Abstract
Background
It is widely accepted that cognitive processes, such as learning and memory, are affected in depression, but the molecular mechanisms underlying the interactions of these 2 disorders are not clearly understood. Recently, glycogen synthase kinase-3 beta (GSK-3β)/β-catenin signaling was shown to play an important role in the regulation of learning and memory.
Methods
The present study used a rat model of depression, chronic unpredictable stress, to determine whether hippocampal GSK-3β/β-catenin signaling was involved in learning and memory alterations.
Results
Our results demonstrated that chronic unpredictable stress had a dramatic influence on spatial cognitive performance in the Morris water maze task and reduced the phosphorylation of Ser9 of GSK-3β as well as the total and nuclear levels of β-catenin in the hippocampus. Inhibition of GSK3β by SB216763 significantly ameliorated the cognitive deficits induced by chronic unpredictable stress, while overexpression of GSK3β by AAV-mediated gene transfer significantly decreased cognitive performance in adult rats. In addition, chronic unpredictable stress exposure increased the expression of the canonical Wnt antagonist Dkk-1. Furthermore, chronic administration of corticosterone significantly increased Dkk-1 expression, decreased the phosphorylation of Ser9 of GSK-3β, and resulted in the impairment of hippocampal learning and memory.
Conclusions
Our results indicate that impairment of learning and memory in response to chronic unpredictable stress may be attributed to the dysfunction of GSK-3β/β-catenin signaling mediated by increased glucocorticoid signaling via Dkk-1.
Keywords: chronic unpredictable mild stress, depression, glycogen synthase kinase-3 beta, learning and memory, β-catenin
Significance Statement.
Growing evidence indicates the concurrence and interrelationship of depression and cognitive impairments. However, the detailed molecular mechanisms underlying the interactions of these 2 disorders have not been fully understood. Recently, glycogen synthase kinase-3 beta (GSK-3β)/β-catenin signaling was shown to play an important role in the regulation of learning and memory. The present study provides the first evidence that impairment of learning and memory in response to chronic unpredictable stress (CUS) may be attributed to the dysfunction of GSK-3β/β-catenin signaling mediated by increased glucocorticoid signaling via Dkk-1. Understanding the mechanisms that underlie hippocampal damage in response to stress/glucocorticoids may shed new light on the pathophysiology of mood disorders and stress-related cognitive dysfunctions and may lead to the identification of new therapeutic targets.
Introduction
Depression, with 10% to 20% lifetime prevalence, is one of the most common psychiatric illness that involves the disturbance of mood (Wong and Licinio, 2001). It is not only life threatening but also has a negative impact on cognitive processes, especially learning and memory (Dolan, 2002; Trivedi and Greer, 2014; Dillon, 2015; McFarland and Vasterling, 2017; Pan et al., 2017). Growing evidence has shown that patients suffering from major depression often experience memory deficits even after the remission of mood symptoms (Airaksinen et al., 2004; Weiland-Fiedler et al., 2004; Reppermund et al., 2009). Furthermore, rodents that experience repeated stress demonstrate deficits in tasks assessing learning or memory (Song et al., 2006). However, the detailed molecular mechanisms underlying the interactions of these 2 disorders are not clearly understood.
Glycogen synthase kinase-3 (GSK-3) is a serine/threonine protein kinase. Four different phosphorylated regions have been described in GSK-3, and phosphorylation of regulatory serine residues (Ser21 in GSK-3α and Ser9 in GSK-3β) correlates with the inhibition of its kinase activity (Hughes et al., 1993; Wang et al., 1994). The GSK-3β/β-catenin pathway has been studied extensively in the context of the canonical Wnt pathway, which is an important regulator of mammalian neural development (Logan and Nusse, 2004; Ciani and Salinas, 2005). In the Wnt/β-catenin pathway, Wnt ligands bind to the Frizzled receptors and the co-receptors LRP5/6, which leads to the phosphorylation of Disheveled (Dvl) (Wharton, 2003). The activation of Dvl leads to the inhibition of GSK3β, which allows β-catenin to be stabilized, to accumulate in the cytoplasm and to translocate to the nucleus where it activates the transcription of T-cell factor/lymphoid enhancer factor (TCF/LEF) target genes, including the cell-cycle regulatory genes cyclin D1 and c-myc (Logan et al., 2004) and genes important for synaptic plasticity and memory (Arrázola et al., 2009). Wnt signaling is not only modulated by the presence or absence of Wnt ligands but also by antagonists such as the secreted Dickkopf (Dkk) glycoproteins, which bind to LRP5/LRP6, thereby preventing its interaction with Wnt ligands (Niehrs, 2006).
It is now widely accepted that the GSK-3β/β-catenin pathway plays an important role in the regulation of learning and memory (Maguschak and Ressler, 2008, 2011; King et al., 2013; Liu et al., 2017). In vivo activation of Wnt signaling increases excitatory synaptic transmission and improves episodic memory in adult wild-type mice (Vargas et al., 2014). Fortress et al. report that learning rapidly activates GSK-3β/β-catenin signaling in the dorsal hippocampus and suggest that canonical Wnt signaling is necessary for hippocampal memory consolidation (Fortress et al., 2013). Moreover, inhibition of GSK-3β facilitates the induction of long-term potentiation, which is the best characterized molecular and cellular component of the plasticity thought to underlie learning and memory, in the hippocampal CA1 and dentate gyrus regions (Hooper et al., 2007). However, it remains unclear whether GSK-3β/β-catenin signaling is involved in the deficits of learning and memory related to depression.
In the present study, we aimed to investigate whether the GSK-3β/β-catenin signaling would be related to the learning and memory changes in a rat chronic unpredictable stress (CUS) model, one of the most valid and relevant rodent models of depression (Willner, 2005; Duric et al., 2010; Banasr et al., 2010). In addition, we explored the mechanisms involved in regulation of the GSK-3β/β-catenin signaling pathway induced by CUS.
Materials and Methods
Animals
Experiments were performed on adult male Sprague-Dawley rats (Experimental Animal Center, Shanghai Medical College of Fudan University) weighing 200 g. Animals were housed 4/cage with food and water available ad libitum. All rats were kept on a 12-h-light/-dark cycle (lights on at 7 am) in the same colony room, with temperature (21°C±2°C) and humidity (55%±5%) remaining constant. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Jiang Su Animal Care and Use Committee.
CUS Procedure and Drug Treatment
Animals were exposed to a variable sequence of mild and unpredictable stressors for 35 days as previously described (Xi et al., 2011). The CUS procedure contained 9 different stressors randomly arranged day and night across 35 consecutive days: 20 h of food and water deprivation, 18 h of water deprivation, 17 h of 45° cage tilt, overnight illumination, 21 h of wet cage, 5 minutes of swimming in water at 4°C, 30 minutes on a 160-Hz rocking bed, 1-minute tail pinch, and 2-h immobilization. The behavioral tests were operated and scored by trained and experienced observers who were blind to the condition of the animals. Three weeks after the beginning of the CUS, rats received SB216763 (2 mg/kg, i.p.) or saline treatment every other day for another 2 weeks. The dose of SB216763 and alternate day treatment schedule used in this experiment was selected based on previous study (Mao et al., 2009). SB216763 has been previously reported to cross the blood-brain barrier after i.p. injection (Selenica et al., 2007).
Sucrose Preference Test
The sucrose preference test was performed as previously described (Xi et al., 2011). The animals were allowed to consume water and 1% sucrose solution for 1 h after 20-h food and water deprivation. The positions of the 2 bottles (right/left) were varied randomly across animals and were reversed after 30 minutes. The sucrose preference was calculated according to the following ratio: sucrose preference (%)=[sucrose intake (g)/sucrose intake (g) + water intake (g)]×100%.
Forced Swim Test
The forced swim test was conducted as previously described (Xi et al., 2011). Briefly, rats were forced to swim individually in a cylindrical glass container (40 cm height, 30 cm diameter), which contained tap water (25°C±1°C) of 28 cm depth. Rats underwent a preswimming session of 15 minutes followed 24 hours later by a swimming test of 5 minutes. All test sessions were recorded by a video camera from the opposite side of the cylinder. The time spent with minimal activity to keep respiration, passively floating in the water, was measured as immobility.
Morris Water Maze Test
The Morris water maze test was performed as previously described (Xi et al., 2011). The water maze was a 160-cm diameter black circular pool filled with opaque water (30 cm depth) at 25°C ±1°C. An escape platform (11 cm diameter) was placed in the middle of one of the quadrants (1 cm below the water surface). The behavior of the animal was monitored by a video camera mounted in the ceiling above the center of the pool. In the acquisition trials, the rats were trained 120 seconds per trial and 4 trials per day starting at 4 different positions with 30-minute intervals for consecutive 4 days. Each trial began with the rat in the pool facing the sidewalls. If the rat failed to escape within 120 seconds, it was guided to the platform by the experimenter. When the rat escaped onto the platform, the rat was allowed to stay on the platform for 30 seconds before being returned to its home cage. The hidden platform was removed on day 5, and memory retrieval was examined by a probe trial that lasted for 180 seconds. The escape latency in the acquisition trials, the number of crossings over the platform location, and the time spent in the target quadrant during the probe test were recorded by a computerized video tracking system.
Western Blotting
After the behavioral test, hippocampal tissues were immediately frozen on dry ice after dissection and stored at -80°C. Western blotting was performed according to a standard protocol. Nuclear and cytoplasmic proteins were extracted using the NE-PER Nuclear Protein Extraction Kit (Thermo). In brief, 100-mg samples were resuspended in 1000 μL cytoplasmic extraction reagent I and homogenized with a probe sonicator. The mixture was incubated in an ice bath for 10 minutes. Then, 55 μL cytoplasmic extraction reagent II was added to the mixture, violently vortexed for 5 seconds, and incubated in an ice bath for 1 minute. The solution was then centrifuged at 16000 g for 10 minutes at 4°C. After removal of the supernatant, 500 μL of nuclear protein extraction reagent was added to the nuclear precipitate and vortexed on the highest setting for 15 seconds every 10 minutes for a total of 40 minutes. The mixture was centrifuged at 16000 g for 15 minutes at 4°C, and protein concentrations in the supernatant were detected by the Bradford method. Equal quantities of protein were loaded onto a 10% polyacrylamide gel containing 0.2% SDS for separation. The separated proteins were transferred onto a PVDF membrane (Millipore) and incubated overnight at 4°C with the following primary antibodies: GSK-3α (1:1000, Cell Signaling); phospho-Ser21-GSK-3α (1:1000, Abcam); GSK-3β (1:1000, Cell Signaling); phospho-Ser9-GSK-3β (1:1000, Cell Signaling); β-catenin (1:2000, BD Bioscience); α-tubulin (1:2000, Invitrogen); Wnt1 (1:1000, Abcam); Wnt3a (1:1000, Abcam); Wnt7a (1:1000, Abcam); Dkk-1(1:500, Santa Cruz Biotechnology). After washing, the membranes were incubated with a secondary antibody solution (goat anti-mouse, or goat anti-rabbit IgG-HRP, 1:5000, Santa Cruz) at room temperature for 2 hours followed by detection using the enhanced chemiluminescence method.
Construction and Preparation of Recombinant AAV
The rat GSK-3β cDNA was amplified from a rat hippocampal cDNA library and subcloned into an AAV2/8 backbone, which was generated from a pAAV-MCS-EGFP vector by digesting with EcoRI/NheI. The control plasmid expressed only EGFP. The virus was generated with a triple-transfection, helper-free method as previously described (Zolotukhin et al., 1999). Briefly, human embryonic kidney-293 cells were cultured in DMEM with 10% FBS and plated at a density of 8×106 cells in T-75 flasks. The following day, the cells were transfected with pAAV-GSK-3β, pHelper, and pAAV-RC plasmids (Gene Chem) with a standard calcium phosphate method. After 48 hours, cells were harvested and lysed (3 freeze/thaw cycles in dry ice-ethanol and 37°C baths). Then benzonase was added to the mixture (50 U/mL, final concentration), and the lysate was incubated for 30 minutes at 37°C, centrifuged for 20 minutes at 3700 g, and filtered through a 0.45-μm sterile syringe filter. Subsequently, viruses were purified using a modified caesium chloride centrifugation. The virus was then titered using an AAV ELISA kit (Progen) and stored at -80°C.
Stereotaxic Surgery and Infusions
Rats were anesthetized with Nembutal (i.p. 55 mg/kg) and mounted in a rat stereotaxic apparatus. Bilateral viral injections were performed with coordinates -4.3 mm (anterior/posterior), -2.0 mm (lateral), and -4.2 mm (dorsal/ ventral) relative to the bregma (Duric et al., 2010). Each hippocampal hemisphere was infused with a total of 2 μL of purified virus over a 15-minute period followed by 5 minutes of rest. Behavioral tests were performed 4 weeks after virus infusion. After behavioral testing, animals were perfused with phosphate-buffered saline followed by 400 mL of 4% paraformaldehyde (dissolved in 0.1 M PBS, pH 7.4). Brains were immediately removed from the skull, postfixed overnight, followed by an incubation overnight in PBS containing 30% sucrose at 4°C. Brains were cut into 25-μm sections using a microtome to allow for staining with GFP (1:1000, Abcam) and β-catenin (1:1000, BD Bioscience).
Corticosterone Administration
Corticosterone (CORT, Sigma) was administered at a dose of 40 mg/kg (Gregus et al., 2005) suspended in 0.9% (w/v) physiological saline with 2% (v/v) polyoxyethylene glycol sorbitan monooleate (Sigma-Aldrich). Control animals were injected with vehicle (physiological saline). All injections were delivered s.c. once per day between 9:00 am and 11:30 am for 21 consecutive days. The acute CORT treatment group received a vehicle injection once per day for 20 days, followed by a single CORT injection on day 21. The chronic CORT treatment group received a CORT injection once per day for 21 consecutive days, and the control group received a vehicle injection along the same time course. The sucrose preference test, forced swim test and Morris water maze test were carried out 24 hours after the last injection.
Measurement of Plasma CORT
Rats were killed by rapid decapitation the next morning (8:00 am to approximately 10:00 am) after the Morris water maze test. Blood samples were collected into EDTA in microcentrifuge tubes on ice, centrifuged at 800 g for 14 minutes at 4°C, and the plasma was collected and centrifuged further at 800 g for 7 minutes at 4°C. Plasma was stored at -80°C until analysis. Plasma CORT was analyzed by radioimmunoassay using the ImmuChem Corticosterone Double Antibody RIA kit (catalog no. 07-120102, MP Biomedicals). The assay sensitivity was 0.8 μg/dL and the intra- and inter-assay CVs were 6.8% and 7.6%, respectively.
Statistical Analysis
All data are expressed as the mean±SEM. Paired Student’s t test was used to compare 2 experimental groups. Considering the acquisition trials of Morris water maze test were carried out on 4 consecutive days, repeated-measures ANOVA was initially performed. In all other cases, 1-way or 2-way ANOVA was used. Posthoc analyses were performed by the Bonferroni’s test for selected or multiple comparisons when P<.05.
Results
Impairment of Spatial Cognitive Performance Induced by CUS
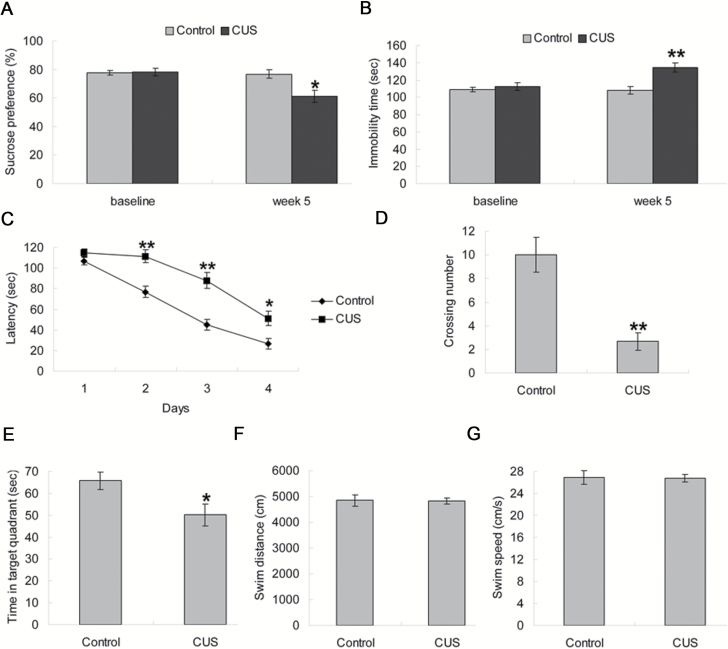
Before CUS, there were no significant differences among the groups exposed to the sucrose preference test (P>.05) and the forced swimming test (P>.05). After CUS for 5 weeks, stressed rats showed a significant decrease in sucrose preference (P<.05; Figure 1A) and a significant increase in immobility time (P<.01; Figure 1B).
Figure 1.
Effects of chronic unpredictable stress (CUS) on behavioral tests. (A) Results of sucrose preference in sucrose preference test. (B) Immobility time in forced swimming test. (C) In the acquisition trials of the Morris water maze test, CUS rats showed longer escape latency during training days 2 to 4. (D–E) In the probe trial, CUS impaired memory retrieval as indicated by fewer crossing times over the platform position and less time spent in the target quadrant. (F–G) There was no significant difference of swim distance and swim speed among groups. Data are presented as mean±SEM (n=6/group). *P<.05, **P<.01 vs control group.
Figure 1C showed the average escape latency onto a hidden platform in the acquisition trials of the Morris water maze test. The curves were similar between groups, with increasingly shorter latency on consecutive days. There was a significant effect of day [F(3, 40)=81.971, P<.001] and CUS [F(1, 40)=61.964, P<.001] on latency to find the platform. On further day-by-day analysis, the CUS group latencies were significantly longer than the control group on day 2 (P<.01), day 3 (P<.01), and day 4 (P<.05), while no significant difference in swimming velocity was observed between the 2 experimental groups (data not shown). In the probe trial, the CUS group displayed fewer crossings (P<.01; Figure 1D) and less time swimming in the target quadrant (P<.05; Figure 1E) compared with the control group. During the period of memory retrieval, the swim distance and swim speed were similar among groups (both P>.05; Figure 1F–G).
Effects of CUS on the GSK-3β/β-Catenin Signaling Pathway
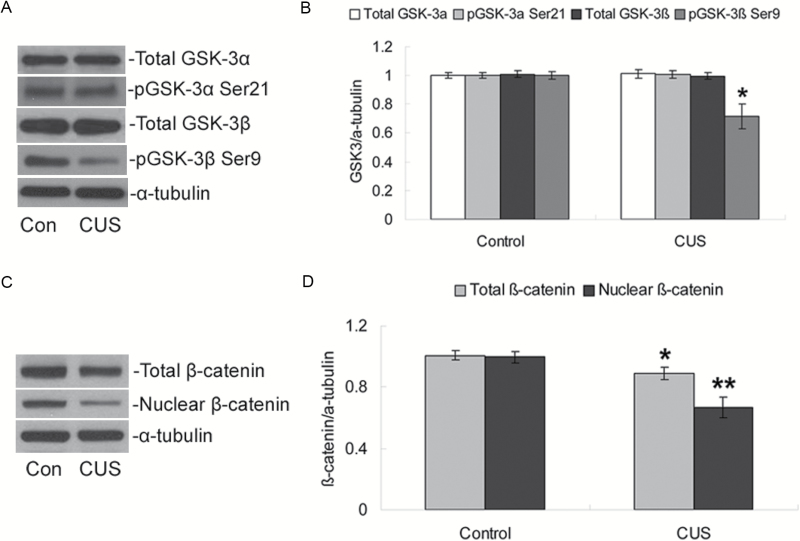
CUS exposure had no significant effect on the total protein levels of either GSK-3α or GSK-3β (both P>.05; Figure 2A–B) in the hippocampus. We further examined the phosphorylation state of GSK-3 and found that phosphorylation only on the Ser9 residue of GSK-3β was significantly decreased after CUS exposure compared with the control group (P<.05; Figure 2A–B), while phosphorylation on Ser21 of GSK-3α was not significantly changed (P>.05).
Figure 2.
Effects of chronic unpredictable stress (CUS) on GSK-3β/β-catenin expression. (A) Representative western blotting of total GSK-3α, phospho-Ser21-GSK-3α, total GSK-3β, phospho-Ser9-GSK-3β, and α-tubulin proteins. (B) Quantification of western-blotting signals of GSK3 and α-tubulin proteins. (C) Representative western blotting of total β-catenin, nuclear β-catenin, and α-tubulin proteins. (D) Quantification of western blotting signals of β-catenin and α-tubulin proteins. Data were ratios compared with α-tubulin protein. Values represent means±SEM (n=6/group). * P<.05, ** P<.01 vs control group.
Because phosphorylation on Ser9 inactivates GSK-3β, a reduction in the inactivation of GSK-3β decreases cytosolic levels of β-catenin and its translocation from the cytoplasm to the nucleus. Therefore, the effects of CUS exposure on the total cellular level of β-catenin and the nuclear level of β-catenin were measured. Figure 2C showed that both total cellular levels and nuclear levels of β-catenin were significantly decreased compared with the control group (P<.05 for total β-catenin, P<.01 for nuclear β-catenin; Figure 2D).
CUS-Induced Cognitive Impairment Is Reversed by GSK-3β Inhibition
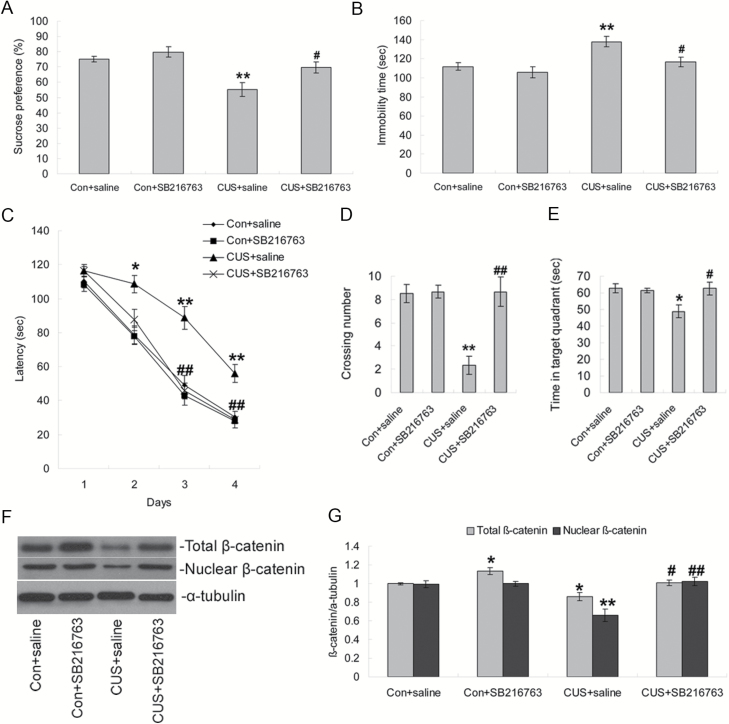
To study the role of GSK-3β/β-catenin signaling in the cognitive function of rats exposed to CUS, we used SB216763, a specific chemical inhibitor of GSK3β. ANOVA analysis revealed significant effects of CUS [F(1, 20)=18.714, P<.001] and SB216763 [F(1, 20)=7.831, P=.01] on the sucrose preference. Posthoc analysis showed that the sucrose preference of CUS+saline animals was significantly decreased compared with control+saline rats (P<.01), and this was reversed by chronic SB216763 administration (P<.05) (Figure 3A). In the forced swimming test, similar effects of CUS [F(1, 20)=13.031, P=.002] and SB216763 [F(1, 20)=7.219, P=.014] were demonstrated on the immobility time, with longer immobility in CUS+saline (P<.01, compared with control+saline group) and a reversal of this effect in CUS+SB216763 (P<.05, compared with CUS+saline group) (Figure 3B).
Figure 3.
Influence of GSK-3β inhibition on behavior tests in chronic unpredictable stress (CUS) rats. (A–B) Effects of CUS and SB216763 treatment on sucrose preference and immobility time in forced swimming test. (C) SB216763 treatment restored the CUS-induced longer latencies in the acquisition trials of Morris water maze test. (D–E) In the probe trial, SB216763 treatment restored the CUS-induced fewer crossing times over the platform position and less time spent in the target quadrant. (F) Western-blotting analysis showing the effects of SB216763 treatment on hippocampal β-catenin expression. (G) Quantification of western-blotting signals of β-catenin and α-tubulin proteins. Data are presented as mean±SEM (n=6/group). *P<.05, **P<.01 vs control+saline group, #P<.05 vs CUS+saline group, ##P<.01 vs CUS+saline group.
We next assessed learning and memory performance in the Morris water maze test. In the acquisition trials, there was a significant effect of day [F(3, 80)=202.115, P<.001], CUS [F(1, 80)=40.840, P<.001] and SB216763 [F(1, 80)=23.846, P<.001] on latency to find the platform. The CUS+SB216763 group showed significantly shorter latencies than the CUS+saline group on day 3 (P<.01), and day 4 (P<.01, Figure 3C) in day-by-day analysis. In the probe trial, ANOVA revealed main effects for CUS and SB216763 treatment on crossing times [F(1, 20)=12.468, P=.002 for CUS; F(1, 20)=13.852, P=.001 for SB216763], but no significant effect on the time in target quadrant [F(1, 20)=4.258, P=.052 for CUS; F(1, 20)=3.826, P=.065 for SB216763]. Posthoc tests demonstrated that SB216763 administration significantly reversed the decreased crossings (P<.01; Figure 3D) and time swimming in the target quadrant (P<.05; Figure 3E) induced by CUS. There were no differences in the swim distance and swim speed among the groups (data not shown). Western-blotting analysis of whole-hippocampal homogenates showed that chronic SB216763 administration significantly increased total cellular β-catenin (P<.05) but had no significant effect on the levels of nuclear β-catenin compared with the control+saline group (Figure 3F–G). Furthermore, SB216763 significantly prevented the CUS-induced decrease of both total cellular levels and nuclear levels of β-catenin compared with the CUS+saline group (P<.05 for total β-catenin, P<.01 for nuclear β-catenin; Figure 3F–G). Further ANOVA analysis showed significant effects for CUS and SB216763 treatment on both total cellular levels [F(1, 20)=16.723, P=.001 for CUS; F(1, 20)=18.863, P<.001 for SB216763] and nuclear levels of β-catenin [F(1, 20)=11.670, P=.003 for CUS; F(1, 20)=16.987, P=.001 for SB216763].
Cognitive Impairment of Viral GSK-3β Expression
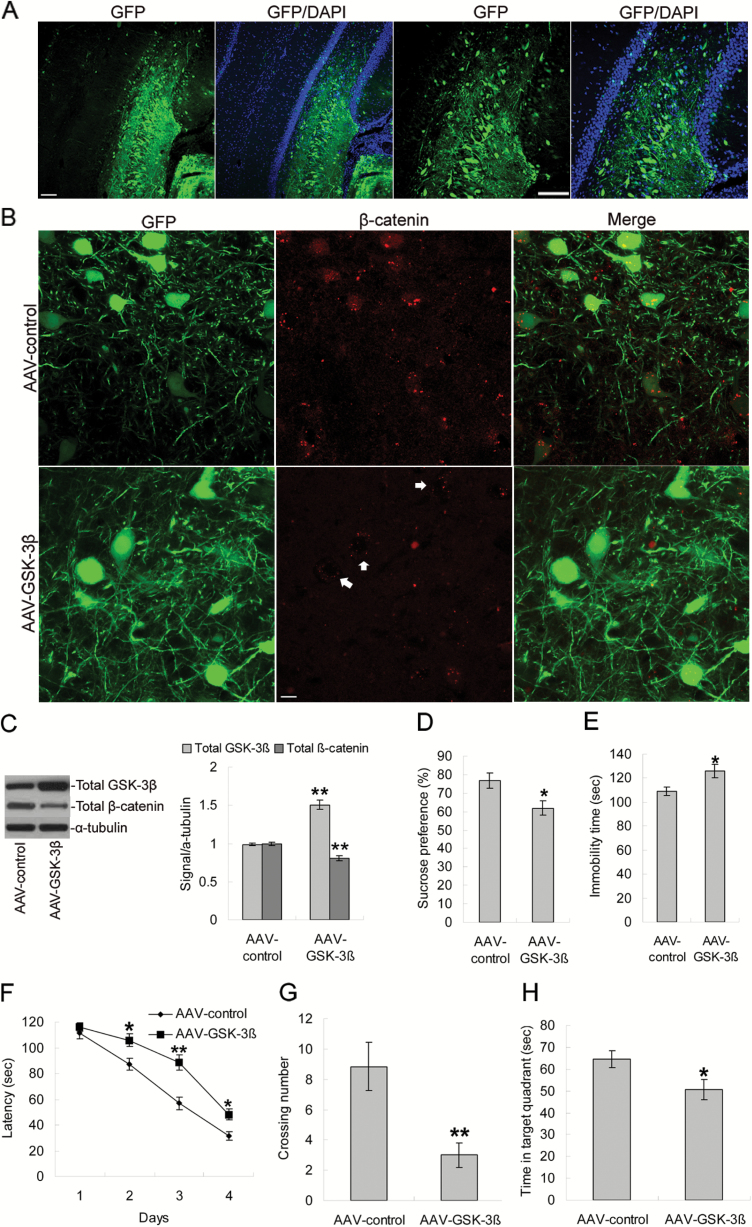
To directly determine the influence of increased expression of GSK-3β on memory and depression behaviors in rats, an AAV vector was designed to express GSK-3β as well as the marker GFP to allow for detection of infected neurons. AAV-control or AAV-GSK-3β was bilaterally injected into the dorsal hippocampus of adult rats, although we also observed GFP+ cells outside hippocampus, probably as a result of the virus traveling up the cannula track (Figure 4A). To verify the expression and function of AAV-GSK-3β, we performed immunofluorescence to detect both GFP, expressed by virus-infected neurons, and β-catenin, a downstream target of GSK-3β. We found that AAV-GSK-3β injection significantly decreased levels of β-catenin colocalization with GFP compared with that in AAV-control rats (Figure 4B). We confirmed this in a separate cohort by Western-blotting analysis of rat hippocampus. AAV-GSK-3β infusion significantly increased GSK-3β levels (P<.01) and decreased total cellular level of β-catenin compared with AAV-control rats (P<.01), demonstrating that GSK-3β overexpression caused functional activation of GSK-3β/β-catenin pathway in the hippocampus (Figure 4C).
Figure 4.
Influence of GSK-3β overexpression on behavior tests in rats. (A) Rats received bilateral intrahippocampal infusions of AAV-control or AAV-GSK-3β-GFP. Representative images of GFP protein expression in dorsal hippocampus. Blue DAPI staining showed the nuclei. Scale bars=100 μm. (B) Representative colocalization images from dorsal hippocampal neurons positive for GFP and β-catenin. Scale bars=25 μm. (C) Western-blotting analysis showing the effects of AAV-GSK-3β injection on the expression of GSK-3β and β-catenin in hippocampus. (D–E) Effects of AAV-GSK-3β infusion on sucrose preference and immobility time in forced swimming test. (F) AAV-GSK-3β infusion rats showed longer escape latency during training days 2 to 4 in the acquisition trials of Morris water maze test. (G–H) In the probe trial, AAV-GSK-3β infusion rats showed fewer crossing times over the platform position and less time spent in the target quadrant. Data are presented as mean±SEM (n=6/group). *P<.05, **P<.01 vs AAV-control group.
Four weeks after virus infusion, AAV-GSK-3β rats showed a significant decrease in sucrose preference (P<.05; Figure 4D) and a significant increase in immobility time (P<.05; Figure 4E). Animals infused with AAV-GSK-3β displayed longer latencies on day 2 (P<.05), day 3 (P<.01), and day 4 (P<.05, Figure 4F) in the acquisition trials of the Morris water maze test relative to AAV-control-infused animals. In the probe trial, the AAV-GSK-3β group displayed decreased crossings (P<.01; Figure 4G) and less time swimming in the target quadrant (P<.05; Figure 4H) compared with the AAV-control group, with no changes in swim distance or swim speed (data not shown).
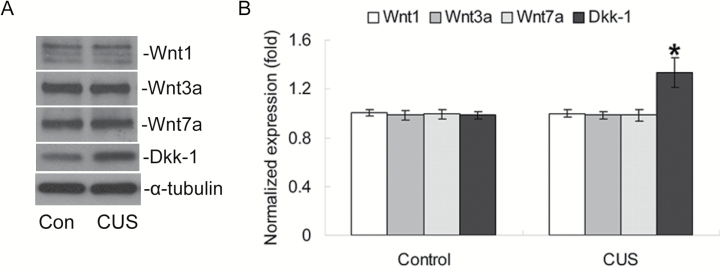
Effects of CUS on Wnt Ligands and Antagonists
Because GSK-3β and β-catenin are the key downstream regulators in canonical Wnt signaling, we next investigated the effects of CUS exposure on protein levels of Wnt ligands and antagonists. Western blotting (Figure 5A) indicated that there were no significant changes in the levels of Wnt1, Wnt3a or Wnt7a, which are classified as canonical Wnt ligands, compared to the control group (all P>.05; Figure 5B). However, there was a significant increase (P<.05; Figure 5B) in the expression of Wnt signaling antagonist Dkk-1.
Figure 5.
Effects of chronic unpredictable stress (CUS) on Wnt ligand and antagonist expression. (A) Representative western blotting of Wnt1, Wnt3a, or Wnt7a, Dkk-1, and α-tubulin proteins. (B) Quantification of western-blotting signals of Wnt ligands, antagonists, and α-tubulin proteins. Data were ratios compared with α-tubulin protein. Values represent means±SEM (n=6/group). *P<.05 vs control group.
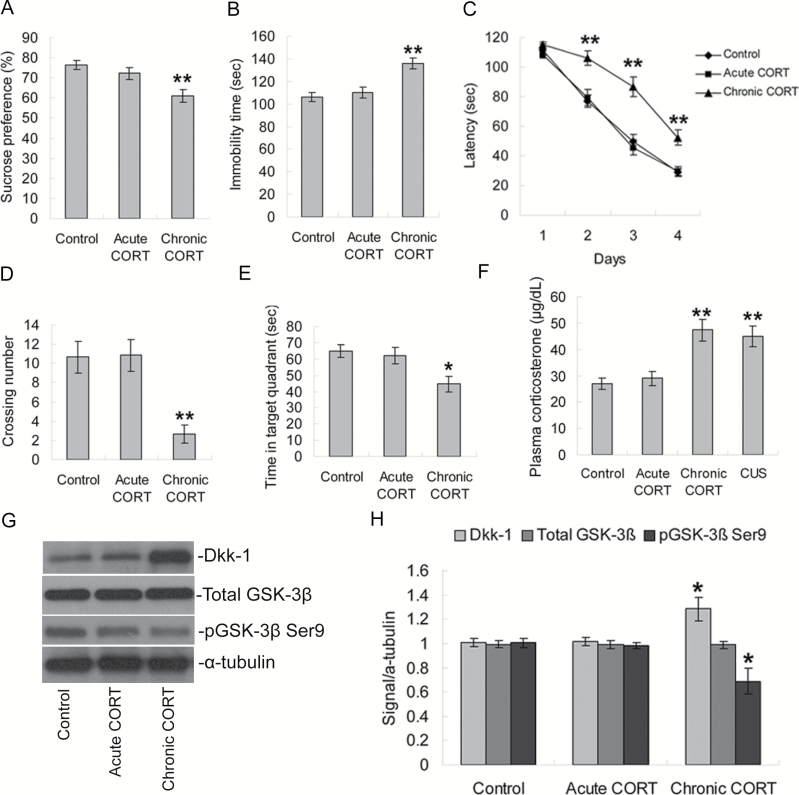
Chronic Treatment of Corticosterone Induces Dkk-1 Expression and Cognitive Impairment
To further examine the effects of CUS exposure on the regulation of Dkk-1, we hypothesized that stress-induced increases in CORT might be the key mediator. Figure 6A and B showed that chronic, but not acute, CORT treatment rats showed a significant decrease in sucrose preference (P<.01) and a significant increase in immobility time (P<.01). Morris water maze test analysis showed that acute CORT treatment had no significant effect on the latencies in acquisition trials compared with the control group (all P>.05; Figure 6C). However, chronic CORT treatment significantly elevated the latencies in acquisition trials on day 2 (P<.01), day 3 (P<.01), and day 4 (P<.01; Figure 6C) compared with the control group. In the probe trial, acute CORT treatment had no significant effect on the crossing times or the time in the target quadrant (both P>.05; Figure 6D–E), while the chronic CORT treatment group displayed decreased crossings (P<.01; Figure 6D) and less time swimming in the target quadrant (P<.05; Figure 6E) compared with the control group. During the memory retrieval phase, the swim distance and swim speed were similar among groups (data not shown).
Figure 6.
Effects of corticosterone (CORT) treatment on behavior tests and Dkk-1 expression in rats. (A–B) Effects of CORT treatment on sucrose preference and immobility time in forced swimming test. (C) Effects of CORT treatment on escape latency during training days 2 to 4 in the acquisition trials of Morris water maze test. (D–E) In the probe trial, rats treated with chronic CORT showed fewer crossing times over the platform position and less time spent in the target quadrant. (F) Effects of CORT treatment and CUS on plasma corticosterone levels. (G) Western-blotting analysis showing the effects of CORT treatments on hippocampal total GSK-3β, phospho-Ser9-GSK-3β, and Dkk-1 expression. (H) Quantification of western-blotting signals of total GSK-3β, phospho-Ser9-GSK-3β, Dkk-1, and α-tubulin proteins. Data are presented as mean±SEM (n=6/group). *P<.05, **P<.01 vs control group.
We further examined the levels of plasma CORT of all 4 groups of rats. Compared with the control group, acute CORT treatment had no significant effect on plasma corticosterone (P>.05; Figure 6F), while the chronic CORT treatment group and CUS group displayed higher levels of CORT (both P<.01; Figure 6F). In addition, Western-blotting analysis showed that chronic, but not acute, CORT treatment significantly increased hippocampal levels of Dkk-1 (P<.05) and decreased phosphorylation of Ser9 on GSK-3β (P<.05; Figure 6G–H), whereas both treatments had no significant effect on the levels of total GSK-3β (both P>.05).
Discussion
The results of the present study demonstrated that exposure to CUS had a dramatic influence on spatial cognitive performance in the Morris water maze task and decreased the phosphorylation of Ser9 of GSK-3β as well as the total and nuclear levels of β-catenin in the hippocampus. Inhibition of GSK3β by SB216763 significantly ameliorated the cognitive deficits induced by CUS, while overexpression of GSK3β by AAV-mediated gene transfer significantly decreased cognitive performance in adult rats. Moreover, CUS exposure increased the expression of the canonical Wnt antagonist Dkk-1. Furthermore, chronic administration of CORT, the key mediator of stress-induced depressive-like behavioral changes and synaptic dysfunction, significantly increased Dkk-1 expression, decreased the phosphorylation of Ser9 of GSK-3β, and resulted in impairment of hippocampal learning and memory. These results suggest that elevated CORT levels could play a role in the regulation of the GSK-3β/β-catenin signaling that underlies learning and memory deficits in CUS.
It is now widely accepted that cognitive dysfunctions including attention, executive function, and memory persist in patients suffering from major depression (Morimoto and Alexopoulos, 2013; Trivedi et al., 2014; Dillon, 2015; Gałecki et al., 2015). An effect size analysis of cognitive functioning in 726 patients with major depressive disorder, conducted using meta-analytic principles, found that the type of memory task most affected by depression was recollection (Zakzanis et al., 1998). Patients suffering from chronic major depression display volume reductions of the hippocampus (Campbell et al., 2004; Koolschijn et al., 2009), a region important for memory formation. The present experiments showed a deficit of spatial memory in rats exposed to CUS, supporting the hypothesis that depressed subjects show differential impairment on memory tasks that are dependent on the hippocampus. Our results are consistent with a previous report showing a deficit of spatial memory in the water maze task following chronic stress or learned helplessness in mice (Song et al., 2006) and the findings of spatial memory deficits in other animal models of depression (Sun and Alkon, 2004; Wright et al., 2006; Bondi et al., 2008).
The GSK-3β/β-catenin pathway has been shown to be regulated by chronic stress. Prenatal chronic mild stress significantly increased the expression of hippocampal GSK-3β (Li et al., 2014). Decreased levels of phosphorylated GSK-3β and β-catenin in the hippocampus have been demonstrated in rats subjected to forced swim stress for 14 consecutive days (Liu et al., 2012). In addition, chronic restraint stress significantly decreased phosphorylation levels of Ser9 of GSK-3β in the prefrontal cortex (Huang et al., 2015). Furthermore, GSK-3β/β-catenin signaling has been implicated in both the pathophysiology and treatment of depression (Crofton et al., 2017; Xu et al., 2017). For example, increases in GSK-3β activity have been found in the prefrontal cortex of postmortem depressed suicide victims (Karege et al., 2012). The GSK-3β gene may play a role in determining the regional gray matter volume differences of the right hippocampus and bilateral superior temporal gyri in patients with recurrent major depressive disorder (Inkster et al., 2009). Okamoto et al. reported that GSK-3β/β-catenin signaling in the hippocampus is regulated by different classes of antidepressant therapies, including SSRIs, SNRIs, dual 5-HT/NE reuptake inhibitors, and chronic electroconvulsive shock (Okamoto et al., 2010). Our findings that CUS exposure decreased the phosphorylation of Ser9 of GSK-3β as well as the total and nuclear levels of β-catenin in the hippocampus are consistent with previous studies.
On the other hand, abnormal Wnt/GSK-3β/β-catenin signaling has been implicated in the pathophysiology of learning and memory deficits. Pharmacological stabilization of β-catenin with LiCl resulted in enhanced learning, whereas genetic deletion of Ctnnb1 (encoding β-catenin) in the amygdala resulted in deficient learning (Maguschak et al., 2008). Furthermore, activation of the canonical Wnt signaling pathway in hippocampus not only improves episodic memory and enhances long-term potentiation in adult wild-type mice but also rescues memory loss and improves synaptic dysfunction in APP/PS1-transgenic mice (Vargas et al., 2014), a model of Alzheimer’s disease, which is characterized by a progressive deterioration of cognitive function (Toledo and Inestrosa, 2010). In agreement with previous studies, the present study demonstrated that inhibition of GSK3β by SB216763 improved the cognitive deficits in the Morris water maze task induced by CUS, while overexpression of GSK3β in the hippocampus decreased cognitive performance in adult rats.
The possible mechanisms of GSK-3β/β-catenin signaling in regulating learning and memory are as follows. First, this signaling pathway has been shown to be involved in the regulation of hippocampal long-term potentiation (Chen et al., 2006; Hooper et al., 2007; Franklin et al., 2014), which is an activity-dependent enhancement of synaptic strength and is considered one of the physiological mechanisms that underlies learning and memory in the hippocampus (Citri and Malenka, 2008). Importantly, β-catenin, present at pre- and postsynaptic terminals, associates with the cytoplasmic domain of cadherin and directly links to the actin cytoskeleton through α-catenin (Gumbiner, 1996). Alterations of cadherin-catenin complexes are thought to influence synaptic size and strength (Murase et al., 2002), suggesting direct participation in synaptic remodelling. Furthermore, GSK-3β/β-catenin has an important role in the regulation of synaptic neurotransmission in hippocampal neurons (Ahmad-Annuar et al., 2006; Cerpa et al., 2008). In addition, as a key component of the Wnt signaling pathway, β-catenin may activate TCF/LEF target genes that are important for neurogenesis, synaptic plasticity, and neuronal death and survival (Clevers, 2006; Hui et al., 2015). Further work is required to determine the mechanisms associated with learning and memory impairments in response to stress.
Growing evidence indicates the concurrence and interrelationship of depression and cognitive impairments (Kuzis et al., 1997; Payne et al., 1998; Zubenko et al., 2003). However, the detailed molecular mechanisms underlying the interactions of these 2 disorders have not been fully understood. It has been suggested that decreased brain derived neurotrophic factor and cAMP-response element-binding protein levels in hippocampus could be involved (Song et al., 2006). In the present study, our results showed that CUS exposure impaired spatial cognitive performance and decreased the phosphorylation of Ser9 of GSK-3β and β-catenin levels in hippocampus, while inhibition of GSK-3β significantly ameliorated the cognitive deficits induced by CUS, indicating an important function of GSK-3β/β-catenin signaling in the interactions between these 2 disorders.
We further investigated the levels of Wnt ligands and antagonists to explore the mechanism of the phosphorylation of GSK-3β caused by CUS. The results showed no significant differences in the levels of Wnt1, Wnt3a, or Wnt7a but did show significantly increased expression of Dkk-1. Although previous studies support a critical role for Wnt ligands and antagonists in learning and memory (Tabatadze et al., 2012; Fortress et al., 2013), our study suggested that only Dkk1 might be involved in the regulation of learning and memory impairments induced by CUS, reflecting differences between Wnt ligands and antagonists in response to chronic stress.
Because the elevated activity of the hypothalamic-pituitary-adrenal axis has key implications in the pathogenesis of several stress-related psychiatric illnesses (de et al., 2005), we hypothesize that the mechanisms underlying the increased Dkk-1 expression induced by CUS may involve glucocorticoid signaling. A recent study has shown that dexamethasone, a glucocorticoid hormone receptor agonist, induces an upregulation of Dkk-1 in human neural stem/progenitor cells (Moors et al., 2012). In addition, mild restraint stress and CORT treatment enhanced the expression of Dkk-1 in the hippocampus of mice, while stress-induced hippocampal damage does not occur in mice that lack a Dkk-1 gene transcriptional enhancer (Doubleridge) (Matrisciano et al., 2011). Importantly, the Dkk-1 gene promoter contains at least 3 glucocorticoid-responsive elements, and the induction of Dkk-1 by dexamethasone mainly resulted from the activation of transcription through glucocorticoid-responsive elements in the Dkk-1 gene promoter in human osteoblasts (Ohnaka et al., 2004). Our results of increased Dkk-1 expression after chronic administration of CORT are consistent with these previous findings.
The action of glucocorticoids on target tissues is mediated by interactions with the glucocorticoid receptor (GR) or mineralocorticoid receptor (MR). Although the mechanism of CORT in increasing the expression of Dkk-1 is not clear, studies implicate GR-dependent regulation. The upregulation of Dkk-1 in primary cultured hippocampal neurons induced by CORT was attenuated by the GR blocker mifepristone but not by spironolactone, which blocks MR (Matrisciano et al., 2011). In addition, our hypothesis is also consistent with the evidence that excessive activation of GRs produces neurotoxic effects in the hippocampus, while activation of MRs is neuroprotective (Crochemore et al., 2005).
We acknowledge that the current studies on learning and memory deficits of CUS rats do not necessarily extrapolate to cognitive declines in depressed patients, which are involved in diminished ability to think and concentrate or indecisiveness, with devastating effects on executive functions, short- and long-term learning, and memory. In addition, effects of CUS on Wnt/GSK-3β/β-catenin signaling pathway were studied in the whole hippocampus; further studies will be needed to determine which subregions of hippocampus are specific to these effects.
In summary, our results suggest that learning and memory deficits resulting from long-term stress exposure are associated with the GSK-3β/β-catenin signaling pathway that links the upregulation of Dkk-1 induced by chronic CORT treatment. Understanding the mechanisms that underlie hippocampal damage in response to stress/glucocorticoids may shed new light on the pathophysiology of mood disorders and stress-related cognitive dysfunctions and may lead to the identification of new therapeutic targets.
Funding
This research was supported by National Natural Science Foundation of China (81201051, Guangjun Xi; 81401619, Jiaojie Hui), Natural Science Foundation of Jiangsu Province (BK2012097, Guangjun Xi), and Medical Young Talents Program of Jiangsu Province (QNRC2016191, Guangjun Xi; QNRC2016178, Jiaojie Hui).
Statement of Interest
None.
Acknowledgments
The authors thank the members of the Ying lab (Department of Cell and Neurobiology, University of Southern California) and Dr Gaoshang Chai (Wuxi Medical School, Jiangnan University) for technical assistance.
References
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC(2006)Signaling across the synapse: a role for wnt and dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol 174:127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen E, Larsson M, Lundberg I, Forsell Y(2004)Cognitive functions in depressive disorders: evidence from a population-based study. Psychol Med 34:83–91. [DOI] [PubMed] [Google Scholar]
- Arrázola MS, Varela-Nallar L, Colombres M, Toledo EM, Cruzat F, Pavez L, Assar R, Aravena A, González M, Montecino M, Maass A, Martínez S, Inestrosa NC(2009)Calcium/calmodulin-dependent protein kinase type IV is a target gene of the wnt/beta-catenin signaling pathway. J Cell Physiol 221:658–667. [DOI] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G(2010)Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA(2008)Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33:320–331. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM(2004)Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 161:598–607. [DOI] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alfaro I, Farías GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC(2008)Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem 283:5918–5927. [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ(2006)Activity-dependent synaptic wnt release regulates hippocampal long term potentiation. J Biol Chem 281:11910–11916. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC(2005)Wnts in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci 6:351–362. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC(2008)Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41. [DOI] [PubMed] [Google Scholar]
- Clevers H.(2006)Wnt/beta-catenin signaling in development and disease. Cell 127:469–480. [DOI] [PubMed] [Google Scholar]
- Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF(2005)Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry 10:790–798. [DOI] [PubMed] [Google Scholar]
- Crofton EJ, Nenov MN, Zhang Y, Scala F, Page SA, McCue DL, Li D, Hommel JD, Laezza F, Green TA(2017)Glycogen synthase kinase 3 beta alters anxiety-, depression-, and addiction-related behaviors and neuronal activity in the nucleus accumbens shell. Neuropharmacology 117:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F(2005)Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475. [DOI] [PubMed] [Google Scholar]
- Dillon DG.(2015)The neuroscience of positive memory deficits in depression. Front Psychol 6:1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ.(2002)Emotion, cognition, and behavior. Science 298:1191–1194. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS(2010)A negative regulator of MAP kinase causes depressive behavior. Nat Med 16:1328–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Schram SL, Tuscher JJ, Frick KM(2013)Canonical Wnt signaling is necessary for object recognition memory consolidation. J Neurosci 33:12619–12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin AV, King MK, Palomo V, Martinez A, McMahon LL, Jope RS(2014)Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragilexmice. Biol Psychiatry 75:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałecki P, Talarowska M, Anderson G, Berk M, Maes M(2015)Mechanisms underlying neurocognitive dysfunctions in recurrent major depression. Med Sci Monit 21:1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE(2005)Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 156:105–114. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM.(1996)Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345–357. [DOI] [PubMed] [Google Scholar]
- Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S(2007)Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci 25:81–86. [DOI] [PubMed] [Google Scholar]
- Huang P, Li C, Fu T, Zhao D, Yi Z, Lu Q, Guo L, Xux(2015)Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav Brain Res 288:1–10. [DOI] [PubMed] [Google Scholar]
- Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR(1993)Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. Embo J 12:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J, Zhang J, Kim H, Tong C, Ying Q, Li Z, Mao X, Shi G, Yan J, Zhang Z, Xi G(2015)Fluoxetine regulates neurogenesis in vitro through modulation of GSK-3beta/beta-catenin signaling. Int J Neuropsychopharmacol 18. doi: 10.1093/ijnp/pyu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkster B, Nichols TE, Saemann PG, Auer DP, Holsboer F, Muglia P, Matthews PM(2009)Association of GSK3beta polymorphisms with brain structural changes in major depressive disorder. Arch Gen Psychiatry 66:721–728. [DOI] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R, Malafosse A(2012)Protein levels of beta-catenin and activation state of glycogen synthase kinase-3 beta in major depression. A study with postmortem prefrontal cortex. J Affect Disord 136:185–188. [DOI] [PubMed] [Google Scholar]
- King MR, Anderson NJ, Guernsey LS, Jolivalt CG(2013)Glycogen synthase kinase-3 inhibition prevents learning deficits in diabetic mice. J Neurosci Res 91:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS(2009)Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 30:3719–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzis G, Sabe L, Tiberti C, Leiguarda R, Starkstein SE(1997)Cognitive functions in major depression and parkinson disease. Arch Neurol 54:982–986. [DOI] [PubMed] [Google Scholar]
- Li M, Li X, Zhang X, Ren J, Jiang H, Wang Y, Ma Y, Cheng W(2014)Effects of prenatal chronic mild stress exposure on hippocampal cell proliferation, expression of GSK-3α, β and NR2B in adult offspring during fear extinction in rats. Int J Dev Neurosci 35:16–24. [DOI] [PubMed] [Google Scholar]
- Liu E, Xie AJ, Zhou Q, Li M, Zhang S, Li S, Wang W, Wang X, Wang Q, Wang JZ(2017)GSK-3β deletion in dentate gyrus excitatory neuron impairs synaptic plasticity and memory. Sci Rep 7:5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Dang W, Jianting M, Su C, Wang H, Chen Y, Tan Q(2012)Citalopram alleviates chronic stress induced depression-like behaviors in rats by activating GSK3Β signaling in dorsal hippocampus. Brain Res 1467:10–17. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R(2004)The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810. [DOI] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ(2008)Beta-catenin is required for memory consolidation. Nat Neurosci 11:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ(2011)Wnt signaling in amygdala-dependent learning and memory. J Neurosci 31:13057–13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH(2009)Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3BETA/beta-catenin signaling. Cell 136:1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Busceti CL, Bucci D, Orlando R, Caruso A, Molinaro G, Cappuccio I, Riozzi B, Gradini R, Motolese M, Caraci F, Copani A, Scaccianoce S, Melchiorri D, Bruno V, Battaglia G, Nicoletti F(2011)Induction of the wnt antagonist dickkopf-1 is involved in stress-induced hippocampal damage. Plos One 6:e16447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland CP, Vasterling JJ(2017)Prospective memory in depression: review of an emerging field. Arch Clin Neuropsychol. doi: 10.1093/arclin/acx118. [DOI] [PubMed] [Google Scholar]
- Moors M, Bose R, Johansson-Haque K, Edoff K, Okret S, Ceccatelli S(2012)Dickkopf 1 mediates glucocorticoid-induced changes in human neural progenitor cell proliferation and differentiation. Toxicol Sci 125:488–495. [DOI] [PubMed] [Google Scholar]
- Morimoto SS, Alexopoulos GS(2013)Cognitive deficits in geriatric depression: clinical correlates and implications for current and future treatment. Psychiatr Clin North Am 36:517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM(2002)Depolarization drives beta-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35:91–105. [DOI] [PubMed] [Google Scholar]
- Niehrs C.(2006)Function and biological roles of the dickkopf family of wnt modulators. Oncogene 25:7469–7481. [DOI] [PubMed] [Google Scholar]
- Ohnaka K, Taniguchi H, Kawate H, Nawata H, Takayanagi R(2004)Glucocorticoid enhances the expression of dickkopf-1 in human osteoblasts: novel mechanism of glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun 318:259–264. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, Newton SS, Duman RS(2010)Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry 68:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Grovu RC, Cha DS, Carmona NE, Subramaniapillai M, Shekotikhina M, Rong C, Lee Y, McIntyre RS(2017)Pharmacological treatment of cognitive symptoms in major depressive disorder. CNS Neurol Disord Drug Targets 16:891–899. [DOI] [PubMed] [Google Scholar]
- Payne JL, Lyketsos CG, Steele C, Baker L, Galik E, Kopunek S, Steinberg M, Warren A(1998)Relationship of cognitive and functional impairment to depressive features in alzheimer’s disease and other dementias. J Neuropsychiatry Clin Neurosci 10:440–447. [DOI] [PubMed] [Google Scholar]
- Reppermund S, Ising M, Lucae S, Zihl J(2009)Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med 39:603–614. [DOI] [PubMed] [Google Scholar]
- Selenica ML, Jensen HS, Larsen AK, Pedersen ML, Helboe L, Leist M, Lotharius J(2007)Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the postnatal rat model of tau hyperphosphorylation. Br J Pharmacol 152:959–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K(2006)Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav 83:186–193. [DOI] [PubMed] [Google Scholar]
- Sun MK, Alkon DL(2004)Induced depressive behavior impairs learning and memory in rats. Neuroscience 129:129–139. [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Tomas C, McGonigal R, Lin B, Schook A, Routtenberg A(2012)Wnt transmembrane signaling and long-term spatial memory. Hippocampus 22:1228–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC(2010)Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an appswe/PSEN1DELTAE9 mouse model of Alzheimer’s disease. Mol Psychiatry 15:272–85, 228. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL(2014)Cognitive dysfunction in unipolar depression: implications for treatment. J Affect Disord 152-154:19–27. [DOI] [PubMed] [Google Scholar]
- Vargas JY, Fuenzalida M, Inestrosa NC(2014)In vivo activation of wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer’s disease model. J Neurosci 34:2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ(1994)Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem 269:14566–14574. [PubMed] [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Charney DS, Neumeister A(2004)Evidence for continuing neuropsychological impairments in depression. J Affect Disord 82:253–258. [DOI] [PubMed] [Google Scholar]
- Wharton KJ.(2003)Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol 253:1–17. [DOI] [PubMed] [Google Scholar]
- Willner P.(2005)Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J(2001)Research and treatment approaches to depression. Nat Rev Neurosci 2:343–351. [DOI] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD(2006)Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur J Neurosci 24:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Hui J, Zhang Z, Liu S, Zhang X, Teng G, Chan KC, Wu EX, Nie B, Shan B, Li L, Reynolds GP(2011)Learning and memory alterations are associated with hippocampal N-acetylaspartate in a rat model of depression as measured by 1H-MRS. Plos One 6:e28686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LZ, Xu DF, Han Y, Liu LJ, Sun CY, Deng JH, Zhang RX, Yuan M, Zhang SZ, Li ZM, Xu Y, Li JS, Xie SH, Li SX, Zhang HY, Lu L(2017)BDNF-GSK-3β-β-catenin pathway in the mPFC is involved in antidepressant-like effects of morinda officinalis oligosaccharides in rats. Int J Neuropsychopharmacol 20:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E(1998)On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 11:111–119. [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N(1999)Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 6:973–985. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Zubenko WN, McPherson S, Spoor E, Marin DB, Farlow MR, Smith GE, Geda YE, Cummings JL, Petersen RC, Sunderland T(2003)A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry 160:857–866. [DOI] [PubMed] [Google Scholar]