Abstract
Background
Motor impairments are amongst the earliest and most consistent signs of autism spectrum disorders but are not used as diagnostic criteria. In addition, the relationship between motor and cognitive impairments and their respective neural substrates remain unknown.
Methods
Here, we aimed at determining whether a well-acknowledged animal model of autism spectrum disorders, the valproic acid model, displays motor impairments and whether they may correlate with social deficits and neuronal loss within motor brain areas. For this, pregnant female mice (C57BL/6J) received valproic acid (450 mg/kg) at embryonic day 12.5 and offspring underwent a battery of behavioral analyses before being killed for histological correlates in motor cortex, nigrostriatal pathway, and cerebellum.
Results
We show that while valproic acid male mice show both social and motor impairments, female mice only show motor impairments. Prenatal valproic acid exposure induces specific cell loss within the motor cortex and cerebellum and that is of higher magnitude in males than in females. Finally, we demonstrate that motor dysfunction correlates with reduced social behavior and that motor and social deficits both correlate with a loss of Purkinje cells within the Crus I cerebellar area.
Conclusions
Our results suggest that motor dysfunction could contribute to social and communication deficits in autism spectrum disorders and that motor and social deficits may share common neuronal substrates in the cerebellum. A systematic assessment of motor function in autism spectrum disorders may potentially help the quantitative diagnosis of autism spectrum disorders and strategies aimed at improving motor behavior may provide a global therapeutic benefit.
Keywords: valproic acid, cerebellum, motor cortex, gait, Purkinje cells
Significance Statement
The paper’s main message is that motor impairments in ASD may be indicative of this pathology and may even underlie some of its core cognitive and behavioral features. For this, we conducted a detailed analysis of motor and social behavior in both sexes of a VPA mouse model and correlated these findings to cell loss in specific brain areas such as the cerebellum and motor cortex. Our work brings evidence that motor behavior can be used as a quantitative approach for the diagnosis of ASD and is indicative of social deficits. This opens new avenues in the diagnosis and treatment of ASD targeting the affected brain areas.
Introduction
Autism is one of the most common and disabling neurological disorders of childhood with no commonly implemented diagnostic test or cure available. It affects 3 times more males than females with a prevalence that has been constantly increasing these last decades to reach a proportion of 1 in 68 individuals (CDC, 2014; Loomes et al., 2017). In the current DSM-5 classification, 2 global symptoms are recognized as hallmarks of autism spectrum disorder (ASD): disorders of social communication and restricted and repetitive behaviors (APA, 2013). However, in their initial reports of autistic patients, Kanner and Asperger described early motor deficits, including clumsy gait in what is now recognized as ASD (Kanner, 1943; Asperger, 1944). As such, motor impairments in ASD have been receiving increased attention recently, and study of their contribution to the pathophysiology of ASD is currently a growing field of research. For instance, there are a number of qualitative and quantitative reports in ASD describing impairments in visio-motor and manual dexterity tasks, limb coordination during tasks requiring balance, agility and speed as well as in gait and ataxia (Fatemi et al., 2012). Furthermore, motor impairments may be amongst the earliest signs of some forms of ASD (Ozonoff et al., 2008). Accordingly, the assessment of motor disorders might help the early and quantitative diagnosis of the pathology and the identification of dysfunctional brain regions and circuits in ASD. Such approach may also pave the way for novel therapeutic interventions that might correct motor impairments and potentially the cognitive and social deficits characteristic of ASD.
With this in perspective, the cerebellum has attracted a renewed interest in ASD as a potential brain area at the crossroads of cognitive and motor symptoms characteristic of the disease. The cerebellum is not only critical for the coordination and control of movements but also implicated in higher functions such as cognition, speech, and emotion. Indeed, the cerebellum controls balance and timing and also has an important contribution in facilitating language and executive functions, all behaviors associated with ASD (Fatemi et al., 2012).
Environmental factors acting in utero are suspected to strongly contribute to the etiology of ASD (Arndt et al., 2005). Among these, valproic acid (VPA) exposure during pregnancy to manage epilepsy and mood disorders has been consistently shown to be a major factor associated with developmental defects leading to ASD (Christianson et al., 1994). Hence, rodent models prenatally exposed to VPA have been increasingly used to model ASD with strong construct, face, and, more recently, predictive validity at the structural, behavioral, and pharmacological levels (Nicolini and Fahnestock, 2018). However, most if not all of these studies have solely focused on the social and cognitive aspects of ASD and have neglected motor aspects of the disease.
Here, we used the VPA model of ASD to extensively determine the motor disturbances associated with this pathology, both in males and females, and to identify associated brain histopathology. We then explored correlations between behavioral and postmortem outcomes to identify their potential links and offer a new perspective on the disease by demonstrating that motor disorders are linked with social impairments and may share common neural substrates.
Materials and Methods
Animals
Animal housing and experimental procedures were performed in accordance with the European Union directive (2010/63/EU) and validated by the regional ethical committee (approval no. 2015020415093780). C57BL/6J Mice (Charles River Laboratories) were housed in ventilated cages with access to food and water ad libitum. Room temperature was maintained at 23°C on a 12-h-light/-dark cycle.
Experimental Design and Statistical Analysis
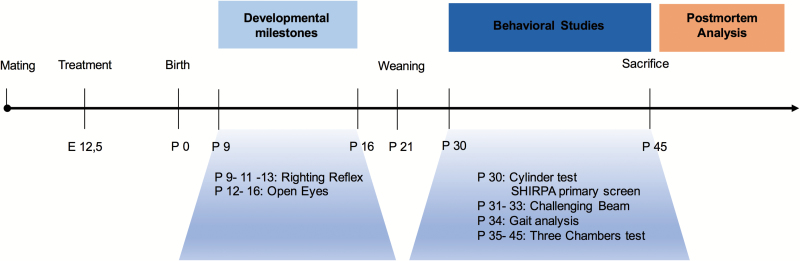
Twenty females and 10 males were used for mating. Four females were placed with a single male and left overnight. Pregnant mice received a single i.p. injection of either VPA (450 mg/kg) or NaCl 0.9% at gestational day 12,5 (E12.5) (Nicolini and Fahnestock, 2018) when the neuronal tube is closing in rodents, followed by neurogenesis (Morriss-Kay et al., 1993). Following mating, pregnant mice were left undisturbed until they gave birth. At weaning (P21), pups from different litters were allocated to the 4 experimental groups depending on prenatal treatment: VPA males (n=34), saline males (n=27), VPA females (n=25), and saline females (n=30). The experiment timeline is presented in Figure 1. The battery of tests was performed in the least stressing and challenging order to avoid potential training and learning effects. Animals were tested during their light cycle, and the experimenter was blind to the treatment until all study and analyses were completed.
Figure 1.
Behavioral experimental plan. For mating, 3 females were transferred to the male cage and the presence of vaginal plug designated gestational day 0 (GD 0). Pregnant dams where treated with either the antiepileptic agent valproic acid (VPA) (450 mg/kg) or NaCl 0.9% on embryonic day 12.5 (E12.5). Sex and age matched pups where separated from the dams on postnatal day 21 (P21) and raised by groups of 4 in a randomized fashion to avoid littermate effects. Comprehensive behavioral screening was performed between P30 and 45. Mice were then killed, brains harvested for histological analysis.
Data are expressed as mean±SEM and analyzed using GraphPad Prism-7 software. Data that followed a normal distribution (all data except grooming and raring behavior) were analyzed using 1- or 2-way ANOVAs whenever appropriate. Upon significant main effects, Tukey’s or Fisher’s LSD multiple comparisons were performed for behavioral or histological measures, respectively. When data did not follow a normal distribution, we conducted nonparametric tests (Kruskal-Wallis) followed by Dunn’s multiple comparison tests. Pearson correlations between behavioral and neuroanatomical readouts were performed using SPSS Statistics version 21 (IBM). For all analyses, P <.05 was considered significant.
Assessment of Developmental Milestones (P9–P16)
During early postnatal life (1–3 weeks), pups were kept in their home cage and righting reflex and eye opening were assessed. To assess righting reflex, mice were placed in the supine position and the time taken to right was monitored 3 times with a 5-minute inter-trial interval. Eye opening was assessed daily at postnatal day (P12) to P16 and scored as either 0=both eyes closed, 1=one eye open, or 2=both eyes open.
SHIRPA Primary Screen (P30)
We implemented the primary SHIRPA screen that serves to identify global disturbances in gait, posture, and muscle tone as well as motor control and coordination abnormalities. Behavior was analyzed in a transparent Plexiglas arena (55×33×22 cm) with 11-×11-cm square grid on the bottom and a 3-mm metal wire crossing diagonally on top. The transfer reaction (time to initiate movement after being placed in the arena and distance covered over 30 seconds) was first evaluated, followed by the wire maneuver test to assess motor coordination and muscle function. Negative geotaxis to assess postural stability and coordination in space was evaluated as the time taken to turn and climb a 45° inclined grid (Rogers et al., 1997).
Spontaneous Activity in the Cylinder (P30)
Spontaneous activity in the cylinder was performed as previously described (Fleming et al., 2013). Mice were put in a transparent Plexiglas cylinder (diameter: 12 cm), and their activity was videotaped for 3 minutes. Number of rearings and time spent grooming were quantified.
Assessment of Motor Coordination on the Challenging Beam (P33)
The challenging beam was performed as described previously (Fleming et al., 2004, 2013). The beam consists of four Plexiglas sections (25 cm length) starting with a width of 3.5 cm and gradually narrowed to 0.5- by 1-cm decrements. Animals were first trained for 2 days to traverse the beam starting at the widest section and ending at the narrowest section that led into the home cage. On the test day, a mesh grid (1-cm squares) was placed over the beam surface. Animals were videotaped while traversing the grid-surfaced beam for 5 trials. Time to traverse, errors, number of steps, and errors per step made by each animal were measured and averaged.
Spatial, Temporal, and Kinetic Gait Parameters (P34)
Gait was analyzed during spontaneous walk using an automated gait analysis system (Viewpoint). The apparatus is made of a 1.5-m-long glass corridor with a dim green light beamed into the glass walkway. The light is reflected downward and a high-speed camera captures footprints’ spatial and kinetic parameters. Each mouse was assessed individually for 3 consecutive runs. The following parameters were analyzed: (1) stride length: distance between 2 consecutive placements of the same paw, (2) limb base of support: distance between 2 pair prints at contact during each step cycle, and (3) pair gap: gap between the placement of the 2 trailing feet, which measures spatial coordination between the 2 pairs.
Assessment of Sociability in the Three Chambers Test (P35–P45)
Social interaction was assessed using the 3-chambers test (Moy et al., 2004). The apparatus consists of a Plexiglas box (60×45×22 cm) partitioned into 3 chambers with retractable doorways. The first phase (PHASE-I) comprises 2 identical nonsocial stimuli (inverted wire cups) placed in the opposite chambers. The second phase (PHASE-II) comprises a nonsocial stimulus and a social stimulus (a naïve mouse with no previous contact with the tested animal). Each phase was of 10 minutes, during which time spent in each chamber was recorded. Subsequently, a sociability index (SI) was calculated as follows: (time exploring social chamber – time exploring nonsocial chamber) / (time exploring social chamber + time exploring nonsocial chamber).
Tissue Processing and Immunohistochemistry
After behavioral assays (P45), males and females from each group were randomly selected for histopathological analysis. Mice were deeply anesthetized with ketamine/xylazine (120/20 mg/kg) and transcardially perfused with 0.9% saline at 37°C followed by 4% paraformaldehyde at 4°C. Brains were post-fixed in 4% paraformaldehyde at 4°C for 24 hours before cryoprotection in 30% sucrose at 4°C for 48 hours. Serial 50 µm (cerebellum) and 40 µm (striatum, cortex, and substantia nigra) free-floating sections were collected and stored at -20°C until use.
Purkinje cells (PC) within the cerebellum, dopaminergic neurons within the substantia nigra, and neurons in the striatum and the motor cortex were quantified. Every fourth cerebellar section was mounted on gelatin-coated slides, PC were identified by cresyl violet staining as previously described (Woodruff-Pak, 2006), and their phenotype was further confirmed with calbindin immunohistochemistry (1:2500; Cb-38a, Swant). However, calbindin was not used for PC quantification, as VPA treatment can lead to reduced calbindin protein expression (Main and Kulesza, 2017). Every 6th section throughout the entire striatum, substantia nigra, and motor cortex was selected and processed for either neuronal nuclei antigen (NeuN) to quantify neurons in the striatum and the motor cortex or for tyrosine hydroxylase (TH) immunoreactivity to quantify dopaminergic neurons in the substantia nigra pars compacta (SNc). Sections were incubated for 90 minutes in a blocking solution (3% bovine serum albumin, 0.3% Triton X-100 in PBS 1 M, pH 7.4). Rabbit anti-NeuN (1:500; Ab177487, abcam) or mouse anti-TH (1:5000; 22941, Immunostar) was applied overnight at 4°C. Biotinylated anti-rabbit or anti-mouse IgG was used as secondary antibody (1:250; BA-1000 and BA-9200, Vector laboratories) for 1 hour at room temperature. Signal was amplified with an ABC Elite kit and revealed with diaminobenzidine (Vector Laboratories). Sections were mounted on gelatin-coated slides and processed for cresyl violet counterstaining.
Stereology
Stereological estimates were performed using the optical fractionator method and systematic random sampling to obtain the total number of cerebellar PC, motor cortex neurons, striatal neurons, and dopaminergic nigral neurons. Each region of interest was outlined based on the mouse brain atlas (Franklin and Paxinos, 2008) at 2.5× objective and neurons were counted at 40× objective using Mercator Software (Explora Nova). Upper and lower guard zones of 1 µm were set at the top and bottom of the section to exclude lost profiles and each neuron or visible nucleus was counted (See Supplementary Table 1).
Results
VPA Mice Showed Delayed Postnatal Development
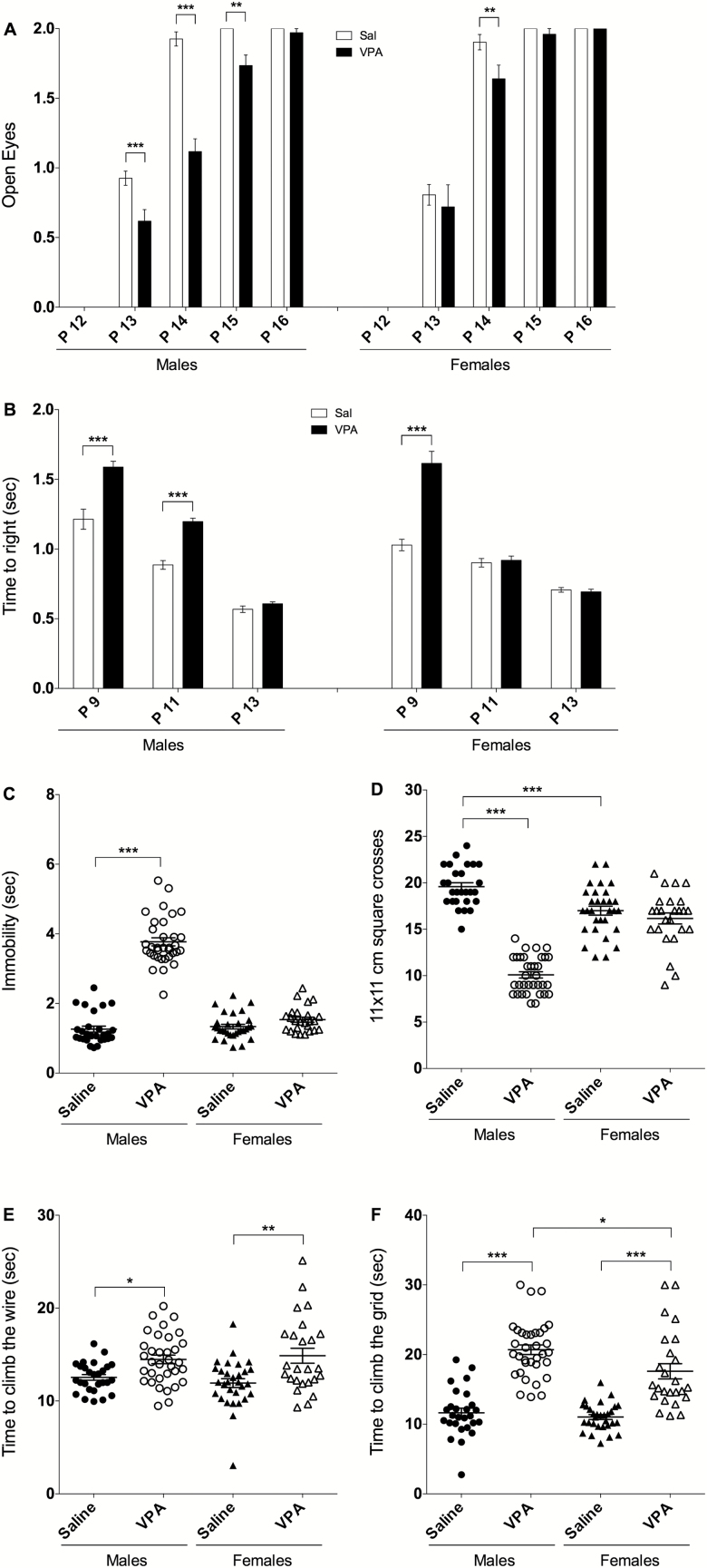
Delays in eye opening and righting reflex were assessed at P9–P16 as indicative of general postnatal development. For eye opening (Figure 2A), 2-way ANOVA revealed a significant effect of treatment [F(3, 560)=22.60, P<.0001], age [F(4, 560)=830.9, P<.0001], and treatment × age interaction [F(12, 560)=6.623, P<.0001]. VPA males showed a significant delay at P13 (P<.001), P14 (P<.001), and P15 (P<.01). Delay appeared earlier and persisted longer in VPA males compared with VPA females, which showed a delay only at P14 (P<.001). Regarding the righting reflex, 2-way ANOVA showed a significant effect of treatment [F(3, 336)=31.05, P<.0001], age [F(2, 336)=323.3, P<.0001], and treatment × age interaction [F(6, 336)=18.64, P<.0001] (Figure 2B). VPA males showed a significant latency in righting at P9 and P11 compared with saline (P<.0001), and VPA females displayed an increase in the latency only at P9 (P<.0001). Thus, VPA prenatal exposure induces significant developmental delays in males compared with saline and that are more pronounced than in VPA females.
Figure 2.
Valproic acid (VPA) prenatal treatment affects several developmental and behavioral paradigms in young pups and that are more pronounced in males than females. (A) VPA mice displayed significant delayed eye opening and (B) latency to righting in comparison with saline. Eye opening was assessed daily at postnatal day (P12) to P16 and scored as 0=both eyes closed, 1=one eye open, or 2=both eyes open. (C) On P30, only VPA males showed a significant increase in immobility (freezing) when transferred to the SHIRPA arena as well as (D) a significant decrease in their locomotor activity. (E) VPA mice show a significant increase in the time climbing the wire and (F) time to climb the grid during the negative geotaxis test in comparison with saline. VPA males (n=34/34), saline males (n=27/27), VPA females (n=25/25), and saline females (n=30/30). Data are expressed as mean±SEM; 2-way ANOVA followed by Tukey’s posthoc were performed (*P<.05, **P<.01, ***P<.001).
VPA Mice Showed Altered Behavior in the SHIRPA Primary Screen
VPA mice showed altered behavior in different SHIRPA parameters tested at P30 (Figure 2C–F). Time spent immobile following transfer to the SHIRPA arena was assessed, and 2-way ANOVA revealed a significant effect of treatment [F(1, 112)=214.2, P<.0001], sex [F(1, 112)=136.7, P<.0001], and treatment × sex interaction [F(1, 112)=155.6, P<.0001] (Figure 2C). Only VPA males showed a 3-fold increase in immobility compared to saline (P<.0001). Directly after transfer to the SHIRPA arena, spontaneous locomotor activity was assessed and an effect of treatment [F(1, 112)=129.4, P<.0001], sex [F(1, 112)=14.64, P<.001], and treatment × sex interaction [F(1, 112)=90.78, P<.0001] were found (Figure 2D). VPA males showed a significant decrease in locomotor activity vs saline males (-50%, P<.0001) while no difference was observed in females.
For the wire maneuver test, 2-way ANOVA revealed a significant effect of treatment [F(1, 112)=21.40, P<.0001], and posthoc analysis showed that both VPA males and females were different from saline as they spent respectively +15% (P<.05) and +25% (P<.001) more time to climb (Figure 2E). Prenatal exposure to VPA also affected negative geotaxis with a significant effect of treatment [F(1, 112)=16.62, P<.0001] on time needed to turn on the grid that was only observed in VPA females (P<.001). In addition, treatment [F(1, 112)=117.0, P<.0001] and sex [F(1, 112)=6.557, P=.0118] had an effect on the time needed to climb the grid (Figure 2F). Both VPA males and females spent significantly more time than controls to climb the grid (+78% and +59% respectively, P<.0001). This increase in time to climb was more pronounced in VPA males compared with VPA females (P<.05).
VPA Mice Showed Core ASD Symptoms
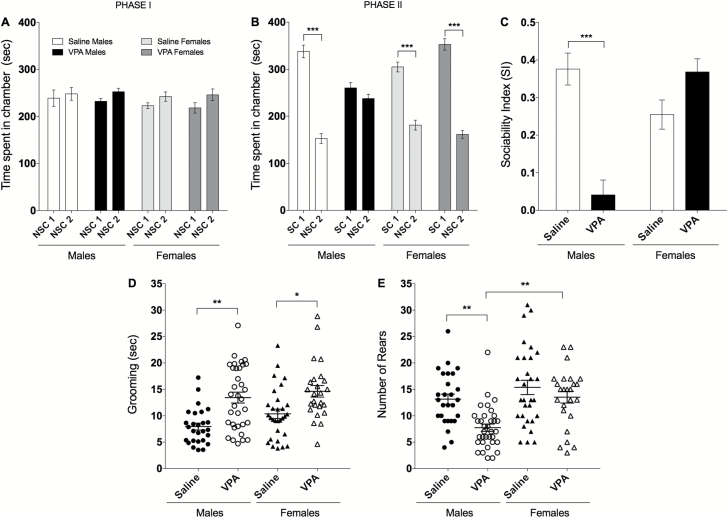
The 3-chambers test was used to assess social behavior in 5- to 6-week-old male and female mice prenatally exposed to VPA or saline. None of the treatment groups, regardless the sex, showed a spontaneous preference for any of the chambers during the 10-minute habituation (PHASE I) (Figure 3A). As expected, there was a significant effect of treatment on PHASE II when a mouse was introduced to one of the chambers [F(1, 128)=243.8, P<.0001]. Indeed, while saline males spent more time in the social chamber than in the empty chamber (69% vs 31%, P<.0001), VPA males spent similar time in both chambers (Figure 3B). This effect was not observed in females that spent more time in the social chamber than in the empty chamber, regardless of the treatment. The effect of VPA on sociability of male mice was even more striking when results were expressed as SI, with a significant effect of treatment [2-way ANOVA F(1, 64)=6.285, P<.05], sex [F(1, 64)=5.453, P<.05] and treatment × sex interaction [F(1, 64)=25.75, P<.0001] (Figure 3C). Posthoc analysis showed that VPA males have a 7-fold decrease in sociability (P<.0001) compared with saline males, while females had similar SI regardless of the treatment.
Figure 3.
Valproic acid (VPA) prenatal treatment induced core symptoms of autism spectrum disorder (ASD) mainly in males. (A–C) Social behavior analysis. VPA males (n=27/34), saline males (n=10/27), VPA females (n=16/25), and saline females (n=15/30). Data are expressed as mean±SEM; 2-way ANOVA followed by Tukey’s posthoc were performed (*P<.05, **P<.01, ***P<.001). (A) Social interaction was assessed using the 3-chambers test. None of the treatment groups showed preference to any of the chambers during the 10-minute habituation (PHASE I). (B) VPA prenatally treated male mice spent less time in the social chamber and more time in the nonsocial chamber relative to controls, (C) they also show a significant decrease in sociability index. (D–E) Grooming and rearing behavior. VPA males (n=34/34), saline males (n=27/27), VPA females (n=25/25), and saline females (n=30/30). (D) VPA mice presented repetitive behavior and (E) a significant decrease in rearing exploratory behavior. Data did not follow a normal distribution and was analyzed using a non-parametric test (Kruskal-Wallis) followed by Dunn’s multiple comparison test.
Repetitive and restricted behaviors are among the core symptoms and the earliest signs of ASD (APA, 2013). As the grooming and rearing data do not follow a normal distribution, Kruskal-Wallis nonparametric test was conducted. Time spent grooming in the cylinder test was significantly affected by treatment [P<.0001] (Figure 3D) in both VPA males (+69%, P<.001) and females (+41%, P<.05) compared with their saline counterparts (Dunn’s multiple comparison test). Additionally, Kruskal-Wallis test revealed a significant change (P<.0001) in rearing behavior (Figure 3E) as VPA males showed a significant reduction in rearing behavior compared with saline (-41%, P<.001) and with VPA females (-50%, P<.001) (Dunn’s multiple comparison test).
Both VPA Males and Females Show Significant Deficits in Motor Coordination and Gait
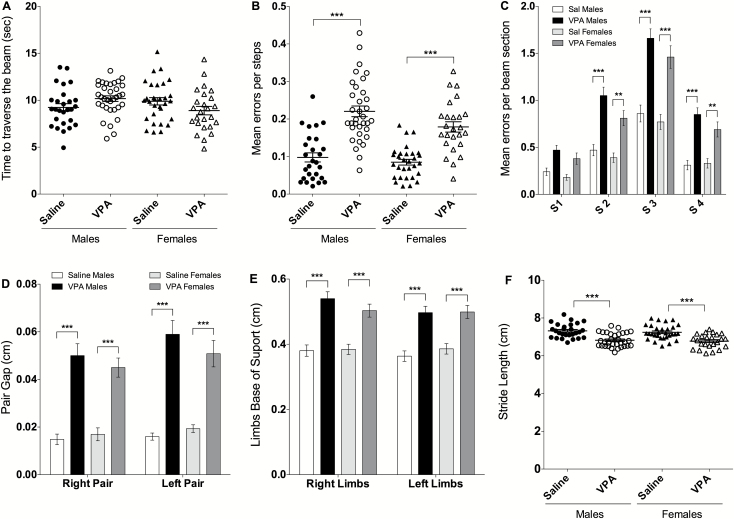
On the challenging beam test, the time needed to traverse the beam was not affected by the treatment [F(1, 112)=0.0045, P=.9462] (Figure 4A). However, the treatment affected errors per step [F(1, 112)=67.04, P<.0001] as both VPA males and females showed a significant increase in errors per step compared with saline (+125% and +110%, respectively, P<.0001; Figure 4B). There was also a significant effect of beam section [F(3, 448)=101.0, P<.0001], group [F(3, 448)=60.61, P<.0001] and beam section × group interaction [F(9, 448)=3.135, P<.0011] (Figure 4C). Indeed, male and female VPA mice made more errors on the second, third, and fourth beam sections compared with their saline counterparts (P<.0001 each).
Figure 4.
Valproic acid (VPA) prenatal treatment induced motor coordination deficits and abnormal gait in both males and females. (A) VPA treatment did not affect the time needed to traverse the beam for any group of mice. (B) VPA males and females showed similar and significant increase in the number of errors per steps in comparison to saline. (C) VPA mice showed significant deficits on the second, third, and fourth sections of the beam. (D) Gait analysis indicated that VPA males and females have a significant increase in both right and left pair gap, (E) right and left limb base of support as well as (F) a significant decrease in stride length in comparison with saline controls. For challenging beam: VPA males (n=34/34), saline males (n=27/27), VPA females (n=25/25), and saline females (n=30/30). For the gait analysis: For the challenging beam, VPA males (n=32/34), saline males (n=27/27), VPA females (n=25/25), and saline females (n=30/30). Data are expressed as mean of 5 trials±SEM, 2-way ANOVA followed by Tukey’s posthoc were performed (*P<.05, **P<.01, ***P<.001).
Gait analysis indicated that VPA treatment did not affect paw area, speed, or regularity of the run in males or females. However, there was a significant effect of treatment [F (3, 220)=49.86, P<.0001] on pair gap (Figure 4D). Indeed, when compared with saline, VPA males and females showed significant increase in right (+2.3-fold, P<.0001) and left pair gap (+1.62-fold, P<.01). In addition, there was a significant effect of treatment [F(3, 220)=33.74, P<.0001] on limb base of support (Figure 4E). Posthoc analysis showed that both VPA males and females have a significant increase in both right and left limbs base of support when compared with saline counterparts (+29 and +40%, respectively, P<.0001), indicating that a significant ataxic gait is present in both males and females following prenatal exposure to VPA. Similarly, there was an effect of VPA [F(1, 109)=46.39, P<.0001] on stride length (Figure 4F), with a decreased stride length in both VPA males and females (-6.5%, P<.0001) compared with their respective saline controls. Altogether, these results indicate major deficits in gait in both VPA males and females.
Cell Loss in Motor Brain Areas of VPA Mice
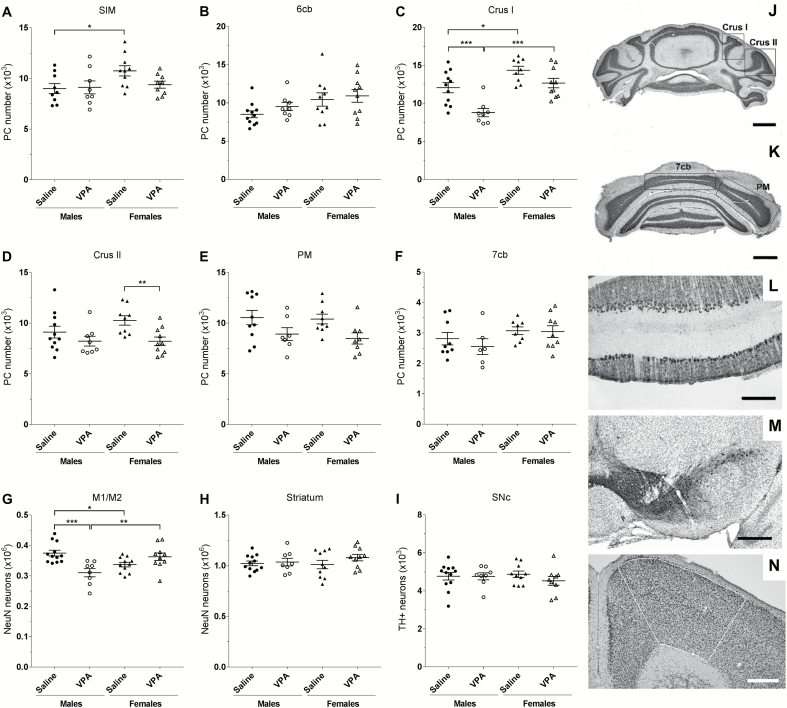
In the cerebellum, we focused our analyses on lobules VI and VII that were found to be the most affected in ASD (Courchesne et al., 1994; Skefos et al., 2014) (Figure 5). In lobule VI (SIM, 6cb), there was no effect of treatment on PC cell number (Figure 5A–B). However, there was an effect of treatment in lobule VII depending on the sub-lobules (Crus I, Crus II, PM, and 7cb, Figure 5J–K) and sex of the VPA-treated mice. Two-way ANOVA revealed a significant effect of treatment [F(1, 34)=16.28, P<.001] and sex [F(1, 34)=25.3, P<.0001] in Crus I. Posthoc analysis revealed a significant PC loss in VPA males in Crus I (-28%, P<.001) and a trend in VPA females (P=.0594) compared with saline (Figure 5C). There was also a significant effect of treatment in Crus II [F (1, 34)=8.502, P<.01] where VPA females, but not males, showed a significant reduction of the number of PC (-20%, P<.01) (Figure 5D). These findings indicate that prenatal VPA treatment caused sex-dependent and specific regional loss of PC in the hemisphere part of the lobule VII.
Figure 5.
Loss of neurons in mice prenatally exposed to VPA is sex and region specific. (A–F) Stereological Purkinje cell (PC) count after Nissl staining on coronal section of cerebellum. (J–K) Photos of the different sub-lobules of the lobule VII, scale bars=1 mm. (L) Illustration of the monolayer organization of PC in the cerebellar cortex after DAB-calbindin immunolabeling, scale bars=200 µm. No difference in PC number was found within the hemispheric part of the lobule VI, SIM (A), or in the vermal part of the lobule VI, 6cb (B). Significant loss of PC was found in the sub-lobule Crus I of the lobule VII in VPA males (C), whereas VPA females showed PC cell loss in the sub-lobule Crus II (D) of the lobule VII. (E) No effect of treatment on the number of PC in the hemispheric sub-lobule PM, or (F) in the vermal sub-lobule 7cb of the lobule VII was found. (G) A decrease in the number of NeuN-stained neurons in the M1/M2 motor cortex was found in VPA males (outlined area on N, scale bars=400 µm). (H) There was no effect of treatment on number of NeuN-stained neurons in the striatum. (I) No change was found in the number of neurons expressing tyrosine hydroxylase (TH) in the substantia nigra pars compacta (SNc) after DAB-TH immunolabeling on ventral mesencephalic coronal sections (M scale bars=400 µm). n(A)=(saline males/VPA males/saline females/VPA females)=9/8/10/9; n(B)=11/8/10/10; n(C)=11/8/9/10; n(D)=11/8/9/10; n(E)=10/7/9/8; n(F)=9/6/8/9; n(G)=12/8/11/10; n(H)=12/8/10/9; n(I)=12/8/10/10. Statistical test: 2-way ANOVA with posthoc Fisher’s LSD test. *P<.05, **P<.01, ***P<.001
To determine whether deficits in motor behavior may be related to a loss of cortical neurons, we quantified NeuN immunoreactive neurons in the primary and secondary motor cortex. Two-way ANOVA revealed a significant sex × treatment interaction [F(1, 37)=17.61, P<.001], and posthoc analysis showed a significant cortical cell loss in VPA males (-17%, P<.001; Figure 5G). This loss was not observed in females (P=.0916), indicating that VPA treatment induces sex-dependent alterations in number of neurons in the motor cortex.
We then explored the effect of VPA treatment on the nigro-striatal pathway, known to be involved in motor control and production of movement. Prenatal VPA exposure did not affect the number of striatal neurons (Figure 5H) or the number of dopaminergic neurons in the SNc (Figure 5I), indicating that motor deficits cannot be attributed to striatal or nigral neuronal loss.
Relationships between Behavioral and Histopathological Findings
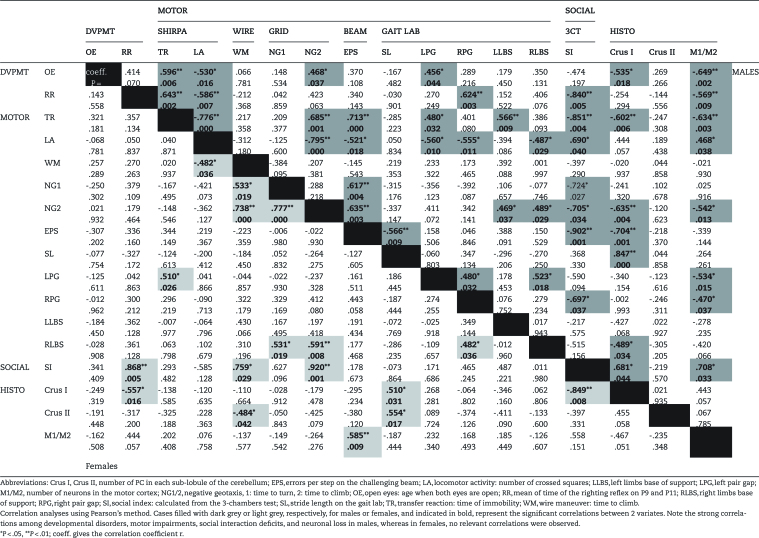
Correlation analyses were performed to determine potential links between behavioral and histological readouts that may be affected by VPA exposure (Table 1). Interestingly, more correlations were observed in males (46/136) than in females (17/136) (Fisher exact test: P<.0001). In males, developmental delays observed at P9-16 correlated with motor impairments at a more advanced age (P30–34). For example, the delay of open eyes correlated with the increase of the time spent to climb the grid and with the increase of the left pair gap. The longer righting reflex correlated with the increase of the right pair gap and both delay of open eyes and righting reflex correlated with an increased immobility time in the transfer reaction and a decreased locomotor activity. Moreover, the righting reflex also correlated with the SI. Interestingly, the SI correlated with several motor impairments such as the number of errors per step, time to climb the grid, right pair gap, immobility time, and locomotor activity. Altogether, these data indicate that in VPA-exposed males, the severity of motor impairments correlates with decreased sociability. In addition, neuronal loss in males appears to be linked to a number of behavioral parameters. Indeed, the number of PC in Crus I correlated with motor impairments (errors per step, stride length, distance between the right pair print) and social deficits. The number of neurons in the motor cortex correlated with the time of righting reflex and with the distance of right and left pair gap. These two histological parameters also both correlated with the delay of eye opening, time of immobility and to climb the grid, and finally with the SI.
Table 1.
Interrelations of the Screened Shifts Behavior and the Anatomical Changes
Collectively, this analysis revealed that, in males: (1) developmental delays correlate with motor impairments later assessed, with social interaction deficits and with neuronal loss in Crus I and motor cortex; (2) motor disturbances correlate with social interactions deficits and neuronal loss; (3) neuronal loss also correlate with the decrease of the SI. Finally, (4) developmental delays, motor impairments, sociability and neuronal loss are all inter-related in males.
In females, the time to turn and climb the grid and time to climb the wire are all correlated. Performances on the grid are also correlated with the distance between the right pair print, which also correlates with the right pair gap. PC loss in Crus II correlated with the time to climb the wire and with the stride length. Interestingly, the number of PC in Crus I also correlated with stride length and developmental delay observed with the righting reflex test. However, there was no correlation between PC counts in Crus I and II and the number of errors per step.
Discussion
Increasing evidence indicates that motor impairments are present in children with ASD (Ming et al., 2007). However, their contribution to the overall clinical expression of the disease remains undetermined. Standardized and quantifiable procedures to explore motor disorders may permit early and reproducible diagnosis of this disease. For instance, 5-year-old ASD children show lower gross and fine motor scores, greater praxis errors, lower movement rates, and greater movement variability (Kaur et al., 2018). Focusing on motor behavior may also allow a better identification of the underlying brain circuitry involved and that may be common to behavioral hallmarks of ASD (Stoodley et al., 2017).
Here, we show that prenatal exposure to VPA (1) provoked early developmental deficits and delayed central nervous system maturation; (2) produced ASD social core features in males but not in females; (3) resulted in deficits in motor coordination and gait in males and, to a lower extent, in females; (4) induced cerebellar PC loss in specific lobules and in relation to sex; and (5) decreased the number of motor cortex neurons only in males. In addition, we show that motor behavior is directly correlated with social deficits and is indicative of the extent of cell loss within the cerebellum and the motor cortex.
The VPA rodent model of ASD is widely used due to its proven construct, face, and, more recently, predictive validity (Nicolini and Fahnestock, 2018). This model stems directly from clinical observations showing that prenatal VPA exposure increases up to 10-fold the risk to develop ASD (Moore et al., 2000; Rasalam et al., 2005). Injection of VPA to pregnant rodent females systematically induces ASD-like syndromes in the offspring (Rodier et al., 1996) such as impaired social interaction (Schneider and Przewłocki, 2005; JW Kim et al., 2014; KC Kim 2014), repetitive behavior (Gandal et al., 2010; JW Kim et al., 2014), and delayed motor development (Schneider and Przewłocki, 2005; Main and Kulesza, 2017). Few studies explored motor performances in this model and reported impaired swimming performance (Schneider and Przewłocki, 2005) and righting reactions (Wagner et al., 2006).
Here, and in agreement with previous results, VPA prenatally exposed males and females displayed a delay in eye opening (Roullet et al., 2010) and an increased latency to righting (Iijima et al., 2016), indicating that postnatal development and motor reflexes are affected. VPA males displayed reduced rearing in the cylinder test, increased immobility, and reduced locomotor activity in the SHIRPA arena. This is in line with previous findings obtained in older animals showing reduced locomotor activity following prenatal VPA treatment (Kataoka et al., 2013; Yamaguchi et al., 2017) and with clinical studies showing that children with ASD spend less time actively exploring their environment (Pierce and Courchesne, 2001).
Motor stereotypies, repetitive and incontrollable motor behaviors, are among the core symptoms of ASD (APA, 2013). In rodents, grooming behavior is often suggested to reflect repetitive/stereotypic behavior (Kalueff et al., 2016). In our study, VPA mice show increased grooming, confirming previous reports with the same animal model (Gandal et al., 2010; Castro et al., 2017). Motor coordination was further explored using the challenging beam test, usually performed in animal models of Parkinson’s disease (Fleming et al., 2004, 2013). Both VPA males and females showed a significant increase in the number of errors made during the beam crossing, indicating major motor coordination deficits. This is line with recent findings in chronically treated rats with VPA showing altered beam walking and motor coordination on a rotarod (Main and Kulesza, 2017).
Gait disturbances and motor development delays were reported in ASD patients (Yirmiya and Charman, 2010). We found altered stride length, pair base of support, and pair gap in our VPA mice models, in line with recent findings with mice bearing a TSC1 genetic knock-out (Tsai et al., 2012) or 15q11-13 duplication (Piochon et al., 2014) they also in line with recent findings reported in rats exposed to VPA (Main and Kulesza, 2017). Interestingly, analyses of family videos have shown that, within a year, movements and postures of children that will later be diagnosed with ASD were asymmetric (Esposito et al., 2009). Karmel et al. (2010) showed that a decrease in arm tone is present at the age of 1 month in infants who will later develop ASD (Karmel et al., 2010). Furthermore, the presence or absence of fine motor delay at 14 months could predict the developmental trajectory of children who develop ASD (Landa et al., 2013), and 70% of high-risk ASD babies who showed early motor retardation were found to later develop a communication deficit (Bhat et al., 2012). Motor deficits may even aggravate social deficits via an impaired ability to properly interact with the environment and with peers. For instance, Crus I, which is affected in ASD and in the VPA animal model as described below, is involved in visuospatial, motor, and cognitive processing and is thus critical to interpreting the gestures of others but also to guide skilled behavior and imitation, all essential for normal social interaction and all affected in ASD (Marko et al., 2015; Nebel et al., 2016).
Beyond motor behavior, the present study points to a major sex-difference in the effect of VPA exposure on social behavior. We confirm previous findings in male mice prenatally exposed to VPA showing deficits in social behavior (Roullet et al., 2010; JW Kim et al., 2014) and we further report that, in contrast, VPA-exposed females developed normal social interaction skills. This suggests that clinical and preclinical exploration based exclusively on social and cognitive readouts may miss some of the neurodevelopmental consequences of VPA exposure in females.
Postmortem and brain imaging studies have consistently identified the cerebellum as one of the most abnormal brain region associated with ASD, and postmortem brains analyses showed a specific loss of cerebellar PC (Bailey et al., 1998; Wegiel et al., 2014). Here we show a loss of PC within the hemispheric part of lobule VII, in Crus I in males and in Crus II in females. The extent and regionally restricted loss is comparable to those found in postmortem ASD brains (Skefos et al., 2014; Wegiel et al., 2014). Interestingly, PC loss in Crus I was found to be correlated with decreased social interaction in VPA males in accordance with the known cognitive role of this sub-lobule in communication and speech in human, both altered in ASD (Stoodley et al., 2012).
The cerebellum is reciprocally connected to areas involved in motor control such as the striatum, the motor, and premotor cortex (Kelly and Strick, 2003; Bostan et al., 2010). Our results show no change in the number of striatal or dopamine nigral neurons of VPA mice, in accordance with postmortem analysis in the striatum of ASD patients (Wegiel et al., 2014). In contrast, the number of cortical neurons was decreased in VPA males. This resonates with a previous study showing altered cellular morphology in the motor cortex neurons of a VPA rat model of autism (Snow et al., 2008). All together our study indicates that the teratogenic effects of VPA on ASD symptoms extend beyond expression of social skills deficits, mainly observed in males, to reach motor deficits and specific cell loss that are observed in both males and females. As such, some forms of ASD may be equally expressed in both sexes but underdiagnosed in women who develop better social coping strategies than male ASD patients (Tierney et al., 2016).
This is the first study showing strong interrelationships among development, sociability, motor performances, and neuronal loss in any mouse model of ASD. Our study indicates that motor impairments are directly correlated to social interaction deficits and to cell loss. Moreover, the more profound cell loss, the more deficits are observed both in social interactions and motor behavior.
Thus, we point out here that motor impairment is a relevant and easily implementable biomarker of ASD severity and suggest that a biomechanical assessment of the children’s motor abilities may be a precious adjunct tool for the diagnosis of the pathology. Our findings also suggest that focusing on motor behavior and its histopathological correlates may pave the way towards the development of therapeutic strategies aimed at specific brain areas such as the cerebellum and the motor cortex.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Funding
This work was supported by grants from the Fondation pour la Recherche Médicale. T.A.S. was awarded a scholarship from the Association of Specialization and Scientific Guidance (Lebanon). O.H. was supported by a fellowship from the Institut National de la Santé et de la Recherche Médicale (INSERM) and the region Poitou-Charentes. The University of Poitiers and INSERM provided infrastructural support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Statement of Interest
None.
Supplementary Material
Acknowledgments
We thank the staff of the PREBIOS animal facility (University of Poitiers-France), Marcello Solinas, Marianne Benoit-Marand, Afsaneh Gaillard, and Laurie Galvan for kind advice and Denis Couratin for technical support.
References
- APA (2013)Diagnostic and statistical manual of mental disorders, 5th ed Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Arndt TL, Stodgell CJ, Rodier PM(2005)The teratology of autism. Int J Dev Neurosci 23:189–199. [DOI] [PubMed] [Google Scholar]
- Asperger H.(1944)Die autistisehen psychopathen im kindesalter. Arch Psychiatr Nurs 117:76–136. [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P(1998)A clinicopathological study of autism. Brain 121:889–905. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ(2012)Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behav Dev 35:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL(2010)The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A 107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro K, Baronio D, Perry IS, Riesgo RDS, Gottfried C(2017)The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr Neurosci 20:343–350. [DOI] [PubMed] [Google Scholar]
- CDC (2014)Health, United States, 2014: with special feature on adults aged 55–64. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Christianson AL, Chesler N, Kromberg JG(1994)Fetal valproate syndrome: clinical and neuro-developmental features in two sibling pairs. Dev Med Child Neurol 36:361–369. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, Schreibman L(1994)Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR Am J Roentgenol 162:123–130. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P, Maestro S, Muratori F(2009)An exploration of symmetry in early autism spectrum disorders: analysis of lying. Brain Dev 31:131–138. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, et al. 2012) Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11:777–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF(2004)Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci 24:9434–9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Ekhator OR, Ghisays V(2013)Assessment of sensorimotor function in mouse models of Parkinson’s disease. J Vis Exp doi: 10.3791/50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G(2008)The mouse brain in stereotaxic coordinates. Cambridge, MA: Academic Press. [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ(2010)Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiatry 68:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima Y, Behr K, Iijima T, Biemans B, Bischofberger J, Scheiffele P(2016)Distinct defects in synaptic differentiation of neocortical neurons in response to prenatal valproate exposure. Sci Rep 6:27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC(2016)Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 17:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L.(1943)Autistic disturbances of affective contact. Nerv Child 2:217–250. [PubMed] [Google Scholar]
- Karmel BZ, Gardner JM, Meade LS, Cohen IL, London E, Flory MJ, Lennon EM, Miroshnichenko I, Rabinowitz S, Parab S, Barone A, Harin A(2010)Early medical and behavioral characteristics of NICU infants later classified with ASD. Pediatrics 126:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Takuma K, Hara Y, Maeda Y, Ago Y, Matsuda T(2013)Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int J Neuropsychopharmacol 16:91–103. [DOI] [PubMed] [Google Scholar]
- Kaur M, M Srinivasan S, N Bhat A(2018)Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without autism spectrum disorder (ASD). Res Dev Disabil 72:79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL(2003)Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Seung H, Kwon KJ, Ko MJ, Lee EJ, Oh HA, Choi CS, Kim KC, Gonzales EL, You JS, Choi DH, Lee J, Han SH, Yang SM, Cheong JH, Shin CY, Bahn GH(2014)Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. Plos One 9:e104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Lee DK, Go HS, Kim P, Choi CS, Kim JW, Jeon SJ, Song MR, Shin CY(2014)Pax6-dependent cortical glutamatergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Mol Neurobiol 49:512–528. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Faherty A(2013)Developmental trajectories in children with and without autism spectrum disorders: the first 3 years. Child Dev 84:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, Mandy WPL(2017)What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 56:466–474. [DOI] [PubMed] [Google Scholar]
- Main SL, Kulesza RJ(2017)Repeated prenatal exposure to valproic acid results in cerebellar hypoplasia and ataxia. Neuroscience 340:34–47. [DOI] [PubMed] [Google Scholar]
- Marko MK, Crocetti D, Hulst T, Donchin O, Shadmehr R, Mostofsky SH(2015)Behavioural and neural basis of anomalous motor learning in children with autism. Brain 138:784–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC(2007)Prevalence of motor impairment in autism spectrum disorders. Brain Dev 29:565–570. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, Dean JC(2000)A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet 37:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay G, Ruberte E, Fukiishi Y(1993)Mammalian neural crest and neural crest derivatives. Ann Anat 175:501–507. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN(2004)Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3:287–302. [DOI] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, Choe AS, Barber AD, Pekar JJ, Mostofsky SH(2016)Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol Psychiatry 79:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolini C, Fahnestock M(2018)The valproic acid-induced rodent model of autism. Exp Neurol 299:217–227. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, Macari S, Hepburn S, Rogers SJ(2008)Gross motor development, movement abnormalities, and early identification of autism. J Autism Dev Disord 38:644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Courchesne E(2001)Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry 49:655–664. [DOI] [PubMed] [Google Scholar]
- Piochon C, Kloth AD, Grasselli G, Titley HK, Nakayama H, Hashimoto K, Wan V, Simmons DH, Eissa T, Nakatani J, Cherskov A, Miyazaki T, Watanabe M, Takumi T, Kano M, Wang SS, Hansel C(2014)Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat Commun 5:5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC(2005)Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol 47:551–555. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J(1996)Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 370: 247–261. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE(1997)Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8:711–713. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wollaston L, Decatanzaro D, Foster JA(2010)Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neuroscience 170:514–522. [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewłocki R(2005)Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30:80–89. [DOI] [PubMed] [Google Scholar]
- Skefos J, Cummings C, Enzer K, Holiday J, Weed K, Levy E, Yuce T, Kemper T, Bauman M(2014)Regional alterations in purkinje cell density in patients with autism. Plos One 9:e81255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow WM, Hartle K, Ivanco TL(2008)Altered morphology of motor cortex neurons in the VPA rat model of autism. Dev Psychobiol 50:633–639. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD(2012)Functional topography of the cerebellum for motor and cognitive tasks: an fmri study. Neuroimage 59:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, Gibson JM, Kelly E, Meng F, Cano CA, Pascual JM, Mostofsky SH, Lerch JP, Tsai PT(2017)Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci 20:1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney S, Burns J, Kilbey E(2016)Looking behind the mask: social coping strategies of girls on the autistic spectrum. Res Autism Spectr Disord 23:73–83. [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M(2012)Autistic-like behaviour and cerebellar dysfunction in purkinje cell tsc1 mutant mice. Nature 488:647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK(2006)A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord 36:779–793. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Flory M, Kuchna I, Nowicki K, Ma SY, Imaki H, Wegiel J, Cohen IL, London E, Wisniewski T, Brown WT(2014)Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum, amygdala and cerebellum. Acta Neuropathol Commun 2:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS.(2006)Stereological estimation of purkinje neuron number in C57BL/6 mice and its relation to associative learning. Neuroscience 141:233–243. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Hara Y, Ago Y, Takano E, Hasebe S, Nakazawa T, Hashimoto H, Matsuda T, Takuma K(2017)Environmental enrichment attenuates behavioral abnormalities in valproic acid-exposed autism model mice. Behav Brain Res 333:67–73. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Charman T(2010)The prodrome of autism: early behavioral and biological signs, regression, peri- and post-natal development and genetics. J Child Psychol Psychiatry 51:432–458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.