Abstract
Loudness dependence of auditory evoked potentials (LDAEP) is a widely used EEG-based biomarker for central serotonergic activity. Serotonin has been shown to be associated with different psychiatric disorders such as depression and schizophrenia. Despite its clinical significance, the underlying neurochemical mechanism of this promising marker is not fully understood, and further research is needed to improve its validity. Other neurotransmitters might have a significant impact on this measure. Thus, we assessed the inhibitory action through individual GABA/H20 concentrations and GABA/glutamate ratios by means of magnetic resonance spectroscopy at 3T in healthy subjects. The measurements were assessed in the primary auditory cortex to investigate the association with the LDAEP, whose generators are mainly in the primary auditory cortex. For the first time, this study examines the link between GABAergic neurotransmission and LDAEP, and the data preliminary show that GABA may not contribute to the generation of EEG-based LDAEP.
Keywords: 1H-MRS, proton magnetic resonance spectroscopy/GABA, gamma-aminobutyric acid /EEG /LDAEP, loudness dependence of auditory evoked potentials
Introduction
The loudness dependence of auditory evoked potentials (LDAEP) is an EEG phenotype that reflects response inhibition in signal processing. Many studies stated that LDAEP is associated with central serotonergic (5-HT) activity, and it is therefore considered as a valid biomarker of central neurotransmitter actions in humans (Hegerl and Juckel, 1993). This biomarker is well established in clinical research and has also been linked to response prediction to antidepressants in clinical samples (Gallinat et al., 2000; Tenke et al., 2017).
To further advance the implementation of this biomarker in routine clinical practice, the validity might be improved by better understanding the processes involved. So far, an inferred relationship between LDAEP and other neurotransmitters such as dopamine and glutamate has been investigated (Hitz et al., 2012; Kenemans and Kähkönen, 2011; Teichert, 2017). However, the influence of GABA is still unresolved. There are several reasons why GABA has to be taken into account: first, there is evidence that intensity tuning in primary auditory cortex (PAC) is determined by the balance of interplay between excitatory and inhibitory synaptic inputs in neuronal networks (Wu et al., 2011). GABAergic interneurons play a key role in such canonical circuits (Ojima, 2011). Second, high neural density of GABA-A receptors has been found in the PAC (La Fougère et al., 2011). Third, 5-HT action mainly modulates GABAergic and glutamatergic neurotransmission (Ciranna, 2006).
Thus, a direct involvement of GABA in the generation of the LDAEP is hypothesized. We hypothesize that GABA levels in the brain are positively associated with LDAEP values. The study by O’Neill et al. (2007) showed that administration of high-dose glycine (activates the NMDA receptors) did not result in an increased LDAEP, as expected, but a reduced LDAEP. The authors stated that this could be due to a strong inhibitory function of GABAergic interneurons on the pyramidal cells, which are relevant for the generation of LDAEP. In the present study, magnetic resonance spectroscopy (MRS) was used to investigate GABA cortical concentrations and inhibitory action (GABA/glutamate) in the auditory cortex of healthy subjects and was correlated with individual LDAEP.
Methods
Fifteen healthy subjects were included in the study (male, right-handed, mean age 27±3.15 years ranging from 19–31 years). None of the participants or their first-degree relatives had a history of neuropsychiatric disorders (Mini International Neuropsychiatry Interview). They reported no history of drug or alcohol abuse, smoking, or metabolic disorders. Subjects were instructed to consume no alcohol or any pharmaceuticals 48 hours beforehand and no caffeine 12 hours before measurements. 1H-MRS and EEG measurements for each subject were taken on the same day.
Subjects were in a supine position with their eyes open in a quiet room adjacent to the recording apparatus and were asked to avoid facial muscle movements throughout the auditory stimulus presentation sequence and the recording. As attention to the auditory stimuli has been shown to modulate the LDAEP (Baribeau and Laurent, 1987), a silent movie was shown to them for distraction and the stimuli were presented in randomized order and points in time that precluded preparatory state. The auditory stimuli were generated and attenuated by a digital signal processor (TDT System 3, Tucker–Davis Technologies). Sinus tones (1000 Hz, 40-millisecond duration with 10-millisecond rise and fall time) of 5 intensities (60, 70, 80, 90, 100 dB sound pressure level) were presented with plastic tubes and ear plugs (delay of 20 ms was subtracted). Pseudo-random SOA was between 2 and 3 seconds in steps of 17 milliseconds. Scalp electrode impedances were kept below 10 kV.
Preprocessing and averaging was performed using Brain Vision Analyzer (version 2.02, Brain Products). Data were down-sampled from 1000 Hz to a rate of 250 Hz, re-referenced to an average reference, and band-pass filtered of 0.16 to 40 Hz (48/12 dB/octave, zero phase). Continuous data were visually inspected for excessive muscle activity, ICA analysis (extended infomax) was applied to remove eye artefacts, and trials with amplitudes exceeding ±100 μV were automatically rejected. Before averaging, the first responses of each of the 5 intensities were excluded to reduce short-term habituation effects. Segmented epochs (−200 to +400 ms) were averaged across conditions (baseline correction from −100 to 0 ms).
Dipole source analysis (DSA) was computed in BESA (version 6.1, MEGIS). Based on the grand average over all subjects 2 dipole models were set: one with 2 regional sources in the PAC (one for each hemisphere) for lower intensities (60, 70, and 80 dB) and another one with 3 regional sources. Based on the results of an earlier study by our group (Wyss et al., 2014), we additionally added one regional source in the premotor cortex for higher intensities (90 and 100 dB).
The peak-to-peak N1/P2 amplitudes were used to quantify differences in the responses to the different tone intensities. In addition to the DSA (nAm) approach, we analyzed single electrode estimation at Cz (μV), as recommended by our group (Hagenmuller et al., 2011), to facilitate across-study comparisons of LDAEP values. Stimuli presentation, EEG assessment, and analysis were according to an earlier study of our group (Wyss et al., 2013).
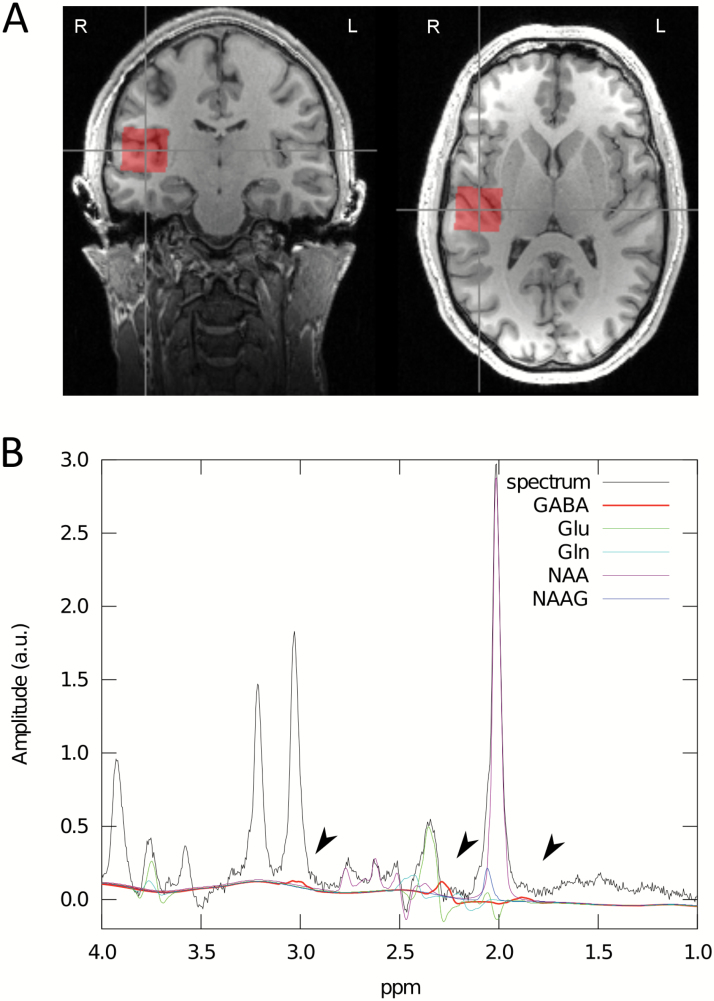
Single-voxel 1H-MRS was performed on a 3T scanner (Trio, Siemens) with a 32-channel phase array coil for receive and a birdcage body coil for transmit. Anatomical T1-weighted images were acquired with a 3D Magnetization Prepared RApid Gradient Echo whole brain acquisition for voxel placement. Figure 1 illustrates voxel position and representative spectra. The concentration of the metabolites was assessed using a standard point resolved spectroscopy sequence optimized for GABA measurement (see Napolitano et al., 2013) using the following parameters: TE1=14 milliseconds, TE=105 milliseconds, TR=2.5 seconds, NA=128, RF pulse centered at 2.4 ppm, 16 step phase cycling. Before the spectroscopy measurements, the static magnetic field was homogenized by running FASTESTMAP iteratively to ensure that the full–width at half maximum of the reference water peak was <0.05 ppm. All metabolite concentrations were quantified with LCModel version 6.3-0I using a basis set simulated with GAMMA. According to this model, the basis set analysis of nonedited spectra included the following metabolites: 2- hydroxyglutaric acid, alanine, aspartate, creatine, GABA, glucose, glutamate, glutamine, glycerophosphocholine, glutathione, lactate, myo-inositol, N-acetylaspartate, N-acetylaspartyglutamate, phosphocreatine, phosphorylcholine, scyllo-inositol, and taurine. The fraction ratios of the grey and white matter and cerebrospinal fluid in the measurement volume were assessed. All metabolite levels were compensated by these fraction ratios and corrected for eddy-currents using the water peak for reference. The toolbox provides two estimates of GABA, one relative to water (GABA/H2O) and one relative to creatine (GABA/Cr). GABA/H2O was used due to the superior signal-to-noise ratio (De Graaf, 2013).
Figure 1.
The voxel of interest (size=2.5×2.5×2.5 cm3) used for magnetic resonance spectroscopy (MRS) was placed by a trained operator at the left and right primary auditory cortex. The voxel included portions of insula, parietal, and frontal operculum (A). A typical spectrum acquired using the optimized standard point resolved spectroscopy sequence for a single subject. Fits for selected metabolites are shown. GABA’s resonance peaks at 3.00, 2.28, and 1.89 ppm (indicated by arrows) (B).
Results
Reliable GABA and glutamate concentrations were detected with Cramer-Rao lower bound (CRLB) <20 % in the right PAC (mean CRLB for GABA 12.33±1.49, for glutamate 3.2±0.56). CRLB is a well-known error estimation method in metabolite quantification, where low CRLBs are associated with reduced noise level and reduced spectral overlap, with <20% being reported as reliable (De Graaf, 2013). CRLB values in the left PAC were not satisfying, and these data were therefore not included in the analysis (mean for GABA 16.6±6.15, for glutamate 3.73±1.16). An inhibitory control index was calculated dividing GABA by the values of glutamate (Harada et al., 2011).
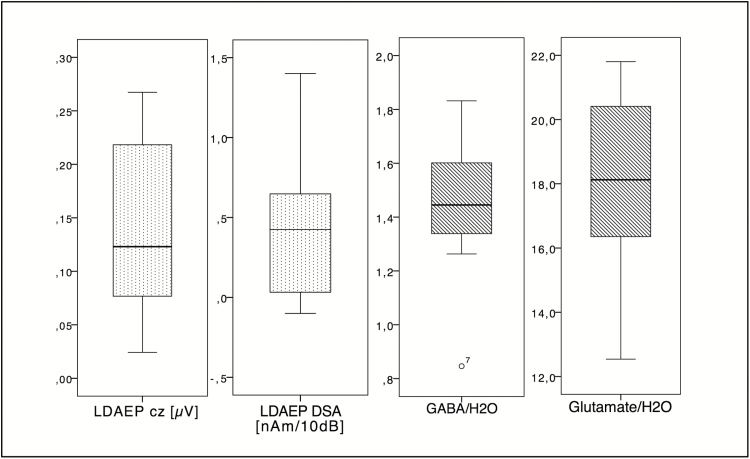
LDAEP values were normally distributed (mean for LDAEP Cz 0.14±0.08; for LDAEP DSA 0.41±0.48). The metabolite concentrations in the right PAC showed individual variability between the subjects (mean for GABA 1.45±0.24, for glutamate 18.25±2.68) (Figure 2).
Figure 2.
The distribution of the variables measured in the right primary auditory cortex (PAC) is summarized by boxplots.
Linear regression analysis was used to test the association between LDAEP values and GABA concentration (SPSS version 23 for Mac). LDAEP of the right hemisphere and from Cz-estimation were entered as the dependent variables. Variables of partial volume effects (GMWM ratio and cerebrospinal fluid) were used as covariates. Results are shown in Table 1. It revealed no significant (P<.05) association between LDAEP values and GABA or inhibitory action.
Table 1.
Summary of values from the linear regression analysis. The inhibitory control index was calculated as follows: the values of GABA divided by the values of glutamate (Glu). N=15. Internal reference=water (H2O).
| EEG phenotype | Metabolite | Δ R2 | Effect size f2 |
Power | B | t | P | CI for B |
|---|---|---|---|---|---|---|---|---|
| LDAEP right | GABA | 0.021 | 0.021 | 0.082 | -0.173 | -0.512 | .619 | -0.919-0.572 |
| Glu | 0.033 | 0.034 | 0.102 | -0.241 | -0.648 | .530 | -1.060-0.577 | |
| Inhibitory control index (GABA/Glu) | 0.000 | - | - | 0.004 | 0.013 | .988 | -0.655–0.664 | |
| LDAEP Cz | GABA | 0.148 | 0.174 | 0.321 | 0.460 | 1.792 | .101 | -0.105–1.025 |
| Glu | 0.086 | 0.094 | 0.196 | 0.389 | 1.296 | .222 | -0.272–1.051 | |
| Inhibitory control index (GABA/Glu) | 0.071 | 0.076 | 0.168 | 0.279 | 1.156 | .272 | -0.252–0.809 |
Discussion
This study is important for a better understanding of the neurochemical mechanisms of the electrophysiological biomarker LDAEP. Our data show that inter-individual differences of auditory signal processing represented by means of LDAEP values were not associated to the variability of GABA or glutamate levels within auditory cortex.
Although GABA and glutamate have an influence on auditory processing, especially on intensity tuning, according to various studies (Kähkönen, 2006; Wu et al., 2011), an association could not be demonstrated by MRS measurements used here. This could be because MRS assesses GABA concentrations in all kinds of neurons rather than specific synaptic release of neurotransmitters (Stagg et al., 2011; Duncan et al., 2014). In addition, it should be noted that several mechanisms, for example, synaptic-level cycle rates influence the dynamic regulation of neurotransmission, which are not depicted here (Duncan et al., 2014). Techniques such as PET, which quantify GABA-A receptor density, offer an alternative approach to investigate the excitatory and inhibitory drive in the auditory cortex (Frankle et al., 2015).
The underlying principle of the LDAEP is that a reduced slope of this function can be considered protective for the information processing system (Hegerl and Juckel, 1993). This is explained by the fact that serotonergic neurons, placed in the raphe nuclei, activate gabaergic interneurons in the pyramidal cells of the primary auditory cortex via 5-HT2 receptors. Alternatively, a modulating influence via 5-HT2 and 5-HT1-A receptors in the PAC is supposed. The lack of a significant relationship between GABA measured in the auditory cortex and LDAEP, however, may be due to the fact that the protective inhibition has already occurred in more basal structures, such as hippocampal formations (Ciranna, 2006). Further studies on the interaction between serotonin and GABA may be of interest for further work.
Moreover, it should be noted that direct evidence from challenge studies with GABA, which could help explain the present results, is lacking in healthy volunteers. On the other hand, studies in patients taking GABAerge medication show that they have both lower LDAEP levels (measured at Cz) (Ostermann et al., 2012) and cortical GABA levels measured by MRS after administration (Goddard et al., 2004). These results are limited to the occipital cortex, as this area was preferably examined for increased measurement quality. To date, there are no comparable studies on GABA assessed by MRS in the auditory cortex. Moreover, there is evidence that the synchronous activation of different brain areas differs between healthy subjects and patients, which generally limits the generalizability of EEG measurements related to a specific brain area (Huang et al., 2003).
Statement of Interest
No funding body was involved in the design of experiment, data collection and analysis, interpretation of the results or preparation, and submission of the manuscript. The authors declare that they have no conflict of interest.
Acknowledgments
C.W. is funded by the Swiss National Science Foundation (SNSF grants P1ZHP3_148704 and PBZHP3_143640) and EMDO Foundation (EMDO Stiftung Zürich, grant no. 784). N.J.S. is funded in part by the Helmholtz Alliance ICEMED - Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Network Fund of the Helmholtz Association. N.J.S. acknowledges funding from the BMBF and Siemens in the 9.4T MR-PET project (grant no. 13N9121). C.W., N.J.S., and I.N. are also funded in part through the European Union Seventh Framework Programme project TRIMAGE (grant no. 602621).
References
- Baribeau JC, Laurent JP(1987)The effect of selective attention on augmenting/intensity function of the early negative waves of aeps. Electroencephalogr Clin Neurophysiol Suppl 40:68–75. [PubMed] [Google Scholar]
- Ciranna L.(2006)Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol 4:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf RA.(2013)In vivo NMR spectroscopy: principles and techniques. West Sussex, England: John Wiley & Sons. [Google Scholar]
- Duncan NW, Wiebking C, Muñoz-Torres Z, Northoff G(2014)How to investigate neuro-biochemical relationships on a regional level in humans? Methodological considerations for combining functional with biochemical imaging. J Neurosci Methods 221:183–188. [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Prasad KM, Mason NS, Paris J, Himes ML, Walker C, Lewis DA, Narendran R(2015)In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. Am J Psychiatry 172:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Bottlender R, Juckel G, Munke-Puchner A, Stotz G, Kuss HJ, Mavrogiorgou P, Hegerl U(2000)The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology (Berl) 148:404–411. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Appel M, Rothman DL, Gueorguieva R, Behar KL, Krystal JH(2004)Impaired GABA neuronal response to acute benzodiazepine administration in panic disorder. Am J Psychiatry 161:2186–2193. [DOI] [PubMed] [Google Scholar]
- Hagenmuller F, Hitz K, Darvas F, Kawohl W(2011)Determination of the loudness dependence of auditory evoked potentials: single-electrode estimation versus dipole source analysis. Hum Psychopharmacol 26:147–154. [DOI] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, Matsuda T(2011)Non-invasive evaluation of the gabaergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord 41:447–454. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G(1993)Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry 33:173–187. [DOI] [PubMed] [Google Scholar]
- Hitz K, Heekeren K, Obermann C, Huber T, Juckel G, Kawohl W(2012)Examination of the effect of acute levodopa administration on the loudness dependence of auditory evoked potentials (LDAEP) in humans. Psychopharmacology (Berl) 221:389–396. [DOI] [PubMed] [Google Scholar]
- Huang MX, Edgar JC, Thoma RJ, Hanlon FM, Moses SN, Lee RR, Paulson KM, Weisend MP, Irwin JG, Bustillo JR, Adler LE, Miller GA, Canive JM(2003)Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clin Neurophysiol 114:835–850. [DOI] [PubMed] [Google Scholar]
- Kähkönen S.(2006)Magnetoencephalography (MEG): a non-invasive tool for studying cortical effects in psychopharmacology. Int J Neuropsychopharmacol 9:367–372. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Kähkönen S(2011)How human electrophysiology informs psychopharmacology: from bottom-up driven processing to top-down control. Neuropsychopharmacology 36:26–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougère C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A(2011)Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. Neuroimage 56:951–960. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Kockenberger W, Auer DP(2013)Reliable gamma aminobutyric acid measurement using optimized PRESS at 3 T. Magn Reson Med 69:1528–1533. [DOI] [PubMed] [Google Scholar]
- Ojima H.(2011)Interplay of excitation and inhibition elicited by tonal stimulation in pyramidal neurons of primary auditory cortex. Neurosci Biobehav Rev 35:2084–2093. [DOI] [PubMed] [Google Scholar]
- O’Neill BV, Croft RJ, Leung S, Oliver C, Phan KL, Nathan PJ(2007)High-dose glycine inhibits the loudness dependence of the auditory evoked potential (LDAEP) in healthy humans. Psychopharmacology (Berl) 195:85–93. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Uhl I, Köhler E, Juckel G, Norra C(2012)The loudness dependence of auditory evoked potentials and effects of psychopathology and psychopharmacotherapy in psychiatric inpatients. Hum Psychopharmacol 27:595–604. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H(2011)What are we measuring with GABA magnetic resonance spectroscopy?Commun Integr Biol 4:573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert T.(2017)Loudness- and time-dependence of auditory evoked potentials is blunted by the NMDA channel blocker MK-801. Psychiatry Res 256:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, et al. (2017)Demonstrating test-retest reliability of electrophysiological measures for healthy adults in a multisite study of biomarkers of antidepressant treatment response. Psychophysiology 54:34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GK, Tao HW, Zhang LI(2011)From elementary synaptic circuits to information processing in primary auditory cortex. Neurosci Biobehav Rev 35:2094–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss C, Hitz K, Hengartner MP, Theodoridou A, Obermann C, Uhl I, Roser P, Grünblatt E, Seifritz E, Juckel G, Kawohl W(2013)The loudness dependence of auditory evoked potentials (LDAEP) as an indicator of serotonergic dysfunction in patients with predominant schizophrenic negative symptoms. Plos One 8:e68650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss C, Boers F, Kawohl W, Arrubla J, Vahedipour K, Dammers J, Neuner I, Shah NJ(2014)Spatiotemporal properties of auditory intensity processing in multisensor MEG. Neuroimage 102:465–473. [DOI] [PubMed] [Google Scholar]