Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a form of pulmonary arterial hypertension (PAH) in which the pulmonary thrombus fails to resolve, resulting in occlusion and remodelling of pulmonary arteries.1 Timely diagnosis is critical since it is potentially curable by pulmonary thromboendarterectomy. Twenty five per cent of cases do not have a history of thromboembolic event. The diagnosis should be considered in the diagnostic work-up of PAH despite lack of history of episodes of thromboembolism. Here we are reporting a case of CTEPH with multiple systemic to pulmonary collaterals delineated by angiogram and CT.
Keywords: venous thromboembolism, pulmonary hypertension
Background
Chronic thromboembolic pulmonary hypertension (CTEPH) is more common than generally recognised. The cumulative incidence is 2%–4% after the first episode of acute pulmonary embolism (PE).2 Despite extensive pulmonary artery occlusion, patients usually remain asymptomatic. Many of the patients do not have a history of PE, which results in the diagnosis being overlooked.3 The condition becomes apparent when pulmonary arterial hypertension (PAH) progresses, causing dyspnoea, hypoxaemia and right ventricular (RV) dysfunction, eventually leading to death from decompensated right heart failure.
Case presentation
A 35-year-old man with no reported family history presented with a 10-day history of progressive shortness of breath. He had no history of PE or deep vein thrombosis. However, he was diagnosed with extrahepatic portal vein thrombosis following episodes of haematemesis from 3 years, resulting in recurrent oesophageal varices requiring banding on several occasions. Physical examination revealed blood pressure of 100/60 mm Hg, pulse rate of 88 beats/min, respiration rate of 16 breaths/min, jugular venous distention, ascites, a narrow split second heart sound, accentuation of pulmonic closure and a palpable RV heave.
Investigations
Blood tests revealed D-dimer of 6.04 µg/mL and pro-B type natriuretic peptide (BNP) of 6000 pg/mL. ECG showed right axis deviation and right ventricle hypertrophy. Venous Doppler of both lower limbs was normal. Initial transthoracic echocardiography showed enlarged right chambers with right overload and moderate tricuspid regurgitation (figure 1, video 1), with severe PAH (figure 2). Although dilated there was no evidence of thrombus in the main, right and left pulmonary arteries (figure 3). Suprasternal view demonstrated the presence of multiple collaterals from the aorta (figure 4, video 2). Chest CT revealed eccentric filling defect in the interlobar artery and segmental divisions with evidence of irregularities, abrupt cut-off and web formation. In addition collaterals were seen arising from the left internal mammary artery (LIMA) and the right internal mammary artery (RIMA) and the descending thoracic aorta heading towards the hila (figure 5). A diagnosis of CTEPH was thus made.
Figure 1.
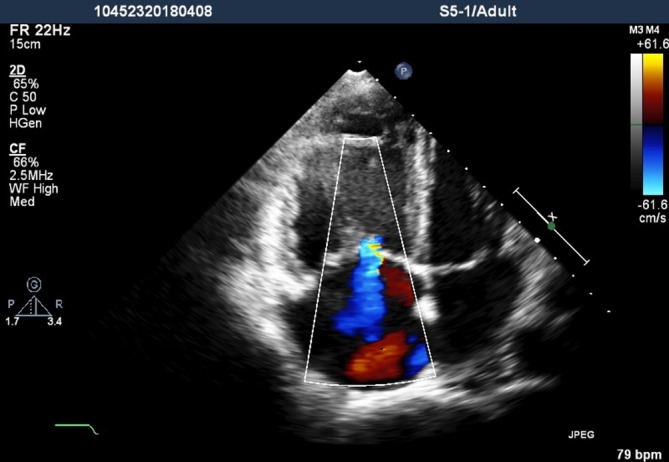
Transthoracic echocardiography apical four-chamber view showed right ventricular and atrial dilatation and moderate tricuspid valvular regurgitation.
Video 1.
Transthoracic echocardiography apical four-chamber view showed right ventricular and atrial dilatation and moderate tricuspid valvular regurgitation.
Figure 2.
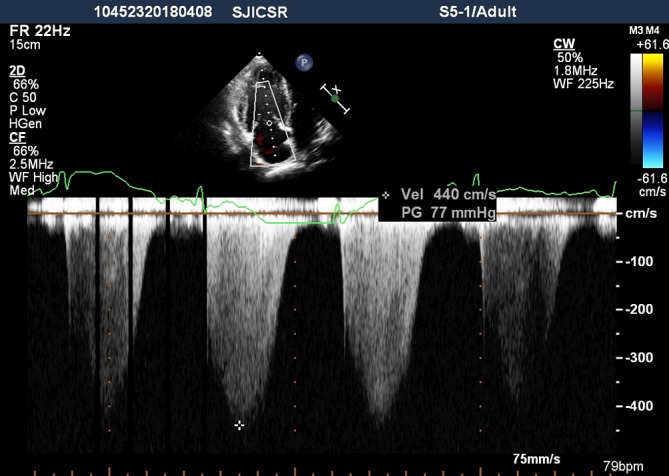
Continuous-wave Doppler signal showed the peak tricuspid valvular regurgitation velocity was 4.4 m/s and the peak pressure gradient was estimated at 77 mm Hg.
Figure 3.
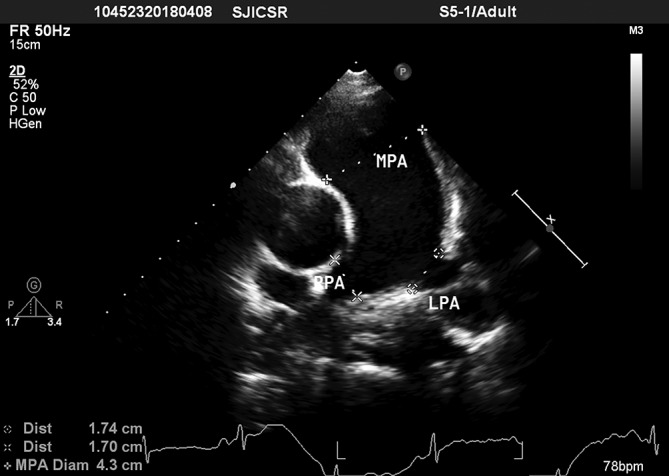
Basis short-axis view showed dilated main, right and left pulmonary arteries. LPA, left pulmonary artery; MPA, main pulmonary artery; RPA, right pulmonary artery.
Figure 4.
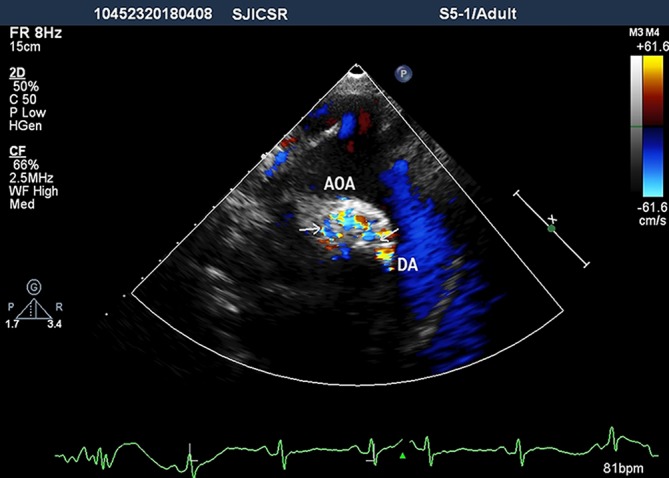
Suprasternal view demonstrating multiple collaterals (arrows). AOA, aortic arch; DA, descending aorta.
Video 2.
Suprasternal view demonstrating multiple collaterals (arrows). AOA, aortic arch; DA, descending aorta.
Figure 5.
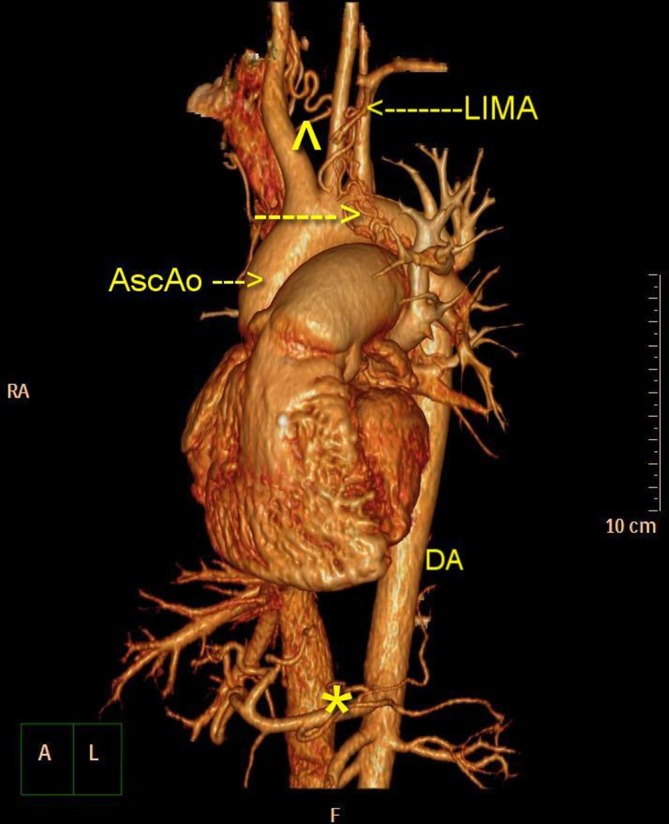
Chest CT showed multiple collaterals from LIMA, right internal mammary artery and the descending aorta. The asterisk, arrowhead and arrows are collaterals. AscAo, ascending aorta; DA, descending aorta; LIMA, left internal mammary artery; RA, right atrium.
Right heart catheterisation was performed, which revealed a mean pulmonary artery pressure of 90 mm Hg with a normal pulmonary capillary wedge pressure. Selective catheterisation of RIMA, LIMA and the descending aorta further defined the collaterals arising from these vessels and supplying the lungs (figures 6–8, videos 3–5).
Figure 6.
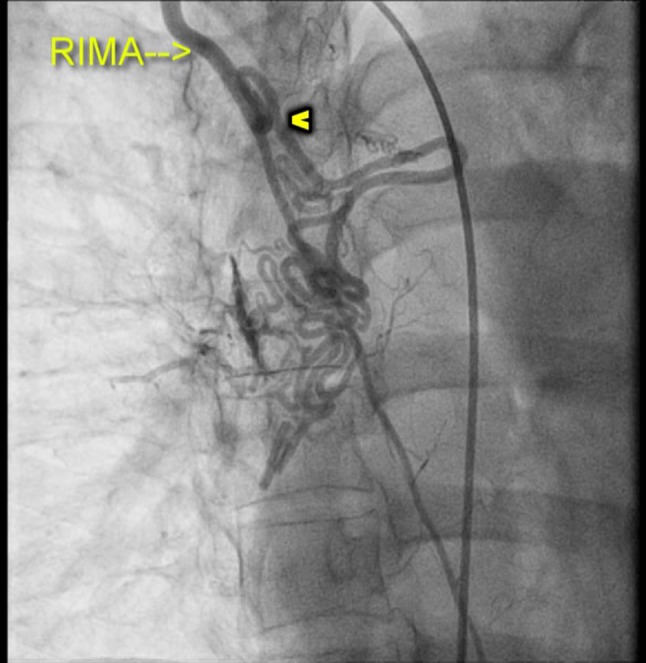
Selective angiogram of the right internal mammary artery (RIMA) demonstrating the collaterals (arrowhead).
Figure 7.
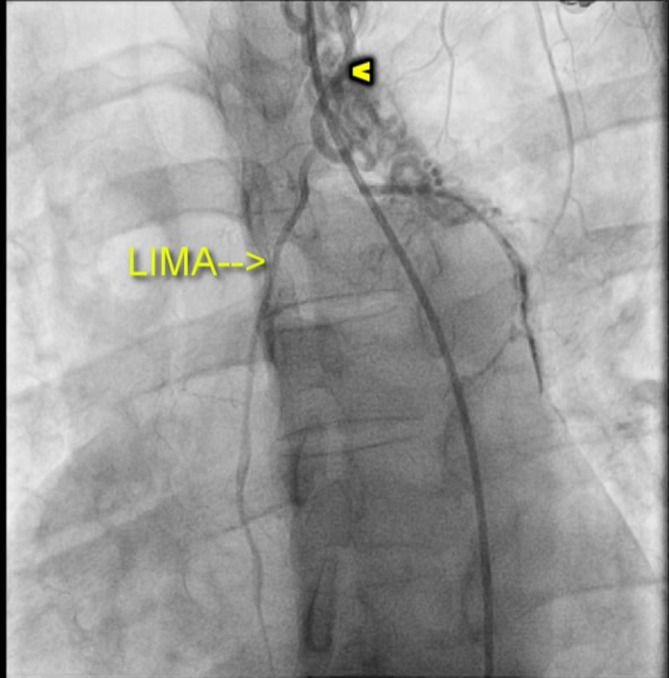
Selective angiogram of the left internal mammary artery (LIMA) demonstrating the collaterals (arrowhead).
Figure 8.
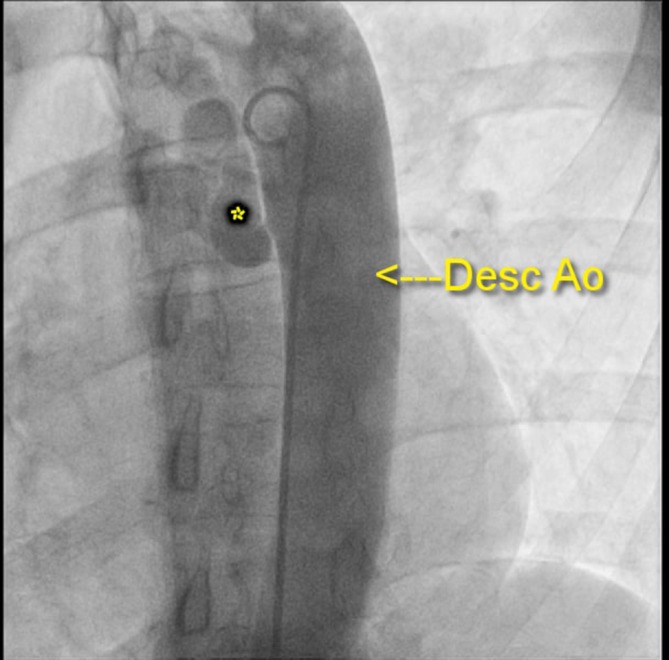
Aortogram demonstrating collaterals (asterisk) from the descending aorta (Desc Ao).
Video 3.
Selective angiogram of the right internal mammary artery demonstrating the collaterals.
Video 4.
Selective angiogram of the left internal mammary artery demonstrating the collaterals.
Video 5.
Aortogram demonstrating collaterals from the descending aorta.
Investigations for thrombophilia were advised, but the patient refused due to financial disadvantage.
Treatment
The patient was advised anticoagulant therapy. He was discharged on warfarin.
Outcome and follow-up
We started our patient on anticoagulants and are awaiting the period of 3 months to assess recovery and decide when surgery can be done.
Discussion
PAH is defined as an increase of mean pulmonary arterial pressure ≥25 mm Hg at rest. CTEPH is a distinct subgroup of pulmonary hypertension.4 CTEPH is a distinct subgroup of pulmonary hypertension with a unique aetiology and potential cure by surgery. Twenty five per cent of cases do not have a history of PE.5 This creates a dilemma to distinguish de novo acute central event from those with acute embolic event complicating an undiagnosed CTEPH. It is unknown which patients are predisposed to developing CTEPH after an acute episode of PE. The following factors appear to increase the risk of developing CTEPH: major thromboembolic events, evidence of RV dysfunction, documented thrombophilia and persistent abnormalities of lung perfusion on follow-up testing.6–8
Initial investigation is usually ventilation/perfusion (V/Q) lung scan, which helps in differentiating CTEPH from other forms of pulmonary hypertension. In CTEPH, the V/Q scan is almost always assessed as ‘high probability’ with multiple mismatched segmental perfusion defects. A normal or low probability scan effectively rules out the diagnosis.9 Pulmonary angiography has been replaced by CT pulmonary angiogram (CTPAG). CTPAG shows the absence or sudden loss of contrast-filled vessels. The filling defects are usually eccentric in the vessel lumen in CTEPH, whereas in acute PE these are central. Magnetic resonance angiography avoids radiation and nephrotoxic agent and is increasingly being used to assess this condition. The definitive treatment for CTEPH is pulmonary thromboendarterectomy (PEA). Some are not candidates for PEA either due to comorbidities making them poor surgical candidates or because the obstruction is inaccessible due to distal location. PAH-specific drug treatment and, in rare cases, balloon angioplasty may be considered in such patients. Finally, lung (or heart-lung) transplantation may be considered in patients who are not candidates for PEA or if significant PAH persists despite PEA.
Learning points.
The prevalence and incidence of chronic thromboembolic pulmonary hypertension are underestimated.
Nearly half of the patients may not have a history of pulmonary embolism.
The diagnosis should be considered in the diagnostic work-up of pulmonary hypertension.
Ventilation/perfusion scan is the screening investigation of choice.
Pulmonary thromboendarterectomy is the treatment of choice when possible.
Footnotes
Contributors: SKK performed the angiogram. AB drafted the paper. SB and PSS proof-read and finalised the draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kim NH, Lang IM. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2012;21:27–31. 10.1183/09059180.00009111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257–64. 10.1056/NEJMoa032274 [DOI] [PubMed] [Google Scholar]
- 3.Dentali F, Donadini M, Gianni M, et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res 2009;124:256–8. 10.1016/j.thromres.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 4.D’Armini AM. Diagnostic advances and opportunities in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015;24:253–62. 10.1183/16000617.00000915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011;124:1973–81. 10.1161/CIRCULATIONAHA.110.015008 [DOI] [PubMed] [Google Scholar]
- 6.Remy-Jardin M, Louvegny S, Remy J, et al. Acute central thromboembolic disease: posttherapeutic follow-up with spiral CT angiography. Radiology 1997;203:173–80. 10.1148/radiology.203.1.9122389 [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro A, Lindmarker P, Johnsson H, et al. Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation 1999;99:1325–30. 10.1161/01.CIR.99.10.1325 [DOI] [PubMed] [Google Scholar]
- 8.Wartski M, Collignon MA. Incomplete recovery of lung perfusion after 3 months in patients with acute pulmonary embolism treated with antithrombotic agents. J Nucl Med 2000;41:1043–8. [PubMed] [Google Scholar]
- 9.McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart 2007;93:1152–8. 10.1136/hrt.2004.053603 [DOI] [PMC free article] [PubMed] [Google Scholar]


