Abstract
Heated tobacco products (HTPs) are new tech devices that release nicotine and other volatile compounds into an inhalable aerosol by heating the tobacco. At their operating temperatures, tobacco combustion is unlikely.
The aim of this randomized cross-over study was to measure the exposure levels of the combustion marker, carbon monoxide in the exhaled breath (eCO) of subjects after use of two HTPs and to compare these levels with participants’ own brand of cigarettes.
A total of 12 healthy smokers who reported smoking ≥10 conventional cigarettes per day for at least 5 years took part in the study. Product administration consisted of a first round of 10 puffs, which was followed by an identical second round after a 5 min pause in between rounds. After obtaining a baseline eCO value, this measure was recorded at 5, 10, 15, 30, and 45 min after the first puff of the first round. In contrast to combustible cigarettes, no eCO elevations were observed in the exhaled breath after use of the HTPs under investigation in any of the study participants.
Keywords: Heated tobacco products, Tobacco-heating products, Combustion, Exhaled breath carbon monoxide
Introduction
The health risks associated with cigarette smoking are well established [1]. The harmful effects of smoking result from chronic exposure to thousands of toxic chemicals and carcinogens in cigarette smoke following the combustion of tobacco in the cigarette.
In conventional cigarettes, tobacco combustion is known to release nicotine together with a multitude of harmful and potentially harmful chemical constituents (HPHCs) including carbon monoxide (CO) [2]. Conversely, products that do not require combustion to deliver nicotine (e.g. nicotine replacement therapies - NRTs, smokeless tobacco, electronic cigarettes) are less likely to release much HPHCs, including CO. For this reason, non-combustible nicotine sources have been proposed for smoking harm reduction [3].
Heated tobacco products (HTPs) - the newest addition to the smoking harm reduction approaches - are an emerging class of nicotine delivery devices that do not burn tobacco. HTPs generally consist of a holder (a battery and an electronically controlled heating element) and a rod (containing processed tobacco, glycerine, and other additives). Newer generation HTPs release nicotine and other volatile compounds by heating the tobacco rod at temperatures not exceeding 350 °C. At these temperatures, tobacco combustion is unlikely and as a consequence far fewer chemical toxicants (including CO) are formed [4]. However, earlier generation HTPs that were marketed as less harmful alternatives to combustible tobacco still produced high levels of carcinogens and more CO than conventional cigarettes [5]. This is in contrast to electronic cigarettes (ECs), which heat a tobacco-free nicotine solution and do not elevate CO levels [6–8].
Studies of subjects who have switched from smoking to the newest generation of HTPs show substantial exposure reductions to a wide range of smoke chemicals [9–12]. However, surprisingly, in two of these studies, when exposure to CO was evaluated by measurement in exhaled breath, a significant elevation was detected [13, 14]. It is possible that combustion can still take place in some HTPs designs.
The aim of this randomized cross-over study was to measure the exposure levels to the combustion marker, carbon monoxide in the exhaled breath (eCO) of subjects after use of two HTPs and to compare these levels with participants’ own brand cigarettes. Smokers will be able to make better choices if they are guided by findings of independent studies focused on the relative reduction in exposure risk after switching to HTPs.
Methods
A total of 12 healthy smokers (6 Males, 6 Females; mean age of 28.6 yrs), smoking ≥10 conventional cigarettes per day (cig/day) for at least 5 years took part in this randomized cross-over trial. At screening participants provided a baseline eCO ≥ 10 ppm (ppm) and were provided training for at least 30 min. The training focused on use of both HTPs as per manufacturers’ recommendations.
Participants were randomized to use either iQOS (iQos device with Marlboro Heets, Philip Morris International), or GLO (Glo device with Kent Neostik, British American Tobacco), or their own brand conventional cigarettes on three separate study days (separated by at least 48 h). The randomization sequence was computer-generated.
Subjects were asked to refrain from smoking for at least 12 h prior to each study visit. As per eligibility criteria, smoking abstinence was verified upon arrival by eCO measurements ≤ 10 ppm obtained from a single expiratory breath using a hand-held eCO meter (Micro CO; Micro Medical Ltd., UK) according to the manufacturer’s recommendations. Product administration was like that described by Vansickel et al. [6] and consisted of a first round of 10 puffs with a 30-s interpuff interval (puff number and interpuff interval were monitored by study staff) followed by an identical second round after a 5 min pause in between rounds. Fully charged HTPs were used during study sessions. After obtaining a baseline value, eCO was recorded at 5, 10, 15, 30, and 45 min after the first puff of the first round.
Results
Figure 1 illustrates time trends of eCO levels, separately for each product. Baseline eCO levels were similar among study days and all were below 5 ppm. As expected, large significant within-subject effect (i.e. time, P < 0.0001) was found for changes in eCO following participants’ use of their own brand of traditional cigarettes as compared to either HTP. No significant changes in eCO levels were observed after using the two HTPs under investigation; their median eCO level (95%CI) reaching a max peak at 45 min for GLO with 4.5 (3.9;5.2) ppm and at 15 min for iQOS, with 4.9 (4.1;5.6) ppm, respectively. Repeated-measures ANOVA post-hoc comparisons showed significant differences between-product effect (iQOS/GLO vs own brand cigarette; P < 0.0001; iQOS vs GLO; P = NS) (Table 1).
Fig. 1.
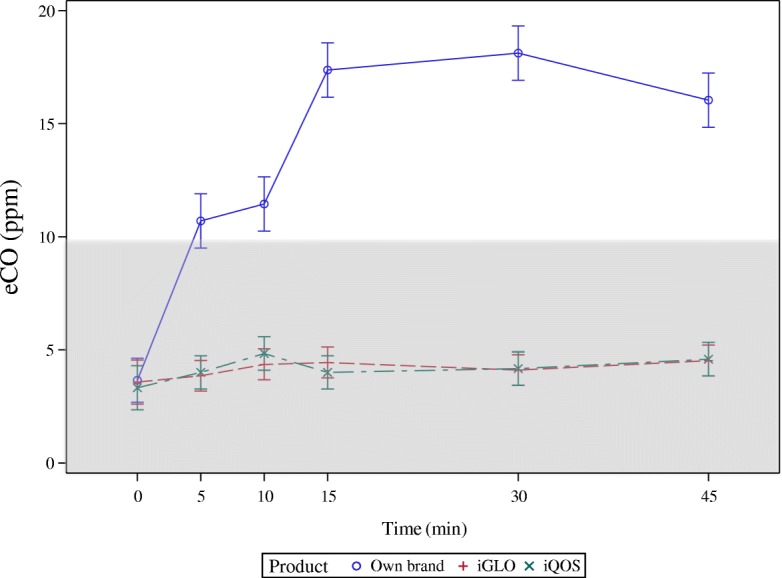
Time trends of eCO levels, separately for each tobacco product use. Time-trends (means ±95% confidence intervals) of exhaled carbon monoxide (eCO) measured at baseline (BL), and at 5, 10, 15, 30 and 45 min after using iQOS (X), GLO (+), and own brand cigarettes (open circle). The model used to produce the repeated measures ANOVA had product and time-point as main effects and it was adjusted by age, sex and baseline measurements. The within-product effect was significant only for own brand cigarette (p < 0.0001). The between-product effect was significantly different for both HTPs compared to own brand cigarette (p < 0.0001), but no different when iQOS was compared to GLO. Shaded area delineates commonly accepted eCO reference ranges for non smokers
Table 1.
Differences of Least Squares Means between Products
| Effect | Product | Product | Estimate | Standard Error | DF | t Value | Pr > |t| | Alpha | Lower | Upper |
|---|---|---|---|---|---|---|---|---|---|---|
| Product | Own brand | GLO | 10.4726 | 0.3126 | 162 | 33.51 | <.0001 | 0.05 | 9.8554 | 11.0899 |
| Product | Own brand | iQOS | 10.4072 | 0.3198 | 162 | 32.54 | <.0001 | 0.05 | 9.7757 | 11.0387 |
| Product | GLO | iQOS | −0.06542 | 0.2270 | 162 | −0.29 | 0.7736 | 0.05 | −0.5138 | 0.3829 |
Discussion
This is the first independent study investigating eCO levels after use of two recently marketed HTPs. We observed no eCO elevations during inhalational testing with HTPs under investigation in any of the study participants. Our findings concur with findings from e-cigarette studies [6–8] as well as from manufacturer and independent data on HTPs. Findings from tobacco industry reports indicate negligible CO emission (0,436 mg/stick and 0,223 mg/stick for iQOS and GLO, respectively; compared to 30.2 mg/tobacco cigarette, that is 69,3 and 135,4 times less) [13, 14]. Independent analytical chemistry data confirms substantial CO level reductions [15, 16] in HTP aerosol emissions compared to combustible cigarettes. Very large reductions were also reported for other tobacco combustible toxicants including TSNAs, PAHs and aldehydes [15, 16]. In another recent independent report, the yields of the carbonyl compounds formaldehyde, acetaldehyde, acrolein, and crotonaldehyde were 80–96% lower compared to combustible cigarettes [17]. Similarly, the emissions of the volatile and semi-volatile compounds benzene, 1,3-butadiene, isoprene, styrene, and toluene were reduced by 97–99%. It should be noted that smoking machine protocols are standardized methods aimed to assess emissions, but not human exposure. In our study we investigated human CO exposure, by measuring and comparing CO levels in the exhaled breath from smokers puffing their own brand, IQOS and GLO. Dissimilarity in absolute CO values reflects different methodologies used for analysis of emissions and assessment of exposures [18]. Nonetheless, the very large reductions reported in CO emissions [13–17] are fully consistent with our findings in human exhaled breath. Interestingly, in a recent paper about GLO aerosol emission [19] when the tobacco stick was ignited and smoked as a conventional cigarette a sharp increase in CO, NO and CO2 was observed, thereby confirming that it is the burning (and not the heating) of the tobacco that results in combustion. We conclude that there is no combustion in iQOS and GLO. Nonetheless, we cannot exclude the presence of toxins generated through pyrolysis in the aerosol of these HTPs.
When interpreting the study findings, many factors need to be considered. First, findings from a small study should be interpreted with caution. Yet reassuring, a power analysis of the collected data indicated that 12 subjects were sufficient to detect differences of 3 ppm or larger, with a power of 80%. Therefore, our randomized crossover trial was more than adequately powered to detect eCO differences between combustible and non-combustible tobacco products. Moreover, eCO elevations larger than 3 ppm were never reported in any of the 12 subjects during HTP use, and most of the eCO changes measured were within the margin of error of the CO monitoring device. Nonetheless, residual combustion (of no clinical significance) cannot be discounted when human exposure studies with low sensitivity measurements are used. Second, the results of the study are product specific under acute laboratory condition of use and cannot be extended to other HTPs or to the same products under investigation after prolonged use. Third, our results appear to support tobacco industry data and may therefore be considered with reluctance. It is important that data reported by the industry is independently verified, as with this investigation.
In relation to the wider implications of this study, it is our opinion that non-combustible nicotine sources - that are significantly less harmful than conventional cigarettes - can be a viable solution for those who, for whatever reason, cannot or do not want to give up nicotine, or who want to cut back on smoking or quit altogether. Therefore, switching to combustion-free products has the potential to act as a gateway out of smoking. The personal preference for a particular product (e.g. e-cigarette vs HTP vs smokeless tobacco products) can play a critical role in increasing the likelihood of successfully abstaining from cigarette smoking. In any case, former smokers already using and smokers intending to use HTPs should receive correct information about their risk-benefit ratio. As for e-cigarettes, health professionals should consider all the options available to a smoking patient and opt for the ones that provide the greatest probability of quitting for good, including HTPs. For many smokers, one possible outcome may be switching to combustion-free products use for the long-term, while tolerating the small residual risk in return for a higher likelihood of success for smoking cessation and tobacco harm reduction.
Acknowledgments
Authors wish to thank LIAF – Lega Italiana Anti Fumo (Italian acronym for Italian Anti Smoking League) - for purchasing from retailers in Italy and Switzerland products used in this study as well as participants for all their time and effort.
Funding
Supported by university grant no. 21040100 of “Ricerca Scientifica Finanziata dall’Ateneo di Catania”.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CO
Carbon monoxide
- CPCT
Centro per la prevenzione e cura del tabagismo
- eCO
Exhaled breath carbon monoxide
- ECs
Electronic cigarettes
- GLO
Glo device with Kent Neostik, British American Tobacco
- HTPs
Heated tobacco products
- iQOS
iQos device with Marlboro Heets, Philip Morris International
- NRT
Nicotine replacement therapies
- PAHs
Polycyclic aromatic hydrocarbons
- TSNAs
Tobacco-specific nitrosamines
Authors’ contributions
RP and PC conceived and designed the study. PC, MM, and GP contributed to participants’ recruitment and performed the tests. BB, RP and PC analyzed and interpreted the data. RP and PC wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the local ERB (reference no. 80/A/2017/P0) and subjects gave written informed consent prior to participation in the study.
Consent for publication
"Not applicable"
Competing interests
R. Polosa is full-time employee of the University of Catania, Italy. In relation to his work in the area of tobacco control, RP has received lecture fees and research funding from Pfizer and GlaxoSmithKline, manufacturers of stop smoking medications. He has also received support from The Consumer Advocates for Smoke-free Alternatives (CASAA) for publication and open access costs of one paper. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl., and Health Diplomat (consulting company that delivers solutions to global health problems with special emphasis on harm minimization). Lectures fees from a number of European electronic cigarette industry and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vapers advocacy no-profit organizations. He is also currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti Smoking League) and for The Consumer Advocates for Smoke-free Alternatives (CASAA); Chair of the European Technical Committee for standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). GP is full-time employee of the Catania University Hospitals Trust (MCAU Ospedale Cannizzaro, Catania, Italy). BB is full-time employee of the Catania University Hospitals Trust (ARNAS Garibaldi, Catania, Italy). PC and MM are part-time employees of the University of Catania; their research contracts are supported by university grant no. 21040100 of “Ricerca Scientifica Finanziata dall’Ateneo di Catania”. The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010. [PubMed]
- 2.Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. Boca Raton (FL): CRC Press, Taylor & Francis Group; 2009. [Google Scholar]
- 3.Polosa R, Rodu B, Caponnetto P, Maglia M, Raciti C. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J. 2013;10:10–19. doi: 10.1186/1477-7517-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlotzhauer WS, Chortyk OT. Recent advances in studies on the pyrosynthesis of cigarette smoke constituents. J Anal ApplPyrol. 1987;12:193–222. [Google Scholar]
- 5.Fagerstrom KO, Hughes JR, Rasmussen T, Callas PW. Randomised trial investigating effect of a novel nicotine delivery device (eclipse) and a nicotine oral inhaler on smoking behaviour, nicotine and carbon monoxide exposure, and motivation to quit. Tob Control. 2000;9:327–333. doi: 10.1136/tc.9.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomark Prev. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagna D, Cibella F, Caponnetto P, Amaradio MD, Caruso M, Morjaria JB, Malerba M, Polosa R. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Investig. 2016;46:698–706. doi: 10.1111/eci.12651. [DOI] [PubMed] [Google Scholar]
- 9.Haziza C, de La Bourdonnaye G, Merlet S, Benzimra M, Ancerewicz J, Donelli A, et al. Assessment of the reduction in levels of exposure to harmful and potentially harmful constituents in Japanese subjects using a novel tobacco heating system compared with conventional cigarettes and smoking abstinence: a randomized controlled study in confinement. Regul Toxicol Pharmacol. 2016;81:489–499. doi: 10.1016/j.yrtph.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Gale N, McEwan M, Eldridge AC, Fearon IM, Sherwood N, Bowen E, et al. Changes in Biomarkers of Exposure on Switching From a Conventional Cigarette to Tobacco Heating Products: A Randomized, Controlled Study in Healthy Japanese Subjects. Nicotine Tob Res. 2018. 10.1093/ntr/nty104. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 11.Sakaguchi C, Kakehi A, Minami N, Kikuchi A, Futamura Y. Exposure evaluation of adult male Japanese smokers switched to a heated cigarette in a controlled clinical setting. Regul Toxicol Pharmacol. 2014;69:338–347. doi: 10.1016/j.yrtph.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Tricker AR, Jang IJ, Martin Leroy C, Lindner D, Dempsey R. Reduced exposure evaluation of an electrically heated cigarette smoking system. Part 4: eight-day randomized clinical trial in Korea. Regul Toxicol Pharmacol. 2012;64:S45–S53. doi: 10.1016/j.yrtph.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Jaccard G, Tafin Djoko D, Moennikes O, Jeannet C, Kondylis A, Belushkin M. Comparative assessment of HPHC yields in the tobacco heating system THS2.2 and commercial cigarettes. Regul Toxicol Pharmacol. 2017;90:1–8. doi: 10.1016/j.yrtph.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Forster M, Fiebelkorn S, Yurteri C, Mariner D, Liu C, Wright C, et al. Assessment of novel tobacco heating product THP1.0. Part 3: comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul Toxicol Pharmacol. 2018;93:14–33. doi: 10.1016/j.yrtph.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Auer R, Concha-Lozano N, Jacot-Sadowski I, Cornuz J, Berthet A. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050–1052. doi: 10.1001/jamainternmed.2017.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekki K, Inaba Y, Uchiyama S, Kunugita N. Comparison of Chemicals in Mainstream Smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH. 2017;39:201–207. doi: 10.7888/juoeh.39.201. [DOI] [PubMed] [Google Scholar]
- 17.Mallock N, Böss L, Burk R, Danziger M, Welsch T, Hahn H, et al. Levels of selected analytes in the emissions of “heat not burn” tobacco products that are relevant to assess human health risks. Arch Toxicol. 2018;92:2145–2149. doi: 10.1007/s00204-018-2215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caruso M, Polosa R. Perplexing conclusions concerning heat-not-burn Tobacco Cigarettes. JAMA Intern Med. 2017;177:1699. [DOI] [PubMed]
- 19.Eaton D, Jakaj B, Forster M, Nicol J, Mavropoulou E, Scott K, et al. Assessment of tobacco heating product THP 1.0. Part 2: product design, operation and thermophysical characterisation. Regul Toxicol Pharmacol. 2018;93:4–13. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


