Abstract
Background
Enterohemorrhagic Escherichia coli (EHEC) O26:H11/H−, the most common non-O157 serotype causing hemolytic uremic syndrome worldwide, are evolutionarily highly dynamic with new pathogenic clones emerging rapidly. Here, we investigated the population structure of EHEC O26 isolated from patients in several European countries using whole genome sequencing, with emphasis on a detailed analysis of strains of the highly virulent new European clone (nEC) which has spread since 1990s.
Results
Genome-wide single nucleotide polymorphism (SNP)-based analysis of 32 EHEC O26 isolated in the Czech Republic, Germany, Austria and Italy demonstrated a split of the nEC (ST29C2 clonal group) into two distinct lineages, which we termed, based on their temporal emergence, as “early” nEC and “late” nEC. The evolutionary divergence of the early nEC and late nEC is marked by the presence of 59 and 70 lineage-specific SNPs (synapomorphic mutations) in the genomes of the respective lineages. In silico analyses of publicly available E. coli O26 genomic sequences identified the late nEC lineage worldwide. Using a PCR designed to target the late nEC synapomorphic mutation in the sen/ent gene, we identified the early nEC decline accompanied by the late nEC rise in Germany and the Czech Republic since 2004 and 2013, respectively. Most of the late nEC strains harbor one of two major types of Shiga toxin 2a (Stx2a)-encoding prophages. The type I stx2a-phage is virtually identical to stx2a-phage of EHEC O104:H4 outbreak strain, whereas the type II stx2a-phage is a hybrid of EHEC O104:H4 and EHEC O157:H7 stx2a-phages and carries a novel mutation in Stx2a. Strains harboring these two phage types do not differ by the amounts and biological activities of Stx2a produced.
Conclusions
Using SNP-level analyses, we provide the evidence of the evolutionary split of EHEC O26:H11/H− nEC into two distinct lineages, and a recent replacement of the early nEC by the late nEC in Germany and the Czech Republic. PCR targeting the late nEC synapomorphic mutation in ent/sen enables the discrimination of early nEC strains and late nEC strains in clinical and environmental samples, thereby facilitating further investigations of their geographic distribution, prevalence, clinical significance and epidemiology.
Electronic supplementary material
The online version of this article (10.1186/s12864-018-5045-7) contains supplementary material, which is available to authorized users.
Keywords: Shiga toxin, O26, Enterohemorrhagic Escherichia coli (EHEC), New European clone
Background
Enterohemorrhagic Escherichia coli (EHEC) O26:H11/H− is the most common non-O157 EHEC serotype causing diarrhea and its systemic complication, the hemolytic uremic syndrome (HUS) worldwide [1–16]. In Europe, the proportion of EHEC O26 among all EHEC patients´ isolates has been increasing during past years [1] and in some countries, EHEC O26 is a more frequent cause of HUS than EHEC O157:H7 [12, 16]. HUS caused by EHEC O26 can be as severe as that caused by EHEC O157:H7 [13, 17–20] and there is no significant difference in the long-term outcome of HUS caused by EHEC O26 and EHEC O157 [18]. In addition to sporadic cases, multiple outbreaks caused by EHEC O26 have been reported throughout the world [15, 21–30].
The majority of EHEC O26 clinical isolates carry genes encoding Shiga toxins (Stx) of two major types including Stx1a and/or Stx2a [9, 26, 27, 31–33]. However, a subset of strains contain genes encoding Stx subtypes such as Stx2d [34, 35], Stx2b [15], or Stx2f [36]. The presence of stx2a and/or production of Stx2a alone is a predictor for a severe disease including progression of the infection to HUS [31]. The stx2a-harboring EHEC O26 belong to two major multilocus sequence types (STs) ST21 or ST29, whereas strains harboring stx1a alone or together with stx2a belong to ST21 [9, 31]. The ST29 clade is considerably heterogeneous and consists of several clonal lineages which differ by stx2 alleles and plasmid gene profiles [9, 31, 37, 38]. A new highly virulent ST29 clone harboring stx2a only was first reported in Germany in the mid-1990s [33] and since then it has rapidly spread throughout Europe [31]. Strains of this lineage termed a “new German clone” or a “new European clone” [31, 38] exhibit a particular plasmid virulence gene profile characterized by the presence of EHEC-hlyA (encoding EHEC hemolysin) and etpD (encoding a type II effector), while katP (encoding catalase-peroxidase) and espP (encoding serine protease EspP) genes are absent [31, 33]. In 2015, a distinct ST29 clonal lineage harboring stx2a or stx2d and lacking the plasmid-borne virulence genes (EHEC-hlyA, katP, espP, etpD) was reported in France as a “new French clone” [35, 38]. Recently, yet another ST29 clone, which harbors stx2a only and differs from both the European and French clone by plasmid virulence gene profile (EHEC-hlyA+, katP-, espP+, etpD-) emerged in Japan [9]. This Japanese clone (designated ST29C1 clade) is phylogenetically unrelated to the new European clone (ST29C2 clade) and the French clone (ST29C3 clade) [9].
Although EHEC O26 are the most common cause of pediatric HUS in the Czech Republic [12], the clonal structure of these strains has not been investigated in detail. The aim of this study was to analyze the phylogenetic relationships and clonal structure of EHEC O26 isolated from patients in the Czech Republic by using whole genome sequencing (WGS) with a particular focus on ST29 strains. To put these data into a global context, the genomic sequences of the Czech isolates were compared with those of EHEC O26 isolated in other European countries and with E. coli O26 genomes present in GenBank.
Results
Whole genome-based clonal structure of EHEC O26 population
Initially, we performed WGS of 16 EHEC O26 strains isolated from patients with HUS or diarrhea without HUS in the Czech Republic during the period of 2006–2016. The STs and plasmid gene profiles were determined in silico from draft genome sequences. This revealed the presence of both major O26 phylogenetic lineages, ST21 (6 isolates) and ST29 (10 isolates), among Czech isolates (Additional file 1: Table S1). Five of the six ST21 strains harbored stx2a only, and one contained stx1a only; all of them displayed a plasmid-borne virulence gene profile (EHEC-hlyA+, katP+, espP+, etpD-) previously identified in ST21 strains [31] (Additional file 1: Table S1). Nine of the ten ST29 EHEC O26 Czech strains corresponded to the new European clone (nEC) as defined in [31], i.e., they contained stx2a as the sole stx gene and the plasmid virulence gene profile EHEC-hlyA+, katP-, espP-, and etpD+ (Additional file 1: Table S1). Remarkably, the remaining ST29 strain (15–496) harbored the plasmid gene profile typical for the nEC, but combined with the presence of stx1c gene only instead of stx2a (Additional file 1: Table S1). This is, to the best of our knowledge, the first report of stx1c genotype in strains of the nEC. To gain a more detailed insight into the population structure of the nEC, we performed additional WGS of a collection of European nEC isolates including 16 strains originating from Germany (n = 11), Italy (n = 3), and Austria (n = 2). For comparison, genomic sequences of E. coli O26 available in the GenBank database were included in this analysis, making up the final set of total 159 E. coli O26 isolates whose genetic relationships were examined (Additional file 1: Table S1).
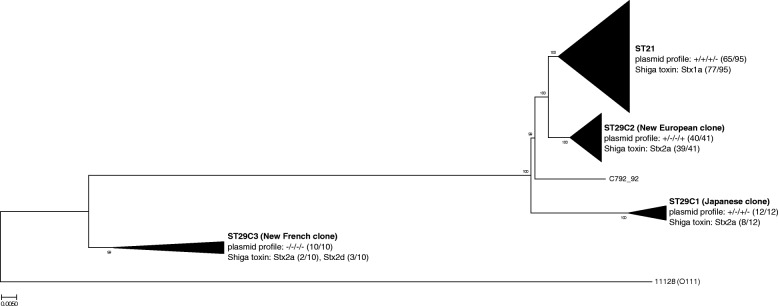
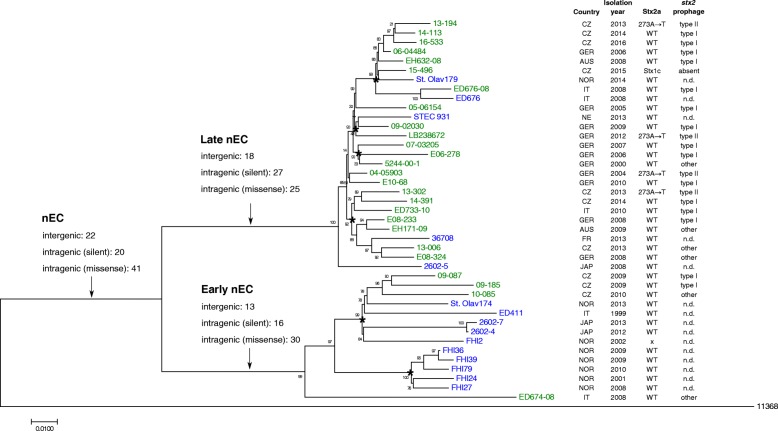
A genome-wide single nucleotide polymorphism (SNP)-based phylogram was constructed using the maximum likelihood model to provide high-resolution inference of E. coli O26 phylogeny (Fig. 1, Additional file 2: Figure S1). This phylogram clearly identified four major lineages, whose characteristics (i.e. ST, plasmid gene profile, and predominant Stx type) were in accordance with previous reports [9, 37–39]. Strikingly, the phylogenetic analysis demonstrated that strains of the nEC (syn. ST29C2 [9]) split into two distinct clusters (Fig. 2). This is in accordance with the observation of two distinct pulse-field gel electrophoresis (PFGE) clusters among a large, representative collection of European ST29 strains isolated between 1996 and 2012 in the original description of the nEC [31]. Retrospectively, we noted tentative differences between the two ST29 PFGE clusters with respect to the distribution of isolation dates of the corresponding strains. First strains of PFGE cluster B were isolated as early as in 1996 and their majority (53.6%) until 2004. In contrast, strains of the PFGE cluster C started to emerge, with a single exception, since 2004 [31]. We therefore propose to name the WGS lineages homologous to the PFGE clusters B and C as “early” and “late” nEC, respectively (Fig. 2, Additional file 3: Table S3). This designation is further supported by a detailed analysis of a temporal shift between the nEC lineages, as shown below.
Fig. 1.
Global population structure of E. coli O26 based on whole genome phylogeny. The major lineages are named according to the current convention [9, 39]; strain C792_92 did not group within any lineage. The proportions of strains carrying plasmid gene profile characteristic for particular lineage (EHEC-hlyA/katP/espP/etpD) are denoted in parentheses. Predominant type of Shiga toxin and its proportion among strains of each particular lineage are denoted in parentheses. Core genome phylogeny was constructed from 12,205 variable bases among 3,718,610 validated homologous nucleotide positions. Phylogenetic tree was constructed with FastTree using approximately Maximum Likelihood phylogeny model [60]. Genomic sequence of E. coli O111:H− strain 11128 (stripped of plasmids, prophages and other horizontally acquired regions [68]) was used as an outgroup. For additional information about particular strains, see Additional file 1: Table S1. For a complete (unreduced) phylogram, see Additional file 2: Figure S1
Fig. 2.
Detailed whole-genome phylogeny of E. coli O26 nEC (ST29C2). Numbers and categories of SNPs synapomorphic for early nEC, late nEC or nEC in general (Additional file 4: Table S2) are indicated above particular branches. Strains sequenced in this study are denoted in green, strains whose genomic sequences were obtained from other sources are denoted in blue. Nodes supported by both FastTree and Neighbor-Joining methods of tree inference are denoted with asterisks. Countries of origins are abbreviated as follows: AUS, Austria; CZ, Czech Republic; FR, France; GER, Germany; IT, Italy; JAP, Japan; NE, Netherlands; NOR, Norway. The type of stx2a-converting prophage present in particular strain is indicated as in Fig. 4; strains exhibiting significant deviations from coverage pattern and/or mutational pattern characteristic for type I or type II prophages are denoted as “other”. Types of stx2a-converting prophages were not determined for whole genome assemblies obtained from other sources (n.d.). Amino acid change in Stx2a (273A → T) specific for type II prophages is denoted. Core genome phylogeny was constructed from 1,258 variable bases among 4,332,095 validated homologous nucleotide positions. Phylogenetic tree was constructed with FastTree using approximately Maximum Likelihood phylogeny model [60]. Genomic sequence of E. coli O26:H11 strain 11368 (stripped of plasmids, prophages and other horizontally acquired regions [68]) was used as an outgroup
Evolution, diversification and spread of the nEC
To infer which genetic events underlied the evolutionary establishment of the nEC and its subsequent split into the “early” and “late” lineages, we sought mutations characteristic for particular lineages, i.e., SNPs absent in other E. coli O26 phylogroups including ST21 and ST29 non-nEC strains (synapomorphic mutations; for details, see Methods). The sets of synapomorphic mutations detected by this approach (Additional file 4: Table S2) provide an unambiguous genetic definition of the particular nEC lineages. The total numbers of SNPs synapomorphic for early nEC, late nEC and nEC as a whole were 59, 70, and 83, respectively (Fig. 2). This justifies that the early nEC and late nEC should be considered distinct phylogenetic entities. Next, we focused on parallel evolution of missense synapomorphic mutations (which are likely to influence function of encoded proteins) within the nEC. Three cases where a single gene underwent multiple amino acid changes during various phases of nEC evolution were detected: cation transporter CusA and hypothetical proteins YehI and YggM accumulated two mutations each. Four operons accumulated two missense mutations each: glgBXCAP (glycogen metabolism), wza-wzb-wzc (capsular polysaccharide synthesis), hisJQMP (histidine transport) and gcl-hyi-glxR-ybbW-allB-ybbY-glxK (purine metabolism) (Additional file 4: Table S2). Notably, three mutations in fimbrial biogenesis proteins (ElfG, YqiG, and HtrE) accounted for 12% of 25 missense mutations synapomorphic for the late nEC (Additional file 4: Table S2). Since fimbriae are determinants of bacterial adherence, their modification might affect persistence of late nEC strains in mammal hosts or even pathogenesis in infected humans.
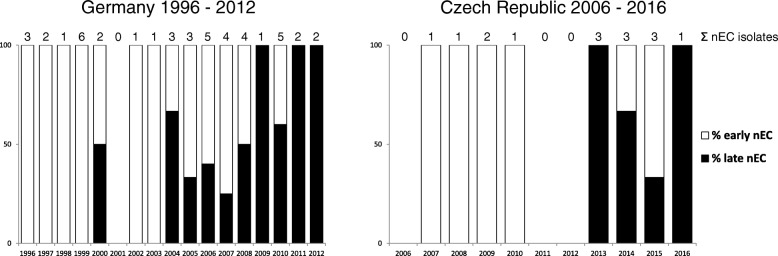
To rapidly differentiate between early nEC and late nEC strains, we designed a simple SNP-based PCR, which discriminates a mutation in the sen/ent gene (encoding Shigella flexneri enterotoxin 2 [40]), which is synapomorphic for the late nEC (Additional file 4: Table S2) (see Methods). This PCR was applied to screen all nEC isolates originating from Germany and the Czech Republic (45 and 15 isolates, respectively; Additional file 3: Table S3). Despite the limited numbers of isolates, a trend of the early nEC decline with a concomitant increase in the late nEC proportion was observed in both countries (Fig. 3). This suggests that the two lineages might occupy the same niche and compete with each other. The emergence of the late nEC in Germany (regularly isolated since 2004) preceded its first occurrence in the Czech Republic (2013) by nine years (Fig. 3). Strains belonging to the late nEC were also isolated in several other European countries including Austria (2008), Italy (2008, 2010), France (2013), Netherlands (2013) and Norway (2014), as well as in Japan (2008) (Fig. 2), which documents the pan-European and inter-continental distribution of this lineage.
Fig. 3.
Annual incidence of early nEC and late nEC isolates in two Central European countries. The charts depict proportions of early nEC and late nEC among nEC isolates (E. coli O26, ST29, EHEC-hlyA+, katP-, espP-, etpD+) collected in Germany and Czech Republic during time periods indicated. Early nEC and late nEC isolates were discriminated by the sen/ent SNP-specific PCR (see Methods and Additional file 3: Table S3)
nEC strains harbor different stx2a-converting prophages
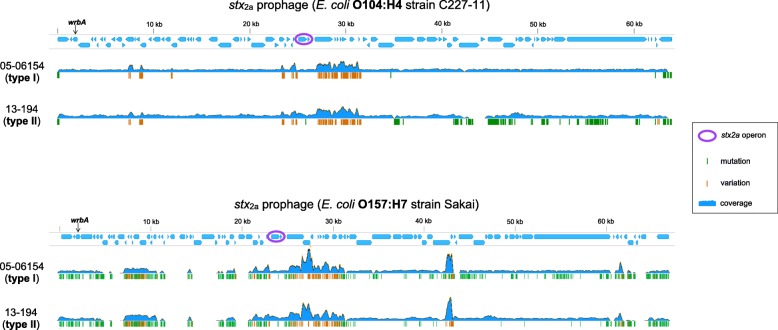
Shiga toxins encoded by stx genes are responsible for systemic complications of EHEC infections such as HUS. The stx genes are located in the genomes of lambdoid prophages (stx-converting phages) which are very heterogeneous (reviewed in [41]). A recent report [38] demonstrated that stx2a-converting prophages of EHEC O26 nEC strains were virtually identical with the stx2a-prophages present in the highly virulent E. coli O104:H4 German outbreak strain [42], while other O26 lineages harbored different stx-converting prophages [38]. To gain a deeper characterization of these elements in the early nEC and late nEC strains, we analyzed stx-converting prophages in all nEC genomes sequenced in this study, utilizing short sequencing reads and complete prophage sequences as references (Fig. 4). All stx2a-converting prophages in nEC strains were integrated into the wrbA gene which is also the site of phage integration in E. coli O104:H4 [43] and EHEC O157:H7 prototype strains Sakai [44] and EDL933 [45]. In agreement with Delannoy et al. [38], our results demonstrate that stx2a-converting prophages virtually identical with E. coli O104:H4 prophage (termed here “type I”) are predominant among nEC isolates. Unexpectedly, another distinct type of stx2a-converting prophage (termed here “type II”) was identified in four late nEC strains (Fig. 2). While the left half (5´-portion) of the type II prophage sequence was identical with O104:H4 prophage, the right half (3´-portion) diverged significantly (Fig. 4). The consensus sequences obtained by mapping of sequencing reads to the O104:H4 prophage reference were extracted from the divergent regions and searched against the BLAST database of complete E. coli genomes. The most closely related sequences were retrieved for stx2a-converting prophages from EHEC O157:H7 [46]; the high level of homology was confirmed by re-mapping of the sequencing reads to the O157:H7 prophage reference (Fig. 4). The presence of two DNA segments highly homologous to either O104:H4 or O157:H7 stx2a-converting prophages suggests a role of recombination in the evolution of type II prophages. This is in agreement with the view of recombination as the most common genetic event contributing to the mosaic structure and thus to the high genetic diversity of EHEC stx-converting phages [38, 41].
Fig. 4.
Different stx2a-converting prophages in EHEC O26 nEC strains. Visualization of sequencing reads mapped onto stx2a-converting prophages from E. coli O104:H4 strain C227–11 (GenBank accession: CP011331; top) and E. coli O157:H7 strain Sakai (GenBank accession: NC_002695; bottom). Strains 05–06154 and 13–194 represent the two distinct patterns of stx2a-converting prophages (type I and type II, respectively), which were detected among EHEC O26 nEC strains (see Fig. 2). Green vertical lines represent true mutations with respect to reference sequence. Orange vertical lines represent hybrid positions containing variant bases, which coincide with regions of abnormally high sequencing coverage (plausibly representing contributions of sequencing reads from other lambdoid prophages [68])
Remarkably, the type II prophages carry a single point mutation in the left half of their sequence, which is otherwise identical among type I, type II and O104 stx-converting prophages (Fig. 4). This mutation, which changes alanine (A) 273 into threonine (T) 273 in Stx2a A subunit (Fig. 2), is novel among the known diversity of Stx2 proteins and complements other mutations of A273 to amino acids with hydroxylated side chains (serine [S]and tyrosine [Y]- Additional file 5: Figure S2).
Stx production and cytotoxicity of nEC strains
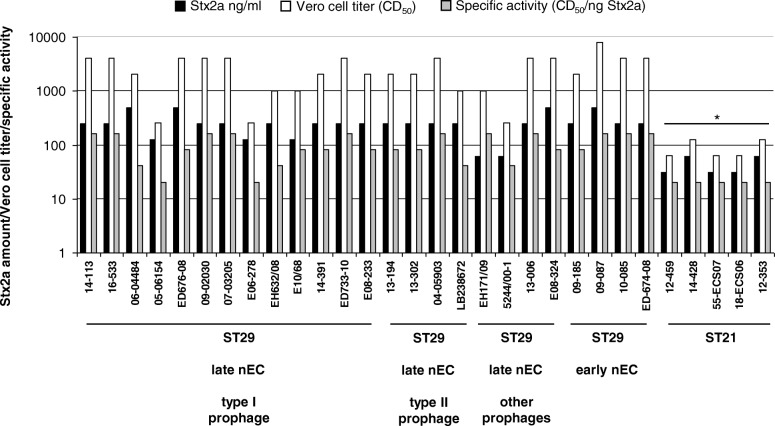
To determine whether or not the A273 → T273 mutation in Stx2a A subunit encoded by the type II stx2a-prophages influenced the toxicity of the resulting Stx2a protein, we compared the amounts, Vero cell cytotoxicity titers, and specific activities (CD50/ng toxin) of Stx2a produced by nEC strains harboring the type II prophages with those of strains harboring the type I or other stx2a-converting prophages. No significant differences were found (Fig. 5), indicating that the A273 → T273 mutation in the type II stx2a-prophage had no effect on Stx2a biological activity. Moreover, comparison of Stx2a amounts, cytotoxicity titers, and Stx2a specific activities between strains of the late nEC and early nEC did not reveal any differences in these characteristics within the ST29 nEC group (Fig. 5). However, all these characteristics of ST29 nEC strains were significantly higher than those of Stx2a-producing strains of ST21 clonal group (Fig. 5).
Fig. 5.
Stx2a production in nEC strains. The chart depicts Stx2a amounts, cytotoxicity titers, and specific activities of Stx2a produced by EHEC O26 ST29 late nEC strains harboring different types of stx2a-converting phages (as denoted in Fig. 2), ST29 early nEC strains and ST21 Stx2a-producing strains. The data are means from four measurements. *p < 0.01 for comparisons between the indicated characteristics of ST21 strains and ST29 strains (one-way ANOVA)
Discussion
Using genome-wide SNP-based analysis, we demonstrate that EHEC O26 strains belonging to the nEC are not phylogenetically homogeneous, but consist of two distinct lineages. This finding extends the present knowledge about the clonal structure of these pathogens and confirms previous reports [9, 31, 35, 39] that E. coli O26:H11/H− are, from the evolutionary point of view, highly dynamic with a potential for novel virulent clones to emerge rapidly. Based on their consecutive appearance and spread within Germany and the Czech Republic, we propose to term these lineages “early” nEC and “late” nEC, respectively. nEC was reported to have arisen in Germany during the 1990s [31, 33]; currently, both nEC lineages are widely distributed in Europe and were also isolated in Japan (Fig. 2), which highlights that nEC strains are capable of rapid spreading across countries and continents. Given the genetic distinctness of the early and late nEC, their expansions are likely to have occurred independently. Although the spread via routes common to other EHEC (i.e. via livestock and/or food trade) seems to be most likely, E. coli O26 corresponding to the nEC have only rarely been isolated from cattle and other animals [47, 48]. Moreover, none of 10 E. coli O26 nEC isolates (ST29C2 clade) present among 520 whole genome sequences analyzed by Ogura et al. [39], which represent a global population of human and animal E. coli O26, originated from cattle or other animals. Thus, reservoirs and possible ways of the worldwide spread of E. coli O26 nEC in general and of the late nEC in particular need to be further elucidated. This is particularly important because of the increasing frequency of the late nEC strains as causes of human diseases in some European countries during last years (Fig. 3). To create a basis for effective measures to control further spread of these pathogens, screening of ST29 E. coli O26 isolates from cattle and other animals, using the sen/ent SNP-based PCR developed in this study, combined with plasmid gene profiling might be a useful and simple tool for identification of strains of the early and late nEC. Altogether, the emergence of the late nEC both throughout and outside Europe supports a continuous evolution of E. coli O26:H11/H− and a high propensity of new clones to spread [9, 35, 39].
Analysis of stx2a-converting prophages in nEC strains (Fig. 4) identified a predominant prophage (designated type I), which displayed a nearly complete sequence identity with the stx2a-converting prophages of EHEC O104:H4 strain, which caused the large devastating outbreak of HUS in Germany in 2011 [43]. Type I prophage is widespread across the nEC diversity (Fig. 2), implicating a single acquisition during early evolution of nEC (as also suggested in [38]). Based on available bibliographic data, the emergence of EHEC O26 nEC in the 1990s [31, 33] predates the isolation of Stx2a-producing EHEC O104:H4 [32, 43, 49], which was first isolated in 2001 [32]. This allows to speculate that EHEC O26 belonging to the nEC might have served as donors of stx2a-converting phages for enteroaggregative E. coli O104:H4 in the final step of evolution of the highly virulent, Stx2a-producing EHEC O104:H4 outbreak strain. Alternatively, EHEC O26 nEC and EHEC O104:H4 outbreak strain might have acquired their stx2a-converting phages independently from a common source. Beutin et al. [50] proposed that stx2a-converting prophages of EHEC O104:H4 might have originated from stx2a-converting phages present among bovine E. coli of various serotypes, with which they share nucleotide sequences of two specific DNA fragments, virion morphology, DNA restriction patterns, chromosomal integration site, and superinfection immunity [50]; however, this hypothesis needs to be confirmed by complete sequence analyses of the bovine phages.
Four late nEC strains were found to harbor a stx2a-converting prophage whose sequence consisted of two regions highly homologous to EHEC O104:H4 and EHEC O157:H7 stx2a-phages, respectively (designed type II; Fig. 4). These strains were scattered throughout the whole-genome phylogenetic tree and their most closely related strains harbored type I prophages (Fig. 2). This provides evidence that horizontal, rather than vertical pattern of type II prophages transfer was prevalent in late nEC evolution, thus suggesting an ongoing dissemination of type II phages in the E. coli O26 population. Notably, Stx2a encoded by type II prophages contain a novel amino acid change (A273 → T273). This mutation does not affect the potency of the toxin as demonstrated by its specific activity for Vero cells, which is similar to that of strains harboring type I prophages (Fig. 5). However, the localization of the mutation suggests a possible connection with Stx2a maturation. A273 follows immediately after arginine [R] 272; cleavage of Stx by the Golgi protease furin takes place precisely between these two residues [51, 52]. The cleavage generates the enzymatically active form of Stx2a A subunit [53]. The furin site where A273 binding takes place is a small hydrophilic pocket [54], which could explain the propensity of A273 for substitutions with hydroxyl-containing amino acids (Additional file 5: Figure S2). If there are any biological effects conferred by this Stx2a mutation specific for type II prophages, remains to be elucidated.
Conclusions
Using genome-wide SNP-based analysis, this study presents the evidence of the split of the EHEC O26:H11/H− nEC, which emerged in Germany in the 1990s and has spread throughout Europe [31], into two cryptic, yet distinct clones (early nEC and late nEC, Fig. 2). For EHEC O26, the combination of MLST, plasmid gene profiling and PCR-based stx subtyping has been regarded as sufficient for clone discrimination [9, 31]. The fact that strains of the early nEC and late nEC are indistinguishable by this approach (Fig. 1) emphasizes the necessity of using highly discriminatory methods such as the genome-wide SNP-based analysis to distinguish the most closely related epidemic lineages. For practical purposes, the SNP-based PCR developed in this study which targets a mutation in the sen/ent gene synapomorphic for the late nEC, represents a rapid and easy tool for distinguishing the early and late nEC strains in clinical microbiological laboratories and field studies. This will, in turn, enable further investigations of the geographic distribution of these pathogens, their clinical significance, and the epidemiology of human infections they cause.
Our results confirm that EHEC O26 nEC strains and E. coli O104:H4 German outbreak strain [43] share the same type of stx2a-converting prophage [38]. An additional stx2a-converting prophage (type II), present in a subset of the late nEC strains, displays several peculiarities (hybrid sequence, novel missense mutation in stx2a, multiple horizontal transmissions among late nEC) which warrant further investigation.
In summary, the discriminatory power of whole genome sequencing allows for accurate reconstruction of evolutionary events which accompanied the emergence of novel clonal lineages of EHEC O26.
Methods
Database mining
Escherichia coli complete or draft genomic sequences were retrieved from the GenBank database [55] on January 31st, 2017. The precompiled genome neighbors of E. coli strain St. Olav179 (a member of late nEC, accession JZED00000000 [56]) were extracted. Strains with symmetric identity below 92.0% were discarded since they typically represented serotypes other than O26. Among remaining strains, those belonging to non-O26 serotypes were discarded. In addition, genomic sequences of strains included in later studies which focused on genomic evolution of E. coli O26 [9, 38] were included in the dataset. In total, 127 strains from public sources were collected for genomic comparisons (Additional file 1: Table S1).
Whole-genome sequencing
Sixteen EHEC O26:H11/H− strains were isolated from patients with HUS (n = 10) or with bloody (n = 4) or non-bloody (n = 2) diarrhea without HUS in the Czech Republic between 2006 and 2016. Additional 16 EHEC O26:H11/H− nEC strains sequenced in this study originated from Germany (n = 11), Italy (n = 3), and Austria (n = 2) and were isolated from patients with HUS (n = 10) or non-bloody diarrhea (n = 6) (Additional file 1: Table S1). The Czech and German strains were from the authors´ laboratories. The Italian and Austrian isolates were kindly provided by Stefano Morabito (European Union Reference Laboratory for E. coli, Instituto Superiore di Sanità, Rome, Italy) and Dorothea Orth-Höller (Division of Hygiene and Medical Microbiology, Innsbruck Medical University, Austria), respectively. WGS was performed as described previously [57]. Briefly, after inoculation of a single colony into nutrient broth (Heipha, Eppelheim, Germany) and overnight incubation (37 °C), genomic DNA was extracted using a MagAttract HMW DNA kit (Qiagen, Hilden, Germany) with the addition of lysozyme (Sigma-Aldrich, Taufkirchen, Germany) following the manufacturer’s instructions. Subsequently, the sequencing libraries were prepared from the genomic DNA using the Nextera sample preparation kit (Illumina, Inc., San Diego, CA, USA) for a 150-bp or 250-bp paired-end sequencing run on a single MiSeq instrument (Illumina) in accordance with the manufacturer’s recommendations. Libraries were scaled to reach 100-fold sequencing coverage. Subsequent quality trimming and de novo assembly were performed using the default parameters of CLC Genomics Workbench software (CLC bio, Arhus, Denmark) and the CLC Genomics Workbench de novo assembler (CLC bio). The draft genomes of strains sequenced in our study were annotated using the NCBI prokaryotic genome annotation pipeline [58] and are available in the GenBank under the accession numbers listed in Additional file 1: Table S1.
Bioinformatic analysis
Phylogeny reconstruction
Genome-wide SNP-based phylogeny was chosen for robust inference of E. coli O26 evolutionary history. Genomic sequences were uploaded to the CSIPhylogeny v1.4 website (https://cge.cbs.dtu.dk/services/CSIPhylogeny/) and automatically processed with default settings. SNP analysis was carried out using a set of algorithms as described in [59]; FastTree [60] was used for phylogram construction. Phylogenetic trees were visualized using MEGA7 [61]. Genomic sequences of E. coli O111:H− strain 11128 and E. coli O26:H11 strain 11368 were included as reference genomes for EHEC O26 and nEC SNP-based phylogeny, respectively.
In silico analysis of molecular diagnostic markers
The presence of pathogenicity-related genes (plasmid genes EHEC-hlyA, katP, espP and etpD, Shiga toxin genes stx1a and stx2a) was assessed using TBLASTN [62] integrated in Geneious R9.1 [63], using protein query sequences from E. coli O26 strain 11368 (GenBank accession: AP010953) or E. coli O157 strain Sakai (GenBank accession: NC_002695). Presence or absence and identity of encoded proteins with respect to query sequences are listed in Additional file 1: Table S1.
Synapomorphic SNPs determination
Genomic sequences of several strains representing the genomic diversity of E. coli O26 (early nEC: St. Olav174, FHI24, FHI27; late nEC: ED676, St. Olav179, 36708, STEC931; ST21: ED180, ED729, STEC1117, CVM9942, non-nEC ST29: C792_92, CFSAN025102, 200C-3689, 34827) were aligned with Progressive MAUVE [64]. Presumptive synapomorphic SNPs (i.e. SNPs specific for early nEC, late nEC or nEC in general) were manually identified among the SNPs reported by MAUVE intergenomic comparison tool, based on their exclusive occurrence in corresponding lineages. For the definite validation, BLAST search [65] was performed against all genomic sequences included in the study (Additional file 1: Table S1); SNPs were considered synapomorphic if they were present in all members of a particular lineage and absent in all remaining O26 strains (Additional file 4: Table S2).
Stx-converting phage analysis
The paired-end sequencing reads were mapped onto stx2a-converting prophage sequences present in E. coli O104:H4 strain C227–11 (GenBank accession: CP011331) and E. coli O157:H7 strain Sakai (GenBank accession: NC_002695) using Geneious R9 platform [63]. The mapping setting was as follows: max. 20% gaps per read, max. 10% mismatches per read. SNPs (true mutations) were called with coverage ≥5 and frequency ≥ 90% (with respect to the reference). Variable sites were called where a mixture of bases was present in assembly of sequencing reads (coverage ≥5, frequency of minor variant ≥30%).
Late nEC-discriminating PCR
A SNP-based PCR was used as a quick screening method to detect a missense mutation in the sen/ent gene (encoding Shigella flexneri enterotoxin 2), specific for late nEC (102S → Y; Additional file 4: Table S2). The following primers were designed using Primer/BLAST [66]: forward primer sen-F (5´-TCTAAATGGAAAGGTTAGTGATTGC-3′) and reverse primer sen-RG (5´-GGTTATATATAACGCTTCCCCAAG-3′). In sen-RG, the 3′-terminal guanine is not complementary to the late nEC-specific SNP. The predicted amplicon length was 233 bp. PCR reactions were performed in CFX96 Touch PCR Detection System (Bio-Rad) using reagents from Top-Bio (Czech Republic), primers obtained from Generi Biotech (Czech Republic) and bacterial lysates (boiling method) as DNA templates. PCR conditions were: 94 °C for 5 min, followed by 30 cycles of denaturation (94 °C for 30 s), annealing (53 °C for 60 s), and extension (72 °C for 60 s) and a final extension step at 72 °C for 5 min. One early nEC and one late nEC strain were used as controls in each PCR. After electrophoretic separation on agarose gel and ethidium bromide staining, early nEC strains produced strong amplicons, while late nEC strains displayed substantially weaker amplicons due to a reduced pairing of 3′ guanine with template DNA containing the SNP (Additional file 3: Table S3). All nEC strains analyzed by WGS in this study were tested with the late nEC-discriminating PCR; 100% correlation between the sen/ent-specific PCR and WGS results was achieved (Additional file 3: Table S3).
Detection of Stx production and cytotoxicity
VTEC RPLA
VTEC RPLA (Verotoxin-producing E. coli reverse passive latex agglutination) assay (Denka Seiken Company, Ltd., Tokyo, Japan) was performed, according to the manufacturer’s instructions, with serially diluted sterile supernatants prepared from overnight shaken broth cultures by centrifugation (15 min, 4000 rpm, 4 °C) and subsequent filtration through 0.22 μm syringe filters (P-LAB, Prague, Czech Republic). Stx1a and Stx2a titers were expressed as reciprocals of the supernatants´ dilutions that produced a clear agglutination of latex particles sensitized with anti-Stx1a and anti-Stx2a antibody, respectively. Stx1a and Stx2a in the supernatants were quantified by comparison of titers produced by the supernatants with those of Stx2a (VT2) and Stx1a (VT1) standards with known toxin concentrations.
Vero cell assay
Vero-B4 cells (ACC-33; German collection of microorganisms and cell cultures, Braunschweig, Germany) were maintained in Dulbecco’s modified Eagle medium (DMEM) with 4.5 g/l of glucose and glutamine (Lonza, Cologne, Germany) supplemented with 10% of fetal bovine serum (Sigma-Aldrich, Prague, Czech Republic). In the cytotoxicity assay [67], semiconfluent Vero cell monolayers grown in 96-well plates were incubated with two-fold dilutions of supernatants prepared as above for 72 h. After removing medium with detached cells, remnant adherent cells were fixed (2% formalin), stained (0.13% crystal violet), washed, and after eluting of crystal violet with 50% ethanol, absorbance (OD570) was measured by FLUOstar Omega (BMG Labtech, Ortenberg, Germany). Cytotoxicity titers were expressed as reciprocal values of the sample dilutions that killed 50% of cells (CD50). Specific activities of Stx in supernatants were expressed as CD50/ng of Stx.
Statistical analysis
Data on Stx2a amounts, cytotoxicity titers, and specific activities of Stx2a produced by different groups of strains were analyzed with one-way analysis of variance (ANOVA). p values < 0.05 were considered significant.
Additional files
Table S1. Summary information about 159 E. coli O26 isolates whose genomic sequences were included for comparative analysis. Presence/absence of molecular diagnostic markers and their identity with query sequences are indicated. (DOCX 144 kb)
Figure S1. Core genome-derived phylogeny of 159 E. coli O26 isolates. The phylogram (inferred using the CSIPhylogeny pipeline [59]) represents the full version of contracted phylogram presented in Fig. 1. (PDF 32 kb)
Table S3. Discrimination of early nEC and late nEC strains by sen/ent SNP-specific PCR, WGS and PFGE. All nEC strains characterized in this study by WGS (Fig. 2) and/or by sen/ent SNP-specific PCR (Fig. 3) are listed, together with results obtained by either method and PFGE clustering [31] (n.a., not available). Representative gel image which demonstrates the performance of the sen/ent SNP-specific PCR in differentiating early- and late nEC strains is included. (XLSX 69 kb)
Table S2. SNPs synapomorphic for the early nEC, late nEC or nEC as whole. Missense mutations in fimbrial proteins are denoted in bold. (XLS 112 kb)
Figure S2. Alignment of all unique Stx2 protein sequences present in the GenBank. Residue A273 is marked with a red rectangle on the top of alignment. (PDF 6180 kb)
Acknowledgements
We thank Dr. Stephano Morabito (European Union Reference Laboratory for E. coli, Istituto Superiore di Sanità, Rome, Italy) and Dr. Dorothea Orth-Höller (Division of Hygiene and Medical Microbiology, Innsbruck Medical University, Austria) for providing us with EHEC O26 strains isolated in Italy and Austria, respectively. We are indebted to Drs. Nozomi Ishijima and Sunao Iyoda (Department of Bacteriology, National Institute of Infectious Diseases, Tokyo, Japan) for providing us with the draft genome assemblies of Japanese EHEC O26 strains. We are grateful to Dr. Michal Malina (Department of Paediatrics, 2nd Faculty of Medicine, Charles University and Motol University Hospital, Prague) for his advice and help during the preparation of this project and to Dr. Ondrej Soucek (Laboratory of Vera Vavrova/VIAL, Department of Paediatrics, 2nd Faculty of Medicine, Charles University and Motol University Hospital, Prague) for help with spectrophotometry. A skillful technical assistance of Veronika Horova (NIPH Prague), Ralph Fischer and Andrea Lagemann (Institute for Hygiene, Münster) is greatly appreciated.
Funding
This study was supported by the Grant Agency of the Charles University (194215) and by the Ministry of Health of the Czech Republic (funding project Conceptual development of research organization “The National Institute of Public Health - NIPH 75010330”). PD and JN acknowledge financial support from Czech Health Research Council of Ministry of Health of the Czech Republic, grant no. 15-28017A. AM acknowledges funding from the German Research Foundation (DFG), grant no. ME3205/2–1. The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All draft genome sequences were submitted to the GenBank database of National Center for Biotechnology Information (Bioproject accession number: PRJNA470577).
Authors´ contributions
JN, LK, MB, PD and KB designed the study. LK and AM performed whole-genome sequencing. JN and LK performed bioinformatic analysis. LK, HH, MM and MB performed laboratory experiments and analyzed the data. MM, HK, AF, AM and MB collected the strains. PD and KB supervised the project. JN, LK, MM and MB drafted the manuscript. All authors have critically revised the manuscript and approved it for submission and publication.
Ethics approval and consent to participate
The use of the Czech isolates was approved by the Ethical Committee of the University Hospital Motol, Prague, Czech Republic. Written informed consent for enrolment in the study was obtained from parents of all patients. The isolates from Germany and other countries available in the collection of the Institute of Hygiene, University of Münster, were used in accordance with guidelines approved by the Ethical Committee of the Medical Faculty of the University of Münster and of the Ärztekammer Westfalen-Lippe. The informed consent of the participants was not required because the data were analyzed anonymously.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lucia Karnisova, Email: lucia.karnisova@fnmotol.cz.
Monika Marejkova, Email: monika.marejkova@szu.cz.
Hana Hrbackova, Email: hana.hrbackova@szu.cz.
Alexander Mellmann, Email: mellmann@uni-muenster.de.
Helge Karch, Email: hkarch@uni-muenster.de.
Angelika Fruth, Email: FruthA@rki.de.
Pavel Drevinek, Email: pavel.drevinek@lfmotol.cuni.cz.
Kveta Blahova, Email: kveta.blahova@lfmotol.cuni.cz.
Martina Bielaszewska, Email: martina.bielaszewska@szu.cz.
Jaroslav Nunvar, Email: jaroslav.nunvar@lfmotol.cuni.cz.
References
- 1.The European Union summary report on trends and sources of zoonoses Zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15(12):e05077. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne L, Jenkins C, Launders N, Elson R, Adak GK. The epidemiology, microbiology and clinical impact of Shiga toxin-producing Escherichia coli in England, 2009-2012. Epidemiol Infect. 2015;143(16):3475–3487. doi: 10.1017/S0950268815000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caprioli A, Scavia G, Morabito S. Public health microbiology of Shiga toxin-producing Escherichia coli. Microbiol Spectr. 2014;2(6) [DOI] [PubMed]
- 4.Elliott EJ, Robins-Browne RM, O'Loughlin EV, Bennett-Wood V, Bourke J, Henning P, Hogg GG, Knight J, Powell H, Redmond D, et al. Nationwide study of haemolytic uraemic syndrome: clinical, microbiological, and epidemiological features. Arch Dis Child. 2001;85(2):125–131. doi: 10.1136/adc.85.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espié E, Grimont F, Mariani-Kurkdjian P, Bouvet P, Haeghebaert S, Filliol I, Loirat C, Decludt B, Minh NN, Vaillant V, et al. Surveillance of hemolytic uremic syndrome in children less than 15 years of age, a system to monitor O157 and non-O157 Shiga toxin-producing Escherichia coli infections in France, 1996-2006. Pediatr Infect Dis J. 2008;27(7):595–601. doi: 10.1097/INF.0b013e31816a062f. [DOI] [PubMed] [Google Scholar]
- 6.Gerber A, Karch H, Allerberger F, Verweyen HM, Zimmerhackl LB. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997-2000, in Germany and Austria: a prospective study. J Infect Dis. 2002;186(4):493–500. doi: 10.1086/341940. [DOI] [PubMed] [Google Scholar]
- 7.Gould LH, Mody RK, Ong KL, Clogher P, Cronquist AB, Garman KN, Lathrop S, Medus C, Spina NL, Webb TH, et al. Increased recognition of non-O157 Shiga toxin-producing Escherichia coli infections in the United States during 2000-2010: epidemiologic features and comparison with E coli O157 infections. Foodborne Pathog Dis. 2013;10(5):453–460. doi: 10.1089/fpd.2012.1401. [DOI] [PubMed] [Google Scholar]
- 8.Hedican EB, Medus C, Besser JM, Juni BA, Koziol B, Taylor C, Smith KE. Characteristics of O157 versus non-O157 Shiga toxin-producing Escherichia coli infections in Minnesota, 2000-2006. Clin Infect Dis. 2009;49(3):358–364. doi: 10.1086/600302. [DOI] [PubMed] [Google Scholar]
- 9.Ishijima N, Lee KI, Kuwahara T, Nakayama-Imaohji H, Yoneda S, Iguchi A, Ogura Y, Hayashi T, Ohnishi M, Iyoda S. Identification of a new virulent clade in enterohemorrhagic Escherichia coli O26:H11/H- sequence type 29. Sci Rep. 2017;7:43136. doi: 10.1038/srep43136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Käppeli U, Hächler H, Giezendanner N, Beutin L, Stephan R. Human infections with non-O157 Shiga toxin-producing Escherichia coli, Switzerland, 2000-2009. Emerg Infect Dis. 2011;17(2):180–185. doi: 10.3201/eid1702.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karch H, Tarr PI, Bielaszewska M. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol. 2005;295(6–7):405–418. doi: 10.1016/j.ijmm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Marejková M, Bláhová K, Janda J, Fruth A, Petráš P. Enterohemorrhagic Escherichia coli as causes of hemolytic uremic syndrome in the Czech Republic. PLoS One. 2013;8(9):e73927. doi: 10.1371/journal.pone.0073927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollock KG, Bhojani S, Beattie TJ, Allison L, Hanson M, Locking ME, Cowden JM. Highly virulent Escherichia coli O26 Scotland. Emerg Infect Dis. 2011;17(9):1777–1779. doi: 10.3201/eid1709.110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivas M, Miliwebsky E, Chinen I, Roldán CD, Balbi L, García B, Fiorilli G, Sosa-Estani S, Kincaid J, Rangel J, et al. Characterization and epidemiologic subtyping of Shiga toxin-producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodborne Pathog Dis. 2006;3(1):88–96. doi: 10.1089/fpd.2006.3.88. [DOI] [PubMed] [Google Scholar]
- 15.Smith AM, Bosco KJ, Nicol MP, Kleynhans J, McCulloch M, Duze ST, Ismail A, Allam M, Tau NP, Keddy KH. Genome sequence for Shiga toxin-producing Escherichia coli O26:H11, associated with a cluster of hemolytic-uremic syndrome cases in South Africa, 2017. Genome Announc. 2017;5(38):e00989-17. [DOI] [PMC free article] [PubMed]
- 16.Tozzi AE, Caprioli A, Minelli F, Gianviti A, De Petris L, Edefonti A, Montini G, Ferretti A, De Palo T, Gaido M, et al. Shiga toxin-producing Escherichia coli infections associated with hemolytic uremic syndrome, Italy, 1988-2000. Emerg Infect Dis. 2003;9(1):106–108. doi: 10.3201/eid0901.020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricotti GC, Buonomini MI, Merlitti A, Karch H, Luzzi I, Caprioli A. A fatal case of hemorrhagic colitis, thrombocytopenia, and renal failure associated with verocytotoxin-producing, non-O157 Escherichia coli. Clin Infect Dis. 1994;19(4):815–816. doi: 10.1093/clinids/19.4.815. [DOI] [PubMed] [Google Scholar]
- 18.Rosales A, Hofer J, Zimmerhackl LB, Jungraithmayr TC, Riedl M, Giner T, Strasak A, Orth-Höller D, Würzner R, Karch H, et al. Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin Infect Dis. 2012;54(10):1413–1421. doi: 10.1093/cid/cis196. [DOI] [PubMed] [Google Scholar]
- 19.Zieg J, Dusek J, Marejkova M, Limrova P, Blazek D, Pavlicek P, Grega M, Janda J, Blahova K. Fatal case of diarrhea-associated hemolytic uremic syndrome with severe neurologic involvement. Pediatr Int. 2012;54(1):166–167. doi: 10.1111/j.1442-200X.2011.03533.x. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerhackl LB, Rosales A, Hofer J, Riedl M, Jungraithmayr T, Mellmann A, Bielaszewska M, Karch H. Enterohemorrhagic Escherichia coli O26:H11-associated hemolytic uremic syndrome: bacteriology and clinical presentation. Semin Thromb Hemost. 2010;36(6):586–593. doi: 10.1055/s-0030-1262880. [DOI] [PubMed] [Google Scholar]
- 21.Dallman TJ, Byrne L, Launders N, Glen K, Grant KA, Jenkins C. The utility and public health implications of PCR and whole genome sequencing for the detection and investigation of an outbreak of Shiga toxin-producing Escherichia coli serogroup O26:H11. Epidemiol Infect. 2015;143(8):1672–1680. doi: 10.1017/S0950268814002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germinario C, Caprioli A, Giordano M, Chironna M, Gallone MS, Tafuri S, Minelli F, Maugliani A, Michelacci V, Santangelo L, et al. Community-wide outbreak of haemolytic uraemic syndrome associated with Shiga toxin 2-producing Escherichia coli O26:H11 in southern Italy summer 2013. Euro Surveill. 2016;21(38) [DOI] [PMC free article] [PubMed]
- 23.Hines JZ, Bancroft J, Powell M, Hedberg K. Case finding using syndromic surveillance data during an outbreak of Shiga toxin-producing Escherichia coli O26 infections, Oregon 2015. Public Health Rep. 2017;132(4):448–450. doi: 10.1177/0033354917708994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanayama A, Yahata Y, Arima Y, Takahashi T, Saitoh T, Kanou K, Kawabata K, Sunagawa T, Matsui T, Oishi K. Enterohemorrhagic Escherichia coli outbreaks related to childcare facilities in Japan, 2010-2013. BMC Infect Dis. 2015;15:539. doi: 10.1186/s12879-015-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luna-Gierke RE, Griffin PM, Gould LH, Herman K, Bopp CA, Strockbine N, Mody RK. Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol Infect. 2014;142(11):2270–2280. doi: 10.1017/S0950268813003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misselwitz J, Karch H, Bielazewska M, John U, Ringelmann F, Rönnefarth G, Patzer L. Cluster of hemolytic-uremic syndrome caused by Shiga toxin-producing Escherichia coli O26:H11. Pediatr Infect Dis J. 2003;22(4):349–354. doi: 10.1097/01.inf.0000059338.38673.ae. [DOI] [PubMed] [Google Scholar]
- 27.Moran-Gilad J, Rokney A, Danino D, Ferdous M, Alsana F, Baum M, Dukhan L, Agmon V, Anuka E, Valinsky L, et al. Real-time genomic investigation underlying the public health response to a Shiga toxin-producing Escherichia coli O26:H11 outbreak in a nursery. Epidemiol Infect. 2017;145(14):2998–3006. doi: 10.1017/S0950268817001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scavia G, Gianviti A, Labriola V, Chiani P, Maugliani A, Michelacci V, Minelli F, Tozzoli R, Caprioli A, Morabito S. A case of haemolytic uraemic syndrome (HUS) revealed an outbreak of Shiga toxin-2-producing Escherichia coli O26:H11 infection in a nursery, with long-lasting shedders and person-to-person transmission, Italy 2015. J Med Microbiol. 2018;67(6):775-82. [DOI] [PubMed]
- 29.Severi E, Vial F, Peron E, Mardh O, Niskanen T, Takkinen J. Community-wide outbreaks of haemolytic uraemic syndrome associated with Shiga toxin-producing Escherichia coli O26 in Italy and Romania: a new challenge for the European Union. Euro Surveill. 2016;21(49) [DOI] [PMC free article] [PubMed]
- 30.Werber D, Fruth A, Liesegang A, Littmann M, Buchholz U, Prager R, Karch H, Breuer T, Tschäpe H, Ammon A. A multistate outbreak of Shiga toxin-producing Escherichia coli O26:H11 infections in Germany detected by molecular subtyping surveillance. J Infect Dis. 2002;186(3):419–422. doi: 10.1086/341457. [DOI] [PubMed] [Google Scholar]
- 31.Bielaszewska M, Mellmann A, Bletz S, Zhang W, Köck R, Kossow A, Prager R, Fruth A, Orth-Höller D, Marejková M, et al. Enterohemorrhagic Escherichia coli O26:H11/H-: a new virulent clone emerges in Europe. Clin Infect Dis. 2013;56(10):1373–1381. doi: 10.1093/cid/cit055. [DOI] [PubMed] [Google Scholar]
- 32.Mellmann A, Bielaszewska M, Köck R, Friedrich AW, Fruth A, Middendorf B, Harmsen D, Schmidt MA, Karch H. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerg Infect Dis. 2008;14(8):1287–1290. doi: 10.3201/eid1408.071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang WL, Bielaszewska M, Liesegang A, Tschäpe H, Schmidt H, Bitzan M, Karch H. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26 strains. J Clin Microbiol. 2000;38(6):2134–2140. doi: 10.1128/jcm.38.6.2134-2140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delannoy S, Mariani-Kurkdjian P, Bonacorsi S, Liguori S, Ison SA, Fach P. Draft genome sequences of human-pathogenic Escherichia coli O26:H11 strains carrying the stx2 gene only and circulating in France. Genome Announc. 2015;3(4) [DOI] [PMC free article] [PubMed]
- 35.Delannoy S, Mariani-Kurkdjian P, Bonacorsi S, Liguori S, Fach P. Characteristics of emerging human-pathogenic Escherichia coli O26:H11 strains isolated in France between 2010 and 2013 and carrying the stx2d gene only. J Clin Microbiol. 2015;53(2):486–492. doi: 10.1128/JCM.02290-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grande L, Michelacci V, Bondì R, Gigliucci F, Franz E, Badouei MA, Schlager S, Minelli F, Tozzoli R, Caprioli A, et al. Whole-genome characterization and strain comparison of VT2f-producing Escherichia coli causing hemolytic uremic syndrome. Emerg Infect Dis. 2016;22(12):2078–2086. doi: 10.3201/eid2212.160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bletz S, Bielaszewska M, Leopold SR, Köck R, Witten A, Schuldes J, Zhang W, Karch H, Mellmann A. Evolution of enterohemorrhagic Escherichia coli O26 based on single-nucleotide polymorphisms. Genome Biol Evol. 2013;5(10):1807–1816. doi: 10.1093/gbe/evt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delannoy S, Mariani-Kurkdjian P, Webb HE, Bonacorsi S, Fach P. The mobilome; a major contributor to Escherichia coli stx2-positive O26:H11 strains intra-serotype diversity. Front Microbiol. 2017;8:1625. doi: 10.3389/fmicb.2017.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogura Y, Gotoh Y, Itoh T, Sato MP, Seto K, Yoshino S, Isobe J, Etoh Y, Kurogi M, Kimata K, et al. Population structure of Escherichia coli O26 : H11 with recent and repeated stx2 acquisition in multiple lineages. Microb Genom. 2017;3(11) [DOI] [PMC free article] [PubMed]
- 40.Nataro JP, Seriwatana J, Fasano A, Maneval DR, Guers LD, Noriega F, Dubovsky F, Levine MM, Morris JG. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect Immun. 1995;63(12):4721–4728. doi: 10.1128/iai.63.12.4721-4728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krüger A, Lucchesi PM. Shiga toxins and stx phages: highly diverse entities. Microbiology. 2015;161(Pt 3):451–462. doi: 10.1099/mic.0.000003. [DOI] [PubMed] [Google Scholar]
- 42.Laing CR, Zhang Y, Gilmour MW, Allen V, Johnson R, Thomas JE, Gannon VP. A comparison of Shiga-toxin 2 bacteriophage from classical enterohemorrhagic Escherichia coli serotypes and the German E. coli O104:H4 outbreak strain. PLoS One. 2012;7(5):e37362. doi: 10.1371/journal.pone.0037362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One. 2011;6(7):e22751. doi: 10.1371/journal.pone.0022751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, Han CG, Ohtsubo E, Nakayama K, Murata T, et al. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001;8(1):11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 45.Perna NT, Plunkett G, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, et al. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature. 2001;409(6819):529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 46.Ogura Y, Mondal SI, Islam MR, Mako T, Arisawa K, Katsura K, Ooka T, Gotoh Y, Murase K, Ohnishi M, et al. The Shiga toxin 2 production level in enterohemorrhagic Escherichia coli O157:H7 is correlated with the subtypes of toxin-encoding phage. Sci Rep. 2015;5:16663. doi: 10.1038/srep16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allerberger F, Friedrich AW, Grif K, Dierich MP, Dornbusch HJ, Mache CJ, Nachbaur E, Freilinger M, Rieck P, Wagner M, et al. Hemolytic-uremic syndrome associated with enterohemorrhagic Escherichia coli O26:H infection and consumption of unpasteurized cow's milk. Int J Infect Dis. 2003;7(1):42–45. doi: 10.1016/S1201-9712(03)90041-5. [DOI] [PubMed] [Google Scholar]
- 48.Zweifel C, Cernela N, Stephan R. Detection of the emerging Shiga toxin-producing Escherichia coli O26:H11/H- sequence type 29 (ST29) clone in human patients and healthy cattle in Switzerland. Appl Environ Microbiol. 2013;79(17):5411–5413. doi: 10.1128/AEM.01728-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou K, Ferdous M, de Boer RF, Kooistra-Smid AM, Grundmann H, Friedrich AW, Rossen JW: The mosaic genome structure and phylogeny of Shiga toxin-producing Escherichia coli O104:H4 is driven by short-term adaptation. Clin Microbiol Infect 2015, 21(5):468.e467–418. [DOI] [PubMed]
- 50.Beutin L, Hammerl JA, Reetz J, Strauch E. Shiga toxin-producing Escherichia coli strains from cattle as a source of the Stx2a bacteriophages present in enteroaggregative Escherichia coli O104:H4 strains. Int J Med Microbiol. 2013;303(8):595–602. doi: 10.1016/j.ijmm.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Garred O, van Deurs B, Sandvig K. Furin-induced cleavage and activation of Shiga toxin. J Biol Chem. 1995;270(18):10817–10821. doi: 10.1074/jbc.270.18.10817. [DOI] [PubMed] [Google Scholar]
- 52.Fagerquist CK, Sultan O. Top-down proteomic identification of furin-cleaved α-subunit of Shiga toxin 2 from Escherichia coli O157:H7 using MALDI-TOF-TOF-MS/MS. J Biomed Biotechnol. 2010;2010:123460. doi: 10.1155/2010/123460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li XP, Tumer NE. Differences in ribosome binding and sarcin/ricin loop depurination by Shiga and ricin holotoxins. Toxins (Basel). 2017;9(4) [DOI] [PMC free article] [PubMed]
- 54.Tian S. A 20 residues motif delineates the furin cleavage site and its physical properties may influence viral fusion. Biochemistry Insights. 2009;2:BCI.S2049. doi: 10.4137/BCI.S2049. [DOI] [Google Scholar]
- 55.Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Ostell J, Pruitt KD, Sayers EW. GenBank. Nucleic Acids Res. 2018;46(D1):D41–D47. doi: 10.1093/nar/gkx1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabrielsen C, Drabløs F, Afset JE. Genome sequences of 11 Shiga toxin-producing Escherichia coli strains. Genome Announc. 2015;3(3) [DOI] [PMC free article] [PubMed]
- 57.Mellmann A, Bletz S, Böking T, Kipp F, Becker K, Schultes A, Prior K, Harmsen D. Real-time genome sequencing of resistant bacteria provides precision infection control in an institutional setting. J Clin Microbiol. 2016;54(12):2874–2881. doi: 10.1128/JCM.00790-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44(14):6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaas RS, Leekitcharoenphon P, Aarestrup FM, Lund O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS One. 2014;9(8):e104984. doi: 10.1371/journal.pone.0104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boratyn GM, Camacho C, Cooper PS, Coulouris G, Fong A, Ma N, Madden TL, Matten WT, McGinnis SD, Merezhuk Y, et al. BLAST: a more efficient report with usability improvements. Nucleic Acids Res. 2013;41(Web Server issue):W29–W33. doi: 10.1093/nar/gkt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain loss and rearrangement. PLoS One. 2010;5(6):e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 66.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bauwens A, Bielaszewska M, Kemper B, Langehanenberg P, von Bally G, Reichelt R, Mulac D, Humpf HU, Friedrich AW, Kim KS, et al. Differential cytotoxic actions of Shiga toxin 1 and Shiga toxin 2 on microvascular and macrovascular endothelial cells. Thromb Haemost. 2011;105(3):515–528. doi: 10.1160/TH10-02-0140. [DOI] [PubMed] [Google Scholar]
- 68.Ogura Y, Ooka T, Iguchi A, Toh H, Asadulghani M, Oshima K, Kodama T, Abe H, Nakayama K, Kurokawa K, et al. Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc Natl Acad Sci U S A. 2009;106(42):17939–17944. doi: 10.1073/pnas.0903585106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary information about 159 E. coli O26 isolates whose genomic sequences were included for comparative analysis. Presence/absence of molecular diagnostic markers and their identity with query sequences are indicated. (DOCX 144 kb)
Figure S1. Core genome-derived phylogeny of 159 E. coli O26 isolates. The phylogram (inferred using the CSIPhylogeny pipeline [59]) represents the full version of contracted phylogram presented in Fig. 1. (PDF 32 kb)
Table S3. Discrimination of early nEC and late nEC strains by sen/ent SNP-specific PCR, WGS and PFGE. All nEC strains characterized in this study by WGS (Fig. 2) and/or by sen/ent SNP-specific PCR (Fig. 3) are listed, together with results obtained by either method and PFGE clustering [31] (n.a., not available). Representative gel image which demonstrates the performance of the sen/ent SNP-specific PCR in differentiating early- and late nEC strains is included. (XLSX 69 kb)
Table S2. SNPs synapomorphic for the early nEC, late nEC or nEC as whole. Missense mutations in fimbrial proteins are denoted in bold. (XLS 112 kb)
Figure S2. Alignment of all unique Stx2 protein sequences present in the GenBank. Residue A273 is marked with a red rectangle on the top of alignment. (PDF 6180 kb)
Data Availability Statement
All draft genome sequences were submitted to the GenBank database of National Center for Biotechnology Information (Bioproject accession number: PRJNA470577).