Abstract
Background
Immune system dysfunction is a hypothesis in the psychopathology of schizophrenia, but the impact of antipsychotic treatment within this system is not clear. The aim of this meta-analysis was to investigate the impact of antipsychotic treatment on cytokine levels in in vivo studies on schizophrenia.
Methods
After a systematic database search, original data were extracted with the help of certain authors. Means and SDs were extracted to calculate standardized mean differences. Cytokine levels were compared in vivo in schizophrenia patients, before and after antipsychotic treatment. Meta-regressions were performed to explore the influence of demographic and clinical variables on cytokine level standardized mean differences. Stratifications by treatment and diagnosis were also performed.
Results
Forty-seven studies were included in this meta-analysis. Proinflammatory cytokine level decreases were found for interleukin-1 β levels (P<.0001) and interferon-γ (P=.01) and a statistical trend towards a decrease in interleukin-6 (P=.08) and tumor necrosis factor-α (P=.07) levels. An antiinflammatory cytokine level increase was found for soluble tumor necrosis factor-R2 (P<.001) and soluble interleukin 2-R (P=.03) levels. A meta-regression analysis found a correlation between interleukin-6 level standardized mean differences and positive schizophrenia symptom score standardized mean differences before and after treatment (P=.01). Stratification by diagnosis or treatment found a possible impact of the kinetics of cytokine levels.
Conclusions
The present meta-analysis provides evidence that antipsychotic treatment has an antiinflammatory effect and could normalize immune balance dysfunction in schizophrenia. Interleukin-6 level normalization could be a marker of illness equilibration and thus used in clinical practice.
Keywords: antipsychotic, cytokine levels, schizophrenia, first episode psychosis, immune inflammation
Introduction
Schizophrenic disorder affects approximately 1% of the general population (Janoutová et al., 2016) and is associated with major functional impact (Green, 2016). The physiopathology of schizophrenia is not well established, but many factors could influence the emergence of this pathology, such as a genetic predisposition (Ripke et al., 2014), obstetrical complications (Geddes et al., 1999; Cannon et al., 2002), or infections (Brown and Derkits, 2010). A relationship with pre- or perinatal neurodevelopmental events (such as obstetrical complications or maternal infections) has been found and could be linked to aberrant hypothalamic-pituitary-adrenal (HPA) axis activation during exposure to psychological stress (Raison and Miller, 2003; Ciufolini et al., 2014; Howes and McCutcheon, 2017). Study results investigating serum cytokine levels appear to contradict some studies that have found an overactivation of the proinflammatory system or T helper 1 (Th1) system (Kim et al., 2004), and others that have found a dysfunction in the normal balance between the proinflammatory system (Th1 system) and the antiinflammatory system or Th2 system (Müller, 2011). Meta-analyses have been performed (Miller et al., 2011; Goldsmith et al., 2016) and they showed that certain cytokines increased in acute exacerbations of schizophrenia and could be considered as state-related markers (interleukin [IL]-1β; IL-6 or transforming growth factor β [TGF-β]), whilst others did not change during acute exacerbation and could be considered as trait markers (IL-12; interferon γ [IFN-γ]; tumor necrosis factor α [TNF-α] and sIL-2R) (Miller et al., 2011).
Currently, antipsychotic drugs are the first choice treatment in schizophrenia. The effect of antipsychotic treatment on the immune system is not yet clear, but preclinical studies have found a possible microglia activation damping effect (Kato et al., 2008; Zhu et al., 2014). Presently, it is not clear whether the kinetics of cytokine levels before and after treatment are caused by antipsychotic drugs or whether they reflect clinical evolution as a state-related marker. To investigate this point, we chose to perform stratification by type of treatment and by diagnosis.
The aim of this meta-analysis was to investigate the impact of antipsychotics on the kinetics of cytokine levels in in vivo schizophrenia studies. A secondary aim was to evaluate the impact of the diagnosis to see whether all antipsychotic drugs had the same impact on the kinetics of cytokine levels. The final aim of this work was to perform a meta-regression analysis including clinical data to assess the putative heterogeneity.
Methods
Data Sources and Study Selection Process
We carried out a search on the MEDLINE and PsycINFO databases in January 2017, without any limits on year of publication, using the key words cytokines or immune inflammation and schizophrenia or first psychotic episode or psychosis. Studies were included if (1) they were published in English in a peer-reviewed journal, (2) they included patients with a diagnosis of schizophrenia based on DSM, III, IV, or V criteria, and (3) they measured in vivo cytokines levels in plasma or serum, before and after antipsychotic treatment. Studies that did not fulfill these 3 criteria were systematically excluded from the analyses. To obtain additional data, an email alert was created after January 2017 on MEDLINE with the same keywords to detect any publications of potential interest. The reference lists of articles identified were reviewed for additional studies. Study selection was performed by one author (B.R.) and verified by another (C.M.).
Data Extraction
When data were not available in the articles, we contacted the corresponding authors (see Acknowledgments) of each included trial by email to improve data collection and obtain the stratified data for each antipsychotic treatment or each diagnosis. If more than one group was available in the article (Borovcanin et al., 2013), we used the method recommended by the Cochrane group (Cochrane 2011): M=(N1 M1+N2 M2)/(N1+N2) and SD=√((N1-1)SD12 +(N2-1)SD22+(N1 *N2/N1+N1)(M12+M12-2 M1 M2))/(N1+ N2-1) (where N1 is the number of patients in the first group assessment, N2 is the number of patients in the second group assessment, M1 is the mean of the first group, M2 is the mean of the second group, SD1 is the standard deviation of the first group, and SD2 is the standard deviation of the second group). For each study, we thus obtained the means and SD for all cytokine levels before and after treatment. Articles written by a given research group were carefully scrutinized to ensure the absence of redundancy among the populations included in the trials.
A set of clinical variables was defined for the meta-regression analysis. We extracted the duration study (in weeks), the means and SDs for age (age variable), the percentages of females (sex variable), the means and SDs for years of illness (duration of illness variable), the mean age at onset of the disorder (onset disorder variable), and the means and SDs for schizophrenia severity scores before and after treatment (total, positive, negative, and general symptom scores). Data extraction was performed by one author (B.R.) and verified by another (C.M.).
Data Analyses
Data analyses were performed using RevMan, version 5.3 (Copenhagen, Denmark; the Nordic Cochrane Centre, Cochrane Collaboration). Effect sizes consisted in the standardized mean differences (SMD) between cytokine levels before and after treatment or between the schizophrenia severity scale scores for total, positive, negative, or general symptoms before and after treatment. According to Cohen’s method, SMDs were calculated as the differences between group means divided by the pooled SDs. All analyses were performed with a random-effect model, which considers both between-study and within-study variability (Der Simonian and Laird, 1986). An effect size was considered significant when the 95% CI excluded 0 and when the P < .05.
Study heterogeneity was estimated with the Q statistic, which was calculated for all analyses and considered significant when P<.1. When a significant level of heterogeneity was reached, the I2 index, an estimate of the total variation across the studies included due to heterogeneity rather than chance, was determined by the equation I2=[(Q – df)/Q] * 100% (Higgins et al., 2003). I2 values of 25, 50, and 75 were indicative of mild, moderate, and marked heterogeneity, respectively, between trials. In addition, to ensure that the overall results were not influenced by a single study, leave-1-out sensitivity analyses, performed by repeating the analyses with the consecutive exclusion of each study, were carried out for each analysis when more than 2 studies were included. Results are given in the supplementary Materials. Finally, we conducted more specific secondary analyses involving the primary diagnoses: first psychotic episode, acute exacerbation of schizophrenia, chronic schizophrenia (studies that included in their methods: chronic schizophrenia patients, patients with a duration of illness >5 years, and no current acute exacerbation of illness were included in our analyses) and resistant schizophrenia (define by an insufficient antipsychotic response or by the Kane’s criteria [Kane et al., 1988]), and the type of antipsychotic treatment used.
Funnel plots, plotting the standard error of each SMD against the SMD calculated for each study included, were drawn up when at least 5 individual studies contributed to an overall result, and their asymmetry was analyzed to assess the possible influence of publication and location biases (Green and Higgins, 2006).
Finally, we conducted meta-regression analyses based on simple linear regression models for assessing the influence of the clinical heterogeneity of the study populations on meta-analysis effect sizes. Regression analyses were performed only when SMDs or heterogeneity were significant and when a reasonable number of data points were available, set at 6 at least.
Results
Article Identification Process
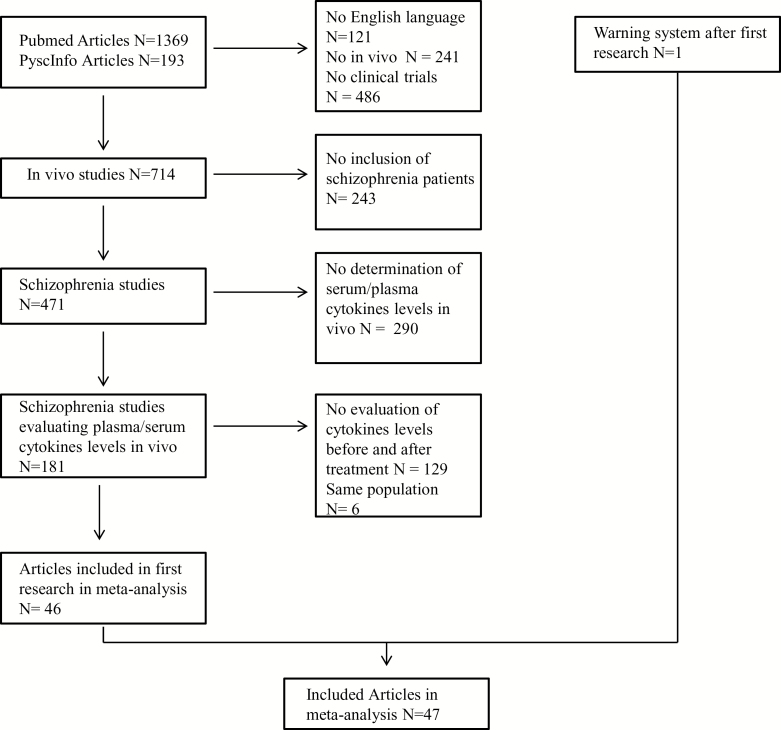
Figure 1 shows the article-selection process and supplemental Table 1 shows details of included studies. The database search found 1562 articles. Forty-six studies identified by database research and 1 other identified by email alert were included. Of these 47 studies, 41 studies specify in their article that they exclude patients (1) with somatic comorbidity that may explain variations in cytokine levels, (2) with an abnormality in standard physical and biological examinations, and (3) taking a treatment that impacts the immune system.
Figure 1.
Article identification process.
All Treatments
All results are summarized in Table 1. After treatment, there was a significant decrease in the following: IL-1 β levels (n=7 studies; test for overall effect: SMD=-0.40, 95% CI: -0.58 to -0.22, P<.0001; test for heterogeneity: χ2=4.5, P=.61, I2=0 %), and IFN-γ levels (n=8 studies; test for overall effect: SMD=-0.38, 95% CI: -0.67 to -0.09, P=.01; test for heterogeneity: χ2=23.02, P=.002, I2=70 %). There was a statistical trend for a decrease in IL-6 levels (n=21 studies; test for overall effect: SMD=-0.22, 95% CI: -0.47 to 0.03, P=.08; test for heterogeneity: χ2=107.13, P<.0001, I2=81 %), in TNF-α levels (n=19 studies; test for overall effect: SMD=-0.32, 95% CI: -0.65 to 0.02, P=.07; test for heterogeneity: χ2=131.95, P<.0001, I2=86 %), and in IL-4 levels (n=7 studies; test for overall effect: SMD=-0.47, 95% CI: -0.99 to 0.06, P=.08; test for heterogeneity: χ2=72.9, P<.0001, I2=92 %). After treatment, a significant increase was observed in sTNF-R2 levels (n=3 studies; test for overall effect: SMD=0.94, 95% CI: 0.52–1.36, P<.0001; test for heterogeneity: χ2=0.49, P=.78, I2=0 %) and in sIL-2R levels (n=11 studies; test for overall effect: SMD=0.26, 95% CI: 0.03–0.49, P=.03; test for heterogeneity: χ2=16.09, P=.1, I2=38 %).
Table 1.
Results for all Treatments
| Cytokine | Numbers of Studies Included | Numbers of Patients before/after Treatments | SMD | 95% CI | P value | Χ2 | P value | I2 (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| IL-1 β | 7 | 241/241 | -0.40 | -.58; -.22 | <.01 | 4.5 | .61 | 0 | Theodoropoulou et al., 2001; Kim et al., 2001, 2000; Haring et al., 2015; Song et al., 2014, 2009; Sobiś et al., 2015 |
| IL-6 | 21 | 763/784 | -0.22 | -.47; 0.03 | .08 | 107.13 | <.01 | 81 | Pollmächer et al., 1996; Frommberger et al., 1997; Schuld et al., 2000; Kim et al., 2001, 2009, 2000; Maes et al., 2000, 1997; Zhang et al., 2004; Pae et al., 2006; Hori et al., 2007; Igue et al., 2011; Lin et al., 2011; Kubistova et al., 2012; Borovcanin et al., 2013; Ding et al., 2014; Song et al., 2014; Haring et al., 2015; Sobiś et al., 2015; Noto et al., 2015; Kao, 2016 |
| sIL-6 R | 4 | 84/84 | -0.17 | -.72; 0.37 | .53 | 8.37 | .04 | 64 | Maes et al. 1995, 1997, 2000; Muller et al. 1997 |
| IL-8 | 4 | 144/144 | -0.17 | -.40; 0.06 | .14 | 0.14 | .99 | 0 | Maes et al. 2002; Zhang 2004; Kubistova et al. 2012; Haring et al. 2015; |
| IL-12 | 4 | 121/121 | 0.13 | -.34; 0.60 | .58 | 9.05 | .03 | 67 | Crespo-Facorro et al. 2008; Kim et al. 2001, 2002; Sobiś et al. 2015 |
| TNF-α | 19 | 583/579 | -0.32 | -.65; 0.02 | .07 | 131.95 | <.01 | 86 | Pollmächer et al., 1996; Monteleone et al., 1997; Hinze-Selch et al., 2000; Schuld et al., 2000; Theodoropoulou et al., 2001; Pae et al., 2006; Baptista et al., 2007; Hori et al., 2007; Sarandol et al., 2007; Kim et al., 2009; Lin et al., 2011; Kubistova et al., 2012; Ajami et al., 2014; Song et al., 2014, 2009; Haring et al., 2015; Noto et al., 2015; Sobiś et al., 2015; Kao, 2016 |
| sTNF-R1 | 5 | 91/91 | 0.35 | -.16; 0.86 | .18 | 11.15 | .02 | 64 | Hinze-Selch et al. 2000; Muller et al. 2004; Pollmacher et al. 1996; Schuld et al. 2000; Sobiś et al. 2015 |
| sTNF-R2 | 3 | 49/49 | 0.94 | 0.52; 1.36 | <.01 | 0.49 | .78 | 0 | Pollmächer et al., 1996; Hinze-Selch et al., 2000; Schuld et al., 2000 |
| IL-2 | 10 | 311/311 | -0.31 | -.03; 0.40 | .39 | 149.82 | <.01 | 94 | Kim et al. 2000, 2001, 2009; Theodoropoulou et al. 2001; Zhang et al. 2004; Pae et al. 2006; Hori et al. 2007; Ajami et al. 2014; Haring et al. 2015; Kao et al. 2016 |
| sIL-2 R | 11 | 263/263 | 0.26 | 0.03; 0.49 | .03 | 16.09 | .10 | 38 | Maes et al., 1995; Pollmächer et al., 1996, 1995; Müller et al., 1997; Schwarz et al., 1998; Hinze-Selch et al., 2000; Schuld et al., 2000; Muller et al., 2004; Sirota et al., 2005; Igue et al., 2011; Szymona et al., 2017 |
| IL-17 | 3 | 248/248 | 0.02 | -.29; 0.34 | .88 | 5.82 | .05 | 66 | Borovcanin et al. 2013; Ding et al. 2014; Noto et al. 2014 |
| IL-1 RA | 6 | 131/131 | -0.03 | -.48; 0.42 | .89 | 16.35 | .006 | 69 | Pollmacher et al. 1996; Maes et al. 1997, 2000; Sirota et al. 2005; Igue et al. 2011; Sobiś et al. 2015 |
| TGF- β | 5 | 303/303 | -0.20 | -.61; 0.21 | .35 | 21.85 | .0002 | 82 | Kim et al. 2004, 2009; Lin et al. 2011; Borovcanin et al. 2013; Sobiś et al. 2015 |
| IL-10 | 8 | 210/210 | 0.09 | -.40; 0.57 | .72 | 39.09 | <.01 | 82 | Maes et al. 2002; Kubistova et al. 2012; Pae et al. 2006; Noto et al. 2014; Ajami et al. 2014; Haring et al. 2015; Sobiś et al. 2015; Kao et al. 2016 |
| IFN-γ | 8 | 380/380 | -0.38 | -.67; -.09 | .01 | 23.02 | .002 | 70 | Borovcanin et al., 2013; Ding et al., 2014; Haring et al., 2015; Sobiś et al., 2015; Kao, 2016; Kim et al., 2001, 2009, 2004 |
| IL-4 | 7 | 399/399 | -0.47 | -.99; 0.06 | .08 | 72.90 | <.01 | 92 | Borovcanin et al., 2013; Haring et al., 2015; Kim et al., 2009, 2004; Noto et al., 2015; Sobiś et al., 2015; Szymona et al., 2017 |
| IL-23 | 2 | 68/68 | -0.01 | -.35; 0.32 | .94 | 0.20 | .65 | 0 | Borovcanin et al. 2015; Sobiś et al. 2015 |
Abbreviations: I2, I2 index, an estimate of the total variation across the studies included; IFN, interferon; IL, interleukin; sIL-6 R, soluble interleukine 6 receptor; sIL-2 R, soluble interleukine 2 receptor; SMD, standard mean difference; sTNF-R1, soluble tumor necrosis factor receptor 1; sTNF-R2, soluble tumor necrosis factor receptor 2; TNF, tumor necrosis factor; TGF, transforming growth factor.
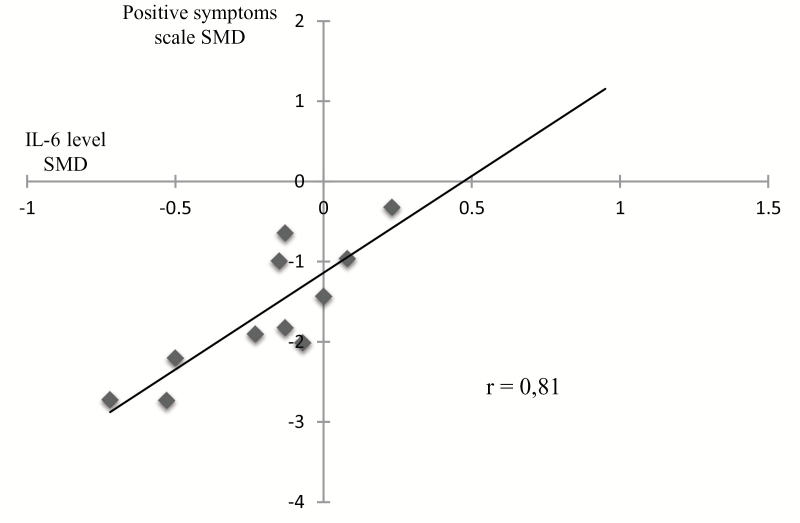
Meta-regression analyses showed a significant positive correlation between IL-6 SMD before and after treatment and positive symptom score SMDs before and after treatment (r=0.81; P=.001) (Figure 2), and a statistical trend between IL-10 SMDs before and after treatment and positive symptom score SMDs before and after treatment (r=0.76; P=.08). No other significant correlation was found in the other meta-regression analyses.
Figure 2.
Correlation between interleukin (IL)-6 standard mean difference (SMD) and positive symptoms scale SMD (r=0.81; P=.001). We found an association between the decrease of IL-6 level and the decrease of positive symptoms after antipsychotic treatment.
Stratification by Treatment
Following risperidone treatment (supplemental Table 2), there was a significant decrease in the following: IL-1 β levels (n=4 studies; test for overall effect: SMD=-0.47, 95% CI: -0.69 to -0.25, P<.0001; test for heterogeneity: χ2=0.80, P=.85, I2=0 %), IL-6 levels (n=7 studies; test for overall effect: SMD=-0.22, 95% CI: -0.39 to -0.05, P=.01; test for heterogeneity: χ2=4.46, P=.61, I2=0 %), IL-2 levels (n=3 studies; test for overall effect: SMD=-0.42, 95% CI: -0.78 to -0.06, P=.02; test for heterogeneity: χ2=0.8, P=.67, I2=0 %), IL-10 levels (n=2 studies; test for overall effect: SMD=-0.83, 95% CI: -1.22 to -0.44, P<.0001; test for heterogeneity: χ2=0.02, P=.89, I2=0 %), and IL-4 levels (n=3 studies; test for overall effect: SMD=-0.67, 95% CI: -0.99 to -0.34, P<.0001; test for heterogeneity: χ2=0.25, P=.88, I2=0 %). A significant increase in IL-12 levels (n=2 studies; test for overall effect: SMD=0.66, 95% CI: 0.18 to 1.14, P=.007; test for heterogeneity: χ2=0.25, P=.62, I2=0 %) was also found after risperidone treatment.
Following olanzapine treatment (supplemental Table 3), there was a significant decrease in IL-2 levels (n=2 studies; test for overall effect: SMD=-1.17, 95% CI: -2.32 to -0.03, P=.05; test for heterogeneity: χ2=4.08, P=.04, I2=76 %) and IFN-γ levels (n=2 studies; test for overall effect: SMD=-1.30, 95% CI: -2.15 to -0.46, P=.003; test for heterogeneity: χ2=0.16, P=.69, I2=0 %).
After quetiapine treatment (supplemental Table 4), no significant increase or decrease in cytokine levels was found.
After aripiprazole treatment (supplemental Table 5), a statistical trend toward a decrease in IL-4 levels (n=2 studies; test for overall effect: SMD=-0.53, 95% CI: -1.15 to 0.09, P=.09; test for heterogeneity: χ2=0.28, P=.60, I2=0 %) was found. A significant increase in IL-10 levels (n=2 studies; test for overall effect: SMD=0.92, 95% CI: 0.28–1.56, P=.005; test for heterogeneity: χ2=0.78, P=.38, I2=0 %) was also found after aripiprazole.
After clozapine treatment (supplemental Table 6), there was a significant increase in the following: sTNF-R1 levels (n=2 studies; test for overall effect: SMD=0.89, 95% CI: 0.43–1.36, P=.0002; test for heterogeneity: χ2=0.20, P=.65, I2=0 %), sTNF-R2 levels (n=2 studies; test for overall effect: SMD=0.98, 95% CI: 0.51–1.46, P<.0001; test for heterogeneity: χ2=0.36, P=.55, I2=0 %), and sIL-2 R levels (n=3 studies; test for overall effect: SMD=0.62, 95% CI: 0.08–1.16, P=.02; test for heterogeneity: χ2=3.21, P=.20, I2=38 %). There was also a statistical trend toward an increase in IL-6 levels (n=3 studies; test for overall effect: SMD=0.44, 95% CI: -0.03 to 0.92, P=.07; test for heterogeneity: χ2=2.82, P=.24, I2=29 %).
After haloperidol treatment (supplemental Table 7), a significant decrease in IL-2 levels was observed (n=2 studies; test for overall effect: SMD=-0.75, 95% CI: -1.11 to -0.38, P<.0001; test for heterogeneity: χ2=0.06, P=.80, I2=0 %).
Stratification by Diagnosis
Among first episode psychosis (FEP) patients (supplemental Table 8), after treatment, there was a significant decrease in IL-1 β levels (n=4 studies; test for overall effect: SMD=-0.36, 95% CI: -0.63 to -0.10, P=.007; test for heterogeneity: χ2=4.14, P=.25, I2=27 %), in IL-6 levels (n=5 studies; test for overall effect: SMD=-0.36, 95% CI: -0.57 to -0.16, P=.0004; test for heterogeneity: χ2=4.61, P=.33, I2=13 %), and in IL-4 levels (n=3 studies; test for overall effect: SMD=-0.45, 95% CI: -0.75 to -0.15, P=.003; test for heterogeneity: χ2=3.38, P=.18, I2=41 %).
In acute exacerbations of schizophrenia (supplemental Table 9), after treatment, there was a significant decrease in IL-1 β levels (n=6 studies; test for overall effect: SMD=-0.41, 95% CI: -0.59 to -0.22, P<.0001; test for heterogeneity: χ2=4.55, P=.47, I2=0 %), in IL-6 levels (n=14 studies; test for overall effect: SMD=-0.44, 95% CI: -0.74 to -0.15, P=.003; test for heterogeneity: χ2=78.67, P<.00001, I2=83 %), in sIL-6 R levels (n=2 studies; test for overall effect: SMD=-0.51, 95% CI: -0.90 to -0.12, P=.01; test for heterogeneity: χ2=0.86, P=.35, I2=0 %), in TNF-α levels (n=12 studies; test for overall effect: SMD=-0.46, 95% CI: -0.89 to -0.03, P=.04; test for heterogeneity: χ2=103.50, P<.00001, I2=89 %), and in IFN-γ levels (n=7 studies; test for overall effect: SMD=-0.41, 95% CI: -0.73 to -0.09, P=.01; test for heterogeneity: χ2=22.62, P<.0001, I2=73 %).
In chronic schizophrenia patients (supplemental Table 10), after treatment, only 2 analyses were performed (IL-6 and IL-1 RA), and they did not show any significant difference in cytokine levels.
In resistant schizophrenia patients (supplemental Table 11), after treatment, a significant increase was observed in IL-6 levels (n=3 studies; test for overall effect: SMD=0.57, 95% CI: 0.20–0.95, P=.003; test for heterogeneity: χ2=1.30, P=.52, I2=0 %) and in sIL-2 R levels (n=2 studies; test for overall effect: SMD=0.85, 95% CI: 0.39–1.32, P=.0003; test for heterogeneity: χ2=0.05, P=.83, I2=0 %).
Discussion
This present meta-analysis shows that the levels of some proinflammatory cytokines decreased after treatment (significant decrease in IL-1 β and IFN-γ levels and a statistical trend toward a decrease in IL-6 and TNF-α levels) and that some antiinflammatory cytokine levels increased after treatment (significant increase in sTNF-R2 and sIL2-R levels). We found that IL-6 level SMDs were correlated to the positive symptoms score SMDs. We also found that, in some cases, the kinetics of cytokine levels could be impacted by diagnosis and/or the type of antipsychotic used.
The main difference from previous meta-analysis on the same topic (Miller et al., 2011; Tourjman et al., 2013; Goldsmith et al., 2016) is the number of studies included and, therefore, the statistical power. We have included 47 studies whereas other meta-analysis included between 12 and 23 studies. A possible explanation of this difference was a difference in the methodology. For example, Goldsmith et al. (2016) had included only acutely ill patients in their analysis. Compared with other meta-analysis (Miller et al., 2011; Tourjman et al., 2013; Goldsmith et al., 2016), inclusion of new studies permit us to perform new comparisons for cytokine levels from other research such as IL-8, IL-23, sTNF-R1, sTNF-R2, which were not previously tested. Many analyses were performed with significantly more studies. For example, for IL-8 level, only 2 studies were included by Tourjman et al., 2013, whereas we included 8 studies or for TNF-α level with 3, 6, and 9 studies included respectively in Miller et al. (2011); Goldsmith et al. (2016) and Tourjman et al. (2013), whereas we included 19 studies. Compared with other meta-analysis (Tourjman et al., 2013; Goldsmith et al., 2016), our results are quite similar, but as we included a larger number of studies, we obtained a larger statistical database. Therefore, we found some difference: we did not find that IL-12 levels were impacted by treatment as contrary to 3 other meta-analyses (Miller et al., 2011; Tourjman et al., 2013; Goldsmith et al., 2016) that found an IL-12 level increase. The significant result found in these 2 meta-analyses could merely be due to the small number of studies included (respectively 2 and 3 studies). We found a significant decrease in IFN-γ level like Tourjman et al. (2013) counter to Goldsmith et al. (2016) and Miller et al. (2011). Finally, other meta-analyses (Miller et al., 2011; Tourjman et al., 2013; Goldsmith et al., 2016) found II-6 level decreased while our results underlined a statistical trend to decrease. It is important to note that in this analysis, 763 patients were included, whereas only 164 and 521 patients were included in Miller et al. (2011) and Goldsmith et al. (2016), respectively.
Our results show a trend to a normalization of the dysfunction in the normal balance between the proinflammatory system (Th1 system) and the antiinflammatory system or Th2 system (Müller, 2011). We found that antipsychotic treatment had an antiinflammatory effect, with a decrease in proinflammatory cytokine levels such as IL-1 β, IFN-γ, IL-6, and TNF-α and an increase in antiinflammatory cytokine levels such as sTNF-R2 or sIL2-R. The increase of sTNF-R2 levels after treatment could illustrate the possible antiinflammatory impact of treatment. In a homeostatic situation, it seems that the production of soluble receptors is auto-regulated by the cytokines themselves (Voss et al., 1989; Cope et al., 1995). We hypothesize that, in schizophrenic patients, the homeostasis situation is disrupted and causes a disturbance of this retro-control. This could be reversed through antipsychotic medications. The possible antiinflammatory involvement could be illustrated by a recent study (Holmes et al., 2016) using positron emission tomography and radioligands that bind to the 18-kDa translocator protein that shows a direct interaction between antipsychotic medication and microglial activation in medicated patients with schizophrenia. The antiinflammatory effect of antipsychotic treatment may produce microglial activation (Holmes et al., 2016). There are 2 possible explanations for this normalization: the direct impact of antipsychotic treatment on the immune system or the indirect effect of antipsychotic treatment on the immune system by the improvement of the clinical symptoms of schizophrenia.
Moreover, we found an association between IL-6 level SMDs and positive symptom schizophrenia score SMDs. A similar correlation between evolution in the general symptom schizophrenia score and IL-6 levels was also found in 2 studies including schizophrenia patients (Frommberger et al., 1997; Pae et al., 2006). Positive correlations were also found between positive symptoms and IL-6 level in one study including ARMS patients (Stojanovic et al., 2014) and one other study including war veterans with schizophrenia (Dimitrov et al., 2013). Another association was found between levels of IL-6 mRNA and positive symptoms in schizophrenia patients (Chase et al., 2016). At the onset of psychosis, a dysfunction of the HPA axis was found in relation to social stress (Ciufolini et al., 2014). Our hypothesis is that microglia activation leads to ventral hippocampus activation, which is also involved in context-related behaviors (Grace, 2012; Howes and McCutcheon, 2017). This activation leads to an overactivation of the ventral tegmental area dopaminergic neuron by activation of the nucleus accubens, which downregulates pallidum ventral inhibition (Grace, 2012; Howes and McCutcheon, 2017). Antipsychotic treatment, by dopaminergic antagonism, could restore the axis balance. IL-6 levels have also been found to decrease in other psychiatric disorders such as major depressive disorder between acute and remission states (Frommberger et al., 1997). In healthy humans, acute psychological stress showed an increased IL-6 plasmatic level (Steptoe et al., 2007). Positive symptoms could therefore be considered an important stress factor independently from the psychiatric diagnosis (e.g., infections associated with immune proinflammatory activation). Theoretically, a glucocorticoid feedback from the inhibitory effects on nuclear factor κβ could enable return to homeostasis after stressor cessation (Raison and Miller, 2003), but it could be impaired in mental illness (Sluzewska, 1999). IL-6 levels could be considered a stress marker and reflect HPA overactivation (Raison and Miller, 2003). IL-6 level normalization could be a marker of illness equilibration and therefore could be used in clinical practice. No other association between cytokine kinetics and demographic variables was found. In particular, we did not find an association between the duration of the study (and therefore the duration of the treatment) and the MDS of cytokine levels. This result was not consistent with the outcome of another study (Song et al., 2014) that investigated the 6-month kinetics of cytokines in patients with schizophrenia under risperidone. In this study (Song et al., 2014), researchers found an initial decrease in IL-6 and IL-1β levels in the first 2 months and a return to baseline after 6-month treatment. In our analysis, most studies included a duration of treatment between 4 and 10 weeks (only 3 studies had a longer duration), which could explain the absence of result of the meta-regression between cytokines SMD and duration of the study. To explain the increase in proinflammatory cytokine levels with long-term treatment, Song et al. (2014) hypothesized that increasing BMI may increase the proinflammatory response. Unfortunately, we were unable to perform an analysis including BMI because of a lack of available data, which did not allow us to establish a relationship between proinflammatory cytokine levels and BMI.
We found that the different types of antipsychotic treatment could influence cytokine levels differently, as with IL-2, IL-10, IFN-γ, or IL-12. One hypothesis is that all antipsychotic drugs have pharmacological specifications, notably on the serotoninergic and noradrenergic systems, which could impact the HPA axis differently and therefore impact the liberation of cytokines in stress response differently (Raison and Miller, 2003). However, other factors could impact results, such as tobacco use, which could decrease IL-2 and IL-6 levels (Zhang et al., 2008), or body mass index (BMI). Obesity has been shown to increase levels of proinflammatory cytokines, such as TNF-α, IFN-γ, or IL-1 β, and decrease levels of antiinflammatory cytokines such as IL-1RA (Chen et al., 2008; Sirota et al., 2015). Some treatments like clozapine, olanzapine, or quetiapine are known to increase weight and cause metabolic syndromes and could cause an increase of proinflammatory cytokine like IL-6. This modification could explain the absence of a significant decrease of IL-6 level after these 3 treatments in our analysis. Baseline IL-6 levels have also been shown to be predictors of the increase in total and LDL cholesterol and therefore a potential predictor of metabolic syndrome (Fernandez-Egea et al., 2011). The meta-analyses concerning aripiprazole treatment, which causes less weight gain than other antipsychotic treatments (Nasrallah et al., 2016), are difficult to interpret due to a lack of data. This meta-analysis confirms the need to consider BMI in different studies investigating the immune system. Another point illustrated by this meta-analysis stratification is a general decrease in heterogeneity when the different antipsychotic treatments are considered separately. For example, the IL-6 level SMD analysis found an I2 at 81% for all treatment analyses, whereas it was 0% for risperidone or olanzapine analyses. This decrease in heterogeneity enables an improvement in analysis quality and therefore in the interpretation of results.
We found a possible implication of the diagnosis in the evolution of cytokine levels. We found some differences between FEP and acute exacerbation of schizophrenia, notably on TNF-α and IFN-γ levels, which show a decrease after treatment in acute episodes but not in FEP. It is important to note that the difference in TNF-α levels in acute episodes is underpinned by only 2 studies (Kim et al., 2009; Ajami et al., 2014). If we exclude one of these 5 studies, the result loses its significance. For IFN-γ levels, interpretation of these 2 results was difficult because of heterogeneity. IL-6 levels showed a decrease after treatment in FEP and in acute exacerbation of schizophrenia but not in chronic schizophrenia or resistant schizophrenia. It is important to note that heterogeneity in the acute exacerbation of schizophrenia is based on only one study (Kim et al., 2009); its exclusion causes an I2 decrease from 83% to 7% but did not change the significance of the result. Our explanation for these results is that the positive symptom score at baseline was low in chronic schizophrenia studies (Igue et al., 2011; Sobiś et al., 2015), so it is possible that the HPA axis was not activated as in acute schizophrenia or in FEP. A similar result was also found in ultra-high-risk schizophrenia, which had positive symptoms significantly lower than in FEP, with an IL-6 level lower than in FEP (Karanikas et al., 2016). These results confirm that IL-6 could be a stress marker, which could reflect HPA activation, and that positive symptoms could be potential stressors that activate this axis. Another factor that could take part in the kinetics of IL-6 levels is illness duration with a possible habituation to psychotic symptoms. For example, Borovcanin et al (2013) investigated IL-6 levels and found an increase in FEP compared with acute schizophrenia (27.85 pg/mL and 16.25 pg/mL, respectively).
Our meta-analysis has several limitations. First, there is a great heterogeneity across analyses, especially in all treatment analyses that are not explained by meta-regression, except for IL-6 levels. A second limitation was the small number of studies included in the analyses, notably in the stratified analyses, which could be responsible for poor statistical power. A final limitation was that the studies analyzed were performed in blood and not in CSF, which could have a more direct link with the CNS. The influence of certain clinical factors such as obesity (Weisberg et al., 2003; Na et al., 2014) could explain the observed variations between peripheral and central cytokines and could explain why peripheral investigation is not necessarily associated with CNS inflammation. The different results obtained in this meta-analysis on peripheral cytokine levels should therefore be interpreted with caution, and further studies on the impact of antipsychotic treatments in CSF cytokine levels may be useful in confirming our results.
In conclusion, this meta-analysis provides evidence for an antiinflammatory effect of antipsychotic treatment. IL-6 levels were found to be stress markers directly correlated with the evolution of psychotic symptoms, and they could be used as markers for illness equilibration. The type of antipsychotic treatment used and/or the diagnosis impact cytokine levels differently and explain in part the heterogeneity found in global analyses.
Supplementary Materials
Supplementary data are available at: International Journal of Neuropsychopharmacology (IJNPPY) online.
Funding
None.
Acknowledgments
We thank all the following authors for their kind collaboration: Dr Liina Haring (Psychiatry Clinic of Tartu University Hospital, Tartu, Estonia) for providing seminal data for the study “Antipsychotic treatment reduces psychotic symptoms and markers of low-grade inflammation in first episode psychosis patients, but increases their body mass index”; Dr Milica Borovcanin (Department of Psychiatry, Faculty of Medical Sciences, University of Kragujevac Svetozara Markovica, Psychiatric Clinic, Clinical Center Kragujevac Zmaj Jovina, Kragujevac, Serbia) for providing data for the studies “Antipsychotics can modulate the cytokine profile in schizophrenia: Attenuation of the type-2 inflammatory response” and “Increase systemic levels of IL-23 as a possible constitutive marker in schizophrenia.” We also thank Amélie Petillion for her help regarding the English language corrections.
Statement of Interest
Amine Benyamina has given lectures for Lundbeck, Mylan, Merck-Serono, and Bristol-Myers Squibb and is a member of the board for Indivior. The other authors declare no conflict of interest.
References
- Ajami A, Abedian F, Hamzeh Hosseini S, Akbarian E, Alizadeh-Navaei R, Taghipour M(2014)Serum TNF-α, IL-10 and IL-2 in schizophrenic patients before and after treatment with risperidone and clozapine. Iran J Immunol 11:200–209. [PubMed] [Google Scholar]
- Borovcanin M, Jovanovic I, Radosavljevic G, Djukic Dejanovic S, Stefanovic V, Arsenijevic N, Lukic ML(2013)Antipsychotics can modulate the cytokine profile in schizophrenia: attenuation of the type-2 inflammatory response. Schizophr Res 147:103–109. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ(2010)Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lönnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjöld-Nordenstam CG(2002)Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 59:35–41. [DOI] [PubMed] [Google Scholar]
- Chase KA, Cone JJ, Rosen C, Sharma RP(2016)The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry 16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DC, Qi LY, Xiu MH, Cao LY, Wang F, Chen S, Wu GY, Kosten TA, Kosten TR, Zhang XY(2008)Elevated serum levels of tumor necrosis factor-alpha in clozapine-associated obesity in chronic schizophrenia. Schizophr Res 106:367–368. [DOI] [PubMed] [Google Scholar]
- Ciufolini S, Dazzan P, Kempton MJ, Pariante C, Mondelli V(2014)HPA axis response to social stress is attenuated in schizophrenia but normal in depression: evidence from a meta-analysis of existing studies. Neurosci Biobehav Rev 47:359–368. [DOI] [PubMed] [Google Scholar]
- Cope AP, Aderka D, Wallach D, Kahan M, Chu NR, Brennan FM, Feldmann M(1995)Soluble TNF receptor production by activated T lymphocytes: differential effects of acute and chronic exposure to TNF. Immunology 84:21–30. [PMC free article] [PubMed] [Google Scholar]
- Der Simonian R, Laird N(1986)Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- Dimitrov DH, Lee S, Yantis J, Valdez C, Paredes RM, Braida N, Velligan D, Walss-Bass C(2013)Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: potential role for IL-17 pathway. Schizophr Res 151:29–35. [DOI] [PubMed] [Google Scholar]
- Ding M, Song X, Zhao J, Gao J, Li X, Yang G, Wang X, Harrington A, Fan X, Lv L(2014)Activation of th17 cells in drug naïve, first episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 51:78–82. [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Miller B, Garcia-Rizo C, Bernardo M, Kirkpatrick B(2011)Metabolic effects of olanzapine in patients with newly diagnosed psychosis. J Clin Psychopharmacol 31:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M(1997)Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci 247:228–233. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Verdoux H, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, Stöber G, Willinger U, Murray RM(1999)Schizophrenia and complications of pregnancy and labor: an individual patient data meta-analysis. Schizophr Bull 25:413–423. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ(2016)A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA.(2012)Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology 62:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF.(2016)Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry 77:8–11. [DOI] [PubMed] [Google Scholar]
- Green S, Higgins J(2006)Cochrane Handbook for Systematic Reviews of Interventions 4.2.6. Chichester, UK: Wiley. [Google Scholar]
- Haring L, Koido K, Vasar V, Leping V, Zilmer K, Zilmer M, Vasar E(2015)Antipsychotic treatment reduces psychotic symptoms and markers of low-grade inflammation in first episode psychosis patients, but increases their body mass index. Schizophr Res 169:22–29. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG(2003)Measuring inconsistency in meta-analyses. Bmj 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS(2016)In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C]®-PK11195 positron emission tomography study. Mol Psychiatry 21:1672–1679. [DOI] [PubMed] [Google Scholar]
- Hori H, Yoshimura R, Yamada Y, Ikenouchi A, Mitoma M, Ida Y, Nakamura J(2007)Effects of olanzapine on plasma levels of catecholamine metabolites, cytokines, and brain-derived neurotrophic factor in schizophrenic patients. Int Clin Psychopharmacol 22:21–27. [DOI] [PubMed] [Google Scholar]
- Howes OD, McCutcheon R(2017)Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry 7:e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igue R, Potvin S, Bah R, Stip E, Bouchard RH, Lipp O, Gendron A, Kouassi E(2011)Soluble interleukin-2 receptor levels correlated with positive symptoms during quetiapine treatment in schizophrenia-spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry 35:1695–1698. [DOI] [PubMed] [Google Scholar]
- Janoutová J, Janácková P, Serý O, Zeman T, Ambroz P, Kovalová M, Varechová K, Hosák L, Jirík V, Janout V(2016)Epidemiology and risk factors of schizophrenia. Neuro Endocrinol Lett 37:1–8. [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H(1988)Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 45:789–796. [DOI] [PubMed] [Google Scholar]
- Kao Y-C, Ko CY, Wang SC, Liu YP(2016)Protective effects of quetiapine on metabolic and inflammatory abnormalities in schizophrenic patients during exacerbated stage. Chin J Physiol 59:69–77. [DOI] [PubMed] [Google Scholar]
- Karanikas E, Griveas I, Ntouros E, Floros G, Garyfallos G(2016)Evidence for increased immune mobilization in first episode psychosis compared with the prodromal stage in males. Psychiatry Res 244:333–338. [DOI] [PubMed] [Google Scholar]
- Kato T, Mizoguchi Y, Monji A, Horikawa H, Suzuki SO, Seki Y, Iwaki T, Hashioka S, Kanba S(2008)Inhibitory effects of aripiprazole on interferon-gamma-induced microglial activation via intracellular Ca2+ regulation in vitro. J Neurochem 106:815–825. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Kim W, Yoon SJ, Go HJ, Choi BM, Jun TY, Kim YK(2001)Effect of risperidone on serum cytokines. Int J Neurosci 111:11–19. [DOI] [PubMed] [Google Scholar]
- Kim YK, Kim L, Lee MS(2000)Relationships between interleukins, neurotransmitters and psychopathology in drug-free male schizophrenics. Schizophr Res 44:165–175. [DOI] [PubMed] [Google Scholar]
- Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ, Leonard BE(2004)Th1, th2 and th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 28:1129–1134. [DOI] [PubMed] [Google Scholar]
- Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B(2009)Cytokine changes and tryptophan metabolites in medication-naïve and medication-free schizophrenic patients. Neuropsychobiology 59:123–129. [DOI] [PubMed] [Google Scholar]
- Kubistova A, Horacek J, Novak T(2012)Increased interleukin-6 and tumor necrosis factor alpha in first episode schizophrenia patients versus healthy controls. Psychiatr Danub 24:S153–S156. [PubMed] [Google Scholar]
- Lin CC, Chang CM, Chang PY, Huang TL(2011)Increased interleukin-6 level in Taiwanese schizophrenic patients. Chang Gung Med J 34:375–381. [PubMed] [Google Scholar]
- Maes M, Bosmans E, Kenis G, De Jong R, Smith RS, Meltzer HY(1997)In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res 26:221–225. [DOI] [PubMed] [Google Scholar]
- Maes M, Bocchio Chiavetto L, Bignotti S, Battisa Tura G, Pioli R, Boin F, Kenis G, Bosmans E, de Jongh R, Lin A, Racagni G, Altamura CA(2000)Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur Neuropsychopharmacol 10:119–124. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B(2011)Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N.(2011)Inflammation and schizophrenia: pathophysiological and therapeutic aspects. Minerva Psichiatr 52:205–218. [Google Scholar]
- Na KS, Jung HY, Kim YK(2014)The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48:277–286. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Newcomer JW, Risinger R, Du Y, Zummo J, Bose A, Stankovic S, Silverman BL, Ehrich EW(2016)Effect of aripiprazole lauroxil on metabolic and endocrine profiles and related safety considerations among patients with acute schizophrenia. J Clin Psychiatry 77:1519–1525. [DOI] [PubMed] [Google Scholar]
- Noto C, Ota VK, Gouvea ES, Rizzo LB, Spindola LMN, Honda PHS, Cordeiro Q, Belangero SI, Bressan RA, Gadelha A, Maes M, Brietzke E(2015)Effects of risperidone on cytokine profile in drug-naive first-episode psychosis. Int J Neuropsychopharmacol 18:pyu042. doi: 10.1093/ijnp/pyu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae CU, Yoon CH, Kim TS, Kim JJ, Park SH, Lee CU, Lee SJ, Lee C, Paik IH(2006)Antipsychotic treatment may alter T-helper (TH) 2 arm cytokines. Int Immunopharmacol 6:666–671. [DOI] [PubMed] [Google Scholar]
- Pollmächer T, Hinze-Selch D, Mullington J(1996)Effects of clozapine on plasma cytokine and soluble cytokine receptor levels. J Clin Psychopharmacol 16:403–409. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH(2003)When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160:1554–1565. [DOI] [PubMed] [Google Scholar]
- Ripke S, et al. (2014)Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuld A, Kraus T, Haack M, Hinze-Selch D, Kühn M, Pollmächer T(2000)Plasma levels of cytokines and soluble cytokine receptors during treatment with olanzapine. Schizophr Res 43:164–166. [PubMed] [Google Scholar]
- Sirota P, Hadi E, Djaldetti M, Bessler H(2015)Difference in inflammatory cytokine production by mononuclear cells from obese and non-obese schizophrenic patients. Acta Psychiatr Scand 132:301–305. [DOI] [PubMed] [Google Scholar]
- Sluzewska A.(1999)Indicators of immune activation in depressed patients. Adv Exp Med Biol 461:59–73. [DOI] [PubMed] [Google Scholar]
- Sobiś J, Rykaczewska-Czerwińska M, Świętochowska E, Gorczyca P(2015)Therapeutic effect of aripiprazole in chronic schizophrenia is accompanied by anti-inflammatory activity. Pharmacol Rep 67:353–359. [DOI] [PubMed] [Google Scholar]
- Song X, Fan X, Li X, Zhang W, Gao J, Zhao J, Harrington A, Ziedonis D, Lv L(2014)Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naïve, first-episode schizophrenia. Psychopharmacology (Berl) 231:319–325. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y(2007)The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 21:901–912. [DOI] [PubMed] [Google Scholar]
- Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, Labad J(2014)Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology 41:23–32. [DOI] [PubMed] [Google Scholar]
- Tourjman V, Kouassi É, Koué MÈ, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, Potvin S(2013)Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res 151:43–47. [DOI] [PubMed] [Google Scholar]
- Voss SD, Hank JA, Nobis CA, Fisch P, Sosman JA, Sondel PM(1989)Serum levels of the low-affinity interleukin-2 receptor molecule (TAC) during IL-2 therapy reflect systemic lymphoid mass activation. Cancer Immunol Immunother 29:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr(2003)Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC(2004)Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry 65:940–947. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Cao LY, Song C, Wu GY, Chen DC, Qi LY, Wang F, Xiu MH, Chen S, Zhang Y, Lu L, Kosten TA, Kosten TR(2008)Lower serum cytokine levels in smokers than nonsmokers with chronic schizophrenia on long-term treatment with antipsychotics. Psychopharmacology (Berl) 201:383–389. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zheng Y, Ding YQ, Liu Y, Zhang X, Wu R, Guo X, Zhao J(2014)Minocycline and risperidone prevent microglia activation and rescue behavioral deficits induced by neonatal intrahippocampal injection of lipopolysaccharide in rats. Plos One 9:e93966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.