0.01) and significant changes over time (Fig. 4A; p < 0.0001). Post hoc testing showed no difference between trained and untrained SCI animals before training was initiated (35 dpi), but a difference between SCI and sham mice (PLSD p < 0.0001).
FIG. 4.
Effect of treadmill training and spinal cord injury (SCI) on mechanical sensitivity assessed by von Frey filaments and a place escape/avoidance paradigm. (A) SCI-induced mechanical hypersensitivity to light filaments (0.16 g) is significantly ameliorated by sensorimotor activity (###p < 0.001 comparing SCI with sham animals; ***p < 0.001, ****p < 0.0001 comparing trained (SCI+T) with untrained SCI mice). (B) No overall group differences are observed with the 0.4 g filament, but changes over time. (C, D) Stronger filaments reveal a significant hypo-responsiveness comparing SCI with sham mice irrespective of training (#p < 0.05; ###p < 0.001; #### p < 0.0001, comparing SCI with sham animals irrespective of training). (E) Graphical representation of the place escape/avoidance (PEAP) set-up. Animals are freely moving in a box consisting of a dark and a light side placed on a wire mesh screen. After a 10 min exploration phase (no stimulus), mice are stimulated with the 0.16 g von Frey filament when present on the dark side of the chamber in a 3 × 5 min test phase. (F) Analysis of the time spent on the dark side reveals no significant difference between treadmill trained SCI (SCI+T) and sham mice (Sham; Sham+T). In contrast, untrained SCI mice spent significant less time on the dark side compared with sham and trained SCI mice to avoid the light mechanical stimulus (two-way analysis of variance, p < 0.001 for group differences; protected least significant difference **p < 0.01; ****p < 0.0001).
Already 2 weeks after training had begun, trained injured mice (SCI+T) showed a significant lower response rate than untrained SCI mice (PLSD p < 0.001). Due to the variability in the sham group, a significant difference between SCI and Sham+T (p < 0.05; not indicated in the figure) but no significant difference between SCI and sham animals was detected at this time. The response rate in the SCI+T group also was reduced after 4 weeks of training compared with untrained SCI animals (PLSD p < 0.0001) returning to pre-injury baseline values, whereas response rates of untrained SCI animals remained higher than baseline values at all time-points (PLSD p < 0.001). Overall, 90% of SCI animals without training showed an increase in response rate to the 0.16 g von Frey filament at 70 dpi versus baseline, whereas only 21% of SCI+T animals showed some increase versus baseline. Applying the same criteria that were used to discriminate Pain from No Pain mice prior to training, 12 of 14 trained SCI mice (86%) reverted to the No Pain category at 70 dpi. Taken together, these data show that SCI-induced mechanical hypersensitivity was significantly ameliorated by sensorimotor activity.
Stimulation of the hindpaws using a 0.4 g von Frey filament revealed no overall group differences (two-way ANOVA p = 0.16) but significant changes over time (p < 0.0001; interaction p < 0.0001; Fig. 4B). Both trained (SCI+T) and untrained injured mice (SCI) showed a slightly increased response rate 35 dpi (PLSD p < 0.05). While the response rate for SCI+T animals significantly decreased below the baseline state (PLSD p < 0.0001) over time, no changes were observed for the SCI group comparing pre-operative to 56 and 70 dpi. Larger-diameter filaments (0.6 g and 1.4 g) indicated a significant hyposensitivity of injured animals compared with sham animals towards these stimuli (Fig. 4C, 4D; two-way ANOVA, p < 0.0001 for groups, p < 0.0001 for time). This behavior was observed 35 dpi and the hyposensitivity for the 1.4 g filament did not improve until the end of the experiment 70 dpi (PLSD p < 0.0001 for 1.4 g at all post-OP time-points). A reduction in the response to the 1.4 g stimulation was observed in all but one animal in the SCI+T group (92.8%) and 100% of animals in the SCI group. The response rate for the 0.6 g filament further declined between 35 and 70 dpi (PLSD p < 0.001 for SCI and p < 0.01 SCI+T). Treadmill training did not affect the hyposensitivity.
PEAP testing behavioral correlates of supraspinal pain processing
To evaluate supraspinal processing of nociception, we used a modified PEAP at 72 dpi.33 Animals were allowed to freely move in a box consisting of a dark and a light side placed on a wire mesh screen (Fig. 4E). After a 2 × 5 min exploration phase (baseline), a 3 × 5 min test phase was initiated, in which the mice received a mechanical stimulus on the plantar surface of the hindpaw (0.16 g von Frey filament) when present in the dark side of the chamber. Thus, mice can make an active choice between the naturally preferred dark environment and pain relief in a rather aversive light surrounding.
Analysis of the time spent on the dark side revealed that SCI mice spent significant less time on the dark side of the chamber (Fig. 4F) during the second and third testing phase, compared with all other groups (two-way ANOVA p < 0.001 for group differences, PLSD p < 0.01 and p < 0.0001, respectively). Considering each interval, the change from baseline (no mechanical stimulus) slightly increased in each interval. In contrast, no significant difference in the time spent on the dark side was detected comparing trained SCI mice with trained or untrained sham mice. Thus, only the untrained injured mice made a “conscious” decision to escape and/or avoid the preferred dark side, where they received a low-intensity mechanical stimulus, in order to stay in a normally less favorable (aversive) bright environment. In line with the von Frey testing using the 0.16 g filament, treadmill training initiated in the subchronic phase after injury ameliorated the mechanical hypersensitivity.
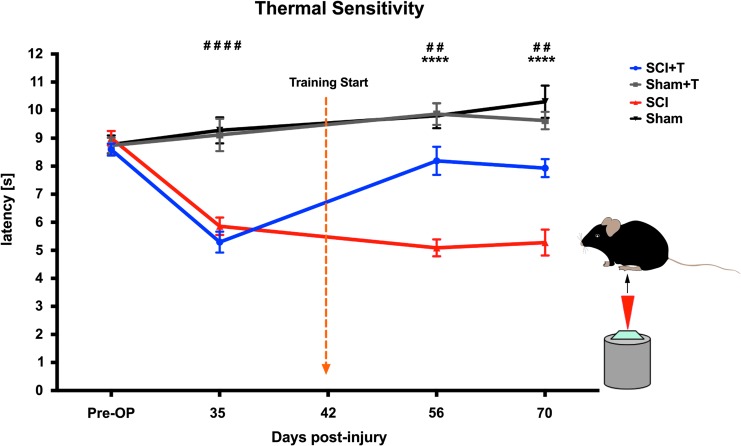
Treadmill training partially normalizes thermal hypersensitivity
Thermal sensitivity testing was performed using the Plantar Test (Hargreaves' method) applied to the hindpaws. Repeated measures ANOVA indicated significant group differences (p < 0.0001) and changes over time (p < 0.0001). Both groups of injured mice developed significant thermal hypersensitivity at 35 dpi (prior to training), compared with their baseline values and to sham mice (PLSD p < 0.0001), which remained stable until the end of the experiment 70 dpi in untrained SCI animals (p < 0.0001 at all time-points, compared with baseline). In all injured animals, the withdrawal latency decreased about 3–4 sec from baseline values at 35 dpi. Interestingly, treadmill training positively influenced thermal hyperalgesia (Fig. 5). The withdrawal latency increased by about 2 sec in trained SCI mice to values between untrained SCI and sham mice and these response rates were not significantly different from baseline values. The significant amelioration in response latency was evident 2 weeks after treadmill training was initiated (56 dpi) and the effect was stable until the end of the experiment (70 dpi; p < 0.0001 comparing SCI+T with SCI animals at both time-points). Sham animals showed no decline in latency but rather a slight increase over time.
FIG. 5.
Treadmill training ameliorates spinal cord injury (SCI)-induced thermal hypersensitivity (Hargreaves' test). SCI mice show a decreased response latency to the heat stimulus developing significant thermal hypersensitivity (analysis of variance, p < 0.0001; protected least significant difference [PLSD] ##p < 0.01; ####p < 0.0001, comparing SCI and SCI+T with sham and sham+T animals), which is partially reversed by treadmill training (PLSD) ****p < 0.0001, comparing trained with untrained SCI mice).
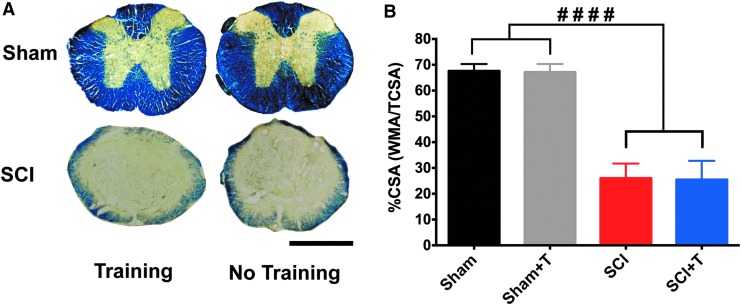
Treadmill training does not influence tissue sparing
To exclude the possibility that differences in lesion size might underlie changes observed in pain sensitivity, coronal sections of the lesion were stained for myelin (eriochrome-cyanine staining; Fig. 6A) and white matter sparing was quantified at the lesion epicenter (Fig. 6B). No difference in lesion size was observed between trained and untrained injured mice. Comparing sham animals with injured mice, the entire gray matter and a large percentage of white matter were lost at the lesion epicenter (p < 0.0001; Fig. 6B).
FIG. 6.
Treadmill training has no effect on tissue sparing. (A) Representative eriochrome cyanine-stained coronal sections of sham and spinal cord injury (SCI) mice. The lesion epicenter shows loss of gray and a large percentage of white matter in trained and untrained SCI mice (scale bar, 500 μm). (B) Quantification of the cross-sectional area (CSA) indicates a similar significant loss (analysis of variance, p < 0.0001, protected least significant difference ####p < 0.0001) in injured animals and no training effect.
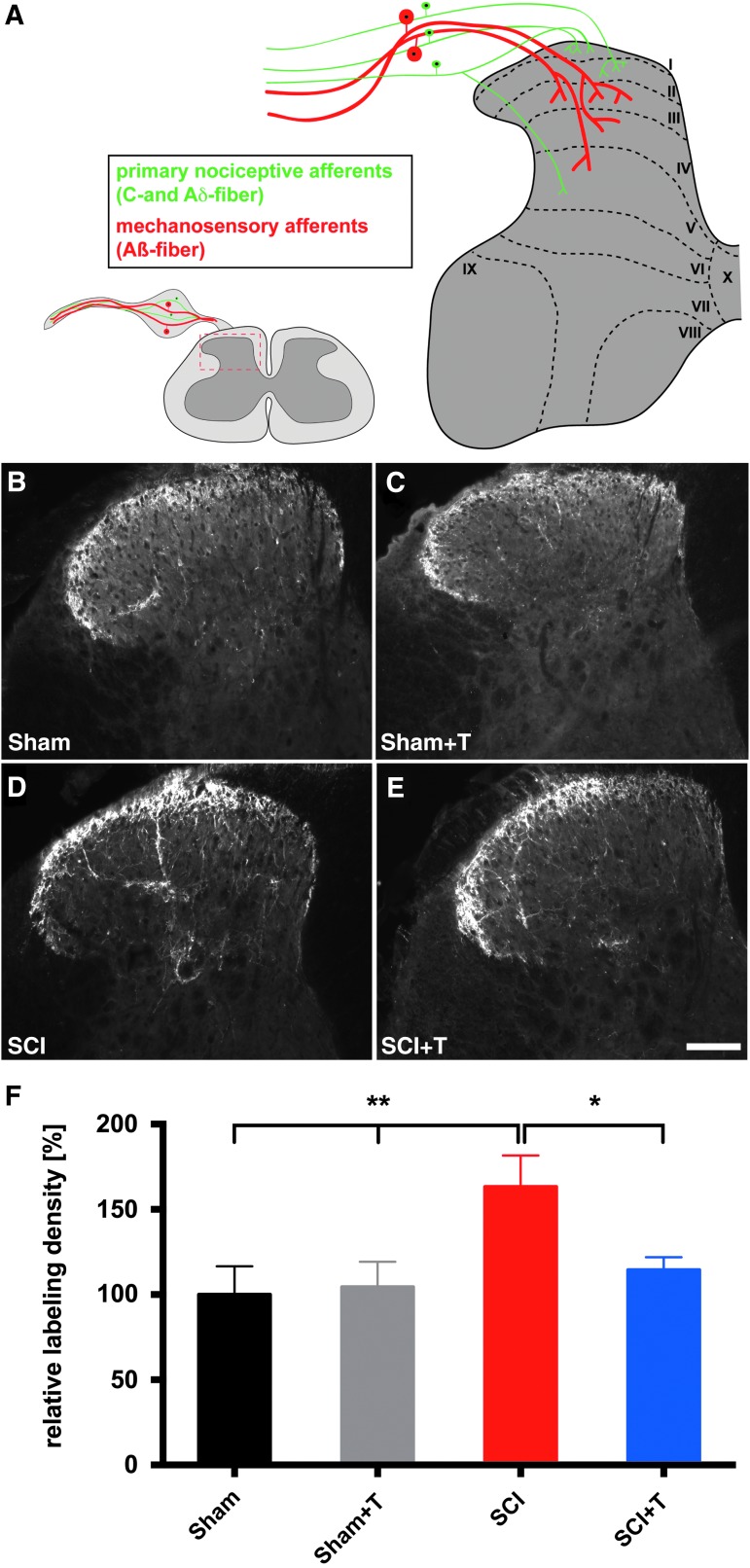
Sensorimotor activity reduces SCI-induced increases in CGRP-labeling density in laminae III-IV
Several studies have suggested that sprouting of C-fibers into deeper dorsal horn laminae parallel the development of pain behavior.21,22,28,36,37 We therefore quantified the CGRP-labeling density in lamina III-IV in the chronic phase after injury after delayed treadmill training using the same method previously used to examine acute effects of injury and training24 (Fig. 7). As stated above, only sham and injured animals that were analyzed for training-mediated effects were included in the histological analysis. Injured animals that did not develop mechanical hypersensitivity prior to initiation of training as defined in Figure 3 were not examined.
FIG. 7.
Increased calcitonin gene-related peptide (CGRP)-labeling density in lamina III-IV of the lumbar (L4-L6) dorsal horn after injury is reduced by sensorimotor activity. (A) Graphical representation of primary sensory afferents projecting into the spinal dorsal horn. Representative images of CGRP-labeled coronal sections of the lumbar (L4-L6) spinal dorsal horn of (B, D) untrained and (C, E) trained sham and spinal cord injury (SCI) mice, respectively (scale bar, 100 μm). Comparing (D) SCI with (B, C) uninjured sham mice reveals an increase in CGRP+ fibers in laminae III-IV. The SCI-induced increase in CGRP-labeling is partially reduced in (E) trained SCI animals. (F) Quantification of the CGRP-labeling density in laminae III-IV of the spinal dorsal horn relative to untrained sham animals indicates an increase in SCI mice compared with sham animals (analysis of variance, p < 0.05; protected least significant difference **p < 0.01). CGRP-labeling density is significantly reduced in trained SCI mice (SCI+T), compared with untrained SCI mice (*p < 0.05). No training effect was observed in sham animals. (SCI, n = 9; SCI+T, n = 10; Sham, n = 11; Sham+T, n = 12)
Similar to our previously reported results at 35 dpi,24 CGRP-labeling density was significantly increased in lamina III-IV of the lumbar dorsal horn (L4-L6) in injured animals. Comparing sham with untrained SCI mice, CGRP-labeling density was increased by approximately 60% (Fig. 7). Statistical analysis (ANOVA) indicated an overall group difference (p = 0.02) and a statistical significant difference between untrained SCI and trained and untrained sham mice (PLSD p < 0.01). Sensorimotor activity using treadmill training initiated in the subchronic/chronic phase after injury significantly reduced CGRP-labeling density in deeper laminae of the lumbar dorsal horn in trained (SCI+T), compared with untrained SCI mice (PLSD p < 0.05; Fig. 7F) to the level that was observed in sham animals. As expected, treadmill training showed no effect on the CGRP-labeling density in sham mice.
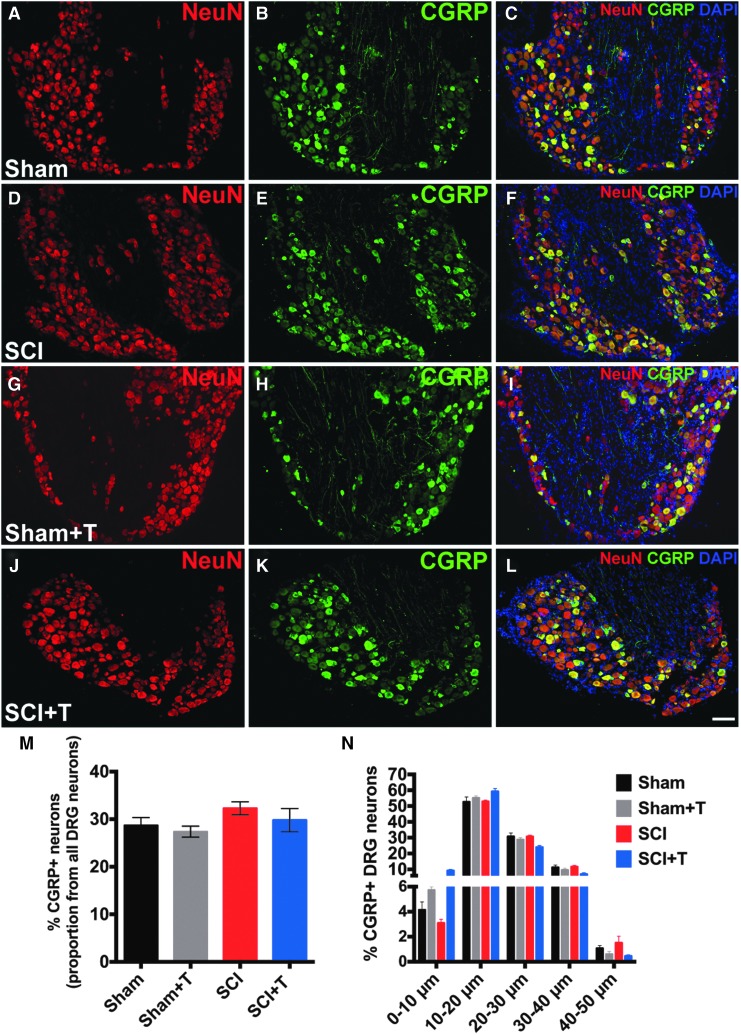
SCI does not change the phenotype of CGRP-expressing L4-L6 DRG neurons
To determine whether the increased CGRP-labeling density in laminae III-IV is due to differences in the number or phenotype of CGRP-expressing neurons, the total number (CGRP+/NeuN+) and the size distribution of CGRP-labeled DRG neurons were evaluated (Fig. 8). Comparing lumbar (L4-6) DRG neurons in sham and SCI animals, neither the proportion of CGRP-expressing neurons (Fig. 8M; p = 0.3) nor the distribution of the size of CGRP-labeled neurons (Fig. 8N; two-way ANOVA, p = 0.85 for groups) changed as a result of SCI. The majority of CGRP+ neurons were within the group of small-to-medium diameter neurons as expected (0–30 μm; ∼88% for sham). Approximately 28–32% of DRG neurons were CGRP+ (CGRP+/NeuN+) consistent with previous reports.38 Qualitatively, immunofluorescent labeling intensity did not differ between DRGs of trained and untrained sham and injured animals (Fig. 8A-L). Together, these data suggest that the observed increase in CGRP-labeling density in the deeper laminae of the dorsal horn is not due to a change in the phenotype or proportion of CGRP-expressing DRG neurons, but rather reflects structural changes in the dorsal horn.
FIG. 8.
Analysis of calcitonin gene-related peptide (CGRP) expression in lumbar (L4-L6) dorsal root ganglia (DRG) neurons. Representative images of CGRP and NeuN labeled DRG neurons of (A-C) sham, (D-F) spinal cord injury (SCI), (G-I) trained sham and (J-L) trained SCI mice (scale bar, 100 μm). (M, N) Quantification of CGRP+/NeuN+ DRG neurons indicates no difference in (M) the proportion of CGRP+ neurons and (N) size distribution comparing sham and SCI mice.
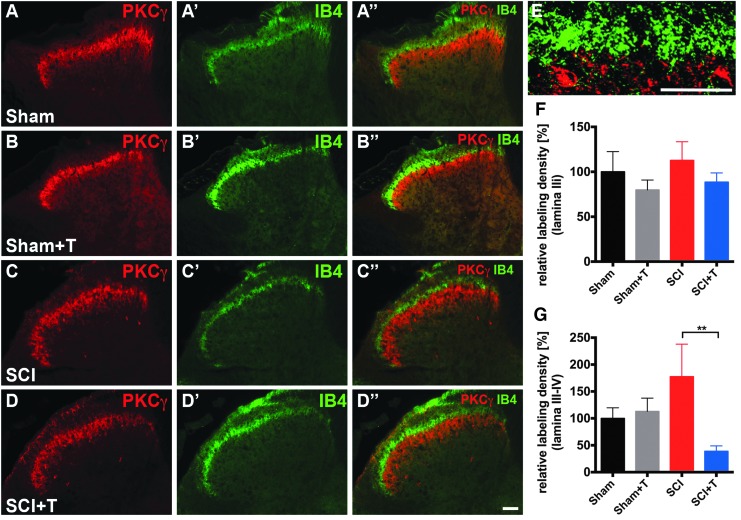
Effects on non-peptidergic IB4+ afferents
In contrast to the increased labeling density of peptidergic CGRP+ fibers in deeper laminae of the dorsal horn, no change in the labeling density of non-peptidergic IB4+ fibers was observed in lamina IIi identified by PKCγ labeling in the lumbar dorsal horn after SCI (Fig. 9). Neither injury nor treadmill training had an effect on non-peptidergic IB4+ C-fiber in lamina IIi (ANOVA p = 0.56; Fig. 9E). The density of IB4-labeling also was measured in laminae III-IV. Statistical analysis indicated a slight but not significant increase of IB4-labeling density after injury and a decrease in labeling in laminae III-IV comparing trained with untrained SCI animals (ANOVA, p < 0.05; PLSD, p < 0.01). While these data suggest a potential effect of treadmill training in the SCI group, these findings are based on extremely sparse and highly variable labeling.
FIG. 9.
Limited effect of spinal cord injury (SCI) and treadmill training on non-peptidergic IB4+ afferents. Representative images of isolectin-B4 (IB4)/protein kinase Cγ (PKCγ) labeled coronal sections of the lumbar (L4-L6) spinal dorsal horn of (A, C) untrained and (B, D) trained sham and SCI mice, respectively (scale bar, 100 μm). (E) Higher magnification of IB4/PKCγ labeling in the dorsal horn at the interface of lamina IIo and IIi. Most of the IB4 labeling is restricted to areas dorsal to PKCγ labeling with some labeling extending into lamina IIi. (Scale bar, 25 μm) (F) IB4 labeling density in lamina IIi identified by PKCγ labeling shows no differences between groups. IB4 labeling in lamina III/IV is very sparse and barely visible. (G) Quantification of the labeling density relative to untrained sham animals indicates a slight but insignificant increase in laminae III-IV in SCI animals compared with sham animals and a training-mediated decrease comparing SCI with SCI+T (analysis of variance, p < 0.05; protected least significant difference **p < 0.01).
Discussion
Previously, we have shown that the development of CNP after a moderate T11 contusion injury in mice can be prevented when treadmill training is initiated 7 days post-injury.24 The current study substantially extends these findings demonstrating that sensorimotor training initiated in the subchronic/chronic phase after a moderate contusion SCI reduces mechanical allodynia and thermal hyperalgesia. In addition, SCI-induced CNP is associated with an increased labeling density of CGRP+ fibers in the lumbar dorsal horn, which is reduced by treadmill training. Thus, sensorimotor activity is still beneficial when initiated after mechanical allodynia and thermal hyperalgesia are established and reduces injury-induced morphological changes in primary nociceptive afferents.
Almost all studies examining the role of activity/training in animal models of SCI pain have only analyzed effects acutely after SCI.19,24,26,27 However, neuropathic pain developing in SCI patients within the first year tends to become chronic2,39 and potential therapies for the reversal of neuropathic pain are highly relevant. We therefore assessed mechanical and thermal sensitivity in the subchronic to chronic phase after SCI (5–10 weeks post-injury) showing persistent mechanical allodynia and thermal hyperalgesia. In contrast to the hyper-responsiveness to small-diameter filaments, noxious mechanical stimuli revealed an injury-induced hypoalgesia reflecting the loss of somatosensory function below the level of injury, a hallmark of SCI.40–43 Taken together, our results indicate that the neuropathic pain characteristics in the acute phase after SCI24 become chronic resulting in a stable phenotype for at least 10 weeks post-injury.
Assessment of pain in rodents mostly depends on stimulus-evoked responses, which are generally accepted as surrogates of neuropathic pain, but might only reflect hyperreflexia,44 especially after SCI. Several paradigms evaluating the affective-motivational component of pain and stimulus-independent spontaneous and ongoing pain have been developed.32,33,45–50 The place escape avoidance paradigm has previously been used in rats to evaluate at-level pain after SCI.31,44 We modified this paradigm and used a normally innocuous von Frey filament (0.16 g) for stimulation rather than a suprathreshold stimulus. As control, sham animals were stimulated with the same small-diameter filament on the dark side. Similar to previous findings in animals with hyperalgesia in peripheral pain models, stimulating the hindpaws with non-noxious intensities elicited an escape/avoidance behavior, which was decreased by treadmill training. Therefore, tactile below-level allodynia is associated with an aversive response involving supraspinal levels and treadmill training-mediated effects are not merely due to changes in spinal reflex circuitries.
Interestingly, delayed treadmill training did not only influence mechanical allodynia as in our previous study examining acute training,24 but also thermal hyperalgesia, suggesting that different mechanisms might underlie thermal hyperalgesia after acute and chronic injury. In other studies evaluating early onset training, thermal hyperalgesia was either not affected after a unilateral cervical contusion injury19 or its development was prevented in a clip-compression SCI in rats.51 Previous studies in rats investigating the effect of delayed training have shown contradictory results.51,52 After an incomplete thoracic SCI, aberrant mechanical and thermal sensation were reversed when treadmill-training was initiated 3 weeks post-injury.51 These data are similar to data of our current study, further confirming effects of delayed activity/training on hindlimb mechanical allodynia and thermal hyperalgesia in a different species with a different injury model. In contrast, forced wheel running initiated 14 or 28 dpi after a unilateral cervical contusion injury in rats did not reduce hindlimb mechanical allodynia.52 Thermal sensitivity was not evaluated in the latter study. It is unclear why a training effect was not detectable in this unilateral cervical injury model, although the same training paradigm was effective when initiated acutely after injury.19 Differences between SCI models affecting the speed and degree of motor recovery, different mechanisms contributing to below-level neuropathic pain after unilateral cervical versus bilateral thoracic injuries, and different training methods might underlie the variable efficacy of exercise on thermal and tactile hyper-responsiveness.
Training is likely to exhibit effects on multiple levels including the cardiovascular, immune, musculoskeletal and nervous systems. Exercise has been shown to promote plasticity of primary afferents, induce changes in the spinal cord circuitry after SCI, and modulate secondary injury processes.53 Thus, mechanisms underlying the observed training effect might represent a complex combination of changes within sensory afferents, spinal cord, and supraspinal levels, as well as immunomodulatory influences. However, for amelioration of mechanical allodynia, rhythmic stimulation of proprioceptive and mechanosensory fibers as well as weight bearing seems to be important as only treadmill but not swim or stand training reduces mechanical allodynia after rat contusion SCI.27 As rhythmic loading of the hindpaws does not stimulate thermal afferents, the observed improvement of thermal hyperalgesia suggests that other mechanisms such as changes in chronic neuroinflammation are involved. Resident microglia are activated after contusion injuries54–57 and activated microglia have been implicated in the maintenance of below-level chronic pain after SCI,17 possibly through release of prostaglandin E2 (PGE2).58 Treadmill training has been shown to reduce microglia activation in a model of neuropathic pain in mice and peripheral nerve injury in rats59,60 and endurance exercise has been shown to reduce PGE2 levels in rats.61 Thus, changes in microglia phenotype could be a potential mechanism underlying the normalization of thermal hyperalgesia.
A well-established correlate of aberrant mechanical and thermal hypersensitivity is the maladaptive sprouting/increased density of nociceptive fibers in laminas III-IV of the dorsal horn.21,22,28,36,37,62 In our previous and current study, SCI resulted in increased CGRP-labeling in deeper laminae of the dorsal horn.24 This might be due to aberrant sprouting of C/Aδ-fibers thereby enhancing activity in lamina III and IV neurons that normally process non-noxious stimuli. Alternatively, changes in CGRP expression could explain an increase in CGRP-labeling density. Phenotypic changes of Aß-fibers to a nociceptive phenotype (CGRP+/substance P+) have been described in inflammatory and nerve injury pain models.63–67 However, our analysis of lumbar DRG neurons comparing sham with SCI animals did not show changes in the number and size distribution of CGRP+ neurons, suggesting that the observed differences in CGRP-labeling in the dorsal horn reflect structural changes rather than expression changes. Importantly, our data indicate that even in the subchronic/chronic phase after SCI, training-induced amelioration of neuropathic pain is associated with reduced CGRP-labeling in laminae III-IV. While recent data also suggest sprouting of non-peptidergic IB4+ C-fibers into lamina IIo and III after SCI,19,27 we did not observe major SCI-induced changes in the labeling pattern of IB4+ fibers and labeling density in deeper laminae was sparse, in line with our previous study,24 and unlikely to be of significance for the observed pain behavior. Differences in species, lesion model, and lesion severity might underlie the conflicting results.
Several cellular and molecular changes that are modulated by exercise might affect peptidergic fibers including the expression of neurotrophic factors.25,68–71 Whether neurotrophic factors have a positive effect on the amelioration of neuropathic pain after peripheral nervous system (PNS) and CNS injuries is controversial. After SCI, reduced levels of brain-derived neurotrophic factor (BDNF) and glial cell line–derived neurotrophic factor (GDNF) correlate with the development of SCI-induced neuropathic pain.19,27 While training restored BDNF and GDNF levels in spinal cord segments corresponding to pain dermatomes and reversed mechanical allodynia,19,27 contrastingly, early-step training has been suggested to induce BDNF-mediated C-fiber sprouting and mechanical hypersensitivity.72 Further, blocking BDNF signaling normalized mechanical hypersensitivity.72 Microglia activated by injury release BDNF and elevated levels of BDNF in the dorsal horn result in the downregulation of KCC2, reduction of inhibitory synaptic transmission and enhanced excitatory input.9,73–75 CGRP+ sensory neurons express the nerve growth factor (NGF) receptor, tropomyosin receptor kinase A (TrkA)76 and upregulation or overexpression of NGF after SCI77,78 induces sprouting of peptidergic fibers as well as hyperalgesia. Since inhibiting TrkA or NGF decreases sprouting of CGRP+ fibers and attenuates mechanical hyperalgesia after SCI, training-mediated changes in NGF expression might underlie the reduced CGRP-labeling density in the dorsal horn.37,79
Interestingly, similar to human patients, and consistent with studies in rats,1,19,35,55 only a subset of injured animals developed chronic signs of mechanical allodynia. As only 20% of injured mice did not develop mechanical allodynia, the small group size does not allow for definitive conclusions about the underlying mechanisms. Lesion size did not differ between animals with and without mechanical allodynia (data not shown). However, exploratory analysis of CGRP-labeling showed a clear trend towards a lower labeling density in No-Pain animals compared with untrained SCI animals and was comparable to trained SCI animals. In contrast to mechanical allodynia, thermal hypersensitivity was observed even in mice that did not develop mechanical allodynia (data not shown). Besides structural differences, inflammatory responses may distinguish SCI animals with and without pain. Transcriptomics after a rat contusion SCI revealed increased expression of astrocytic and pro-inflammatory genes only in the spinal cord of animals that developed pain.55 Additional studies are warranted to examine the underlying mechanisms.
In summary, our data indicate that SCI-induced hypersensitivity is stable in the chronic phase and can be reversed by delayed treadmill training. Mice displaying signs of CNP show increased CGRP-labeling in the dorsal horn that is not due to changes in CGRP expression, suggesting that aberrant plasticity in nociceptive afferents plays a role in the maintenance of SCI-induced pain. Furthermore, normalized CGRP fiber density is evident after training-induced pain amelioration. Thus, sensorimotor activation can control pain in acute and chronic phases of SCI in mice.
Acknowledgments
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 1158), the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative, and the Indiana Clinical and Translational Sciences Institute, funded in part by grant #UL1 TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Behavioral studies were supported by the Interdisciplinary Neuroscience Behavioral Core (INBC) at Heidelberg University.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Finnerup N.B. (2013). Pain in patients with spinal cord injury. Pain 154, S71–S76 [DOI] [PubMed] [Google Scholar]
- 2.Siddall P.J., McClelland J.M., Rutkowski S.B., and Cousins M.J. (2003). A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257 [DOI] [PubMed] [Google Scholar]
- 3.Zeilig G., Enosh S., Rubin-Asher D., Lehr B., and Defrin R. (2012). The nature and course of sensory changes following spinal cord injury: Predictive properties and implications on the mechanism of central pain. Brain 135, 418–430 [DOI] [PubMed] [Google Scholar]
- 4.Baastrup C. and Finnerup N.B. (2008). Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs 22, 455–475 [DOI] [PubMed] [Google Scholar]
- 5.Teasell R.W., Mehta S., Aubut J.A.L., Foulon B., Wolfe D.L., Hsieh J.T.C., Townson A.F., and Short C. (2010). A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch. Phys. Med. Rehabil. 91, 816–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drew G.M., Siddall P.J., and Duggan A.W. (2004). Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain 109, 379–388 [DOI] [PubMed] [Google Scholar]
- 7.Meisner J.G., Marsh A.D., and Marsh D.R. (2010). Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J. Neurotrauma 27, 729–737 [DOI] [PubMed] [Google Scholar]
- 8.Gwak Y.S., Crown E.D., Unabia G.C., and Hulsebosch C.E. (2008). Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain 138, 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y., Zheng J., Xiong L., Zimmermann M., and Yang J. (2008). Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J. Physiol. 586, 5701–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasbargen T., Ahmed M.M., Miranpuri G., Li L., Kahle K.T., Resnick D., and Sun D. (2010). Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Ann. N. Y. Acad. Sci. 1198, 168–172 [DOI] [PubMed] [Google Scholar]
- 11.Leem J.W., Kim H.K., Hulsebosch C.E., and Gwak Y.S. (2010). Ionotropic glutamate receptors contribute to maintained neuronal hyperexcitability following spinal cord injury in rats. Exp. Neurol. 224, 321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boroujerdi A., Zeng J., Sharp K., Kim D., Steward O., and Luo Z.D. (2011). Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain 152, 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlton S.M., Du J., Tan H.Y., Nesic O., Hargett G.L., Bopp A.C., Yamani A., Lin Q., Willis W.D., and Hulsebosch C.E. (2009). Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 147, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., Yang Q., Crook R.J., O'Neil R.G., and Walters E.T. (2013). TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain 154, 2130–2141 [DOI] [PubMed] [Google Scholar]
- 15.Yang Q., Wu Z., Hadden J.K., Odem M.A., Zuo Y., Crook R.J., Frost J.A., and Walters E.T. (2014). Persistent pain after spinal cord injury is maintained by primary afferent activity. J. Neurosci. 34, 10765–10769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwak Y.S., Kang J., Unabia G.C., and Hulsebosch C.E. (2012). Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp. Neurol. 234, 362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hains B.C. (2006). Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 26, 4308–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwak Y.S. and Hulsebosch C.E. (2011). GABA and central neuropathic pain following spinal cord injury. Neuropharmacology 60, 799–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Detloff M.R., Smith E.J., Quiros Molina D., Ganzer P.D., and Houlé J.D. (2014). Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp. Neurol. 255, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagg T. (2006). Collateral sprouting as a target for improved function after spinal cord injury. J. Neurotrauma 23, 281–294 [DOI] [PubMed] [Google Scholar]
- 21.Krenz N.R. and Weaver L.C. (1998). Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience 85, 443–458 [DOI] [PubMed] [Google Scholar]
- 22.Ondarza A.B., Ye Z., and Hulsebosch C.E. (2003). Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp. Neurol. 184, 373–380 [DOI] [PubMed] [Google Scholar]
- 23.Weaver L.C., Verghese P., Bruce J.C., Fehlings M.G., Krenz N.R., and Marsh D.R. (2001). Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J. Neurotrauma 18, 1107–1119 [DOI] [PubMed] [Google Scholar]
- 24.Nees T.A., Tappe-Theodor A., Sliwinski C., Motsch M., Rupp R., Kuner R., Weidner N., and Blesch A. (2016). Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain 157, 687–697 [DOI] [PubMed] [Google Scholar]
- 25.Gómez-Pinilla F., Ying Z., Roy R.R., Molteni R., and Edgerton V.R. (2002). Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 88, 2187–2195 [DOI] [PubMed] [Google Scholar]
- 26.Houle J.D. and Côté M.P. (2013). Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann. N. Y. Acad. Sci. 1279, 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchinson K.J., Gómez-Pinilla F., Crowe M.J., Ying Z., and Basso D.M. (2004). Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 127, 1403–1414 [DOI] [PubMed] [Google Scholar]
- 28.Kalous A., Osborne P.B., and Keast J.R. (2007). Acute and chronic changes in dorsal horn innervation by primary afferents and descending supraspinal pathways after spinal cord injury. J. Comp. Neurol. 504, 238–253 [DOI] [PubMed] [Google Scholar]
- 29.Molteni R., Zheng J.Q., Ying Z., Gómez-Pinilla F., and Twiss J.L. (2004). Voluntary exercise increases axonal regeneration from sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 101, 8473–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., and Lumpp J.E. (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 [DOI] [PubMed] [Google Scholar]
- 31.Baastrup C., Jensen T.S., and Finnerup N.B. (2011). Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res. 1370, 129–135 [DOI] [PubMed] [Google Scholar]
- 32.LaBuda C.J. and Fuchs P.N. (2000). Morphine and gabapentin decrease mechanical hyperalgesia and escape/avoidance behavior in a rat model of neuropathic pain. Neurosci. Lett. 290, 137–140 [DOI] [PubMed] [Google Scholar]
- 33.LaBuda C.J. and Fuchs P.N. (2000). A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp. Neurol. 163, 490–494 [DOI] [PubMed] [Google Scholar]
- 34.Basso D.M., Fisher L.C., Anderson A.J., Jakeman L.B., Mctigue D.M., and Popovich P.G. (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 [DOI] [PubMed] [Google Scholar]
- 35.Detloff M.R., Wade R.E., Jr., and Houle J.D. (2013). Chronic at- and below-level pain after moderate unilateral cervical spinal cord contusion in rats. J. Neurotrauma 30, 884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen M.D., Everhart A.W., Pickelman J.T., and Hulsebosch C.E. (1996). Mechanical and thermal allodyniain chronic central pain following spinal cord injury. Pain 68, 97–107 [DOI] [PubMed] [Google Scholar]
- 37.Christensen M.D. and Hulsebosch C.E. (1997). Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 147, 463–475 [DOI] [PubMed] [Google Scholar]
- 38.McCoy E.S., Taylor-Blake B., Street S.E., Pribisko A.L., Zheng J., and Zylka M.J. (2013). Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 78, 138–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finnerup N.B., Jensen M.P., Norrbrink C., Trok K., Johannesen I.L., Jensen T.S., and Werhagen L. (2016). A prospective study of pain and psychological functioning following traumatic spinal cord injury. Spinal Cord 54, 816–821 [DOI] [PubMed] [Google Scholar]
- 40.Baron R., Binder A., and Wasner G. (2010). Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 9, 807–819 [DOI] [PubMed] [Google Scholar]
- 41.von Hehn C.A., Baron R., and Woolf C.J. (2012). Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73, 638–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eide P.K., Jørum E., and Stenehjem A.E. (1996). Somatosensory findings in patients with spinal cord injury and central dysaesthesia pain. J. Neurol. Neurosurg. Psychiatry 60, 411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krassioukov A., Wolfe D.L., Hsieh J.T.C., Hayes K.C., and Durham C.E. (1999). Quantitative sensory testing in patients with incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 80, 1258–1263 [DOI] [PubMed] [Google Scholar]
- 44.Baastrup C., Maersk-Moller C.C., Nyengaard J.R., Jensen T.S., and Finnerup N.B. (2010). Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain 151, 670–679 [DOI] [PubMed] [Google Scholar]
- 45.Tappe-Theodor A. and Kuner R. (2014). Studying ongoing and spontaneous pain in rodents—challenges and opportunities. Eur. J. Neurosci. 39, 1881–1890 [DOI] [PubMed] [Google Scholar]
- 46.Cobos E.J., Ghasemlou N., Araldi D.i., Segal D., Duong K., and Woolf C.J. (2012). Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153, 876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban R., Scherrer G., Goulding E.H., Tecott L.H., and Basbaum A.I. (2011). Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 152, 990–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews N., Legg E., Lisak D., Issop Y., Richardson D., Harper S., Pheby T., Huang W., Burgess G., Machin I., and Rice A.S. (2012). Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur. J. Pain 16, 485–495 [DOI] [PubMed] [Google Scholar]
- 49.King T., Vera-Portocarrero L., Gutierrez T., Vanderah T.W., Dussor G., Lai J., Fields H.L., and Porreca F. (2009). Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci. 12, 1364–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langford D.J., Bailey A.L., Chanda M.L., Clarke S.E., Drummond T.E., Echols S., Glick S., Ingrao J., Klassen-Ross T., Lacroix-Fralish M.L., Matsumiya L., Sorge R.E., Sotocinal S.G., Tabaka J.M., Wong D., van den Maagdenberg A.M.J.M., Ferrari M.D., Craig K.D. and Mogil J.S. (2010). Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 7, 447–449 [DOI] [PubMed] [Google Scholar]
- 51.Dugan E.A. and Sagen J. (2015). An intensive locomotor training paradigm improves neuropathic pain following spinal cord compression injury in rats. J. Neurotrauma 32, 622–632 [DOI] [PubMed] [Google Scholar]
- 52.Detloff M.R., Quiros-Molina D., Javia A.S., Daggubati L., Nehlsen A.D., Naqvi A., Ninan V., Vannix K.N., McMullen M.-K., Amin S., Ganzer P.D., and Houlé J.D. (2016). Delayed exercise is ineffective at reversing aberrant nociceptive afferent plasticity or neuropathic pain after spinal cord injury in rats. Neurorehabil. Neural Repair 30, 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houle J.D. and Tessler A. (2003). Repair of chronic spinal cord injury. Exp. Neurol. 182, 247–260 [DOI] [PubMed] [Google Scholar]
- 54.Popovich P.G., Wei P., and Stokes B.T. (1997). Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 377, 443–464 [DOI] [PubMed] [Google Scholar]
- 55.Nesic O., Lee J., Johnson K.M., Ye Z., Xu G.Y., Unabia G.C., Wood T.G., McAdoo D.J., Westlund K.N., Hulsebosch C.E., and Perez-Polo J.R. (2005). Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J. Neurochem. 95, 998–1014 [DOI] [PubMed] [Google Scholar]
- 56.Zai L.J. and Wrathall J.R. (2005). Cell proliferation and replacement following contusive spinal cord injury. Glia 50, 247–257 [DOI] [PubMed] [Google Scholar]
- 57.Hains B.C., Klein J.P., Saab C.Y., Craner M.J., Black J.A., and Waxman S.G. (2003). Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 23, 8881–8892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao P., Waxman S.G., and Hains B.C. (2007). Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J. Neurosci. 27, 2357–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cobianchi S., Marinelli S., Florenzano F., Pavone F., and Luvisetto S. (2010). Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience 168, 273–287 [DOI] [PubMed] [Google Scholar]
- 60.López-Álvarez V.M., Modol L., Navarro X., and Cobianchi S. (2015). Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain 156, 1812–1825 [DOI] [PubMed] [Google Scholar]
- 61.Lira F.S., Yamashita A., Carnevali L.C., Gonçalves D.C., Lima W.P., Rosa J.C., Caperuto E.C., Rosa L.F.C., and Seelaender M. (2010). Exercise training reduces PGE2 levels and induces recovery from steatosis in tumor-bearing rats. Horm. Metab. Res. 42, 944–949 [DOI] [PubMed] [Google Scholar]
- 62.Kerr B.J. and David S. (2007). Pain behaviors after spinal cord contusion injury in two commonly used mouse strains. Exp. Neurol. 206, 240–247 [DOI] [PubMed] [Google Scholar]
- 63.Zheng L.F., Wang R., Xu Y.Z., Yi X.N., Zhang J.W., and Zeng Z.C. (2008). Calcitonin gene-related peptide dynamics in rat dorsal root ganglia and spinal cord following different sciatic nerve injuries. Brain Res. 1187, 20–32 [DOI] [PubMed] [Google Scholar]
- 64.Ma W., Ramer M.S., and Bisby M.A. (1999). Increased calcitonin gene-related peptide immunoreactivity in gracile nucleus after partial sciatic nerve injury: age-dependent and originating from spared sensory neurons. Exp. Neurol. 159, 459–473 [DOI] [PubMed] [Google Scholar]
- 65.Neumann S., Doubell T.P., Leslie T., and Woolf C.J. (1996). Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384, 360–364 [DOI] [PubMed] [Google Scholar]
- 66.Noguchi K., Dubner R., De Leon M., Senba E., and Ruda M.A. (1994). Axotomy induces preprotachykinin gene expression in a subpopulation of dorsal root ganglion neurons. J. Neurosci. Res. 37, 596–603 [DOI] [PubMed] [Google Scholar]
- 67.Noguchi K., Kawai Y., Fukuoka T., Senba E., and Miki K. (1995). Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J. Neurosci. 15, 7633–7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beaumont E., Kaloustian S., Rousseau G., and Cormery B. (2008). Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci. Res. 62, 147–154 [DOI] [PubMed] [Google Scholar]
- 69.Côté M.P., Azzam G.A., Lemay M.A., Zhukareva V., and Houlé J.D. (2011). Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J. Neurotrauma 28, 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jung S.Y., Kim D.Y., Yune T.Y., Shin D.H., Baek S.B., and Kim C.J. (2014). Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp. Ther. Med. 7, 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaynman S. and Gomez-Pinilla F. (2005). License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil. Neural Repair 19, 283–295 [DOI] [PubMed] [Google Scholar]
- 72.Endo T., Ajiki T., Inoue H., Kikuchi M., Yashiro T., Nakama S., Hoshino Y., Murakami T., and Kobayashi E. (2009). Early exercise in spinal cord injured rats induces allodynia through TrkB signaling. Biochem. Biophys. Res. Commun. 381, 339–344 [DOI] [PubMed] [Google Scholar]
- 73.Biggs J.E., Lu V.B., Stebbing M.J., Balasubramanyan S., and Smith P.A. (2010). Is BDNF sufficient for information transfer between microglia and dorsal horn neurons during the onset of central sensitization? Mol. Pain 6, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ulmann L., Hatcher J.P., Hughes J.P., Chaumont S., Green P.J., Conquet F., Buell G.N., Reeve A.J., Chessell I.P., and Rassendren F. (2008). Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J. Neurosci. 28, 11263–11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou L.J., Yang T., Wei X., Liu Y., Xin W.J., Chen Y., Pang R.P., Zang Y., Li Y.Y., and Liu X.-G. (2011). Brain-derived neurotrophic factor contributes to spinal long-term potentiation and mechanical hypersensitivity by activation of spinal microglia in rat. Brain Behav. Immun. 25, 322–334 [DOI] [PubMed] [Google Scholar]
- 76.Averill S., McMahon S.B., Clary D.O., Reichardt L.F., and Priestley J.V. (1995). Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur. J. Neurosci. 7, 1484–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown A., Ricci M.J., and Weaver L.C. (2004). NGF message and protein distribution in the injured rat spinal cord. Exp. Neurol. 188, 115–127 [DOI] [PubMed] [Google Scholar]
- 78.Romero M.I., Rangappa N., Li L., Lightfoot E., Garry M.G., and Smith G.M. (2000). Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. 20, 4435–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gwak Y.S., Nam T.S., Paik K.S., Hulsebosch C.E., and Leem J.W. (2003). Attenuation of mechanical hyperalgesia following spinal cord injury by administration of antibodies to nerve growth factor in the rat. Neurosci. Lett. 336, 117–120 [DOI] [PubMed] [Google Scholar]