Abstract
The cellular mechanisms determining the firing patterns of gonadotropin-releasing hormone (GnRH) neurons are presently under intense investigation. In this study, we used GnRH-green fluorescent protein transgenic mice and perforated-patch electrophysiology to examine the role of small conductance calcium-activated potassium (SK) channels in determining the electrical excitability and burst firing characteristics of adult GnRH neurons. After establishing an appropriate protocol for examining the after-hyperpolarization potential (AHP) currents in GnRH neurons, the highly-selective SK channel blocker apamin was used to demonstrate that all GnRH neurons express functional SK channels (35.7±2.7pA, mean decay time constant 2167 ms, apamin IC50 = 9.6 nM) and that this channel underlies ~90% of the AHP in these cells. Current-clamp experiments showed that apamin-sensitive SK channels were tonically active in the majority (74%) of GnRH neurons, with apamin (100 nM) administration resulting in a mean 6.9±0.5mV membrane depolarization. Apamin also elevated the firing rate of GnRH neurons, including increased burst frequency and duration in spontaneously bursting cells, as well as the ability of GnRH neurons to fire action potentials in response to current injection. In GnRH neurons activated by current injection, apamin significantly enhanced the amplitude of the afterdepolarization potential (ADP) following a single action potential and eliminated spike frequency adaptation. Together, these studies show that apamin-sensitive SK channels play a key role in restraining GnRH neuron excitability. Through direct modulation of the AHP, and indirect actions on the ADP, the SK channel exerts a powerful tonic influence upon the firing dynamics of GnRH neurons.
Gonadotropin-releasing hormone (GnRH) neurons are responsible for generating the pulsatile pattern of gonadotropin secretion essential for fertility in all mammals (1). Although it is established that GnRH neurons achieve this by secreting GnRH into the pituitary portal system in a pulsatile manner, the mechanisms enabling this pattern of release remain unclear (1, 2). Nevertheless, taking inference from other pulsatile neuroendocrine systems (3, 4), it seems likely that the ability of GnRH neurons to fire in a bursting fashion will be essential for pulsatile GnRH secretion to occur.
Two recent studies have demonstrated that afterdepolarizing potentials (ADPs) are important in enabling GnRH neurons to fire in a bursting manner (5, 6). The sequential summation of small ADPs, generated following each action potential, results in a depolarized plateau that facilitates repetitive firing by GnRH neurons. The ADP results from sodium influx through tetrodotoxin (TTX)-sensitive, voltage-gated sodium channels in GnRH neurons (6). The other potentials believed to play a major role in shaping burst firing are the medium (~200ms) and slow (seconds) afterhyperpolarization potentials (AHPs). These currents result from activation of Ca2+-sensitive K+ channels following calcium entry through voltage-dependent calcium channels during the action potential or calcium released from intracellular calcium stores (7–10). The AHP typically reduces the probability of subsequent action potentials and controls the frequency and patterning of repetitive firing in neurons (9, 10). Small-conductance calcium-dependent potassium (SK) channels underlie the medium AHP, whereas the molecular identity of the channels responsible for the slow AHP remain to be determined (8, 9). Recent investigations have shown that GnRH neurons express SK mRNA (11, 12) and that apamin, a highly selective SK channel antagonist, inhibits the AHP in rat and mouse GnRH neurons (12, 13). However, the role of SK channels and AHPs in shaping endogenous firing in GnRH neurons remains unexplored.
In the present series of experiments we tested the hypothesis that SK channels are tonically active in GnRH neurons and that they play a role in controlling excitability and burst firing through generation of the AHP. Using a GnRH-GFP mouse model, current- and voltage-clamp perforated-patch recordings were used to (i) establish a protocol for full activation of the AHP in GnRH neurons, (ii) determine the sensitivity of the AHP to the SK antagonist apamin, and (iii) examine the effects of SK blockade on the excitability and patterning of burst firing in GnRH neurons.
Materials and Methods
Animals
Male and female GnRH–GFP mice (14) were housed under 12 h light/dark cycles (lights on at 7:00 A.M.) with ad libitum access to food and water. All experimentation was approved by the University of Otago Animal Welfare and Ethics Committee.
Electrophysiology
Gramicidin-perforated recordings of GnRH neurons were undertaken on 200 μm-thick coronal brain slices containing the rostral preoptic area cut with a vibratome (Leica VT1000S). After cutting with high (6 mM) MgCl2 and low CaCl2 (0.5 mM) artificial cerebrospinal fluid (aCSF), slices were incubated at room temperature in aCSF (in mM: 118 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 11 D-glucose, 10 HEPES, 25 NaHCO3) equilibrated with 95% O2-5% CO2 for at least one hour before being transferred to a submerged recording chamber, where the slices were viewed under a fixed-stage upright microscope (BX51WI; Olympus, Tokyo, Japan). Fluorescent GnRH neurons were identified under brief fluorescence illumination and patched under Nomarski differential interference contrast optics. Apamin (Sigma, St. Louis, MA) was bath-applied for 4 min by inclusion in the aCSF at concentrations of 0.1, 1, 10, 100, 200, and 400 nM. Patch pipettes with resistances between 3–5.5 MOhms were pulled from glass capillaries (id, 1.18 mm; od, 1.5 mm) with a microelectrode puller (Sutter Instruments, Novato, CA). Gramicidin (Sigma, St. Louis, MO) was dissolved in methanol to a concentration of 10 mg/ml, which was diluted in the pipette solution (in mM: 135 CH3KSO4, 4.5 NaCl, 0.1 CaCl2, 2 MgATP, 0.2 Na2GTP, 0.2 Na2ATP, 10 HEPES, 1.1 EGTA, 7 phosphocreatine-tris, 0.1 leuptine, pH 7.3, ~ 285 mOsmol) to a final concentration of 75 μg/ml just before use, then sonicated for 1~2 min. After a giga-seal was established, access resistance was monitored, and experiments in current clamp were begun when access resistance (Ra) reduced to below 100 MOhms (mean±SEM of 49±3 MOhms). All IAHP data were collected while Ra was between 15 and 35 MΩ. Signals (voltage and current) were amplified with a Multiclamp 700B (CV7B; Molecular Devices, Foster City, CA) and sampled on-line with the use of a Digidata 1440A interface (Molecular Devices) connected to a personal computer. Signals were filtered (3 or 10 kHz; Bessel filter of Multiclamp 700B) before being digitized at a rate of 10 kHz. Acquisition and subsequent analysis of the acquired data were performed with the Clampex suite software 10 (Molecular Devices) and Origin pro 7.5 (OriginLab Corporation, Northampton, MA). The resting membrane potentials were the potentials recorded in current clamp without any holding current after whole cell recordings established. The calculated liquid junction potentials was ~ -12mV, with the resting membrane potential not corrected. The input resistances were determined by fitting the linear part of the current-voltage curve (in current clamp between -60 and -90mV). During experiments, access resistance (Ra) was monitored and if it changed by >15%, the cell was discarded. The decay time constant (two thirds from maximal amplitude) of afterhyperpolarization current (IAHP) was determined by exponential fitting to the descending phase of IAHP (complete return to baseline within 20 to 40s), evoked by depolarizing the cell to 40 mV for 600 ms from a holding potential of -60mV (in voltage clamp). Burst analysis was performed with the Clampfit program (15). The Surprise Factor is an index of deviation from Poisson distribution such that a large Surprise Factor represents data further away from a Poisson distribution. A significant membrane depolarization was defined as being >1.5mV change from the baseline. The amplitude of IAHP was calculated as the difference of the baseline and peak of IAHP.
Statistical analysis
Data are expressed as mean±SEM for the number of samples indicated. Unless otherwise indicated, data were analyzed using Student’s t-tests.
Results
In total, 50 GnRH neurons were recorded from 36 adult GnRH-GFP mice (30 males and 6 females with a mean age of 80±1.8 days, n=36). GnRH neurons had a mean resting membrane potential (RMP) of -57.0±0.8 mV (n=50) and input resistance of 1.4±0.1 GΩ (n=50). At RMP, 50% of GnRH neurons were silent, 29% exhibited bursting patterns of action potentials with the remainder (21%) being spontaneously active but not firing in bursts.
Characterization of SK channel-mediated currents in GnRH neurons
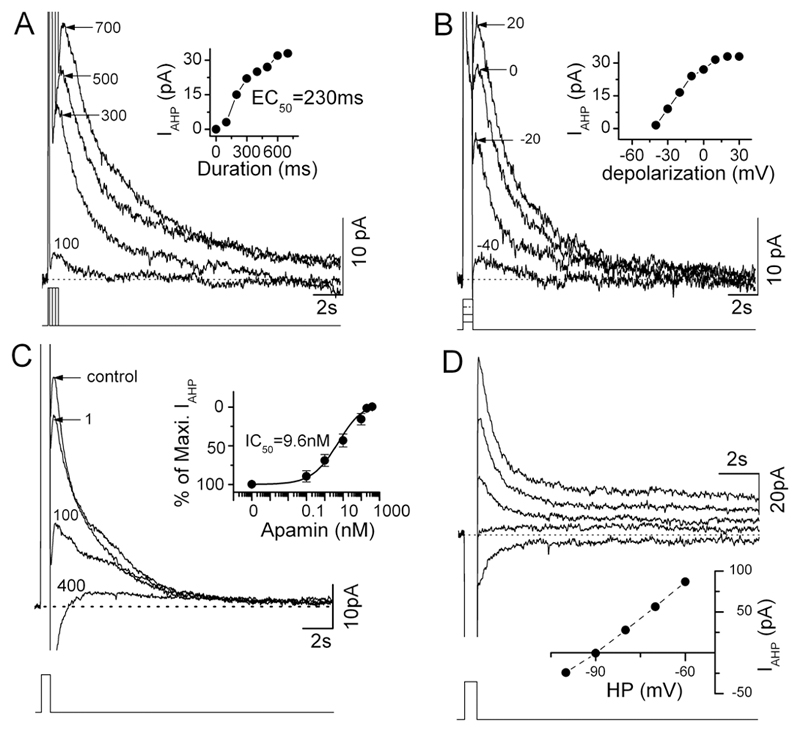
A voltage-clamp protocol to fully activate SK channels in GnRH neurons was first established. The SK channel is voltage-independent but regulated by intracellular calcium levels. Hence, the depolarization of a GnRH neuron will result in calcium influx through voltage-dependent calcium channels, and subsequent activation of SK channels to generate the AHP (12). Initially, five GnRH neurons were held at -60mV and depolarized to 40mV for different durations ranging from 100ms to 700ms in 100ms increments (Fig.1A). The amplitude of the IAHP generated by this procedure was dependent on the voltage step with the mean half-maximal activation achieved at 244±17 ms (n=5; Fig.1A). Next, GnRH neurons were depolarized for 600ms but with the voltage step changed in 10mV increments from -40 to 40mV. This showed that depolarization to 30-40 mV was required to fully activate the IAHP in GnRH neurons (Fig.1B). In subsequent experiments a 600ms, -60 to 40mV depolarizing step was used to ensure maximal activation of the GnRH neuron IAHP. No sex differences were found in evoked IAHP amplitude (diestrous and estrous females, 35.0±5.4pA, n=10; males, 35.9±3.2pA, n=40) or decay time constant (females, 1342.5±26.3ms; males, 2479.7±453.6). In the male group, 5 of the 40 GnRH neurons exhibited exceptionally long decay constants between 9,000-10,000ms. After excluding these cells, the time constant for males was 1607.6±207.4 (n=35). The amplitude (30.9±5.1pA) and decay time constant (1791.2±435.9ms) of the IAHP were found to be similar in whole-cell recordings of GnRH neurons (Ra = 7 -18 MΩ; n=9).
Figure 1. Characteristics of AHP and SK channel-mediated currents in GnRH neurons.
A and B, current traces evoked by a square pulse depolarization using different duration (A, from 100ms to 700ms, increment=100ms) and amplitude (B, from -40mV to 30mV, increment=10mV) currents (shown below). For clarity, only some traces are shown (A, 100, 300, 500, 700ms; B, -40, -20, 0, 20mV). Inset in A, shows plot of currents as a function of the duration of stimulating pulses. Inset in B, shows plot of currents as a function of the amplitude of stimulating pulses. C, current traces induced by the depolarization pulse (-60mV to 40mV for 600ms, indicated below) in the presence of 1, 100 and 400 nM apamin. Inset shows plot of the percentage of maximum AHP current as a function of different apamin concentrations (0.1, 1, 10, 100, 200, and 400 nM). D, apamin-sensitive current traces induced by a depolarization pulse (-60mV to 40mV for 600ms) at different holding potentials (HP, -60mV to -100mV, with 10mV increments) showing a reversal potential of -90mV. From top to bottom, current traces at holding potentials of -60, -70, -80, -90mV and -100mV. The apamin-sensitive currents at different HP were obtained by subtraction of the currents after apamin from those before apamin at corresponding holding potentials.
The effect of the SK blocker apamin on the IAHP was examined by inclusion in the aCSF at concentrations of 0.1, 1, 10, 100, 200, and 400 nM. The current was blocked by apamin in dose-dependent manner, with an IC50 of 9.6nM (n=6, Fig.1C). An apamin-sensitive current was recorded in all 50 GnRH neurons and found to have an average peak amplitude of 35.7±2.7pA (range 9.4 to 86.9pA) and decay time constant of 2167.0±356.7ms (range 665.9 to 10705.7ms, n=50). In most cells, the IAHP had an onset of ~10ms (time from termination of stimulation pulse). The apamin-sensitive current had a reversal potential of -92±2mV (n=4; Fig.1D), consistent with SK channels being selective for potassium ions. Following treatment with 100nM apamin, a residual (~10%) IAHP was found in 42% of GnRH neurons (amplitude 4.0±0.5pA, range 1.4 - 8.5 pA, decay time constant of 9495±1145ms, n=21). These small residual currents, that remained even in the presence of 400nM apamin (n=5), may represent apamin-insensitive SK channels or unidentified channels. Together, these experiments showed that all GnRH neurons express the SK channel and that it is responsible for the great majority of IAHP in these cells.
Apamin-sensitive SK channels determine firing patterns of GnRH neurons
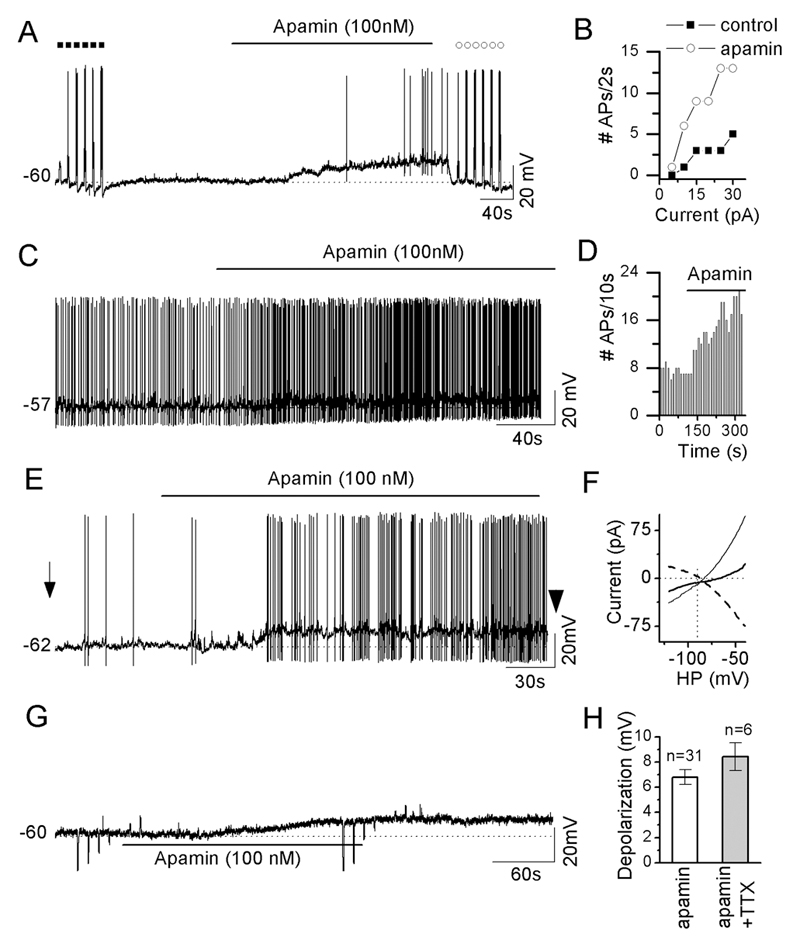
Current-clamp recordings were made from 50 GnRH neurons. Treatment with apamin (100nM) was found to depolarize 31 of 42 (74%) of GnRH neurons by 6.8±0.6mV with the remaining 11 cells not showing any change in resting membrane potential. The effect of apamin on the patterning of GnRH neuron firing was dependent upon the cell’s pre-test firing pattern.
i) Silent GnRH neurons (RMP 58.8±1.1) responded to apamin by depolarization (13 of 21; 62%) with accompanying action potentials in 8 neurons (Fig.2A); three of which occurred in a bursting manner. Current injection protocols in silent GnRH neurons showed that ~3-fold more action potentials were evoked by 5 to 30pA currents in the presence of apamin compared with the control period (n=8; Fig.2B).
Figure 2. SK channels restrain the excitability of GnRH neurons.
A, representative voltage trace of a silent GnRH neuron at rest membrane potential showing apamin-induced depolarization and firing. Filled squares and open circles indicate the pre- and post-, respectively, responses evoked by depolarizing current injections of 2 seconds (from 5 to 30pA, increment of 5pA). B, plot of number of action potentials induced by 2 s depolarizing currents as a function of amplitude of injection currents before and immediately after apamin treatment as indicated in A. C, a representative voltage trace of a spontaneously active, non-bursting cell responding to apamin with a small depolarization and increase in firing frequency. D, histogram showing effect of apamin on firing frequency of cell in C. E, representative voltage trace of a spontaneously-active bursting GnRH neuron in which apamin evokes depolarization and increased burst firing. F, plot of current induced by voltage ramp from -120 to -40mV in cell shown in E, before (left arrow E) and after apamin (right arrowhead E), as a function of holding potentials. The dashed line shows the apamin-induced current obtained by subtraction of currents before and immediately after apamin treatment. G, representative voltage trace of apamin-evoked depolarization in the presence of TTX, kynurenic acid (1 mM) and picrotoxin (0.1 mM). H, histogram summarizing the apamin-evoked depolarization in the absence and presence of TTX (0.5 μM), kynurenic acid (1 mM) and picrotoxin (0.1 mM). Note that the large upward and downward deflections on the traces are responses to the injection of hyperpolarizing or depolarizing currents to test membrane resistance.
ii) 5 of 8 GnRH neurons exhibiting spontaneous activity that was not bursting in nature (RMP 54.9±2.0), responded to apamin with depolarization and an increase in firing frequency that remained non-bursting. (Fig.2C,D).
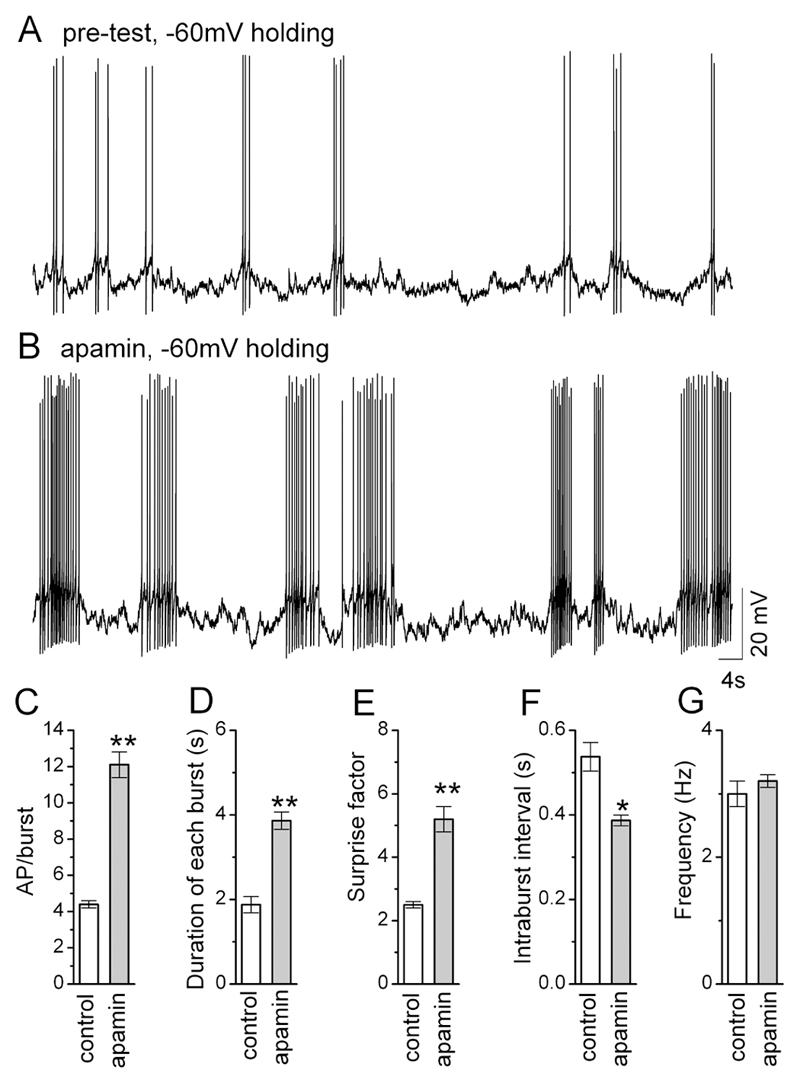
iii) 13 of 13 GnRH neurons exhibiting burst firing (RMP 58.4±1.0) responded to apamin with depolarization accompanied by an increase in burst duration and frequency (Fig.2E & 3) and an increase in input resistance (Fig. 2F). The effect of apamin upon burst firing was clearly observed by holding GnRH neurons back to their control membrane potential in the presence of apamin (Fig.3AB). The blockade of SK channels significantly increased the number of action potentials per burst (p<0.01;Fig.3C), duration of each burst (p<0.01;Fig.3D), the normality of firing within the burst, as determined by the Surprise factor (p<0.01; Fig.3E), while decreasing the intraburst interval (p<0.05; Fig.3F). However, apamin had no effect upon mean firing frequency within the burst (Fig.3G; control bursts n=78; apamin bursts, n=115; Student’s t-test).
Figure. 3. SK channels control burst length in GnRH neurons.
A and B, representative voltage traces from a single GnRH neuron exhibiting burst-firing before (A) and during (B) apamin treatment with holding potential maintained at -60mV. C-G, histograms summarizing the effects of apamin on spontaneous bursting in GnRH neurons (n=12, 78 control bursts and 118 apamin bursts). * P<0.05, ** P<0.001. Note that the cell has been held back to its original holding potential after apamin.
These observations indicated that tonically-active apamin-sensitive SK channels exert a powerful restraining influence on GnRH neuron membrane excitability and firing. Apamin (100nM) was found to have no effect on the amplitude, rise time, decay time and half-width of action potentials indicating that SK channels do not contribute to shaping action potentials in GnRH neurons (n=10, not shown).
The effects of apamin remained in the absence of action potentials and synaptic transmission. Six of 8 (75%) of GnRH neurons pre-treated with 0.5 μM TTX, 1mM kynurenic acid and 0.1mM picrotoxin, were depolarized (8.4±1.1 mV) by 100nM apamin (Fig. 2G) with the remaining 2 cells not showing any change in resting membrane potential. The magnitude of the apamin-induced depolarization was the same with and without synaptic transmission (Fig. 2H, P>0.2, Mann-Whitney tests). These observations indicate that apamin acts directly upon SK channels expressed by GnRH neurons to evoke their effects and that action potentials are not necessary for the tonic SK current.
SK channels influence firing by regulating spike frequency adaptation and ADPs
To examine the ways in which SK channels/AHP determine GnRH neuron excitability, perforated-patch current-clamp experiments were undertaken in which a train of action potential was initiated by depolarizing current injections (amplitude of 5 to 30pA in 5pA increments, duration 2s) in the absence and presence of apamin.
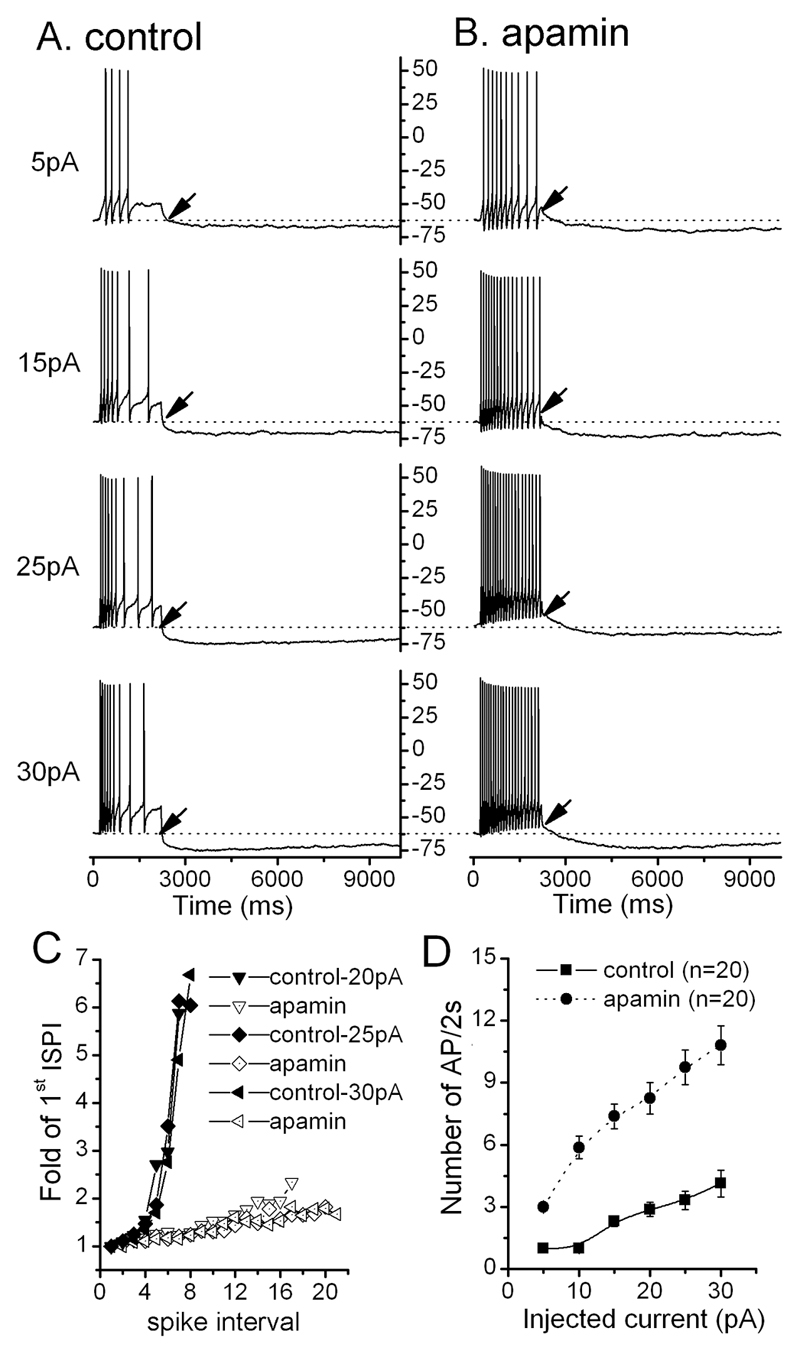
Current injections evoked trains of action potentials that exhibited spike frequency adaptation (SFA), where the inter-spike interval of action potentials increases as the action potential train proceeds (Fig.4A). Typically, the inter-spike interval for the first 4-5 action potentials is similar but then widens markedly for subsequent action potentials (Fig.4C). Apamin almost completely abolished SFA in all GnRH neurons tested (n=20; Fig.4BC) and resulted in more action potentials per train with the increase in frequency of action potentials being proportional to the amplitude of the injected current (Fig. 4D).
Figure. 4. SK channels control spike frequency adaptation (SFA) in GnRH neurons.
A and B, representative voltage traces showing the response of a silent GnRH neuron to 2s-depolarizing current injections of 5, 15, 25 and 30pA, before (A) and after (B) apamin. Note that apamin reduces SFA and that the afterdepolarization potential (ADP) becomes obvious (arrows). C, plot showing interspike interval (ISPI) during a burst in relation to the 1st ISPI. In control conditions (filled symbols), SFA is evident after the 4th spike when the ISPI becomes greatly increased. This is abolished in the presence of apamin (open symbols). D, plot showing number of spikes during 2s of depolarization as a function of injected depolarizing currents before (control-filled square) and after (apamin-filled circle) apamin. Note all points are divided by the number of spikes evoked by 5pA current in control condition.
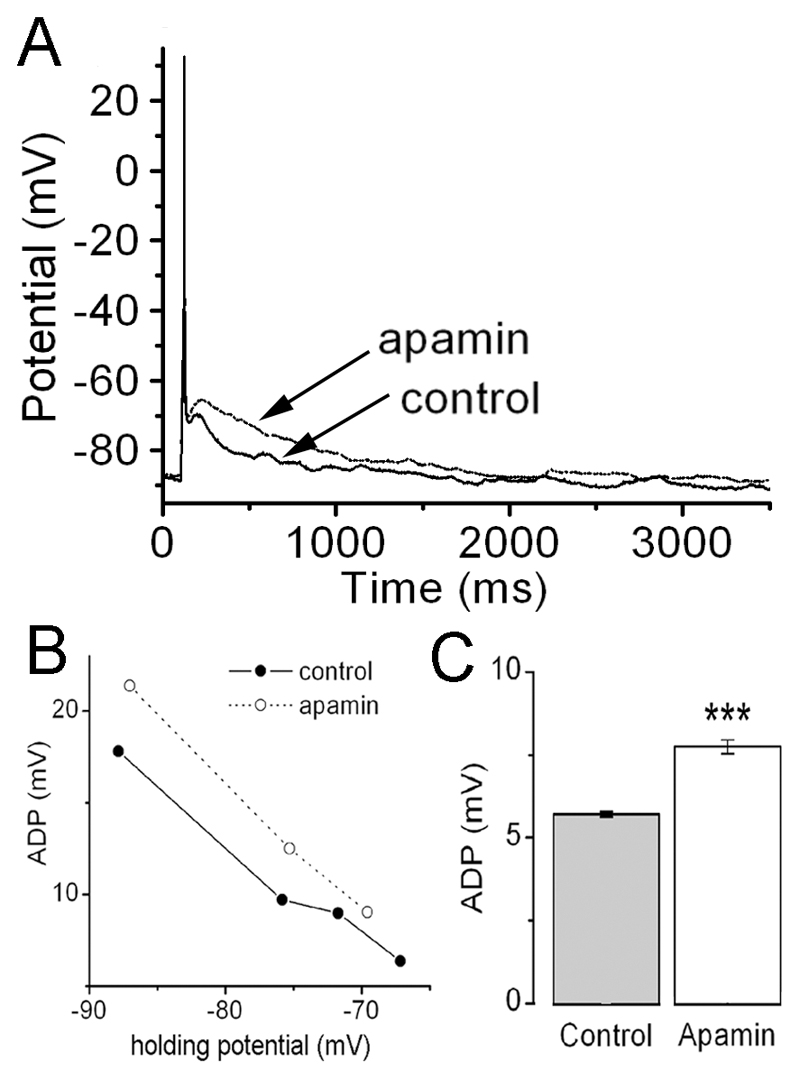
As expected, the AHP was reduced following apamin (Fig.4AB). In contrast, the ADP associated with the action potential train became more obvious in the presence of apamin (arrows, Fig. 4AB). The effect of apamin on ADPs was further examined using a depolarizing current of 50-80pA for 20 ms that typically evokes only a single action potential (Fig.5A). In this situation, the ADP was enhanced following apamin treatment (Fig.5A). The ADP was also found to be voltage dependent and apamin resulted in a consistent increase in ADP amplitude across -70 to -90mV holding potentials (Fig.5B). When held at -70mV, the ADP amplitude was ~35% larger in the presence of apamin (p<0.001; n=10; Fig. 5C).
Figure. 5. SK channels influence afterdepolarization (ADP) amplitude in GnRH neurons.
A, representative voltage trace showing action potentials evoked by a 20ms-depolarizing current injection of 65pA in the presence and absence of apamin. B, plot of ADP amplitude as a function of holding potential in the absence and presence of apamin. C, histogram showing maximum ADP amplitude in the presence and absence of apamin (n=10; ***P<0.001).
Discussion
We report here that all adult GnRH neurons express SK channels and that these channels underlie the great majority of the AHP current in mouse GnRH neurons. It was important in these studies that we first identified the appropriate conditions for evaluating the AHP as the protocol used typically in neurons (depolarizing to 20mV for 100ms), would have evoked only ~ 30% of the peak AHP current (36.1±3.3pA) in mouse GnRH neurons. In 74% of GnRH neurons, the SK channel was found to be active at rest where it was responsible for a potent inhibition of membrane excitability. This resulted from a tonic hyperpolarization of membrane potential and a role in limiting the frequency of action potentials including those occurring within bursts. In addition to direct effects of the SK channel on the AHP, these channels also modulate the amplitude of the ADP, providing a further mechanism for the regulation of burst firing and excitability in GnRH neurons.
Two previous electrophysiological studies have shown that GnRH neurons express SK channels. A study by Kato and colleagues (12) reported that cultured rat GnRH neurons expressed apamin-sensitive SK channels and a similar finding was found recently by Spergel in the mouse (13). Our present study confirms these findings and extends them by determining the functional role of SK channels in modulating the firing patterns of GnRH neurons in situ. The results from all three electrophysiological studies to date indicate that the channels responsible for the AHP in GnRH neurons are somewhat atypical. Elsewhere in the brain, AHP currents of ~200 ms duration (medium AHPs) are mediated by SK channels whereas longer-lasting AHP currents, occurring over 100s ms to seconds (slow AHP), are driven by as yet unidentified channels (8, 9). The GnRH neurons exhibit slow AHPs that, despite being activated within ms of the action potential, last for several seconds and are very clearly dependent upon SK channels. How the millisecond increase in intracellular calcium accompanying the action potential can activate SK channels in GnRH neurons for seconds is unclear. It may, for example, depend upon calcium pump dynamics or represent a particular spatial arrangement between the SK channels and calcium binding proteins (10), as has also been suggested recently for the slow AHP (16), or even the slow dynamics of calcium release from stores in these cells (17).
The identity of the SK channel subunits underlying the AHP in mouse GnRH neurons is unknown. The different SK subunits show differential sensitivity to apamin in the order of SK2>SK3>SK1 (9). Although apamin blocks nearly all (90%, here; 93%,(13)) of the AHP current in mouse GnRH neurons, it has a relatively high IC50 (9.6nM) suggesting that the SK2 subunit is not dominant and that SK channels may even be heteromeric in these cells (9). In contrast, cultured rat GnRH neurons exhibit a lower IC50 (~0.1nM) for apamin and express transcripts for all three SK subunits (12). However, in the rat, only ~70% of the AHP current is blocked by apamin suggesting the existence of another major current or apamin-insensitive SK channel generating AHPs.
It is apparent from the present study that SK channels exert a powerful regulatory influence upon the excitability of GnRH neurons. The channel is tonically active in the great majority of GnRH neurons where it contributes to both the resting membrane potential and the frequency/patterning of firing. Intriguingly, over half (62%) of silent GnRH neurons were depolarized by apamin treatment suggesting the existence of a tonic potassium conductance through the SK channel in these cells. Similarly, apamin continued to depolarize GnRH neurons in the presence of TTX. Thus, it is apparent that an action potential-independent mechanism exists for the calcium activation of SK channels in these cells. In this light, it may be important that over half of adult GnRH neurons have been reported recently to exhibit spontaneous calcium transients originating from store mechanisms (17). In 85% of GnRH neurons exhibiting spontaneous activity, apamin resulted in a marked increase in firing frequency. In addition, apamin treatment resulted in GnRH neurons exhibiting more action potentials to current injection. This “input-output” relationship indicates that the SK channel will also play a significant role in determining the ability of synaptic inputs to activate GnRH neurons.
The powerful effect of the SK channel upon GnRH neuron excitability likely results from its role underlying the great majority of the AHP in GnRH neurons and the effect of the AHP on ADP amplitude. As noted previously (6), the size of the ADP is dependent upon the AHP and the two currents likely counter-act each other in terms of determining post-spike excitability. Chu et al (6) found that blockade of calcium-activated potassium channels resulted in a faster-peaking ADP in GnRH neurons and we show here that blockade of the SK channel, specifically, results in a 35% increase in ADP amplitude in these cells. The absence of the majority of the AHP, and enhancement of the ADP, following SK channel blockade would provide a strong drive towards enhanced burst firing. This is clearly observed in both spontaneously active and stimulated bursting GnRH neurons, where apamin results in much longer duration trains of action potentials within each burst. In stimulated bursts, it is also evident that the SK channel underlies SFA, although this phenomenon was not observed in spontaneously active bursting GnRH neurons where typically only ~4 spikes make up each burst. Studies in other neuronal phenotypes have suggested a role for the SK channel and AHP in the termination of burst firing (8, 9). Although other scenarios exist (18), one possibility for the GnRH neurons is that individual bursts are evoked by the ADP and terminated by the AHP.
Together, these observations indicate that the SK channel exerts an important suppressive influence upon GnRH neuron excitability by (i) tonically hyperpolarizing resting membrane potential, (ii) limiting the duration of action potential bursts, and (iii) reducing the efficacy of excitatory synaptic inputs to these cells. Taking insight from other neuronal cell types (10), it is likely that the net activity of SK channels is regulated not only directly by calcium but also indirectly by alterations in calmodulin phosphorylation status (19) as well as SK channel abundance. In this light it is interesting to note that SK3 mRNA levels in the guinea-pig hypothalamus are increased by estrogen (11), although the apamin-sensitive current charge density was not found to change across the estrous cycle in overnight cultured rat GnRH neurons (12). Future experiments will now need to address the physiological factors modulating this important calcium-activated ion channel in GnRH neurons.
Acknowledgements
The authors wish to thank Dr. Colin Brown for commenting on an early draft of the manuscript. These studies were supported by the New Zealand Marsden Fund, and the UK Wellcome Trust.
Footnotes
Disclosure Statement: The authors have nothing to disclose
References
- 1.Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 1415–1482. [Google Scholar]
- 2.Moenter SM, Anthony DeFazio R, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 3.Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol. 1995;47:291–339. [PubMed] [Google Scholar]
- 5.Kuehl-Kovarik MC, Partin KM, Handa RJ, Dudek FE. Spike-dependent depolarizing afterpotentials contribute to endogenous bursting in gonadotropin releasing hormone neurons. Neuroscience. 2005;134:295–300. doi: 10.1016/j.neuroscience.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci. 2006;26:11961–11973. doi: 10.1523/JNEUROSCI.3171-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 8.Vogalis F, Storm JF, Lancaster B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci. 2003;18:3155–3166. doi: 10.1111/j.1460-9568.2003.03040.x. [DOI] [PubMed] [Google Scholar]
- 9.Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 10.Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–311. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Bosch MA, Kelly MJ, Ronnekleiv OK. Distribution, neuronal colocalization, and 17beta-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in the guinea pig brain. Endocrinology. 2002;143:1097–1107. doi: 10.1210/endo.143.3.8708. [DOI] [PubMed] [Google Scholar]
- 12.Kato M, Tanaka N, Usui S, Sakuma Y. The SK channel blocker apamin inhibits slow afterhyperpolarization currents in rat gonadotropin-releasing hormone neurones. J Physiol. 2006;574:431–442. doi: 10.1113/jphysiol.2006.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spergel DJ. Calcium and Small-Conductance Calcium-Activated Potassium Channels in Gonadotropin-Releasing Hormone Neurons before, during, and after Puberty. Endocrinology. 2007;148:2383–2390. doi: 10.1210/en.2006-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurone in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- 16.Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow afterhyperpolarization in hippocampal pyramidal cells. Neuron. 2007;53:487–493. doi: 10.1016/j.neuron.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jasoni CL, Todman MG, Strumia MM, Herbison AE. Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci. 2007;27:860–867. doi: 10.1523/JNEUROSCI.3579-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006;29:108–115. doi: 10.1016/j.tins.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B. Protein kinase CK2 is coassembled with small conductance Ca2+-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]