Abstract
Objectives
The level of vitamin D is considered to be associated with the development and progression of heart failure (HF). However, it is still unclear whether supplementation of vitamin D could improve ventricular remodelling in patients with HF. This study aimed to systematically evaluate the influence and safety of additional vitamin D supplementation on ventricular remodelling in patients with HF.
Design
This study is a meta-analysis of randomised controlled trials (RCTs).
Setting
The PubMed, EMBASE, CNKI, Cochrane library, Web of Science databases and grey literature were searched for RCTs regarding the effect of vitamin D on ventricular remodelling in patients with HF (from database creation to October 2017). RevMan V.5.3 software was employed for data analysis.
Participants
Seven RCTs with a total of 465 patients, including 235 cases in the vitamin D group and 230 cases in the control group, were included.
Primary and secondary outcome measures
Left ventricular end-diastolic dimension (LVEDD), left ventricular ejection fraction (LVEF) and the incidence of adverse reactions.
Results
Compared with the control group, a decrease in the LVEDD (mean difference (MD)=−2.31 mm, 95% CI −4.15 to −0.47, p=0.01) and an increase in the LVEF (MD=4.18%, 95% CI 0.36 to 7.99, p=0.03) were observed in the vitamin D group. Subgroup analysis also revealed a reduced LVEDD in adults (>18 years) and adolescents (<18 years) of the vitamin D group relative to that in those of the control group. High-dose vitamin D (>4000 IU/day) was more effective at reducing the LVEDD than low-dose vitamin D (<4000 IU/day). Moreover, vitamin D supplementation was more effective at reducing the LVEDD and increasing the LVEF in patients with reduced ejection fraction than in patients without reduced ejection fraction.
Conclusion
Vitamin D supplementation inhibits ventricular remodelling and improves cardiac function in patients with HF.
Trial registration number
CRD42017073893.
Keywords: meta-analysis, vitamin D, ventricular remodeling, cardiac function, heart failure
Strengths and limitations of this study.
The results of this systematic review and meta-analysis are highly dependent on the quality of the included primary research studies, and only randomised controlled trials were included in this study.
Subgroup analyses were performed according to clinical heterogeneity analysis of the included studies.
This was the first review to systematically examine the impact of vitamin D supplementation on ventricular remodelling in patients with heart failure.
The results suggest that vitamin D may be used as adjunctive heart failure medication in heart failure patients with an underlying lack of or insufficiency in vitamin D.
The results need to be interpreted with caution as there were few studies in each subgroup.
Introduction
Heart failure (HF) is the main factor leading to economic loss due to poor prognosis and a high mortality rate.1 In the USA, there are at least 5 000 000 patients with decreased contractile function; meanwhile, there is also the same number of patients with the same disease in Western Europe.2 3 In recent years, the prognosis of HF has improved remarkably, and the 5-year survival rate has increased from 43% to 52%.4 At present, the main treatment methods for HF are still β-receptor blocking agents, ACEI/angiotensin receptor blocker and aldosterone receptor antagonists; although these medications can reduce the incidence of adverse cardiac events and improve cardiac function,5 HF is still a main cause of global mortality. Therefore, there is presently an urgent need for supplementary treatment methods and strategies. Lack of or insufficiency in vitamin D may result in cardiovascular and cerebrovascular diseases.6–10 Many studies have discovered that11–14 there is a remarkable association between lack of vitamin D and the progression of HF. Studies have shown that patients with HF generally lack vitamin D and have a poor prognosis; moreover, supplementation of vitamin D could reduce the mortality rate of patients with HF.15–17 Several studies have shown that vitamin D acts as a negative regulator of the renin–angiotensin–aldosterone system (RAAS)18–20 and modulates myocardial extracellular matrix turnover. Consistently, vitamin D receptor (VDR) knockout mice show increased RAAS activity, which leads to hypertension, cardiac hypertrophy, increased water intake and sodium retention,18 and VDR knockout mice show increased metalloprotease activity, which promotes the destruction of myocardial tissue, leading to ventricular remodelling.21 22 Therefore, lack of vitamin D could result in deterioration of heart function and accelerate myocardial remodelling.
At present, different studies have reported controversial conclusions regarding the influence of vitamin D on ventricular remodelling in patients with HF. Therefore, the present study performed a meta-analysis to further clarify the influence of vitamin D on ventricular remodelling in patients with HF.
Materials and methods
Search strategy
PubMed, EMBASE, Cochrane Central Register of Controlled Trials, CNKI and Web of Science were searched. Grey literature was also retrieved in Opengrey and ProQuest. The reference lists of identified articles and the bibliographies of original articles were also reviewed. The medical subject headings used in the search were as follows: “heart failure”, “vitamin D”, “ventricular remodelling”, “heart function tests” and “randomized controlled trials”. The keywords used in the search were as follows: “cardiac failure”, “myocardial failure”, “heart decompensation”, “left ventricular remodelling”, “ventricular remodelling”, “ventricular myocardial remodelling”, “cholecalciferol”, “vitamin D3” and “controlled clinical trials”. The time frame for the retrieval was from the establishment of the database to 1 October 2017.
Study selection
The inclusion criteria were as follows: (1) randomised controlled trials (RCTs) involving the effect of vitamin D on ventricular remodelling in patients with HF with or without blinding methods or allocation concealment methods; (2) parallel or crossover trials; (3) only the data before the washout period were used in a crossover test; (4) available baseline data and changes in the left ventricular end-diastolic dimension (LVEDD) and left ventricular ejection fraction (LVEF); (5) HF defined as New York Heart Association (NYHA) functional class ≥II or LVEF ≤40%; (6) participants of any gender, age or ethnicity; (7) participants without or changing any micronutrient use except for vitamin D and (8) a minimum of 3 months of therapy was necessary for inclusion in the review to ensure that the intervention had sufficient time to produce a better effect. The exclusion criteria were as follows: (1) only the abstract was reported for reference; (2) studies with duplicated data, including the same group of patients or patients for whom there were updated results available; (3) the study had no outcomes of interest; (4) animal studies and reviews; (5) conference documents and (6) non-RCTs, cohort studiers and retrospective studies.
Data extraction and quality assessment
Data were independently extracted from each study by two authors (JDZ and JJJ) and entered into a structured spreadsheet followed by a cross-check procedure. Disagreements were resolved by consensus or by a third investigator (PD). The following data were extracted from each trial: the first author’s surname, year of publication; demographic and methodological data; total number, mean age, gender distribution and race of enrolled patients; use of or change in drugs for HF; seated LVEDD and LVEF at baseline, when available; number of patients randomly assigned to each intervention; duration of therapy; incidence and type of adverse events; number of dropouts or withdrawals because of adverse events; and change from baseline seated LVEDD and LVEF. Criteria for the RCT risk of bias evaluation listed in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 were adopted, including the following: (1) random sequence generation, (2) allocation concealment, (3) blinding of patients and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data and (6) selective reporting risk. Then, an evaluation system with ‘low risk’, ‘high risk’ and ‘not clear’ was established according to the six criteria described above.23
Outcomes assessed
The primary endpoint was LVEDD, and the secondary endpoints were LVEF and the incidence of adverse reactions.
Data analysis and synthesis
RevMan V.5.3 software was employed for data analysis. Continuous variables are reported as the mean difference (MD) and 95% CI, and the test level was α=0.05. Following clinical heterogeneity analysis of the included studies, statistical heterogeneity was assessed using χ2-based Cochran Q statistic and I2.24 For the Q statistic, p≥0.1 indicates homogeneity among multiple similar studies, and the fixed-effects model was employed for the meta-analysis, while p<0.1 indicates statistically significant heterogeneity, and the random-effects model was used for analysis. For the I2 statistic, I2 <25% indicates low heterogeneity, while I2 >50% indicates moderate to high heterogeneity.25
Results
Selection and description of studies
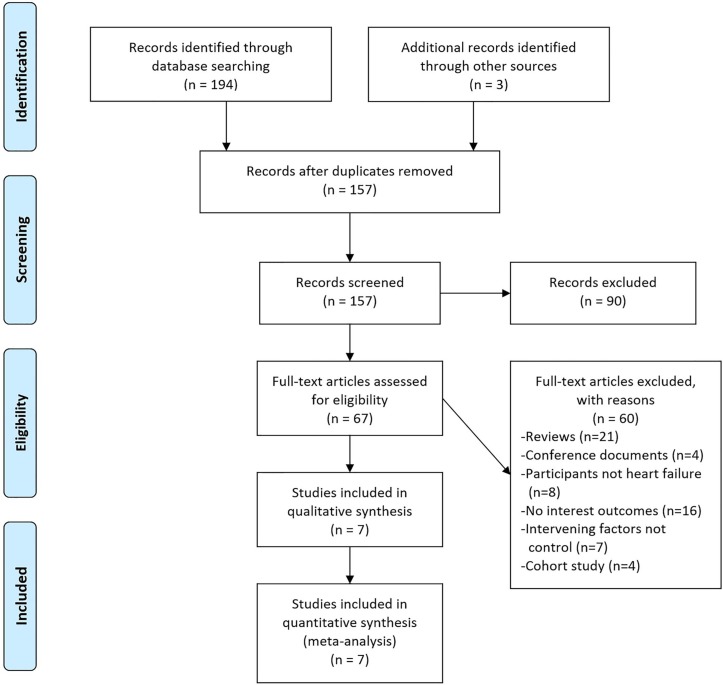
A total of 157 published papers were collected after the initial screening, and eventually, 7 RCTs26–32 with a total of 465 patients, including 235 cases in the vitamin D group and 230 cases in the control group, were included after reviewing the title, abstract and full text, as well as eliminating duplicate documents, non-RCTs and studies that failed to meet the inclusion criteria. See figure 1 for the screening process.
Figure 1.
Flow diagram of study selection.
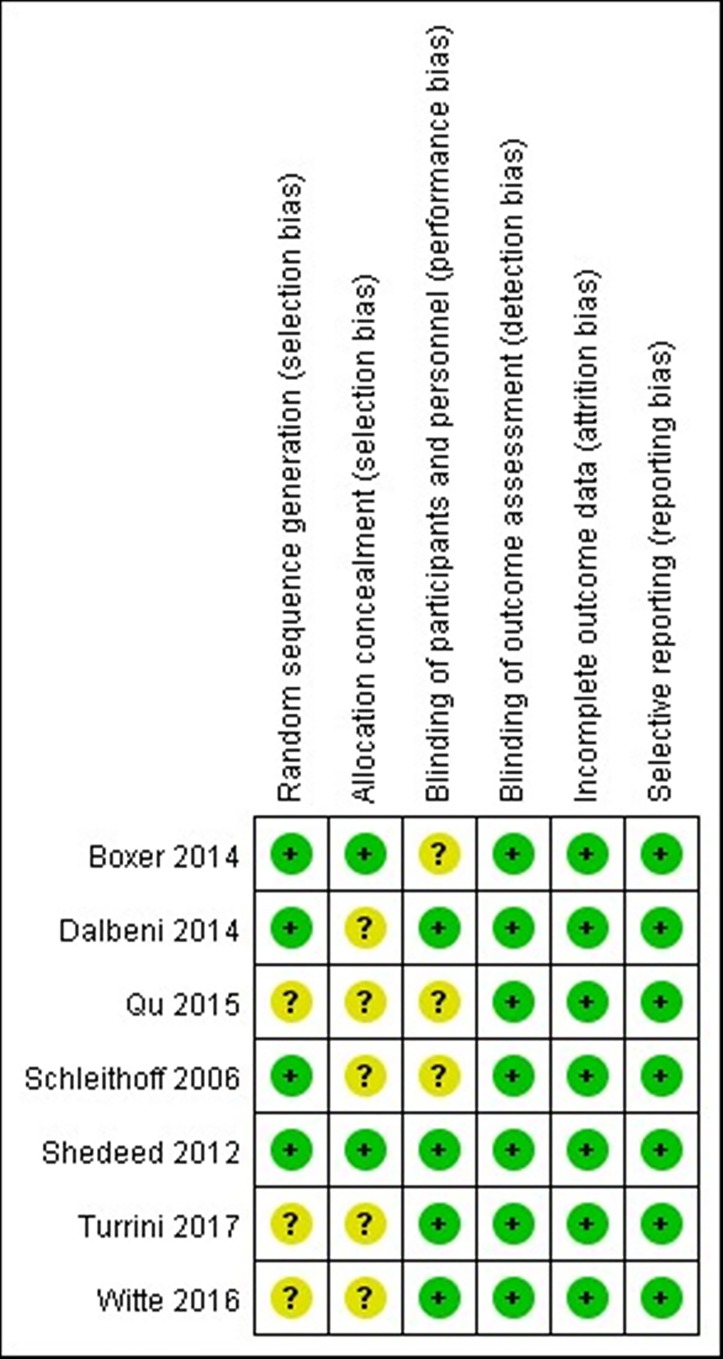
In the seven included studies,26–32 four reported an appropriate randomisation method27–29 32; two adopted allocation concealment29 32 and six used double-blinding.26–30 32 See figure 2 for the evaluation of the methodology of the studies.
Figure 2.
Quality assessment.
Data of the curative effect on the LVEDD were reported in five studies, and the LVEF was reported in all the included studies; two studies mentioned adverse reactions.26 30 Dropout or withdrawal from the research study was covered in all the included studies. The study characteristics are shown in table 1, and the basic information of the included population is shown in table 2.
Table 1.
Study characteristics
| Author | Design | Blinding | Vitamin D dose | Follow-up duration |
Study population | Primary outcome |
| Turrini et al 26 | Prospective RCT | Double-blind | 300 000 IU at baseline 50 000 IU/month | 6 months | Chronic HF, 25(OH)D<20 ng/mL, 60 years<age |
6MWD, echocardiography parameters, hormonal |
| Witte et al 30 | Prospective RCT | Double-blind | 4000 IU/day | 12 months | Chronic HF, NYHA class II–III, LVEF<45%, 25(OH)D<20 ng/mL | 6MWD, echocardiography parameters |
| Qu et al 31 | Prospective RCT | Single-blind | 1000 IU/day | 3 months | NYHA class III–IV | Echocardiography parameters, BNP, 25(OH)D |
| Dalbeni et al 27 | Prospective RCT | Double-blind | 4000 IU/day | 25 weeks | Chronic HF, LVEF<55%, NYHA class>II, 25(OH)D<30 ng/mL, Age>40 years |
Echocardiographic parameters, NYHA class, NT-proBNP |
| Boxer et al 32 | Prospective RCT | Double-blind | 50 000 IU/week | 6 months | Age≥50 years, NYHA class II–IV, 25(OH)D<37.5 ng/mL |
Echocardiographic parameters, serum analysis, urine analysis |
| Shedeed29 | Prospective RCT | Double-blind | 1000 IU/day | 12 weeks | Congestive HF, LVEF<40%, LV>2 SD for age and sex |
Echocardiographic parameters |
| Schleithoff et al 28 | Prospective RCT | Double-blind | 2000 IU/day | 9 months | Chronic HF, NYHA class II–IV |
Survival rates, NT-proBNP, pro-inflammatory and anti-inflammatory cytokines, echocardiographic parameters |
25(OH)D, 25-hydroxyvitamin D; 6MWD, 6-minute walk distance; HF, heart failure; IU, international units; LV, left ventricular; LVEF, left ventricular ejection fraction; NT, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; RCT, randomised controlled trial.
Table 2.
Study population characteristics
| Author | Intervention | Number (n) | Age (years) | Male (%) | LVEF (%) | NYHA class II (%) | NYHA class III (%) | NYHA class IV (%) | LVEDD (mm) | 25(OH)D (ng/mL) | Ischaemic cause (%) | Hypertension (%) |
Diabetes (%) |
| Turrini et al 26 | Vitamin D | 17 | 77±7 | 35.3 | 54.7±13.8 | 64.7 | 35.3 | 0 | 51±1.58 | 9.4±5.2 | 41.2 | 64.7 | 35.3 |
| Placebo | 16 | 79±7 | 43.8 | 49.2±19.1 | 68.7 | 21.3 | 0 | 54±3.48 | 9.6±7.3 | 43.7 | 43.7 | 18.7 | |
| Witte et al 30 | Vitamin D | 80 | 68.5±12.45 | 83.8 | 25.6±10.80 | 92.5 | 7.5 | 0 | 57.6±8.62 | 38.2±24.81 | 55.0 | NR | 21.3 |
| Placebo | 83 | 69.0±13.78 | 74.7 | 26.5±10.62 | 85.5 | 14.5 | 0 | 58.0±6.49 | 36.4±20.24 | 60.2 | NR | 24.1 | |
| Qu et al 31 | Vitamin D | 22 | 70±7 | 59.3 | 34.9±3.8 | NR | NR | NR | NR | NR | NR | 66.7 | 81.5 |
| Blank | 17 | 69±8 | 51.8 | 34.6±3.9 | NR | NR | NR | NR | NR | NR | 63.0 | 85.2 | |
| Dalbeni et al 27 | Vitamin D | 13 | 71.2±6.22 | 84.6 | 39.08±8.00 | 58.8* | 50.0† | 53.9±6.81 | 16.2±6.59 | 76.9 | 100 | NR | |
| Placebo | 10 | 73.4±13.78 | 60.0 | 43.6±7.63 | 41.2* | 50.0† | 50.6±7.04 | 16.0±6.15 | 90 | 100 | NR | ||
| Boxer et al 32 | Vitamin D | 19 | 65.8±10.6 | 48.4 | 39.2±13.2 | 55.0 | 73.0 | 0 | NR | 19.1±9.3 | 25.8 | 83.0 | 51.6 |
| Placebo | 15 | 66.0±10.4 | 54.5 | 36.1±14.5 | 45.0 | 27.0 | 0 | NR | 17.8±9.0 | 30.3 | 84.8 | 42.4 | |
| Shedeed29 | Vitamin D | 42 | 0.86±1.3 | 64.29 | 36.4±2.26 | NR | NR | NR | 32.81±4.6 | 13.4±2.21 | NR | NR | NR |
| Placebo | 38 | 0.93±1.0 | 57.89 | 37.2±2.62 | NR | NR | NR | 30.7±5.2 | 14.0±2.46 | NR | NR | NR | |
| Schleithoff et al 28 | Vitamin D+calcium | 42 | 57±7.41 | 85.25 | 32.5±8.67 | NR | NR | NR | 69.0±8.89 | 14.4±7.85 | 47 | 38 | 20 |
| Placebo+calcium | 51 | 54±8.89 | 80.65 | 33.0±7.56 | NR | NR | NR | 69.0±9.26 | 15.3±7.48 | 40 | 32 | 23 |
*I and II.
†III and IV.
25(OH)D, 25-hydroxyvitamin D; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; NR, not reported; NYHA, New York Heart Association.
The SD for changes from baseline on the LVEF were reported in four studies.27 28 30 32 According to the Cochrane Handbook, the Corr were imputed by averaging the Corr of those four studies, and further imputed the SD for changes from baseline for other studies.26 29 31
The SD for changes from baseline on the LVEDD were reported in three studies.27 28 30 According to the Cochrane Handbook, the Corr were imputed by averaging the Corr of those three studies, and further imputed the SD for changes from baseline for other studies.26 29
Effects of vitamin D on the LVEDD
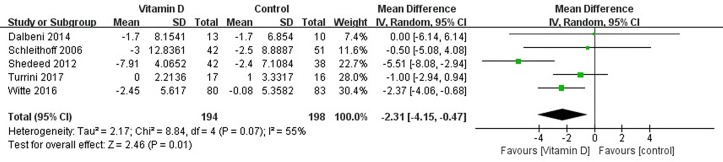
Changes in the LVEDD of patients were reported in five studies,26–30 which showed high levels of heterogeneity among the results of the studies (heterogeneity χ2, p=0.07, I2=55%), thus supporting analysis using the random-effects model. Compared with the control group, a decrease in the LVEDD was observed in the vitamin D group (MD=−2.31 mm, 95% CI −4.15 to −0.47, p=0.01) (figure 3).
Figure 3.
Forest plot showing the effect of vitamin D on left ventricular end-diastolic dimension.
Effects of vitamin D on the LVEF
Changes in the LVEF of patients were reported in all seven studies,26–32 which showed high levels of heterogeneity among the results of the studies (heterogeneity χ2, p<0.001, I2=88%), thus supporting analysis using the random-effects model. Compared with the control group, an increase in the LVEF was observed in the vitamin D group (MD=4.18%, 95% CI 0.36 to 7.99, p=0.03) (figure 4).
Figure 4.
Forest plot showing the effect of vitamin D on left ventricular ejection fraction.
Subgroup analysis
The analysis based on age stratification revealed that compared with the control group both the adults (aged ≥18 years) and non-adults (aged <18 years) with HF in the vitamin D group showed a decrease in the LVEDD (adults: heterogeneity χ2, p=0.65, I2=0%;MD=−1.62 mm, 95% CI −2.83 to −0.42, p=0.008; non-adults: MD=−5.51 mm, 95% CI −8.08 to −2.94, p<0.001) (figure 5). These results, however, need to be interpreted with caution as there was only one study in the subgroup of non-adults.
Figure 5.
Subgroup analysis of effect of vitamin D on left ventricular end-diastolic dimension according to patients’ age.
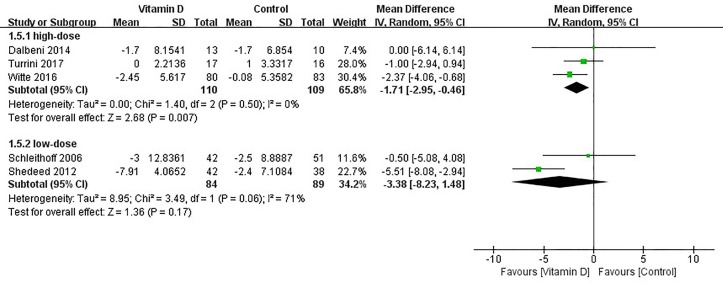
A subgroup analysis was performed according to the dosage of vitamin D. There was an effect from high-dose vitamin D on the reduction of the LVEDD (heterogeneity χ2, p=0.50, I2=0%; MD=−1.71 mm, 95% CI −2.95 to −0.46, p=0.007), but this effect was not seen with low-dose vitamin D treatment (heterogeneity χ2, p=0.06, I2=71%; MD=−3.38 mm, 95% CI −8.23 to 1.48, p=0.17). (figure 6).
Figure 6.
Subgroup analysis of effect of vitamin D on left ventricular end-diastolic dimension (LVEDD) according to dose of vitamin D.
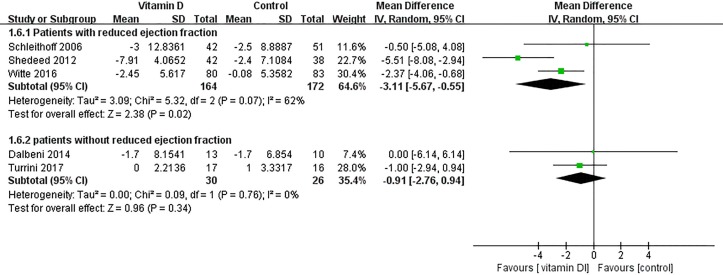
According to patients with or without reduced ejection fraction, subgroup analyses were performed. Vitamin D supplementation was effective at reducing the LVEDD in patients with reduced ejection fraction (patients with reduced ejection fraction: heterogeneity χ2, p=0.07, I2=62%;MD=−3.11 mm, 95% CI −5.67 to −0.55, p=0.02; patients without reduced ejection fraction: heterogeneity χ2, p=0.76, I2=0%;MD=−0.91 mm, 95% CI −2.76 to 0.94, p=0.34) (figure 7). In addition, vitamin D supplementation was effective at increasing the LVEF in patients with reduced ejection fraction (patients with reduced ejection fraction: heterogeneity χ2, p=0.02, I2=73%; MD=6.21%, 95% CI 2.01 to 10.41, p=0.004; patients without reduced ejection fraction: heterogeneity χ2, p=0.002, I2=80%; MD=2.74%, 95% CI −1.96 to 7.45, p=0.25) (figure 8).
Figure 7.
Subgroup analysis of effect of vitamin D on left ventricular end-diastolic dimension according to patients with or without reduced ejection fraction.
Figure 8.
Subgroup analysis of effect of vitamin D on left ventricular ejection fraction according to patients with or without reduced ejection fraction.
Publication bias
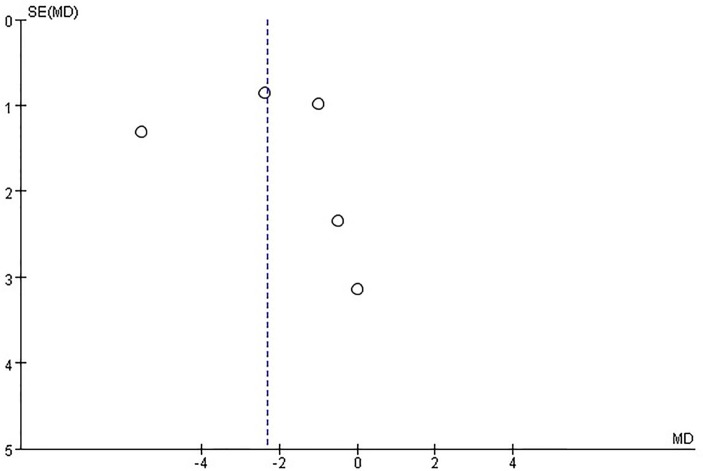
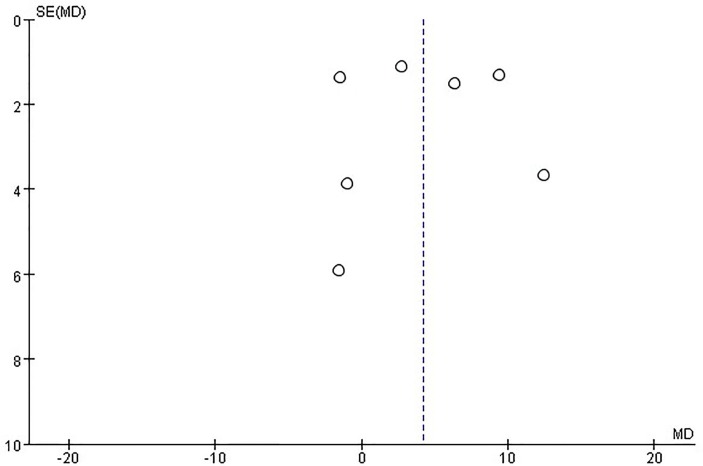
There was significant asymmetry in the funnel plot for the effect of vitamin D on the LVEDD, which may be due to publication bias and other causes (figure 9). On the other hand, no publication bias was found for the effect of vitamin D on the LVEF (figure 10).
Figure 9.
Funnel plots for the effect of vitamin D on left ventricular end-diastolic dimension.
Figure 10.
Funnel plots for the effect of vitamin D on left ventricular ejection fraction.
Adverse event
Two studies26 30 reported adverse events. During the follow-up, no significant adverse event was recorded. No data associated with the incidence of adverse events were recorded in the other studies.
Discussion
At present, different studies have reported controversial conclusions regarding the influence of vitamin D on ventricular remodelling in patients with HF. The results of this study show that compared with the control group supplementation of vitamin D could reduce the LVEDD (MD=−2.31 mm, 95% CI −4.15 to −0.47, p=0.01) and improve the LVEF (MD=4.18%, 95% CI 0.36 to 7.99, p=0.03) among patients with HF. In addition, a more pronounced effect is achieved using high-dose vitamin D and on patients with a reduced ejection fraction.
Most new treatment methods for chronic HF are expensive and require advanced technologies33; in addition, most of these treatment methods have not passed strict phase III clinical trials. For patients with HF, vitamin D is not only cheap but also safe, and these patients may obtain more benefits from vitamin D therapy.34 Vitamin D toxicity is based not only on the dosing but also on circulating 25-hydroxyvitamin D (25(OH)D) levels. The Institute of Medicine35 has set the dosage for vitamin D at 4000 IU daily for healthy adults, and the Endocrine Society15 has set a dosage of 10 000 IU daily for patients who are at risk of having circulating 25(OH)D levels<50 nmol/L. The Institute of Medicine35 considers circulating 25(OH)D levels below 30 nmol/L as deficient, levels between 30 and 49.99 nmol/L as inadequate, levels between 50 and 125 nmol/L as adequate and levels above 125 nmol/L as potentially harmful. In HF, cardiac contraction and diastole are affected due to overload of Ca2+ ions in myocardial cells. Lack of vitamin D may intervene with the functions of Ca2+ in myocardial cells, thus resulting in cardiomyocyte hypertrophy and intra-organisational inflammatory reaction and fibrosis.36 37 Low vitamin D levels can activate the renin–angiotensin system,38 give rise to inflammatory reactions39 and result in endothelial dysfunction.40 The effects of vitamin D on the CV system are additionally mediated through elevated parathyroid hormone (PTH) levels, which are associated with the development of left ventricular (LV) hypertrophy.41 Although there is much evidence showing that a lack of vitamin D could result in poor prognosis among patients with HF, different studies have reported controversial conclusions regarding whether supplementation of vitamin D could benefit patients with HF.
In recent years, some relatively small RCTs have studied the influence of vitamin D on patients with HF. In the 2014 World Heart Failure Conference, Louise et al 42 reported an RCT that performed a 6-month study on 32 patients with HF, and the result showed that supplementation of vitamin D did not improve the LVEF or pro-BNP level. A recent meta-analysis43 also reported that vitamin D supplementation could not improve the LVEF (WMD: 4.11%, 95% CI −0.91 to 9.12, p=0.11) and 6-minute walk distance (6MWD) (WMD: 8.90 m, 95% CI: −48.47 to 66.26, p=0.76) in the treatment of chronic HF. In contrast, this study included three other studies, two of which showed a positive effect from vitamin D supplementation. The present studies have not shown that vitamin D supplementation can improve the 6MWD among patients26 30 44 45; thus, our meta-analysis did not evaluate this parameter. There are probably many factors that affect exercise tolerance, such as physical condition, obesity, habits and environment, and these confounding factors may obscure the weak force from the remodelled ventricle. However, other studies have drawn opposite conclusions. In 2015, Lowry et al 46 reported in the European Society of Cardiology a 12-month RCT, and the result showed that for patients with HF supplementation of vitamin D could improve ventricular remodelling (LVEDD −4.46 mm; p=0.047); in addition, the LVEF showed an increasing trend. A non-RCT47 also showed that supplementation of vitamin D could remarkably increase the LVEF value compared with baseline. Elidrissy et al 48 evaluated 61 cases of children with cardiomyopathy; they concluded, based on the available evidence, that the most likely cause of cardiomyopathy is hypocalcaemia and suggested maternal supplementation of vitamin D during pregnancy and lactation with up to 2000 units of vitamin D and 400 units for their infants for prevention, which was also similar to the results reported by Shedeed.29
Since there are disputes among different study results, we conducted a meta-analysis of relevant RCT studies to further clarify the influence of vitamin D supplementation on ventricular remodelling in patients with HF. Results of this study show that supplementation of vitamin D inhibits myocardial remodelling and improves cardiac function in patients with HF.
Different studies have different results and conclusions, which may be attributed to the different recommended dosages of vitamin D; since there is no standard recommended dosage of vitamin D at present, there are differences in the recommended dosage of vitamin D in different trials, which may affect the study results. However, there has not been a report related to adverse effects from the dosage of vitamin D. Meanwhile, the study results are related to the selected group; for example, the study results of Schleithoff et al 28 showed that there were no remarkable changes in the LVEF and LVEDD, and the participants included in this study were predominantly those with NYHA level 3–4 and a high degree of HF, and there was a high rate of lost to follow-up visit (37%).
The data on changes in the LVEDD showed high levels of heterogeneity among studies. Shedeed29 studied the cause of heterogeneity according to subgroup. There was a difference in the metabolism of vitamin D and cardiac recovery capacity between newborns and adults, which might be the cause of heterogeneity.
Although all trials included in this study are RCTs, there are still many limitations in this study (1) because the current studies found that vitamin D has a weak and uncertain effect on ventricular remodelling and cardiac function in patients with HF and cannot improve exercise tolerance or reduce cardiac mortality, additional large-scale clinical studies are needed. Future research needs to focus on whether different vitamin D dosages would be superior; in addition, different selection criteria need to be defined (eg, ejection fraction, vitamin D threshold, PTH threshold, etc) and additional echocardiographic parameters, the 6MWD and cardiovascular mortality need to be evaluated; (2) this study exhibits heterogeneity, and age stratification and whether there is reduced ejection fraction may be sources of clinical heterogeneity according to the subgroup analysis; (3) different recommended dosages of vitamin D are reported in different trials, which may affect study results; therefore, additional trials are required to explore the relationship between vitamin D dosage and effect; (4) the conclusions need to be interpreted with caution as the extent of detected improvement in the remodelling parameters is close to the range of the inter-observer or intra-observer variability of the echocardiographic method itself. The baseline vitamin D level of patients and the follow-up duration may affect the study results, and except for Dalbeni et al,27 who have mentioned that no change in therapy was made during follow-up, other studies have not reported adjustments in HF medication. Therefore, whether the weak improvement in the remodelling parameters from vitamin D are attributed to other HF drugs is unclear.
In conclusion, this study shows that supplementation of vitamin D inhibits myocardial remodelling in patients with HF and improves their cardiac function. Vitamin D may be used as adjunctive HF medication for patients with HF with an underlying lack of or insufficiency in vitamin D. This result is encouraging and of great clinical interest but still far from practical implications. The main implication is to encourage further research.
Supplementary Material
Footnotes
Contributors: J-DZ: Makes a significant contribution to the concept or design of the work; or the acquisition, analysis or interpretation of work data; drafting work or critically modifying important knowledge content; final approval of the released version; and agreeing to all work Responsible for ensuring proper investigation and resolution of issues related to the accuracy or completeness of any part of the work. JJ J: Participate in writing. P-SD: Serving as a scientific consultant. DZ: Participated in the technical editing of the manuscript. X-MY: Critically reviewed the research proposal. D-LL: Data collected. H-FZ: Data collected.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The study was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committee of Henan University of Science and Technology.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The authors declare that they are willing to share their data directly.
References
- 1. Jin M, Wei S, Gao R, et al. . Predictors of Long-Term Mortality in Patients With Acute Heart Failure. Int Heart J 2017;58:409–15. 10.1536/ihj.16-219 [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, et al. . Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–46. 10.1136/hrt.2003.025270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roger VL, Weston SA, Redfield MM, et al. . Trends in heart failure incidence and survival in a community-based population. JAMA 2004;292:344–50. 10.1001/jama.292.3.344 [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJ, Adamopoulos S, Anker SD, et al. . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–69. 10.1093/eurjhf/hfs105 [DOI] [PubMed] [Google Scholar]
- 6. Sun Q, Shi L, Rimm EB, et al. . Vitamin D intake and risk of cardiovascular disease in US men and women. Am J Clin Nutr 2011;94:534–42. 10.3945/ajcn.110.008763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamez H, Thadhani RI. Vitamin D and hypertension: an update and review. Curr Opin Nephrol Hypertens 2012;21:492–9. 10.1097/MNH.0b013e3283557bf0 [DOI] [PubMed] [Google Scholar]
- 8. Karakas M, Thorand B, Zierer A, et al. . Low levels of serum 25-hydroxyvitamin D are associated with increased risk of myocardial infarction, especially in women: results from the MONICA/KORA Augsburg case-cohort study. J Clin Endocrinol Metab 2013;98:272–80. 10.1210/jc.2012-2368 [DOI] [PubMed] [Google Scholar]
- 9. Sun Q, Pan A, Hu FB, et al. . 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke 2012;43:1470–7. 10.1161/STROKEAHA.111.636910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai CK, Huang J, Lokhandwala A, et al. . The role of vitamin supplementation in the prevention of cardiovascular disease events. Clin Cardiol 2014;37:576–81. 10.1002/clc.22299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schierbeck LL, Jensen TS, Bang U, et al. . Parathyroid hormone and vitamin D–markers for cardiovascular and all cause mortality in heart failure. Eur J Heart Fail 2011;13:626–32. 10.1093/eurjhf/hfr016 [DOI] [PubMed] [Google Scholar]
- 12. Liu L, Chen M, Hankins SR, et al. . Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am J Cardiol 2012;110:834–9. 10.1016/j.amjcard.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 13. Ford JA, MacLennan GS, Avenell A, et al. . Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr 2014;100:746–55. 10.3945/ajcn.113.082602 [DOI] [PubMed] [Google Scholar]
- 14. Wang L, Song Y, Manson JE, et al. . Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012;5:819–29. 10.1161/CIRCOUTCOMES.112.967604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 16. Gotsman I, Shauer A, Zwas DR, et al. . Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail 2012;14:357–66. 10.1093/eurjhf/hfr175 [DOI] [PubMed] [Google Scholar]
- 17. Pourdjabbar A, Dwivedi G, Haddad H. The role of vitamin D in chronic heart failure. Curr Opin Cardiol 2013;28:216–22. 10.1097/HCO.0b013e32835bd480 [DOI] [PubMed] [Google Scholar]
- 18. Li YC, Kong J, Wei M, et al. . 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002;110:e38 10.1172/JCI15219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomaschitz A, Pilz S, Ritz E, et al. . Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta 2010;411:e60 10.1016/j.cca.2010.05.037 [DOI] [PubMed] [Google Scholar]
- 20. Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010;55:1283–8. 10.1161/HYPERTENSIONAHA.109.148619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weber KT, Weglicki WB, Simpson RU. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc Res 2009;81:500e8 10.1093/cvr/cvn261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agarwal M, Phan A, Willix Jr R, et al. . Is vitamin D deficiency associated with heart failure? A review of current evidence. J Cardiovasc Pharmacol Ther 2011;16: 354:e63. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Green S, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, 2011. www.cochrane-hand-book.org (updated Mar 2011).
- 24. Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 26. Turrini F, Scarlini S, Giovanardi P, et al. . Effects of Cholecalciferol Supplementation in Patients with stable heart failure and LOw vITamin D levels (ECSPLOIT-D): a double-blind, randomized, placebo-controlled pilot study. Minerva Cardioangiol 2017;65 doi:10.23736/S0026-4725.17.04340-7 [DOI] [PubMed] [Google Scholar]
- 27. Dalbeni A, Scaturro G, Degan M, et al. . Effects of six months of vitamin D supplementation in patients with heart failure: a randomized double-blind controlled trial. Nutr Metab Cardiovasc Dis 2014;24:861–8. 10.1016/j.numecd.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 28. Schleithoff SS, Zittermann A, Tenderich G, et al. . Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006;83:754–9. 10.1093/ajcn/83.4.754 [DOI] [PubMed] [Google Scholar]
- 29. Shedeed SA. Vitamin D supplementation in infants with chronic congestive heart failure. Pediatr Cardiol 2012;33:713–9. 10.1007/s00246-012-0199-6 [DOI] [PubMed] [Google Scholar]
- 30. Witte KK, Byrom R, Gierula J, et al. . Effects of Vitamin D on Cardiac Function in Patients With Chronic HF: The VINDICATE Study. J Am Coll Cardiol 2016;67:2593–603. 10.1016/j.jacc.2016.03.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jun Q, Zhen W, hong-bo S, et al. . The C linical Effect of V itam in D 3 Supplem entation on C ardiac Function in Patients w ith Ischem ic H eart Failure. Chinese and Foreign M edical R esearch 2015;13:3–5. [Google Scholar]
- 32. Boxer RS, Hoit BD, Schmotzer BJ, et al. . The effect of vitamin D on aldosterone and health status in patients with heart failure. J Card Fail 2014;20:334–42. 10.1016/j.cardfail.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amao R, Imamura T, Nakahara Y, et al. . Reversible Motor Paralysis and Early Cardiac Rehabilitation in Patients With Advanced Heart Failure Receiving Left Ventricular Assist Device Therapy. Int Heart J 2016;57:766–8. 10.1536/ihj.16-153 [DOI] [PubMed] [Google Scholar]
- 34. Witte KK, Byrom R. Micronutrients for chronic heart failure: end of the road or path to enlightenment? JACC Heart Fail 2014;2:318–20. 10.1016/j.jchf.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 35. Institute of Medicine. Dietary Reference Intakes: Calcium and Vitamin D. Washington, DC: National Academies Press, 2011. [PubMed] [Google Scholar]
- 36. Weishaar RE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. II. Direct and indirect effects. Am J Physiol 1987;253:E675–83. 10.1152/ajpendo.1987.253.6.E675 [DOI] [PubMed] [Google Scholar]
- 37. Chen S, Law CS, Grigsby CL, et al. . Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 2011;124:1838–47. 10.1161/CIRCULATIONAHA.111.032680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism 2012;61:450–8. 10.1016/j.metabol.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Leung DY, Richers BN, et al. . Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012;188:2127–35. 10.4049/jimmunol.1102412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caprio M, Mammi C, Rosano GM. Vitamin D: a novel player in endothelial function and dysfunction. Arch Med Sci 2012;8:4–5. 10.5114/aoms.2012.27271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saleh FN, Schirmer H, Sundsfjord J, et al. . Parathyroid hormone and left ventricular hypertrophy. Eur Heart J 2003;24:e60. [DOI] [PubMed] [Google Scholar]
- 42. Schierbeck LLLouise, Madsen JL, Bang UC. The effect of vitamin D supplementation in vitamin D deficient men with heart failure. A randomized controlled trial.:P1686. European journal of heart failure 2014;16:335. [Google Scholar]
- 43. Jiang WL, Gu HB, Zhang YF, et al. . Vitamin D Supplementation in the Treatment of Chronic Heart Failure: A Meta-analysis of Randomized Controlled Trials. Clin Cardiol 2016;39:56–61. 10.1002/clc.22473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boxer RS, Kenny AM, Schmotzer BJ, et al. . A randomized controlled trial of high dose vitamin D3 in patients with heart failure. JACC Heart Fail 2013;1:84–90. 10.1016/j.jchf.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Witham MD, Crighton LJ, Gillespie ND, et al. . The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2010;3:195–201. 10.1161/CIRCHEARTFAILURE.109.907899 [DOI] [PubMed] [Google Scholar]
- 46. Lowry J, Gierula J, Byrom R. Vitamin D supplementation improves the size and function of the left ventricle in patients with heart failure. Eur Heart J 2015;36:665. [Google Scholar]
- 47. Zia AA, Komolafe BO, Moten M, et al. . Supplemental vitamin D and calcium in the management of African Americans with heart failure having hypovitaminosis D. Am J Med Sci 2011;341:113–8. 10.1097/MAJ.0b013e3182058864 [DOI] [PubMed] [Google Scholar]
- 48. Elidrissy AT, Munawarah M, Alharbi KM. Hypocalcemic rachitic cardiomyopathy in infants. J Saudi Heart Assoc 2013;25:25–33. 10.1016/j.jsha.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.