Abstract
The legacy of pre-Columbian land use on modern Amazonian forests has stimulated considerable debate which, until now, has not been satisfactorily resolved due to the absence of integrated studies between pre-Columbian and modern land use. Here we show an abrupt enrichment of edible forest species combined with the cultivation of multiple annual crops in lake and terrestrial fossil records associated with pre-Columbian occupation in the eastern Amazon. Our results suggest that ~4,500 years ago, pre-Columbians adopted a polyculture agroforestry subsistence strategy that intensified with the development of Amazon Dark Earth soils after ~2,000 cal yr B.P. These millennial-scale polyculture agroforestry systems have left an enduring legacy on the modern enrichment of edible plants, demonstrating the important role of past indigenous land management in shaping modern forest ecosystems in the eastern Amazon.
The extent to which pre-Columbian societies altered Amazonian landscapes is one of the most debated topics in botany3,11,13–15, archaeology1,2,5–7, palaeoecology7,12,16–19, and conservation4,20,21. New findings show a disproportionate number of plants, accounting for half of all trees in the Amazon, are hyperdominant22, and domesticated species are five times more likely to be hyperdominant than non-domesticated species13. This is particularly prevalent in archaeological sites, suggesting the effect of pre-Columbian people on modern flora is more pronounced than previously thought13. The pre-Columbian anthropogenic soils known as Amazonian Dark Earths (ADEs) (traditionally called Terras Pretas de Índio), are one of the most distinct lines of evidence of human transformation of Amazonia because these modified soils are indicators of pre-Columbian sedentary occupation23,24. ADEs have been associated with sustained and intensive agriculture in the past, and have been re-utilised by modern farmers because of their extremely high fertility4. Several studies have shown that (i) forests on ADEs have a distinct species composition, exhibiting greater richness and higher abundance of domesticated and edible plants (used as food resources)25, (ii) the more complex the ADE archaeological context (e.g., multi-component sites), the greater the floristic composition of cultivated useful plants in modern home gardens26, and (iii) increased fertility associated with ADEs improves conditions for the establishment and growth of exotic species that are generally more nutrient-demanding than native Amazonian species27. However, the lack of detailed integrated archaeological/palaeoecological studies to connect past land use with modern vegetation have left fundamental questions about land use practices and the impact of ADEs on modern Amazonian ecosystems unresolved. To address these issues we integrate archaeology and archaeobotany records which reflect local-scale vegetation histories with lake and terrestrial palaeoecology which reflect broader regional-scale vegetation histories, combined with palaeoclimate and modern botanical surveys to investigate the impact of the past 4,500 years of human land use in the eastern Amazon (Fig. 1).
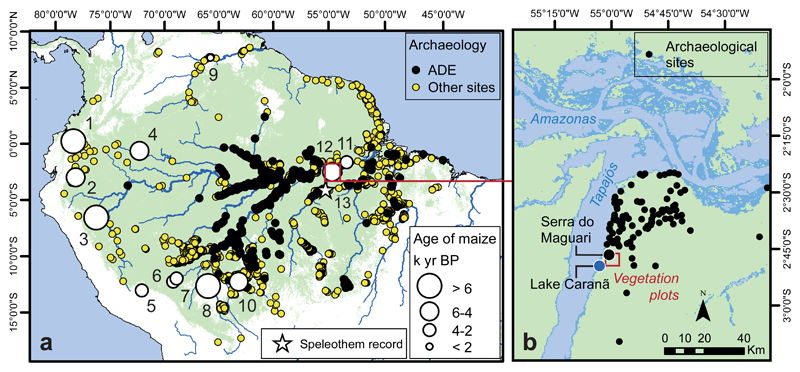
Figure 1. Regional Study Area .
a. Map showing Amazonian pollen, archaeological site 93,94 and records documenting early presence of maize: 1. Lake San Pablo, 2. Lake Ayauch, 3. Lake Sauce, 4. Abeja, 5. Huaypo, 6. Puerto Maldonado, 7. Lake Gentry, 8. Lake Rogaguado, 9. Parmana, 10. Monte Castelo, 11. Geral, 12. Lake Caranã (Supplementary Table S1). 13. Location of Paraíso Cave speleothem record (indicated by star). b. Santarém region showing location of Lake Caranã and the Serra do Maguari archaeological site 50.
Results
The study area located within the protected rainforest of the FLONA Tapajós Reserve, provides an ideal setting because of the presence of extensive archaeological sites, high concentrations of ADE soils, a nearby lake with limited riverine influence, and the existence of a nearby high-resolution palaeoclimate record28. We (i) collected a 210 cm sediment core dating to ~8,500 cal B.P. from Lake Caranã (LC, ~0.7 km in diameter, ~3 m depth; S 02, 50', 08", W55, 02', 33", 5 m a.s.l) for palaeoecological analysis (Methods M1-8); (ii) carried out excavations and sampled three ADE soil profiles at the nearby Serra do Maguari-1 (SDM1) archaeological site (Methods M 9-10), ~5 km NE of LC on the crest of the upper slope of Belterra Plateau (BTP) (S 02, 47', 87", W55, 03', 53", 126 m a.s.l.) (Fig. S4), compiled existing regional archaeological data (Methods M11), and (iii) set up survey plots (three in ADE and three in non-ADE sites) to perform modern vegetation inventories (Supplementary Fig. S6; Methods M12). Due to the extensive landscape level modification of soils in this region, we define non-ADEs plots as less modified soils without ceramics, located at least 150 m from dark ADE soils. As the formation and utilisation of ADEs is closely associated with food production, we focus our analysis on edible plant taxa in the pollen, phytolith and botanical assemblage. We classify edible plants as taxa that are ethnographically used as food resources in the Americas following Clement29, Levis et al13, and Hanelt30. These proxies are compared to the nearby speleothem record of Paraíso Cave (Fig. 2E), which provides a high-resolution record of natural climate variability for the past ~45,000 years28.
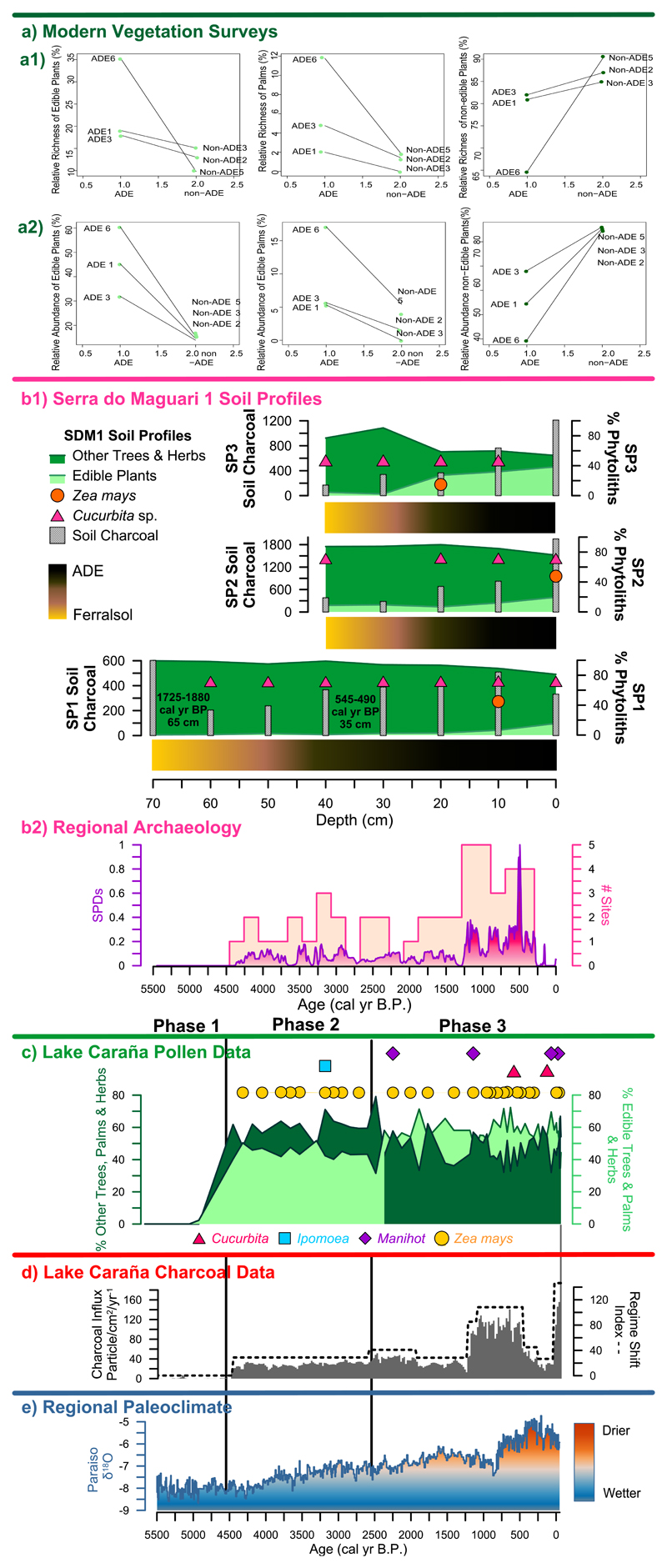
Figure 2.
Figure 2 Compiled Data Summary: a, Comparison of relative richness (left) and relative abundance (right) of edible plants (top), edible palms (middle) and other plants from modern vegetation surveys from the three ADE and the three non-ADE sites. b, The phytolith percentage summary diagram of soil profiles (SP), including edible, other trees and herbs, soil charcoal records, and the ADE soil lithology from SDM1 (top). The SPDs from compiled archaeological sites of the Santarém region are also shown (bottom) (Supplementary Table 3). c–d, A summary of the Lake Caranã pollen data (c), the charcoal influx (grey bars) and regime shift index (dashed line) from Lake Caranã (d) and the Paraíso cave speleothem record (e). The age-depth model for Lake Caranã (c–e) is based on calibrated 14C and 210Pb dates (Supplementary Table 2). Data in e from ref. 28.
We used a combination of 210Pb and AMS-radiocarbon dating techniques to develop a robust chronology for the age-depth model for LC. 210Pb dating was used to constrain the most recent palaeoenvironmental changes (<250 years), while AMS-radiocarbon dating was used to date sediments >200 years (Methods M3). Based on the compiled charcoal, pollen and geochemical data, three main phases are identified at LC representing the past ~8,500 years (Fig. 2 and 3, Supplementary Figs. S1-3, Methods M2-8, and Supplementary Table S2):
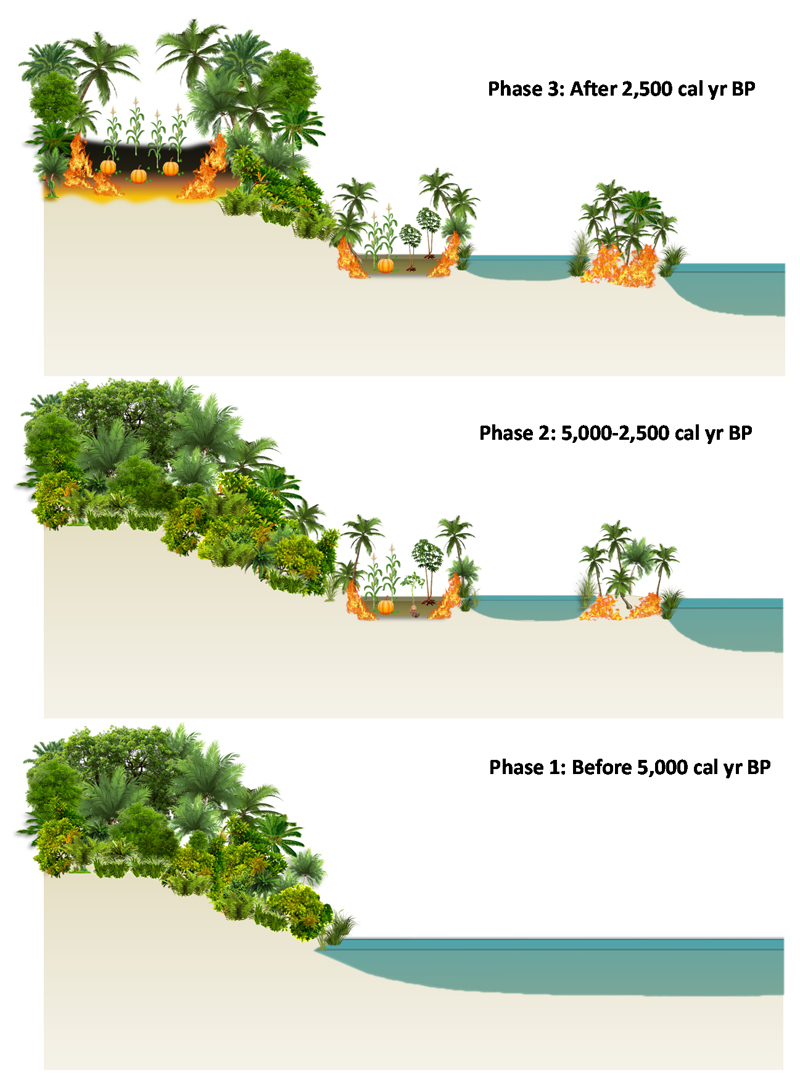
Figure 3.
A conceptual landscape drawing of the changing vegetation and disturbance regimes.
The conceptual drawing was inferred from an analysis of pollen, phytoliths and charcoal from the Lake Caranã core and the SMD1 archaeological site associated with the three phases discussed in the main text
Phase 1 (210-128 cm; Before ~4,500 cal B.P.). Geochemical data (Supplementary Fig. S2 and Methods M3-M5) characterise Phase 1 as a high-energy environment with increased allochthonous inputs, likely associated with increased riverine influence (Supplementary Discussion D1). Pollen concentration is very low during this period (i.e., <100 grains cm-3) and is attributed to poor preservation associated with sandy soils and low organic material (Supplementary Fig. S1-3). Fire activity is low (Fig. 2D) and there is no palaeoecological evidence of human occupation in the near vicinity during this phase despite documented human activity in the region9 (Supplementary Discussion D2). Phase 1 is associated with the wettest period in the past ~45,000 years28.
Phase 2 (128-79 cm; ~4,500-2,500 cal B.P.). Phase 2 begins with the formation of LC following decreased riverine inputs, as indicated by the shift in geochemistry and increase in sediment organics (Supplementary Fig. S2). Pollen is dominated by >50% rainforest taxa (Fig. 2C). ~30% of the total pollen taxa, and the frequency of herbs and grasses remains low throughout the length of the record (<10%). Our record documents the earliest arrival of maize (Zea mays) to the eastern Amazon, which is then present consistently after ~4,300 cal B.P.; combined with sweet potato (Ipomoea batatas) recorded at ~3,200 cal B.P., the assemblage indicates the lake inhabitants practiced polyculture (mixed cropping) including cereal and tubers crops (Fig. 2C and Supplementary Fig. S3). The occurrence of maize pollen is consistent with a temporal gradient of maize dispersal that begins outside Amazonia and reaches the eastern Amazon ~4,300 cal B.P. (Fig. 1A and Supplementary Table S1), ~3,800 years prior to the development of ADE soils at SDM1. The formation of LC is followed by increased charcoal accumulation, indicating low severity fire activity around the lake (Fig. 2D). Although regional climate data document a gradual shift towards drier conditions from ~4,500 cal B.P., which continues into Phase 3 (Fig. 2E), the synchronous onset of fire activity combined with the presence of maize pollen suggests intentional human-caused ignitions associated with local forest clearance for crop cultivation around the lake. The Sum of the calibrated Probability Distributions (SPDs) from dated archaeological contexts in the lower Tapajós indicate an increase in regional scale pre-Columbian activity after ~4,300 cal B.P. (Fig. 2B2, Methods M11, and Supplementary Table S3). During Phase 2, the residents of Lake Caranã were likely hunting and fishing and utilising the seasonally flooded, nutrient-rich soils surrounding the lake shore, practicing agroforestry and exploiting wild plants, combined with low-level fire activity to clear land for polyculture.
Phase 3 (79-0 cm; 2,500 cal yr BP to modern). Phase 3 is characterised by an increase in edible plants (from ~45 to >70% of terrestrial pollen taxa), a decrease in non-edible plants (from ~50 to 30%), followed by the arrival of manioc (Manihot esculenta) ~2,250 cal B.P. (Fig. 2C). The increase in edible plants is not associated with significant change in the regional climate data, suggesting pre-Columbian forest enrichment is the driver of this abrupt change in forest composition. LC exhibits an increase in fire activity ~1,250 to 500 cal B.P., associated with the increase in pre-Columbian activity (Fig. 2B2), coupled with the arrival of squash (Cucurbita sp.) ~600 cal B.P. in the lake pollen.
Phase 3 is contemporaneous with the archaeobotanical data from SDM1, a ~15 ha mounded village with a central plaza surrounded by a mosaic of ADE sites (Supplementary Fig. S4). Mound construction and ADE formation at SDM1 occurred between ~530 and 450 cal B.P. (Supplementary Table S3), and ceramic material at the site is characteristic of the late pre-Columbian Tapajós Period (Supplementary Discussion D2). Herb phytoliths from the three profiles account for ~4 to 18% (Fig. 2B1 and Supplementary Fig. S5). Phytolith data indicate a gradual increase in edible plants (Methods 10, Supplementary Tables 9-10) following the formation of ADE soils that reach the highest levels (>66%) in modern surface soils. Squash is present in the phytolith assemblage at SDM1 from before the formation of the ADE, consistent with the presence of squash in the lake; however, maize does not appear at SDM1 until the ADE has formed. Soil charcoal is present in all three profiles at SDM1 and increases with the formation of ADE soils ~530 cal B.P (Fig. 2B1). The increase in soil charcoal in the ADE layers suggests in-field burning was likely implemented to ameliorate nutrient poor ferralsols on BTP to cultivate nutrient demanding crops, such as maize. Similar practices have been recorded in indigenous Amazonian groups31. Sediment charcoal from the lake however, indicates an overall decrease in fire activity in the watershed at this time, synchronous with the driest regional climate conditions in the past 5,500 years (Fig. 2). Modern fire activity in the eastern Amazon is associated with increased droughts32, thus this decrease in fire suggests pre-Columbian fire management likely suppressed large wildfires during the apex of pre-Columbian activity in the region. This interpretation is further supported by the continued presence of rainforest vegetation at LC (~30 to 45%) and closed canopy forest at SDM1 (~56 to 82%), indicating that large-scale, labour-intensive deforestation did not occur around the lake nor on the plateau over the past 4,500 years. High percentage of trees, increased abundance of palms, low and continuous percentages of herbs, presence of cultigens, and low levels of fire, suggests that the residents of SDM1 were practicing polyculture agroforestry and fire management from ~4,500 cal B.P. The increase of edible plants in the pollen record suggests that the land cleared for cultivation was not abandoned, but instead was managed during early succession to promote edible plant species. Similar cultivation practices are reported in the early accounts of the sixteenth and seventeenth centuries33, as well as in palaeoecological and archaeobotanical studies in other regions of Amazonia34,35.
The persistent enrichment of edible plants and palms in the watershed, which began ~2,500 cal B.P. and progressively increased with the formation of ADE soils at SDM1, demonstrates that millennial-scale polyculture agroforestry systems have an enduring legacy on modern forest composition. Modern botanical inventories indicate a heightened enrichment in relative frequency, relative richness, and relative abundance of edible plants (trees and palms) on vegetation plots on the nutrient rich ADE soils in comparison with non-ADE soils (Fig. 2A, Methods M11, Supplementary Fig. S7, and Supplementary Table S4). These data demonstrate the formative conditions that likely account for the disproportionate hyperdominance of edible species in modern Amazonian forests13,22.
Discussion
Collectively, our study evidences persistent anthropogenic landscapes for the past 4,500 years in the eastern Amazon with a long-lasting effect on the modern hyperdominance of edible species. The low percentage of herbs, permanence of forest cover and increasing charcoal levels at SDM1 are consistent with shaded agroforestry systems that suppress weeds and practice controlled in-field burning as recorded among several indigenous groups today36,37. Our record is consistent with a practice of short cropping/long fallow polyculture agroforestry practiced with inefficient stone axes characterised by a mosaic of patches in different stages of succession, forming a complex landscape that transitions from forest to field and back to forest again3,31,38,39. Maize was initially cultivated along the LC shore and only with the development of human-enriched soils could it be cultivated in the terra firme upland plateau areas. The development of ADEs may have allowed for the expansion of the cultivation of this productive crop beyond lake margins, which likely increased carrying capacity to sustain the large populations recorded in the region during the historic period2.
In the periodisation traditionally adopted for South American archaeology, the emergence of sedentary village life coupled with an increased reliance on staple crops has been associated with the onset of the Formative23,40–42. The validity of the Formative as a cultural horizon in Amazonia is somewhat questionable due to its temporal heterogeneity across the basin as well as the persistence of Formative lifestyles after the arrival of Europeans to the region43. Our data indicates that a diverse polyculture agroforestry system had a more important role in the subsistence base for sedentism and population growth seen during the late Holocene. Additionally, the procurement of aquatic resources (fish, turtles), hunting, and the collection of wild plants likely significantly contributed to the subsistence strategy of eastern Amazonia populations44,45. Through the maintenance of closed canopy forest enrichment of edible plants, with limited clearing for crop cultivation and low-severity fire management, long-term food security was attained despite climate and socio-economic changes. The patterns observed in the LC record are concomitant with key periods of cultural transformation in the lower Tapajós. The abrupt increase in fire activity after ~1,250 cal B.P. followed by the arrival of new cultigens (Cucurbita sp.) ~600 cal B.P. coincide with the development of the Santarém culture, centered at the modern city of Santarém, where the largest site comprised of ~16 ha of ADE is located2. The Santarém polity purportedly comprised an area of 23,000 km2 with sites extending for hundreds of miles along river bluffs and interior plateaus46. During this period, the lower Tapajós concentrated one of the highest population densities in Amazonia47–49. Initially interpreted as a warlike, tribute-based chiefdom that persisted until colonial times47, the degree of centralisation of the Santarém polity has been recently questioned given the absence of differential access to prestige goods or other clear evidences of hierarchy50. Moreover, peripheral sites were shown to be independent of Santarém influence during the early stages of that cultural expansion, although they were ultimately abandoned after ~1,000 cal B.P.50. Irrespective of the social organisation implied, the changes observed in the LC record after ~1,250 cal B.P could reflect changing land management practices aimed to increase subsistence demands associated with the Santarém culture expansion.
As modern deforestation and agricultural plantations expand across the Amazon Basin, coupled with the intensification of drought severity driven by warming global temperatures, these data provide a detailed history of over four millennia of anthropogenic land use that progressively intensified, in the absence of large-scale deforestation, that has a lasting legacy on composition of modern rainforests in the eastern Amazon. These data provide valuable new insights into the vital role indigenous land management practices played in shaping modern ecosystems that can inform ecological benchmarks and future management efforts in the eastern Amazon.
Methods
Palaeoecology Methods
M1. Regional study area
To investigate coupled human-environment systems we designed a multi-proxy approach integrating local (archaeological site/terrestrial palaeoecology) and regional (lake palaeoecology) spatial scales. We selected the Tapajós National Forest (FLONA), located on the eastern side of the white water Tapajós River, ~50 km south of Santarem (Pará state, Brazil), which forms part of the Cretaceous Alter do Chão Formation51. Climate is seasonally dry, inter-tropical humid with a distinct wet-season between January to June. Mean annual rainfall ranges between 1900 to 2200 mm year-1 and average annual temperatures are between 21 and 31 °C52. The vegetation is composed of dense terra firme humid evergreen rainforest53. An understanding of the spatio-temporal nature of the pre-Amazonian Dark Earths (ADE) subsistence strategies was gained by comparing radiocarbon dated lake sediment core data from Lago Caranã (pollen, charcoal, geochemistry, and magnetic susceptibility) with AMS dated archaeobotanical soil profile data (phytoliths) from Serra do Maguari-1 (SDM1), which allowed the reconstruction of pre-Columbian land-use and subsistence strategies for the past 5,000 years. These records provided two distinct spatial scales: pollen and charcoal from the lake sediment core provided watershed scale (<106 m2) vegetation composition54 whereas phytoliths, which are deposited in situ55, represent local-scale (~1 m2) vegetation structure. These data were compared with modern botanical inventory data to evaluate the legacy of pre-Columbian land-use on modern vegetation in the eastern Amazon.
M2. Palaeoecology site selection and core collection
Lake Caranã (LC) (2° 50′ 08″ S, 55° 02′ 33″ W) is a flat bottom lake located on the fluvial terrace on the eastern bank of the Rio Tapajós. LC is located within a small closed basin and is separated from the main river channel (except during extreme flood events) by a depositional sand berm (200 m long, ~3 m tall) located on the NE edge of the lake. A 210 cm sediment core was collected using overlapping drives from a Livingston drive rod piston corer56 with a modified Bolivia surface corer to collect the sediment-water interface. Cores were transported back to the University of Exeter for cold storage. LC was selected because it is located at the base of the Belterra Plateau, which is rich in archaeological sites and ADE soils and today, receives limited sediment inputs from the Tapajós River. LC is thus ideally located to reconstruct changes in human land use around the Belterra Plateau.
M3. Palaeoecological age-depth model
The chronology for the LC sediment core relies on six radiocarbon (14C) dates, 210Pb radionuclide analysis of recent sedimentation and an age-depth model constructed in Bacon v2.257 within R58. Ages for the upper sediments of core LC were modelled using 210Pb radionuclide analyses following standard procedures59. Atmospheric fallout of 210Pb can be used to estimate the age of sedimentary sequences by measuring the rate of its decay across approximately six to nine half-lives, or 130 to 200 years. The addition of 210Pb dating was used in this study to develop a robust chronology for the most recent palaeoenvironmental changes, which also to provides an important validation tool for the youngest part of the age-depth model that otherwise relies on radiocarbon analyses. Radiocarbon ages that are younger than ~250 cal yrs B.P. contain large calibration uncertainties due to a ~200 year plateau in the calibration curve and are of limited use for tightly constraining recent centuries when developing an age-depth model. Activity of 210Pb was determined by measuring alpha decay of its daughter product 210Po as a proxy 60. Sediment subsample was spiked with a 209Po chemical yield tracer, acid digested using sequential HNO3:H2O2:HCl (1:2:1) chemical washes at 90°C, and then extracted from the solution, electroplated onto a silver disc, and measured using an Ortec Octête Plus Integrated Alpha-Spectrometry System at the University of Exeter. The age-depth profile was calculated from the total 210Pb inventory, the 210Pb decay constant (0.03114 yr-1), sample-specific activity and cumulative mass using the constant rate of supply model 59, which provided ten ages for the top 0.17 m of the core with modelled root-mean-square-error 2σ uncertainties (Supplementary Table S2). Bulk sediment organic material was collected from the sediment core for conventional AMS radiocarbon dating61 and sent to Beta Analytic for standard pretreatments and radiocarbon analysis. Radiocarbon ages were calibrated (Supplementary Table S1) within Bacon using IntCal1362 and modelled using Student-t test distributions with wide tails to negate the need of identifying and removing potential outliers in the age-depth model63,64. The use of Bacon and Bayesian statistics to reconstruct the accumulation history at LC allowed us to include every radiocarbon date that was taken throughout the LC core and develop robust estimations of age-depth uncertainty. Age-depth model mean accumulation rate priors in Bacon were calculated using the 14C chronology (acc.mean (accumulation mean) =42) and memory priors were set slightly below default so that the model would capture accumulation rate changes driven by variable sediment delivery from the catchment (mem.strength (memory strength) =2; mem.mean (memory mean) =0.4). Model means and 2 σ age distributions were calculated from millions of Markov chain Monte Carlo age-depth iterations through the core (Supplementary Fig. S1). The distribution of profile iterations identified radiocarbon ages Beta-469035 and Beta-469038 as potential outliers. Rather than omit these data points, they were retained and contributed to the uncertainty distribution of the model. For example, at depths 1.00 ±0.005 m and 1.15 ±0.005 m where a possible reversal occurs, the outliers allow for a greater range of age-depth iterations, which provide age estimations (3562 ±423 and 4555 ±514 cal yr B.P. respectively) with larger uncertainties in comparison to the younger part of the model where the age profile distributions were narrower and showed more certainty.
M4. X-ray fluorescence
X-ray fluorescence (XRF) analysis was conducted using a portable XRF Thermo Scientific Niton 3L3t GOLDD at the University of Reading at a step size of 2000 or 5000 µm. A micro-X-ray beam focused through a flat capillary waveguide was used to irradiate samples to enable both X-radiography and XRF analysis. Data were acquired incrementally at 0.25 cm contiguous intervals by advancing the split core through the X-ray beam65 and results were normalized using z-scores.
M5. Magnetic susceptibility
Magnetic susceptibility (MS) was measured to identify mineralogical variation in the sediments66. The MS of sediments is reflective of the relative concentration of ferromagnetic (high positive MS), paramagnetic (low positive MS), and diamagnetic (weak negative MS) minerals or materials. Typically, sediment derived from freshly eroded rock has a relatively high MS, whereas sediments that are dominated by organic debris, evaporites, or sediments that have undergone significant diagenetic alteration have a low or even negative MS67. Sediment cores were scanned horizontally, end to end through the ring sensor. MS was conducted at 1 cm intervals using a Bartington ring sensor equipped with a 75 mm aperture.
M6. Loss-on-ignition
Organic and carbonate sediment composition was determined by loss-on-ignition (LOI) conducted at 4 cm intervals throughout the core. For each sample, 1 cm3 of sediment was dried in an oven at 100°C for 24 hours. The samples underwent a series of 2 hour burns in a muffle furnace at 550°C and 1000°C to determine the relative percentage of the sample composed of organics and carbonates. Organic composition was determined by weight following standard methodologies68.
M7. Macrocharcoal
The LC sediment core was subsampled for macroscopic charcoal analysis at 0.5 cm intervals from 0 to 210 cm depth. Samples were analyzed for charcoal pieces greater than 125 µm using a modified macroscopic sieving method69. Subsampled material (1 cm3) was treated with 5% potassium hydroxide in a hot water bath for 15 minutes. The residue was sieved through a 125 µm sieve. Macroscopic charcoal (particles >125 μm in minimum diameter) was counted in a gridded petri dish at 40× magnification on a dissecting microscope. Charcoal counts were converted to charcoal influx (number of charcoal particles cm-2 yr-1) and charcoal accumulation rates by dividing by the deposition time (yr cm-1). Charcoal influx data (particles cm-2 yr-1) were used as an indicator of fire severity (the amount of biomass consumed during a fire episode or period of increased burning). A regime shift detection algorithm (RSI) based on sequential t-tests was applied to determine the occurrence of statistically significant shifts in the charcoal influx data70. Shifts were detected in both the mean fluctuations and the variance of raw charcoal counts. The algorithm for the variance is similar to that for the mean, but based on a sequential F-test71. RSI values were plotted against charcoal influx data to identify statistically significant changes in past fire regimes, which were interpreted as indicators of fire intensity changes.
M8. Pollen
The LC sediment core was subsampled for pollen analysis at 2 cm intervals between 0 and 128 cm depth (0 to ca. 5,000 cal B.P.) and at 16 cm intervals between 128 and 205 cm depth (5,000 to 8,500 cal B.P.), due to low pollen preservation (<100 terrestrial pollen grains cm-3) below 128 cm. Subsampled material (1 cm-3) was prepared using standard digestion protocol72, including an additional sieving stage to concentrate large cultigen pollen types such as Z. mays73. Following this sieving stage, equal numbers of exotic Lycopodium clavatum L. tablets74 were added to both the filtrate and residue of the sieved samples allow for direct comparison of cultigen pollen abundance with the standard terrestrial pollen counts73. Large pollen grains (>53 μm) concentrated through the fine-sieving methodology were scanned for Z. mays and other crop taxa producing large pollen such as Manihot esculenta and Ipomoea batatas73. The coarse fractions were counted to a standardized equivalent count of 2,000 Lycopodium grains (~3 to 4 slides). The pollen in the fine fractions was counted to the standard 300 terrestrial grains. Mauritia/Mauritiella were counted and totaled separately due to high concentrations. Larger non-crop pollen that was sieved into the coarse fraction (e.g. Mauritia/Mauritiella), was factored back into the total terrestrial pollen sums using abundance calculations from Lycopodium counts from the fine and coarse fractions using standard methods73. Fossil pollen was identified with reference to the collection of tropical pollen specimens housed at the University of Exeter. Maize pollen grains were distinguished from those of other wild grasses according to defined morphological and size criteria (e.g., grain size: > 80 µm)75. Pollen of Ipomoea batatas type, Manihot and Cucurbita are indistinguishable between that of cultigens (sweet potato, manioc, and squash respectively) and wild relatives, but we are confident that the grains we report come from cultigens since a) wild species of these crops were absent in the botanical survey carried around the lake that represent the catchment area for these large heavy pollen grains, b) the co-occurrence of Ipomoea, Manihot, and Cucurbita pollen, c) their absence at the site before the first signs of human land use, and d) the presence of Cucurbita phytoliths in the soil depth profiles. Therefore we interpret it as evidence for sweet potato, manioc, and squash cultivation. Where possible, members of the Moraceae family were identified to genus level using published pollen reference material and morphological descriptions76. Pollen taxa were grouped into edible trees, palms, and herbs, crops, other trees and herbs in the pollen diagram based on modern botanical classifications13,29,30 (Tables S5-S8). In addition to edible palms (e.g. Mauritia/Mauritiella), we have included in the ‘edible’ category of all the plant taxa identified to the genus level in the pollen record that are ethnographically used as food resources in the Americas30. Over seventy percent of these pollen taxa are present in the modern botanical inventories, thus these pollen genera likely represent edible species in past anthropogenic forests around Lake Caranã (Table S8). This edible plant classification is a conservative estimate since a large proportion of the families in the 'Other Trees and Herbs’ category contain species that are edible, however, these taxa were excluded if they could not be taxonomically identified higher than family level. Pollen percentage data is available in SI. Raw pollen data will be uploaded to Neotoma following publication.
Archaeology Methods
M9. Archaeological site selection
The Serra do Maguari-1 (SDM1) archaeological site (ca. 15 ha) is located on the crest and upper slope of Belterra Plateau (S 02, 47′, 87″, W55, 03′, 53″, 126 m a.s.l.). This site was selected due to its proximity ca. 5 km from LC and the presence of mounded architecture and a mosaic of ADE soils which is representative of the regional archaeology. Additionally, SDM1 is within the watershed of LC and provides a comparison of the local, in-situ vegetation reconstruction from the phytoliths soil profiles with the regional vegetation reconstruction from the pollen data. Together, this paired methodology enables the examination of past human disturbance on multidimensional spatial scales.
M10. Soil phytoliths
The three ADE soil profiles from SDM1 were analyzed at 10 cm intervals. Phytolith extraction followed standard protocols55. Subsampled material (200 g) was deflocculated by shaking for 24 hours in 900 ml warm water with sodium hexametaphosphate (NaPO36). Clays were removed by gravity sedimentation and separated into silt (<50 µm) and sand (>50µm) fractions by wet sieving. Carbonates were removed with 10% HCl and organic matter with nitric acid (HNO3). Phytoliths were floated in a heavy metal solution (ZnBr2) and drawn off by pipette. Slides were mounted using Entellan. Identification was carried out using an Axiovision 40 microscope at 200x (>50 µm) and 500x (<50 µm) magnification, respectively. The identification was based on comparison with the reference collection of the Archaeobotany Laboratory at the University of Exeter and by consulting an extensive comparative literature55,77–83. In addition to the edible palms (Arecaceae), we included in the edible plants category all the phytolith taxa identified to genus level that are ethnographically used as food resources in the Americas13,29,30 (Tables S9-10). Test pit samples were analyzed in 10 cm homogenized sample intervals from 0 to 70 cm at Profile 1, and 0 to 40 cm at profiles 2 and 3. Phytolith percentage data is available in SI. Raw phytolith data will be available at: Travassos, D. 2018. 'Dark Earth Plant Management in the Lower Tapajós. Unpublished PhD Dissertation. Department of Archaeology, University of Exeter.
M11. SPDs and site frequencies
The Sum of the calibrated Probability Distributions (SPDs) is a standard method for representing chronological trends in radiocarbon datasets. SPDs are produced by calibrating each independent date in the sample and adding the results to produce a single density distribution. This has the advantage of including the full range of probabilities associated with calibrated dates, instead of using single point estimates84–88. SPDs were built in OxCal using the Sum function and the IntCal13 calibration curve62,89 with an original dataset of 85 radiocarbon dates from the Lower Tapajós. In order to account for oversampling of some sites and phases within those sites, we applied a binning procedure85,87. Dates within sites were ordered and those occurring within 100 years of each other were grouped into bins and merged with the R_combine function. Timpson et al.87 found that different values for the bin-width did not affect the final shape of the SPD. This procedure is necessary because a sum of the calibrated dates assumes that observations are independent, whereas this is not the case when multiple dates were obtained for single sites or phases within them, as was the case with many sites of the Tapajós. The final filtered dataset contained 52 dates. Despite the decrease in sample size, the filtered SPD is highly correlated with an SPD built with all radiocarbon dates (r2 = 0.991, p < 0.001). In addition to the SPD, a histogram of the number of occupied sites is used as another proxy of human activity, based on the medians of the calibrated dates per 200 year intervals. Although the radiocarbon record is inherently biased by research (privileged dating of certain sites or periods) and taphonomic factors (greater preservation of charcoal towards more recent periods), SPDs have been shown to be a reliable method to assess past population dynamics in relative terms, provided an adequate sample size and measures of chronometric hygiene86,87, which were employed here. The trends in the SPD for the Santarém region are confirmed by cultural changes that provide independent evidence of population dynamics: the initial increase after ~4500 cal BP coincides with the appearance of ADE in the Tapajós90, and the peak after ~1250 cal BP corresponds to the development of the Santarém culture and proliferation of ADE sites in the Belterra plateau48,91,92.
Modern Vegetation Methods
M12. Modern botanical survey
Three pairs of 0.25 ha plots (50 x 50 m) were sampled in ADE and non-ADE sites on the Belterra Plateau (Supplementary Figure S6). The vegetation is classified as modern terra firme forests53. All live trees, palms, and lianas with diameter at breast height (~1.30 m above the ground) larger or equal to 10 cm were measured. Species were identified in the field by taxonomic specialists. Vouchers specimens were collected and transferred to the collections Nova Xavantina Herbarium, Nova Xavantina, Mato Grosso following identification. Botanical inventory data was grouped into cultivated edible plants (trees and palms) based on a revised list of domesticated plants from Clement (1999)29, Levis et al. (2017) 13, and cultivated plants within the Americas - North, Central and South America from Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops30, and other uncultivated trees (Supplementary Tables S5-S7). Relative richness and relative abundance of edible plants, palms and other trees plants were calculated and presented in Fig.2a. The relative abundance indicates the number of individuals of edible plants, edible palms or other plants divided by the total number of individuals found in the plot, and the relative richness is the number of edible plants, edible palms or other plants divided by the total number of species found in the plot. Bar charts for the frequency of edible plants, edible palms and other plants that occur in the vegetation plots and are presented in Supplementary Figs. S7. Data generated or analysed during this study are included in this published article (see supplementary information files).
Supplementary Material
Summary Paragraph.
The legacy of pre-Columbian land use in the Amazonian rainforest is one of the most controversial topics in the social1–10 and natural sciences11,12. Until now, the debate has been limited to discipline-specific studies, based purely on archaeological data8, modern vegetation13, modern ethnographic data3, or a limited integration of archaeological and palaeoecological data12. The lack of integrated studies to connect past land use with modern vegetation has left questions about the legacy of pre-Columbian land use on the modern vegetation composition in the Amazon unanswered11. Here we show persistent anthropogenic landscapes for the past 4,500 years have had an enduring legacy on hyperdominance of edible plants in modern forests in the eastern Amazon. We found an abrupt enrichment of edible plant species in fossil lake and terrestrial records associated with pre-Columbian occupation. Our results demonstrate that through closed-canopy forest enrichment, limited clearing for crop cultivation, and low-severity fire management, long-term food security was attained despite climate and social changes. Our results suggest that in the eastern Amazon the subsistence basis for the development of complex societies began ~4,500 years ago with the adoption of polyculture agroforestry, combining the cultivation of multiple annual crops with the progressive enrichment of edible forest species, and exploitation of aquatic resources. This subsistence strategy intensified with the later development of ADEs, enabling the expansion of maize cultivation to the Belterra Plateau providing a food production system that sustained growing human populations in the eastern Amazon. Furthermore, these millennial-scale polyculture agroforestry systems have an enduring legacy on the hyperdominance of edible plants in modern forests in the eastern Amazon. Together, our data provide a long-term example of past anthropogenic land use that can inform management and conservation efforts in modern Amazonian ecosystems.
Acknowledgments
Funding for this research was supported by the PAST (Pre-Columbian Amazon-Scale Transformations) European Research Council Consolidator Grant to JI (ERC_Cog 616179). Research was conducted under permit 01506.004836/2014-69 from the Instituto do Patrimônio Histórico e Artístico Nacional (IPHAN) and ICMBio permit 106/14-FNT. We thank all residents of Maguarí and Jamaraquá community for their hospitality and help.
Footnotes
Data Availability The botanical and archaeological source data used to support the findings of this study are published as supplementary items along with this paper. The pollen, charcoal, and geochemical data from LC have been made publically available through Neotoma and the Latin American Pollen Database.
Author contributions: JI, SYM, and DPS designed the research; SYM, JI, DA, MR carried out palaeoecological and archaeological fieldwork; EAO carried out botanical inventories; SYM carried out pollen, charcoal, geochemistry and magnetic susceptibility analyses; DA carried out the analysis of archaeological data; RLB built the age-model chronology; JGS compilation and analysis of archaeological radiocarbon dates; CL carried out analysis of modern vegetation and compiled the list of edible plants; SYM and JI led the writing of the paper with inputs from all other authors.
References
- 1.Clement CR, et al. The domestication of Amazonia before European conquest. Proc R Soc London B Biol Sci. 2015;282 doi: 10.1098/rspb.2015.0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaan DP. Sacred Geographies of Ancient Amazonia: Historical Ecology of Social Complexity. Left Coast Press; Walnut Creek, CA: 2012. [Google Scholar]
- 3.Balee W. Cultural Forests of the Amazon. A Historical Ecology of People and Their Landscapes. University of Alabama Press; 2013. [Google Scholar]
- 4.Roberts P, Hunt C, Arroyo-Kalin M, Evans D, Boivin NL. The deep human prehistory of global tropical forests and its relevance for modern conservation. Nat Plants. 2017:1–9. doi: 10.1038/nplants.2017.93. [DOI] [PubMed] [Google Scholar]
- 5.de Souza JG, et al. Pre-Columbian earth-builders settled along the entire southern rim of the Amazon. Nat Commun. 2018;9:1125. doi: 10.1038/s41467-018-03510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iriarte J, et al. Fire-free land use in pre-1492 Amazonian savannas. Proc Natl Acad Sci. 2012;109:6473–6478. doi: 10.1073/pnas.1201461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watling J, et al. Impact of pre-Columbian ‘geoglyph’ builders on Amazonian forests. Proc Natl Acad Sci U S A. 2017;114:1868–1873. doi: 10.1073/pnas.1614359114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heckenberger MJ, et al. Pre-Columbian urbanism, anthropogenic landscapes, and the future of the Amazon. Science. 2008;321:1214–7. doi: 10.1126/science.1159769. [DOI] [PubMed] [Google Scholar]
- 9.Roosevelt AC. The Amazon and the Anthropocene: 13,000 years of human influence in a tropical rainforest. Anthropocene. 2013;4:69–87. [Google Scholar]
- 10.Erickson CL. In: The Handbook of South American Archaeology. Silverman H, Isbell WH, editors. Springer; 2008. pp. 157–183. [Google Scholar]
- 11.Barlow J, Gardner TA, Lees AC, Parry L, Peres CA. How pristine are tropical forests? An ecological perspective on the pre-Columbian human footprint in Amazonia and implications for contemporary conservation. Biol Conserv. 2012;151:45–49. [Google Scholar]
- 12.McMichael CH, et al. Sparse pre-Columbian human habitation in western Amazonia. Science. 2012;336:1429–1431. doi: 10.1126/science.1219982. [DOI] [PubMed] [Google Scholar]
- 13.Levis C, et al. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science. 2017;355:925–931. doi: 10.1126/science.aal0157. [DOI] [PubMed] [Google Scholar]
- 14.McMichael CH, Feeley KJ, Dick CW, Piperno DR, Bush MB. Comment on ‘Persistent effects of pre-Columbian plant domestication on Amazonian forest composition’. Science. 2017;358 doi: 10.1126/science.aan8347. [DOI] [PubMed] [Google Scholar]
- 15.Junqueira AB, et al. Response to Comment on ‘Persistent effects of pre-Columbian plant domestication on Amazonian forest composition’. Science. 2017;358 doi: 10.1126/science.aan8837. [DOI] [PubMed] [Google Scholar]
- 16.Mayle FE, Iriarte J. Integrated palaeoecology and archaeology – a powerful approach for understanding pre-Columbian Amazonia. J Archaeol Sci. 2012:1–11. at < http://linkinghub.elsevier.com/retrieve/pii/S0305440312003913>. [Google Scholar]
- 17.Carson JF, et al. Environmental impact of geometric earthwork construction in pre-Columbian Amazonia. Proc Natl Acad Sci U S A. 2014;111:1–6. doi: 10.1073/pnas.1321770111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitney BS, Dickau R, Mayle FE, Soto JD, Iriarte J. Pre-Columbian landscape impact and agriculture in the Monumental Mound region of the Llanos de Moxos, lowland Bolivia. Quat Res. 2013;80:207–217. [Google Scholar]
- 19.Maezumi SY, Whitney BS, Mayle FE, de Souza J Gregorio, Iriarte J. Reassessing climate and pre-Columbian drivers of paleofire activity in the Bolivian Amazon. Quat Int. 2017 doi: 10.1016/j.quaint.2017.11.053. [DOI] [Google Scholar]
- 20.Willis KJ, Gillson L, Brncic TM. How ‘virgin’ is virgin rainforest? Science. 2004;304:402–403. doi: 10.1126/science.1093991. [DOI] [PubMed] [Google Scholar]
- 21.Iriarte J. In: Tropical Forest Conservation. Long-Term Processes of Human Evolution, Cultural Adaptations and Consumption Patterns. Sanz N, editor. UNESCO; 2017. pp. 140–161. [Google Scholar]
- 22.ter Steege H, et al. Hyperdominance in the Amazonian tree flora. Science. 2013;342:1243092. doi: 10.1126/science.1243092. [DOI] [PubMed] [Google Scholar]
- 23.Heckenberger M, Neves EG. Amazonian archaeology. Annu Rev Anthropol. 2009;38:251–266. [Google Scholar]
- 24.Woods WI, et al. Amazonian dark earths: Wim Sombroek’s vision. Springer; Dordrecht: 2009. [Google Scholar]
- 25.Junqueira AB, Shepard GH, Clement CR. Secondary Forests on Anthropogenic Soils of the Middle Madeira River: Valuation, Local Knowledge, and Landscape Domestication in Brazilian Amazonia. Econ Bot. 2011;65:85–99. [Google Scholar]
- 26.Lins J, Lima HP, Baccaro FB, Kinupp VF, Shepard Glenn H, Jr, Clement CR. Pre-Columbian floristic legacies in modern homegardens of Central Amazonia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Souza NB, Junqueira AB, Struik PC, Stomph T, Clement CR. The role of fertile anthropogenic soils in the conservation of native and exotic agrobiodiversity in Amazonian homegardens. Agrofor Syst. 2017 doi: 10.1007/s10457-017-0137-y. [DOI] [Google Scholar]
- 28.Wang X, et al. Hydroclimate changes across the Amazon lowlands over the past 45,000 years. Nature. 2017;541:204–207. doi: 10.1038/nature20787. [DOI] [PubMed] [Google Scholar]
- 29.Clement CR. 1492 and the loss of Amazonian crop genetic resources. II. crop biogeography at contact. Econ Bot. 1999;53:203–216. [Google Scholar]
- 30.Hanelt P, Büttner R, Mansfeld R. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops (Except Ornamentals) Springer; 2001. [Google Scholar]
- 31.Denevan W. Cultivated Landscapes of Native Amazonia and the Andes. Oxford University Press; 2001. [Google Scholar]
- 32.Chen Y, et al. Forecasting fire season severity in South America using sea surface temperature anomalies. Science. 2011;334:787–91. doi: 10.1126/science.1209472. [DOI] [PubMed] [Google Scholar]
- 33.Heriarte Md. Descripção do Estado do Maranhão, Pará, Corupá e Rio das Amazonas. Impresa do Filho de Carlos Gerold; Vienna: 1874. [Google Scholar]
- 34.Bozarth S, Price K, Woods W, Neves E, Rebellato R. In: Amazonian Dark Earths: Wim Sombroek’s Vision. Woods WI, et al., editors. Springer; Dordrecht: 2009. pp. 163–190. [Google Scholar]
- 35.Herrera LF, Cavelier I, Rodriguez C, Mora S. The Technical Transformation of an Agricultural System in the Colombian Amazon. World Archaeol. 1992;24:98–113. [Google Scholar]
- 36.Schmidt MJ, Heckenberger MJ. In: Amazonian Dark Earths: Wim Sombroek’s Vision. Woods WI, et al., editors. Springer; Dordrecht: 2009. pp. 136–190. [Google Scholar]
- 37.Hecht SB. Amazonian dark earths: origin, properties, management. 2003. p. 355. [Google Scholar]
- 38.Ford A, Nigh R. Origins of the Maya Forest Garden: Maya Resource Management. J Ethnobiol. 2009;29:213–236. [Google Scholar]
- 39.Levis C, et al. How People Domesticated Amazonian Forests. Frontiers in Ecology and Evolution. 2018;5:171. [Google Scholar]
- 40.Willey GR, Phillips P. Method and Theory in American Archaeology. University of Chicago Press; 1958. [Google Scholar]
- 41.Arroyo-Kalin M. The Amazonian Formative: Crop Domestication and Anthropogenic Soils. Diversity. 2010;2 [Google Scholar]
- 42.Heckenberger MJ. In: Comparative Arawakan Histories: Rethinking Language Family and Culture Area in Amazonia. Hill JD, Santos-Granero F, editors. University of Illinois Press; 2002. pp. 99–122. [Google Scholar]
- 43.Neves EG. El Formativo que nunca terminó: la larga historia de estabilidad en las ocupaciones humanas de la Amazonía central. Boletín Arqueol PUCP. 2007:117–142. [Google Scholar]
- 44.Hermenegildo T, O’Connell TC, Guapindaia VLC, Neves EG. New evidence for subsistence strategies of late pre-colonial societies of the mouth of the Amazon based on carbon and nitrogen isotopic data. Quat Int. 2017 [Google Scholar]
- 45.Roosevelt AC, Housley RA, Da Silveira MI, Maranca S, Johnson R. Eighth millennium pottery from a prehistoric shell midden in the Brazilian Amazon. Science. 1991;254:1621–1624. doi: 10.1126/science.254.5038.1621. [DOI] [PubMed] [Google Scholar]
- 46.Roosevelt AC. San Jacinto I: an historical ecological approach to an archaic site in Colombia. Hispanic Am. Hist. Rev. 2007;87:738–740. [Google Scholar]
- 47.Roosevelt AC. In: Complex Polities in the Ancient Tropical World. Ruskin Arthur, Bacus EA, Lucero LG., editors. American Anthropological Association; 1999. pp. 13–33. [Google Scholar]
- 48.Nimuendajú C. Os Tapajó. Bol. Museu Emílio Goeldi. 1948;10:93–106. [Google Scholar]
- 49.Gomes DMC. Politics and ritual in large villages in Santarém, lower Amazon, Brazil. Cambridge Archaeol J. 2017;27:275–293. [Google Scholar]
- 50.Schaan DP. Discussing centre-periphery relations within the Tapajó domain, Lower Amazon. In: Stenborg P, editor. Beyond Waters: Archaeology and Environmental History of the Amazonian Inland. GOTARC; Gothenurg: 2016. pp. 23–36. [Google Scholar]
- 51.Mendes AC, Truckenbrod W, Nogueira A. Faciological analysis of Alter do Chão formation (Cretaceous, Amazon basin), near the town of Óbidos, Pará, Brazil. Revista Brasileira de Geociencias. 2012;42 [Google Scholar]
- 52.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 53.Veloso HP, Rangel-Filho ALR, Lima JCA. Classificação da vegetação brasileira, adapetada a um sistema universal. IBGE, Departamento de recursos naturais e estudos ambientais; 1991. [Google Scholar]
- 54.Sugita S. Pollen representation of vegetation in Quaternary sediments: theory and method in patchy vegetation. J Ecol. 1994;82:881–897. [Google Scholar]
- 55.Piperno DR. Phytoliths: A comprehensive guide for archaeologists and paleoecologists. Altamira Press; 2005. [Google Scholar]
- 56.Wright HE. A square-rod piston sampler for lake sediments. J Sediment Res. 1967;37:975–976. [Google Scholar]
- 57.Blaauw M, Christen JA, Mauquoy D, van der Plicht J, Bennett KD. Testing the timing of radiocarbon-dated events between proxy archives. The Holocene. 2007;17:283–288. [Google Scholar]
- 58.R Foundation for Statistical Computing. R Core Development Team. R: A language and environment for statistical computing. 2014 [Google Scholar]
- 59.Appleby PG. In: Tracking environmental change using lake sediments. Volume 1: Basin analysis, coring and chronological techniques. Last WM, Smol JP, editors. Kluwer Academic Publishers; 2001. [Google Scholar]
- 60.Flynn WW. The determination of low levels of polonium-210 in environmental materials. Anal Chim Acta. 1968;43:221–227. doi: 10.1016/s0003-2670(00)89210-7. [DOI] [PubMed] [Google Scholar]
- 61.Stuiver M, Polach HA. Discussion reporting of 14C data. Radiocarbon. 1977;19:355–363. [Google Scholar]
- 62.Reimer PJ, et al. IntCal13 and Marine13 radiocarbon age calibration curves 0-50,000 years cal BP. Radiocarbon. 2013;55:1869–1887. [Google Scholar]
- 63.Andrés CJ, Pérez SE. A new robust statistical model for radiocarbon data. Radiocarbon. 2009;51:1047–1059. [Google Scholar]
- 64.Blaauw M, Christen JA. Flexible palaeoclimate age-depth models using an autoregresive gamma process. Bayesian Anal. 2011;6:457–474. [Google Scholar]
- 65.Croudace IW, Rindby A, Rothwell RG. ITRAX: description and evaluation of a new multi-function X-ray core scanner. Spec Publ Soc London. 2006;267:51. [Google Scholar]
- 66.Nowaczyk NR. Tracking environmental change using lake sediments. Springer Netherlands; 2001. pp. 155–170. [Google Scholar]
- 67.Reynolds R, Belnap J, Reheis M, Lamothe P, Luiszer F. Aeolian dust in Colorado Plateau soils: Nutrient inputs and recent change in source. Proc Natl Acad Sci. 2001;98:7123–7127. doi: 10.1073/pnas.121094298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dean WE., Jr Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. J Sediment Res. 1974;44:242–248. [Google Scholar]
- 69.Whitlock C, Larsen C. Tracking environmental change using lake sediments. Springer; 2002. pp. 75–97. [Google Scholar]
- 70.Rodionov SN. A sequential algorithm for testing climate regime shifts. Geophys Res Lett. 2004;31 [Google Scholar]
- 71.Rodionov SN. A sequential method for detecting regime shifts in the mean and variance. Large-Scale Disturbances (Regime Shifts) Recover Aquat Ecosyst Challenges Manag Towar Sustain. 2005:68–72. [Google Scholar]
- 72.Faegri K, Iversen J. Textbook of Pollen Analysis. John Wiley; 1989. [Google Scholar]
- 73.Whitney BS, Rushton EA, Carson JF, Iriarte J, Mayle FE. An improved methodology for the recovery of Zea mays and other large crop pollen, with implications for environmental archaeology in the Neotropics. The Holocene. 2012;22:1087–1096. [Google Scholar]
- 74.Stockmarr J. Tablets with spores used in absolute pollen analysis. Pollen Spores. 1971:615–621. [Google Scholar]
- 75.Holst I, Moreno JE, Piperno DR. Identification of teosinte, maize, and Tripsacum in Mesoamerica by using pollen, starch grains, and phytoliths. Proc Natl Acad Sci U S A. 2007;104:17608–13. doi: 10.1073/pnas.0708736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burn MJ, Mayle FE. Palynological differentiation between genera of the Moraceae family and implications for Amazonian palaeoecology. Rev Palaeobot Palynol. 2008;149:187–201. [Google Scholar]
- 77.Dickau R, et al. Differentiation of neotropical ecosystems by modern soil phytolith assemblages and its implications for palaeoenvironmental and archaeological reconstructions. Rev Palaeobot Palynol. 2013;193:15–37. [Google Scholar]
- 78.Watling J, Iriarte J. Phytoliths from the coastal savannas of French Guiana. Quat Int. 2013;287:162–180. [Google Scholar]
- 79.Iriarte J, et al. Late Holocene Neotropical agricultural landscapes: phytolith and stable carbon isotope analysis of raised fields from French Guianan coastal savannahs. J Archaeol Sci. 2010;37:2984–2994. [Google Scholar]
- 80.Piperno DR. Identifying crop plants with phytoliths (and starch grains) in Central and South America: A review and an update of the evidence. Quat Int. 2009;193:146–159. [Google Scholar]
- 81.Iriarte J. Assessing the feasibility of identifying maize through the analysis of cross-shaped size and three-dimensional morphology of phytoliths in the grasslands of southeastern South America. J Archaeol Sci. 2003;30:1085–1094. [Google Scholar]
- 82.Pearsall DM, Chandler-Ezell K, Chandler-Ezell A. Identifying maize in neotropical sediments and soils using cob phytoliths. J Archaeol Sci. 2003;30:611–627. [Google Scholar]
- 83.Piperno DR, Andres TC, Stothert KE. Phytoliths in Cucurbita and other Neotropical Cucurbitaceae and their occurrence in early archaeological sites from the lowland American tropics. J Archaeol Sci. 2000;27:193–208. [Google Scholar]
- 84.Downey SS, Haas WR, Shennan SJ. European Neolithic societies showed early warning signals of population collapse. Proc Natl Acad Sci. 2016;113:9751–9756. doi: 10.1073/pnas.1602504113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldberg A, Mychajliw AM, Hadly EA. Post-invasion demography of prehistoric humans in South America. Nature. 2016;532:232–235. doi: 10.1038/nature17176. [DOI] [PubMed] [Google Scholar]
- 86.Shennan S, et al. Regional population collapse followed initial agriculture booms in mid-Holocene Europe. Nat Commun. 2013;4 doi: 10.1038/ncomms3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Timpson A, et al. Reconstructing regional population fluctuations in the European Neolithic using radiocarbon dates: a new case-study using an improved method. J Archaeol Sci. 2014;52:549–557. [Google Scholar]
- 88.Zahid HJ, Robinson E, Kelly RL. Agriculture, population growth, and statistical analysis of the radiocarbon record. Proc Natl Acad Sci. 2016;113:931–935. doi: 10.1073/pnas.1517650112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramsey CB, Lee S. Recent and planned developments of the program OxCal. Radiocarbon. 2013;55:720–730. [Google Scholar]
- 90.Alves DT. Ocupação Indígena na Foz do Rio Tapajós (1610 a.a. - 1020 d.c.) Novas Edições Acadêmicas, Saarbrücken. 2014:245. [Google Scholar]
- 91.Quinn ER. Excavating “Tapajó” ceramics at Santarém: Their age and archaeological context. Univ. Illinois Chicago; Chicago: 2004. Unpubl Dr Thesis, [Links] [Google Scholar]
- 92.Roosevelt AC. The development of prehistoric complex societies: Amazonia, a tropical forest. Archeol Pap Am Anthropol Assoc. 1999;9:13–33. [Google Scholar]
- 93.IPHAN. National Register of Archaeological Sites. 2018 [Google Scholar]
- 94.WinklerPrins AMGA, Aldrich SP. Locating Amazonian Dark Earths: Creating an interactive GIS of known locations. J Lat Am Geogr. 2010;9:33–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.