Abstract
The dynamics of l-lactate transport across the blood–brain barrier (BBB) and its cerebral metabolism are still subject to debate. We studied lactate uptake and intracellular metabolism in the mouse brain using hyperpolarized 13C magnetic resonance spectroscopy (MRS). Following the intravenous injection of hyperpolarized [1-13C]lactate, we observed that the distribution of the 13C label between lactate and pyruvate, which has been shown to be representative of their pool size ratio, is different in NMRI and C57BL/6 mice, the latter exhibiting a higher level of cerebral lactate dehydrogenase A (Ldha) expression. On the basis of this observation, and an additional set of experiments showing that the cerebral conversion of [1-13C]lactate to [1-13C]pyruvate increases after exposing the brain to ultrasound irradiation that reversibly opens the BBB, we concluded that lactate transport is rate-limited by the BBB, with a 30% increase in lactate uptake after its disruption. It was also deduced from these results that hyperpolarized 13C MRS can be used to detect a variation in cerebral lactate uptake of <40 nmol in a healthy brain during an in vivo experiment lasting only 75 s, opening new opportunities to study the role of lactate in brain metabolism.
Keywords: Hyperpolarization, magnetic resonance spectroscopy, dynamic nuclear polarization, ultrasound, pyruvate, bicarbonate
Introduction
The role of lactate as a source of energy for the mammalian brain has repeatedly been a subject of debate,1−3 but it is established that the level of utilization of lactate by the brain increases with an increase in the lactate plasma concentration following lactate injection or exercise. While glucose is thought to be preferentially taken up by astrocytes,4 a large number of observations show that lactate is predominantly taken up by neurons and transformed, via lactate dehydrogenase (LDH), into pyruvate for mitochondrial oxidation.5 In mice, it has been demonstrated that lactate is metabolized by the intact brain in an activity-dependent manner.3
l-Lactate can cross the blood–brain barrier (BBB) relatively easily and is taken up by cells in the mammalian brain either via monocarboxylate transporters (MCTs) in the plasma membrane or by nonsaturable diffusion.6 There is an equilibration between blood and brain concentrations,1,7 and at high plasma lactate levels, the transport is dominated by nonfacilitated mechanisms.8 Earlier studies in rats9 and in patients10 had shown that this equilibration is not immediate, leaving unsettled the question of the kinetics of transport of lactate through the BBB. After its uptake into brain cells, lactate rapidly equilibrates with pyruvate through the action of LDH. The conversion is a near-equilibrium reaction governed by the relation [pyruvate]/[lactate] = KLDH[NAD+]/([NADH][H+]), where KLDH is the equilibrium constant for lactate dehydrogenase.
13C magnetic resonance spectroscopy (MRS) has been widely used to investigate the kinetics of substrate utilization in cerebral intermediary metabolism, in particular following the fate of infused [13C]glucose and [13C]lactate. However, this technique is limited by poor sensitivity, which precludes directly probing metabolic transformations taking place within the first minute of injection. With the advent of dissolution dynamic nuclear polarization (DNP),11 hyperpolarized (HP) 13C MRS has become a powerful technique for monitoring fast metabolic conversions in vivo by enhancing the sensitivity of MRS signals by ≤4 orders of magnitude.12 It is assumed that the lactate-to-pyruvate ratio derived from hyperpolarized [13C]pyruvate can be a suitable marker of LDH activity.13−15 Studies using hyperpolarized [13C]pyruvate have also shown that the limited transport of pyruvate across the BBB can be a significant constraint for cerebral metabolic studies based on hyperpolarized 13C MRS.16
Several recent studies have shown that hyperpolarized [13C]lactate can also be used to investigate lactate-to-pyruvate conversion in vivo.17−22 Compared to pyruvate, hyperpolarized lactate has the advantage that a bolus injection does not greatly alter its circulating concentration, because lactate has a physiological concentration substantially higher than that of pyruvate in the blood.23
It has previously been demonstrated that the [13C]lactate signal detected in vivo following the injection of hyperpolarized [13C]pyruvate mostly originates from the rapid 13C label exchange catalyzed by LDH and that the net production of lactate is nearly negligible.24,25 The intensity of the lactate signal is therefore expected to be directly related to the endogenous lactate pool size, and this property can be used to detect tumorous tissue, which is known to have a lactate concentration higher than that of healthy tissue.26 The same holds true for the [13C]pyruvate signal measured following the injection of hyperpolarized [13C]lactate, the intracellular pyruvate pool being labeled by exchange catalyzed by LDH.20 With both hyperpolarized 13C-labeled substrates, the detected pyruvate-to-lactate 13C signal ratio is representative of the local pyruvate-to-lactate concentration ratio because LDH governs the equilibrium between these two metabolites.
The aim of this study was to evaluate the rate-limiting role of the BBB on equilibration between the plasma and brain within the first minute following the intravenous injection of hyperpolarized [1-13C]lactate. We first established that the kinetics of the observed cerebral [1-13C]pyruvate signal can provide a measure of cerebral [1-13C]lactate uptake through its rapid intracellular equilibration catalyzed by LDH, by performing hyperpolarized 13C MRS experiments in two mouse strains, namely, C57BL/6 and NMRI, that exhibit differences in Ldha expression. The rate-limiting role of the BBB in lactate transport was then assessed by disrupting the BBB with ultrasound and microbubbles, following a protocol described previously.27
Results and Discussion
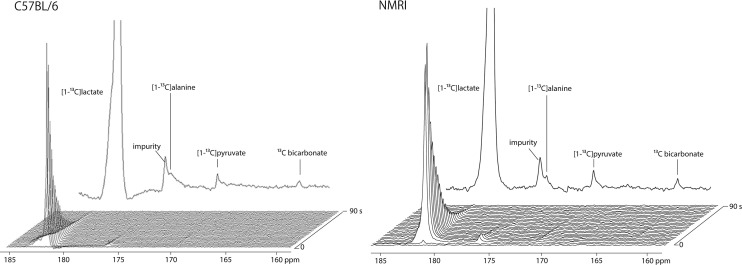
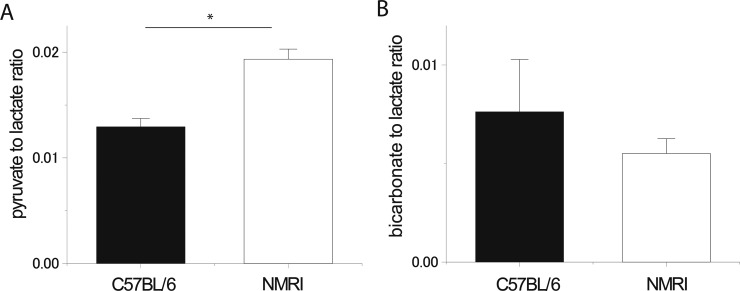
The hyperpolarized 13C magnetic resonance (MR) signals originating from the injected substrate, [1-13C]lactate (183 ppm), and its metabolites, [1-13C]alanine (176 ppm) and [1-13C]pyruvate (171 ppm), were detected in all the experiments (n = 19) (Figure 1). [13C]Bicarbonate (161 ppm) was also observed in nearly all the experiments (18 of 19). The additional peak observed around 176.5 ppm corresponds to an impurity overlapping with the [1-13C]alanine peak and was confirmed by high-resolution 13C nuclear magnetic resonance (NMR) of the purchased solution (data not shown). Compared to that of NMRI mice, the pyruvate-to-lactate ratio was clearly smaller in C57BL/6 mice (p < 0.05), while the bicarbonate-to-lactate ratio was not significantly different between the two strains (Figure 2).
Figure 1.
Representative dynamic 13C MRS spectra measured in a C57BL/6 mouse (left) and a NMRI mouse (right) head following the injection of hyperpolarized [1-13C]lactate. Along with the substrate resonance at 183 ppm, the three expected metabolites were detected: [1-13C]pyruvate at 171 ppm, [13C]bicarbonate at 161 ppm, and [1-13C]alanine at 176 ppm (overlapping with an impurity peak). The delay between each acquisition was set to 3 s, starting 2 s after the beginning of the injection. A sum of spectra 2–26 is shown at the top.
Figure 2.
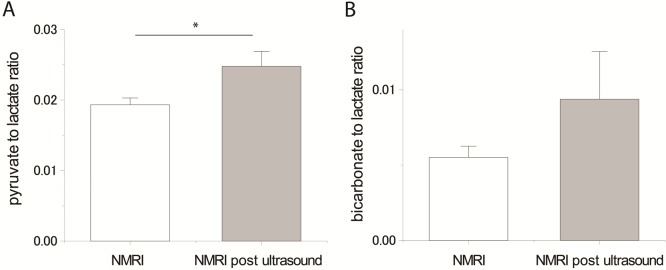
(A) Pyruvate-to-lactate ratio deduced from the hyperpolarized 13C MRS experiments (sum of spectra 2–26) performed in two different mice strains (C57BL/6 and NMRI). The ratio is significantly different for the two groups (p < 0.05). (B) Bicarbonate-to-lactate ratio deduced from the same experiments and for the two groups. The difference was not significant. Error bars represent the mean ± the standard error of the mean (SEM).
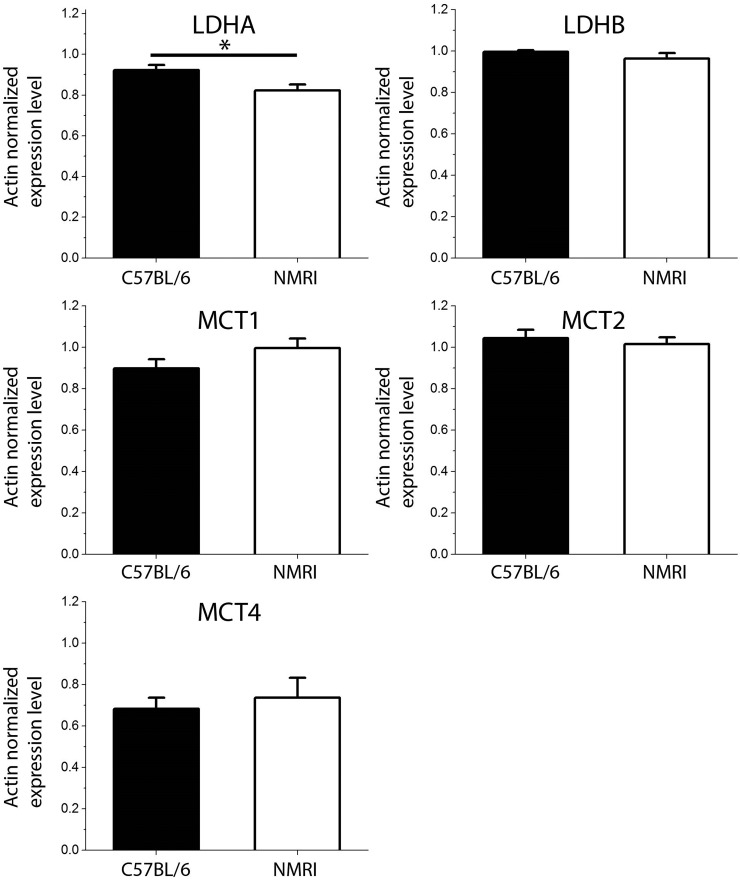
The origin of the higher pyruvate-to-lactate signal ratio observed in NMRI mice as compared to C57BL/6 mice could possibly be a difference in cerebral MCTs between the two strains. The quantitative real-time polymerase chain reaction (PCR) analyses did however not exhibit any significant difference in Mct expression (Figure 3). Because MCT activity was not measured, it is nevertheless not possible to completely rule out the possibility that a difference in cerebral MCTs may have had an impact on the observed pyruvate-to-lactate signal ratios. The results of the quantitative real-time PCR analyses highlighted that the level of Ldha expression in NMRI mice brain was lower than in C57BL/6 mice (p < 0.05), while no significant difference was found in Ldhb expression between the two strains (Figure 3). It was previously proposed that an increased level of expression of Ldha might lead to an increased lactate concentration in the brain.28,29 Although the p value did not reach the level of significance, it was also previously observed in a 1H MRS study that C57BL/6 mice have a lactate brain concentration higher than that of NMRI mice.30
Figure 3.
Quantitative PCR data for C57BL/6 (n = 7) and NMRI (n = 7) mice. Only the Ldha expression level was significantly different between C57BL/6 and NMRI mice brain (p = 0.025).
These observations coupled to the fact that no significant difference in blood lactate concentration was found between C57BL/6 (1.1 ± 0.2 mM) and NMRI (1 ± 0.1 mM) led us to the conclusion that the variation in the pyruvate-to-lactate ratio is most likely not due to a difference in transport kinetics but rather a reflection of the endogenous lactate pool size. Our observation therefore seems to be a confirmation that the pyruvate-to-lactate signal ratio is correlated to the ratio between the two metabolite pool sizes and shows that it is possible to determine variations in lactate pool size using hyperpolarized [1-13C]lactate. Because the detected pyruvate signal originates from cerebral tissue only and we did not observe any difference in lactate blood concentration between the two strains, we could conclude that our observation is related to unequal intracellular concentrations.
To assess the role of the BBB on the kinetics of transport of l-lactate into the brain, we measured the cerebral pyruvate signal pre- and post-ultrasound irradiation. The analysis is based on the assumption that LDH will nearly instantaneously equilibrate the distribution of 13C between the two pools as soon as lactate has entered the brain. This assumption is reasonable given that the transport kinetics across the BBB is at least 1 order of magnitude slower than the apparent rate constant associated with intracellular transport and LDH activity in the rodent brain.16,31 Because the pyruvate-to-lactate ratio determined from our measurements was larger in NMRI mice than in C57BL/6 mice, the study was performed on the former strain. To assess the effect of ultrasound on the BBB, T1-weighted images of the brain were acquired in NMRI mice after administration of either Gd3+ (Figure S1) or Mn2+ (Figure S2). The striking change in contrast observed in the images, particularly in the lateral ventricles that exhibit a hyperintense signal following ultrasound irradiation, confirmed the opening of the BBB after the injection of microbubbles and the application of ultrasound.
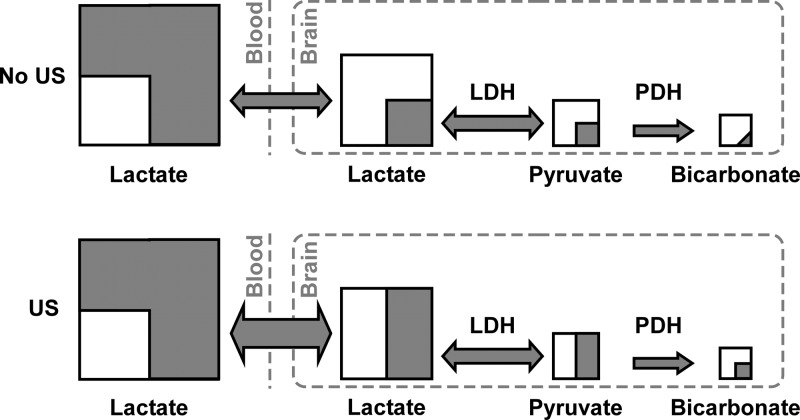
To quantify the increase in the intracellular cerebral lactate concentration after ultrasound application, we propose the following model illustrated in Figure 4. In the absence of ultrasound (US) exposure, the pyruvate-to-lactate 13C signal ratio can be written as follows
| 1 |
where the [1-13C]pyruvate signal (Spyrbrain) originates exclusively from the brain cells. If we assume that the 13C fractional enrichments for both pyruvate and lactate brain pools are equal because of the rapid exchange through LDH
| 2 |
where [pyr] and [lac] are the pyruvate and lactate brain concentrations, respectively. When the brain is exposed to ultrasound irradiation, Slacbrain can be replaced by Slac(1 + δ), where δ is the portion of the 13C signal originating from the increase in brain lactate concentration during the measurement. We obtain
| 3 |
where Slacblood is assumed to be identical in both cases because the decrease in blood lactate concentration due to higher cerebral uptake is negligible and Slac + Slacbrain ≫ δSlac to approximate the denominator from Slacblood + Slac(1 + δ) to Slacblood + Slac. Therefore, the change in 13C signal ratio induced by the application of ultrasound is reduced to
| 4 |
Figure 4.
Schematic representation of the 13C label distribution, across the detected metabolites in the mouse brain exposed to ultrasound (US, bottom) and not exposed to US (top), 75 s after the intravenous injection of [1-13C]lactate. Each square represents a metabolic pool, with the gray part corresponding to the 13C-labeled fraction. The gray arrows represent the 13C label flux between the pools. Note that the relative size of the squares or the gray area within each pool is not scaled to the real pool size ratios or the proportion of the 13C label.
The pyruvate-to-lactate ratio measured in mice exposed to ultrasound irradiation was significantly higher than that in mice that did not experience ultrasound exposure (Figure 5). The 28 ± 3% increase (p < 0.05) post-ultrasound demonstrates that the BBB limits the transport of lactate into the brain. Although the difference in the bicarbonate-to-lactate ratio did not quite reach the level of significance (p = 0.45), most likely because of the low SNR, the observed trend toward increased conversion also points in the direction of an increased lactate transport rate post-ultrasound.
Figure 5.
(A) Pyruvate-to-lactate ratio deduced from the hyperpolarized 13C MRS experiments (sum of spectra 2–26) performed in NMRI mice with or without ultrasound irradiation. The ratio is significantly different for the two groups (p < 0.05). (B) Bicarbonate-to-lactate ratio deduced from the same experiments and for the two groups. The differences were not significant. Error bars represent the mean ± the standard error of the mean.
It has been shown that the intracellular and extracellular hyperpolarized [1-13C]lactate signals can be separated in vitro.32 It was however not possible to achieve the required spectral resolution in the in vivo study presented here. As a consequence, we could not deduce the Michaelis–Menten constants associated with BBB transport. However, because of the rapid exchange catalyzed by LDH, the detection of cerebral pyruvate provides an indirect readout of the transport of lactate into the brain. In fact, the increase in the pyruvate-to-lactate ratio following ultrasound irradiation corresponds to a quantitative measurement of the increased cerebral lactate uptake, δ (see eq 4). We conclude that the application of ultrasound led to an increase of 28 ± 3% of cerebral lactate within the 75 s experiment. It was previously demonstrated that lactate flux through the BBB via nonfacilitated transport is roughly identical to the flux via MCTs in the rat brain across a large lactate plasma concentration range.33 Assuming the uptake via MCTs was not substantially affected by ultrasound, nonfacilitated transport would then account for the nearly 30% increase in lactate uptake after opening the BBB. Note that because the pyruvate and bicarbonate signals originate from the intracellular compartment, their intensity will not be affected by a potential variation in extracellular pH that may result from the opening of the BBB.
Using a Vmax of 300 nmol g–1 min–1 and a KM of 13 mM as the Michaelis–Menten constants for the kinetics of transport of lactate across the BBB,8 we estimate for a 700 mg mouse brain (42 g body weight corresponds to 0.017 × 42 = 0.7 g brain weight for a male34), with 13 mM lactate in the blood, that approximately 150 × 0.7 × 75/60 = 130 nmol of lactate entered the brain during the 75 s experiment. This is consistent with the results obtained in NMRI mice using thermally polarized [3-13C]lactate, where 516 nmol was estimated to be taken up by the brain within 5 min of a bolus injection (with a lactate dose nearly 5-fold larger than that in the study presented here).35 It can therefore be concluded that the method herein presented allows for the detection of a 130 × 0.28 ≅ 36 nmol increase in lactate uptake upon BBB opening by ultrasound, demonstrating the high sensitivity of hyperpolarized 13C MRS in studying cerebral metabolism.36 For future hyperpolarized 13C MR imaging studies aiming at correlating the spatiotemporal evolution of the BBB opening with hyperpolarized 13C MRI, it would be necessary to determine the spatiotemporal delivery of small molecules like [13C]lactate as was done by Choi et al. with Gd-based contrast agents.37
The dynamics of transport of a substrate across the BBB has previously been studied using hyperpolarized 13C MR. Hurd et al. looked at the cerebral metabolism of [1-13C]pyruvate16 and ethyl [1-13C]pyruvate.31 Because the flux through MCTs is on the order of 4 times slower for pyruvate than for lactate,38 and the pyruvate plasma concentration largely exceeded KM, it can be assumed that MCTs were saturated following the injection of hyperpolarized [13C]pyruvate. Consequently, the nonsaturable flux could be considered as the main contribution to transport across the BBB, but the more hydrophobic precursor ethyl [1-13C]pyruvate crossed the BBB much faster and resulted in greater cerebral [1-13C]lactate production. Although the results of this previous study are highly important in the context of hyperpolarized 13C MR because [13C]pyruvate is by far the most widely used substrate and to date the only one used in humans, the authors highlighted that the contribution of extracellular [13C]lactate to the measured brain signal is difficult to estimate. For this reason, injecting hyperpolarized [13C]lactate is an interesting alternative because the signal from its metabolic product pyruvate is purely intracellular. In fact, both pyruvate and lactate concentrations stay near physiological range following the injection of a bolus of hyperpolarized [13C]lactate. The disadvantages of using hyperpolarized [13C]lactate are that the fractional 13C enrichment of brain lactate during the time frame of the experiment is limited and the pool size of pyruvate is small, leading to a sensitivity lower than that in experiments performed with hyperpolarized [13C]pyruvate. It is however worth noting that the bicarbonate signal intensity is not substantially higher in experiments with hyperpolarized [13C]pyruvate with a similar 13C polarization level using the same equipment.39 It can also be mentioned here that the use of sodium lactate instead of lactic acid, which is considerably less stable, greatly simplifies sample preparation and precludes the need for adding sodium hydroxide to reach physiological pH in the hyperpolarized [13C]lactate solution prior to injection. Note finally that alternative hyperpolarized 13C MR approaches based on flow,40 chemical shift,32,41 and diffusion weighting measurements42,43 could be used to study the impact of ultrasound on the dynamics of transport of small molecules across the BBB.
Conclusion
We demonstrated that transport of l-lactate across the BBB can be observed using hyperpolarized [13C]lactate, and we deduced that its transport is limited by the BBB. Our findings show that the equilibration between plasma and brain lactate is not instantaneous and that the BBB can restrict diffusion into the brain by nearly 30% at high plasma lactate concentrations. We also deduced that, although an exact determination of metabolite concentrations is challenging using hyperpolarized 13C, variations of <40 nmol in cerebral lactate uptake can be detected. Our study shows that [1-13C]lactate is a promising molecule for studying cerebral metabolism by hyperpolarized 13C MRS because the injected substrate and its metabolites, namely, pyruvate and bicarbonate, remain and can be detected at near physiological concentrations. Hyperpolarized [13C]lactate could also be potentially considered as a nontoxic alternative to lanthanide-based contrast agents for diagnosing brain tumors through the evaluation of BBB integrity by MRI.
Methods
All experiments were conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines and were approved by the local regulatory body of the Canton Vaud, Switzerland (Service de la consommation et des affaires vétérinaires, Affaires vétérinaires, Canton de Vaud, Switzerland). All animals were purchased from Charles River Laboratories (Châtillon-sur-Chalaronne, France).
Animal Preparation and Injection Protocol
A total of seven C57BL/6J mice (29.1 ± 1.5 g, 10–24 weeks of age, male) and 17 NMRI mice (41.7 ± 1.4 g, 10–24 weeks of age, male) were purchased for the MR experiments. All mice were anesthetized using 1.5–2% isoflurane in air containing 50% oxygen (1 L/min). A 12 cm long homemade catheter was introduced into the femoral vein 60 min before each hyperpolarized 13C MRS experiment. Animal physiology, e.g., body temperature and respiration rate, was monitored and kept stable during the experiments by controlling the temperature of circulating warm water and the amount of isoflurane delivered to the animal. The animals were euthanized in accordance with local guidelines at the end of each hyperpolarized 13C MRS experiment.
Preparation of the Hyperpolarized [1-13C]Lactate Solution
A mixture of a sodium [1-13C]lactate solution [45–55% (w/w) in H2O, 99 atom % 13C] and d8-glycerol [1:1 (w/w)] doped with 50 mM 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) radical was warmed for 15 min in a water bath at 50 °C.17−22 All chemicals were purchased from Sigma-Aldrich (Buchs, Switzerland). A total volume of 0.2 mL of 2 ± 0.5 μL frozen beads was prepared in liquid nitrogen and placed into a polytetrafluoroethylene (PTFE) sample cup described in a previous publication.44 The cup was then loaded into a 5 T home-built DNP polarizer and polarized at 1 ± 0.05 K and 140.18 GHz with a millimeter-wave power output of 50 mW.45 The solid-state 13C polarization buildup was monitored by applying a 5° radiofrequency (rf) pulse every 5 min.
In Vivo Hyperpolarized 13C MRS
All in vivo MR acquisitions were performed with a Direct Drive spectrometer (Agilent, Palo Alto, CA) interfaced to an actively shielded 9.4 T magnet with a 31 cm horizontal bore (Magnex Scientific, Abingdon, U.K.) using a home-built dual-channel surface coil consisting of 12 mm diameter quadrature 1H loops and an 8 mm diameter 13C surface coil. After the animal had been positioned inside the magnet, series of axial, sagittal, and coronal two-dimensional images were acquired using a gradient echo sequence (TR = 50 ms, TE = 3 ms, field of view = 30 × 30 mm, matrix = 128 × 128, flip angle = 30°) from which the volume of interest (VOI) was selected. The static magnetic field was shimmed in a 75 μL (3 mm × 5 mm × 5 mm) voxel to reduce the localized proton line width to 20 Hz using the FAST(EST)MAP protocol.46
After being polarized for 2 h, the frozen beads were rapidly dissolved in 6 mL of superheated D2O and transferred into the separator/infusion pump,44,47 which was located inside the magnet bore, over 2 s. A 13C polarization of 10 ± 2% was measured at the time of injection inside the pump as described in a previous publication.47 The concentration of the infusate, measured afterward in a high-resolution NMR system, was 110 ± 20 mM. Either 200 μL (for seven C57BL/6 mice) or 300 μL (for 12 NMRI mice) of a hyperpolarized [1-13C]lactate solution was injected within 5 s, corresponding to a volume-to-weight ratio of ∼7 μL/g. The blood concentration at the end of the injection was estimated to be 13 ± 3 mM. Starting 5 s after dissolution, 40 single-pulse 13C acquisitions were sequentially recorded every 3 s using 30° adiabatic rf pulses (BIR4) with 1H decoupling during acquisition (WALTZ-16). Localization was achieved by placing the surface coil on top of the mouse head. The adiabatic pulse offset and power were set to ensure a homogeneous 30° excitation of substrate and metabolite resonances within the entire VOI.
After induction of the BBB opening in NMRI mice, hyperpolarized [1-13C]lactate was injected using the same protocol that was used without ultrasound application.
Quantitative Real-Time PCR Analysis and Blood Lactate Measurement
A set of seven C57BL/6J mice (28.4 ± 0.5 g, 10 weeks of age, male) and seven NMRI mice (42.4 ± 1.0 g, 10 weeks of age, male) were subjected to PCR analyses. Quantitative real-time PCR was performed to measure the expression levels of Ldha, Ldhb, Slc16a1 (MCT1), Slc16a7 (MCT2), and Slc16a3 (MCT4) transcripts associated with the metabolism of [1-13C]lactate for both C57BL/6 mice and NMRI mice. The plasma lactate concentration was measured with the lactate oxidase method using a multiassay analyzer (GW7Micro-Stat, Analox Instruments, London, U.K.). For the quantitative PCR measure, mice were rapidly killed with a guillotine and brains extracted; 40 mg of brain cortex was directly mixed in a lysis solution. RNA was extracted using the Maxwell 16 LEV simplyRNA Cells kit (Promega, ref AS1270) following the manufacturer’s instructions. Reverse transcriptions were performed with the High Capacity RNA-to-cDNA Kit (Life Technologies) according to the manufacturer’s instructions. Each reaction was performed in a volume of 20 μL with 400 ng of mRNA. Quantitative determination of the targeted mRNA sequences was performed with the fast real-time PCR Applied Biosystem 7900HT system (Applied Biosystens, Rotkreuz, Switzerland). The following primers (5′–3′) were used: mActinBF031, GCT TCT TTG CAG CTC CTT CGT; mActinbRE94, ATA TCG TCA TCC ATG GCG AAC; mLDHAFo427, TTG TCT CCA GCA AAG ACT ACT GTG T; mLDHARe536, TTT CGC TGG ACC AGG TTG AG; mLDHBFo312, GCA GCA CGG GAG CTT GTT; mLDHBRe389, CAA TCT TAG AGT TGG CTG TCA CAG A; mMCT1Fo1361, AAT GCT GCC CTG TCC TCC TA; mMCT1Re1441, CCC AGT ACG TGT ATT TGT AGT CTC CAT; mMCT2Fo755, CAG CAA CAG CGT GAT AGA GCT T; mMCT2Re830, TGG TTG CAG GTT GAA TGC TAA T; mMCT4Fo320, TCT GCA GAA GCA TTA TCC AGA TCT A; mMCT4Re407, ATG ATG AGG GAA GGC TGG AA. Gene expression data were analyzed using an Excel macro from Frontiers in Genetics (RT-PCR analysis-macro version 1.1). The average quantities were normalized to a normalization factor obtained by calculating the geometric mean of the most stable reference gene.48 For the normalization factor, we tested the β-actin, cyclophilin, hypoxanthine guanine phosphoribosyl transferase, and TATA box binding protein genes and chose β-actin as it was the most stable gene in our study.
Protocol for BBB Opening
The BBB was opened using a previously described method.49 In brief, a circular single-element ultrasound transducer (model A306S-SU, Olympus NDT) with a diameter of 13 mm and a center frequency of 2.25 MHz was used in this study. Using a stereotaxic instrument, the transducer was positioned at its natural focal distance (58 mm) in a column of degassed water (contained by a thin plastic film) placed directly over the mouse brain. The transducer was driven by a 47 dB power amplifier (model RF0510-200, RFPA), which was fed by a periodic pulse sequence from a signal generator (model 33220A, Agilent, Santa Clara, CA). The pulse sequence consisted of bursts of 2.15 MHz sinusoidal pulses with 50000 cycles per burst and a burst period of 64 ms. The pulse amplitude was calibrated to generate negative acoustic pressure peaks of 0.5 MPa at the center of the natural focus of the transducer. The power output of the ultrasonic transducer was calibrated in a water bath using a needle hydrophone (1 mm diameter needle hydrophone probe containing a 28 μm thick gold electroded polyvinylidene fluoride film, Precision Acoustics Ltd.).
Prior to the application of ultrasonic excitation, hair was removed from the mouse scalp using an electric trimmer. Ultrasound gel was placed on the scalp, and the water column was lowered onto the head. A 100 μL bolus of sulfur hexafluoride microbubbles with a phospholipid shell (SonoVue, Bracco Imaging, Milan, Italy) was administered through the femoral vein within 5 s, ∼1 min before ultrasound irradiation. Following microbubble injection, the ultrasound pulse sequence was initiated and maintained for 10 min. Note that to ensure an effective opening of the BBB, we applied a pulse substantially longer (10 min instead of 3 min) than that in the original protocol published by Howles et al.49 We did however not specifically quantify skull attenuation in this study, but it was previously demonstrated that the opening of the BBB in mice can be induced with ultrasound without craniotomy by compensating for the ∼20% attenuation in pressure amplitude.50
The efficacy of the protocol to open the BBB was evaluated via T1-weighted MRI scans using either a gadolinium-based contrast agent, Gadoteridol (ProHance, Bracco Imaging), injected intraperitoneally 20 min before imaging at a concentration of 2 mmol/kg, or a 100 mM MnCl2 solution (Sigma-Aldrich) injected intraperitoneally 30 min before imaging at a concentration of 0.4 mmol/kg.51 We used a three-dimensional spoiled gradient-recalled (SPGR) MRI sequence with the following parameters:49 pulse repetition (TR) = 25 ms, echo time = 6 ms, flip angle = 30°, field of view = 20 × 20 × 16, matrix = 256 × 256 × 32, and number of averages = 4.
Analysis of 13C MRS Data
A nonlinear least-squares quantification algorithm, AMARES, as implemented in the jMRUI software package,52 was used to fit the 13C MRS data. The spectra were corrected for phase and DC offset. Soft constraints were imposed on peak frequencies (171.0–171.2 ppm for pyruvate, 161.0–161.4 ppm for bicarbonate, and 183.2–183.6 ppm for lactate) and line widths (full width at half-maximum = 10–30 Hz), and the relative phases were fixed to zero. The peak areas of [1-13C]lactate, [1-13C]pyruvate, and [13C]bicarbonate were quantified, averaged over 25 acquisitions (sum of spectra 2–26, corresponding to a total acquisition time of 75 s), and used to compute the pyruvate-to-lactate and bicarbonate-to-lactate ratios. Changes in relative metabolite ratios were used as a measure of kinetic rate changes, because it was previously demonstrated that the kinetic rates obtained from fitting the evolution of the signals of hyperpolarized substrates and its metabolic products are directly proportional to the ratios of the summed spectral signals.53 The time range was chosen to include only the spectra with a lactate signal-to-noise ratio (SNR) of >2.
Statistics
Statistical analyses were performed using the OriginPro 9.0G software. p values were computed using an unpaired or paired Student’s t test, where appropriate. For the statistical analysis of multiple groups, one-way analysis of variance was used followed by Tukey’s test. A p value of 0.05 was considered significant. All data are presented as means ± the standard error of the mean unless otherwise stated.
Acknowledgments
The authors thank the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenards and Jeantet Foundations for its support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneuro.8b00066.
Author Contributions
Y.T., M.M., and A.C. designed the study. Y.T., T.C., and J.A.M.B. performed the in vivo experiments. Y.T. and H.A.I.Y. analyzed the data. J.A.M.B. and B.L. made a critical contribution to the analysis of the kinetic data. S.L. performed and analyzed the quantitative PCR measurements. A.C. wrote the manuscript. All authors discussed the results and commented on the manuscript.
This work is part of a project that has received funding from the European Union’s Horizon 2020 European Research Council (ERC Consolidator Grant) under Grant Agreement 682574 (ASSIMILES) and was supported by the Swiss National Science Foundation (Grant PP00P2_133562).
The authors declare the following competing financial interest(s): A.C. is currently employed by General Electric Medical Systems Inc.
Supplementary Material
References
- Boumezbeur F.; Petersen K. F.; Cline G. W.; Mason G. F.; Behar K. L.; Shulman G. I.; Rothman D. L. (2010) The Contribution of Blood Lactate to Brain Energy Metabolism in Humans Measured by Dynamic C-13 Nuclear Magnetic Resonance Spectroscopy. J. Neurosci. 30, 13983–13991. 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G.; Stømstad M.; Rasmussen P.; Jans O.; Zaar M.; Gam C.; Quistorff B.; Secher N. H.; Nielsen H. B. (2009) Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 29, 1121–1129. 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Wyss M. T.; Jolivet R.; Buck A.; Magistretti P. J.; Weber B. (2011) In vivo evidence for lactate as a neuronal energy source. J. Neurosci. 31, 7477–7485. 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby P.; Schmidt E.; Ruminot I.; Gutiérrez R.; Barros L. F.; Deitmer J. W. (2014) Higher Transport and Metabolism of Glucose in Astrocytes Compared with Neurons: A Multiphoton Study of Hippocampal and Cerebellar Tissue Slices. Cereb. Cortex 24, 222–231. 10.1093/cercor/bhs309. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore A. K.; Pellerin L. (2013) Unraveling the complex metabolic nature of astrocytes. Front. Cell. Neurosci. 7, 179. 10.3389/fncel.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen G. M.; Pettigrew K. D.; Patlak C. S.; Paulson O. B. (1994) Blood-Brain-Barrier Permeability Measurements by Double-Indicator Method Using Intravenous-Injection. Am. J. Physiol. 266, H987–H999. 10.1152/ajpheart.1994.266.3.H987. [DOI] [PubMed] [Google Scholar]
- Tofteng F.; Larsen F. S. (2002) Monitoring extracellular concentrations of lactate, glutamate, and glycerol by in vivo microdialysis in the brain during liver transplantation in acute liver failure. Liver Transplantation 8, 302–305. 10.1053/jlts.2002.32283. [DOI] [PubMed] [Google Scholar]
- Knudsen G. (2012) Blood-Brain Barrier Transport of Lactate. In Neural Metabolism in Vivo (Choi I.-Y., and Gruetter R., Eds.) pp 755–761, Springer. [Google Scholar]
- Dager S. R.; Marro K. I.; Richards T. L.; Metzger G. D. (1992) MRS detection of whole brain lactate rise during 1 m sodium lactate infusion in rats. Biol. Psychiatry 32, 913–921. 10.1016/0006-3223(92)90180-8. [DOI] [PubMed] [Google Scholar]
- Dager S. R.; Marro K. I.; Richards T. L.; Metzger G. D. (1992) Localized magnetic resonance spectroscopy measurement of brain lactate during intravenous lactate infusion in healthy volunteers. Life Sci. 51, 973–985. 10.1016/0024-3205(92)90404-D. [DOI] [PubMed] [Google Scholar]
- Ardenkjaer-Larsen J. H.; Fridlund B.; Gram A.; Hansson G.; Hansson L.; Lerche M. H.; Servin R.; Thaning M.; Golman K. (2003) Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 100, 10158–10163. 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comment A.; Merritt M. E. (2014) Hyperpolarized Magnetic Resonance as a Sensitive Detector of Metabolic Function. Biochemistry 53, 7333–7357. 10.1021/bi501225t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. S.; Venkatesh H. S.; Chaumeil M. M.; Brandes A. H.; Vancriekinge M.; Dafni H.; Sukumar S.; Nelson S. J.; Vigneron D. B.; Kurhanewicz J.; James C. D.; Haas-Kogan D. A.; Ronen S. M. (2010) Noninvasive detection of target modulation following phosphatidylinositol 3-kinase inhibition using hyperpolarized 13C magnetic resonance spectroscopy. Cancer Res. 70, 1296–1305. 10.1158/0008-5472.CAN-09-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P.; Grant A.; Tang J.; Vinogradov E.; Wang X.; Lenkinski R.; Sukhatme V. P. (2011) On-target inhibition of tumor fermentative glycolysis as visualized by hyperpolarized pyruvate. Neoplasia 13, 60–71. 10.1593/neo.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P.; Le A.; Vander Jagt D. L.; Tsukamoto T.; Martinez G. V.; Dang C. V.; Gillies R. J. (2013) Evaluation of LDH-A and Glutaminase Inhibition In Vivo by Hyperpolarized 13C-Pyruvate Magnetic Resonance Spectroscopy of Tumors. Cancer Res. 73, 4190–4195. 10.1158/0008-5472.CAN-13-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd R. E.; Yen Y. F.; Tropp J.; Pfefferbaum A.; Spielman D. M.; Mayer D. (2010) Cerebral dynamics and metabolism of hyperpolarized [1-(13)C]pyruvate using time-resolved MR spectroscopic imaging. J. Cereb. Blood Flow Metab. 30, 1734–1741. 10.1038/jcbfm.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen J. M.; Yoshihara H. I.; Takado Y.; Gruetter R.; Comment A. (2014) Hyperpolarized 13C lactate as a substrate for in vivo metabolic studies in skeletal muscle. Metabolomics 10, 986–994. 10.1007/s11306-014-0630-5. [DOI] [Google Scholar]
- Chen A. P.; Kurhanewicz J.; Bok R.; Xu D.; Joun D.; Zhang V.; Nelson S. J.; Hurd R. E.; Vigneron D. B. (2008) Feasibility of using hyperpolarized [1–13C]lactate as a substrate for in vivo metabolic 13C MRSI studies. Magn. Reson. Imaging 26, 721–726. 10.1016/j.mri.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. P.; Lau J. Y. C.; Alvares R. D. A.; Cunningham C. H. (2015) Using [1–13C]lactic acid for hyperpolarized 13C MR cardiac studies. Magn. Reson. Med. 73, 2087–2093. 10.1002/mrm.25354. [DOI] [PubMed] [Google Scholar]
- Kennedy B. W.; Kettunen M. I.; Hu D. E.; Brindle K. M. (2012) Probing lactate dehydrogenase activity in tumors by measuring hydrogen/deuterium exchange in hyperpolarized l-[1-(13)C,U-(2)H]lactate. J. Am. Chem. Soc. 134, 4969–4977. 10.1021/ja300222e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer D.; Yen Y. F.; Josan S.; Park J. M.; Pfefferbaum A.; Hurd R. E.; Spielman D. M. (2012) Application of hyperpolarized [1-(13) C]lactate for the in vivo investigation of cardiac metabolism. NMR Biomed. 25, 1119–1124. 10.1002/nbm.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. M.; Josan S.; Mayer D.; Hurd R. E.; Chung Y.; Bendahan D.; Spielman D. M.; Jue T. (2015) Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J. Exp. Biol. 218, 3308–3318. 10.1242/jeb.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A.; Williamson D. H.; Krebs H. A. (1971) Ketone-body utilization by adult and suckling rat brain in vivo. Biochem. J. 122, 13–18. 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day S. E.; Kettunen M. I.; Gallagher F. A.; Hu D. E.; Lerche M.; Wolber J.; Golman K.; Ardenkjaer-Larsen J. H.; Brindle K. M. (2007) Detecting tumor response to treatment using hyperpolarized (13)C magnetic resonance imaging and spectroscopy. Nat. Med. 13, 1382–1387. 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- Kettunen M. I.; Hu D. E.; Witney T. H.; McLaughlin R.; Gallagher F. A.; Bohndiek S. E.; Day S. E.; Brindle K. M. (2010) Magnetization transfer measurements of exchange between hyperpolarized [1–13C]pyruvate and [1–13C]lactate in a murine lymphoma. Magn. Reson. Med. 63, 872–880. 10.1002/mrm.22276. [DOI] [PubMed] [Google Scholar]
- Kurhanewicz J.; Vigneron D. B.; Brindle K.; Chekmenev E. Y.; Comment A.; Cunningham C. H.; DeBerardinis R. J.; Green G. G.; Leach M. O.; Rajan S. S.; Rizi R. R.; Ross B. D.; Warren W. S.; Malloy C. R. (2011) Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia 13, 81–97. 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meairs S.; Alonso A. (2007) Ultrasound, microbubbles and the blood–brain barrier. Prog. Biophys. Mol. Biol. 93, 354–362. 10.1016/j.pbiomolbio.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Quistorff B.; Grunnet N. (2011) The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging 3, 457–460. 10.18632/aging.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. M.; Oberg J.; Brene S.; Coppotelli G.; Terzioglu M.; Pernold K.; Goiny M.; Sitnikov R.; Kehr J.; Trifunovic A.; Larsson N. G.; Hoffer B. J.; Olson L. (2010) High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc. Natl. Acad. Sci. U. S. A. 107, 20087–20092. 10.1073/pnas.1008189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz A.; Natt O.; Watanabe T.; Boretius S.; Frahm J.; Michaelis T. (2003) Localized proton MRS of cerebral metabolite profiles in different mouse strains. Magn. Reson. Med. 49, 822–827. 10.1002/mrm.10445. [DOI] [PubMed] [Google Scholar]
- Hurd R. E.; Yen Y. F.; Mayer D.; Chen A.; Wilson D.; Kohler S.; Bok R.; Vigneron D.; Kurhanewicz J.; Tropp J.; Spielman D.; Pfefferbaum A. (2010) Metabolic Imaging in the Anesthetized Rat Brain Using Hyperpolarized [1-(13)C] Pyruvate and [1-(13)C] Ethyl Pyruvate. Magn. Reson. Med. 63, 1137–1143. 10.1002/mrm.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukels V.; Jansen K. C.; van Heijster F.; Capozzi A.; van Bentum P. J. M.; Schalken J.; Comment A.; Scheenen T. W. J. (2015) Direct dynamic measurement of intracellular and extracellular lactate in small-volume cell suspensions with 13C hyperpolarised NMR. NMR Biomed. 28, 1040–1048. 10.1002/nbm.3341. [DOI] [PubMed] [Google Scholar]
- LaManna J. C.; Harrington J. F.; Vendel L. M.; Abi-Saleh K.; Lust W. D.; Harik S. I. (1993) Regional blood-brain lactate influx. Brain Res. 614, 164–170. 10.1016/0006-8993(93)91030-V. [DOI] [PubMed] [Google Scholar]
- Fairless A. H.; Dow H. C.; Kreibich A. S.; Torre M.; Kuruvilla M.; Gordon E.; Morton E. A.; Tan J. H.; Berrettini W. H.; Li H. Z.; Abel T.; Brodkin E. S. (2012) Sociability and brain development in BALB/cJ and C57BL/6J mice. Behav. Brain Res. 228, 299–310. 10.1016/j.bbr.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B.; Brathe A. (2000) Cerebral metabolism of lactate in vivo: Evidence for neuronal pyruvate carboxylation. J. Cereb. Blood Flow Metab. 20, 327–336. 10.1097/00004647-200002000-00014. [DOI] [PubMed] [Google Scholar]
- Mishkovsky M.; Comment A. (2017) Hyperpolarized MRS: New tool to study real-time brain function and metabolism. Anal. Biochem. 529, 270–277. 10.1016/j.ab.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Choi J. J.; Pernot M.; Brown T. R.; Small S. A.; Konofagou E. E. (2007) Spatio-temporal analysis of molecular delivery through the blood-brain barrier using focused ultrasound. Phys. Med. Biol. 52, 5509–5530. 10.1088/0031-9155/52/18/004. [DOI] [PubMed] [Google Scholar]
- Rodrigues T. B., Sierra A., Ballesteros P., and Cerdan S. (2012) Pyruvate Transport and Metabolism in the Central Nervous System. In Neural Metabolism in Vivo (Choi I.-Y., and Gruetter R., Eds.) pp 715–753, Springer. [Google Scholar]
- Eichhorn T. R.; Takado Y.; Salameh N.; Capozzi A.; Cheng T.; Hyacinthe J. N.; Mishkovsky M.; Roussel C.; Comment A. (2013) Hyperpolarization without persistent radicals for in vivo real-time metabolic imaging. Proc. Natl. Acad. Sci. U. S. A. 110, 18064–18069. 10.1073/pnas.1314928110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshari K. R.; Sriram R.; Koelsch B. L.; Van Criekinge M.; Wilson D. M.; Kurhanewicz J.; Wang Z. J. (2013) Hyperpolarized 13C-pyruvate magnetic resonance reveals rapid lactate export in metastatic renal cell carcinomas. Cancer Res. 73, 529–538. 10.1158/0008-5472.CAN-12-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram R.; Van Criekinge M.; Hansen A.; Wang Z. J.; Vigneron D. B.; Wilson D. M.; Keshari K. R.; Kurhanewicz J. (2015) Real-time measurement of hyperpolarized lactate production and efflux as a biomarker of tumor aggressiveness in an MR compatible 3D cell culture bioreactor. NMR Biomed. 28, 1141–1149. 10.1002/nbm.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch B. L.; Reed G. D.; Keshari K. R.; Chaumeil M. M.; Bok R.; Ronen S. M.; Vigneron D. B.; Kurhanewicz J.; Larson P. E. Z. (2015) Rapid in vivo apparent diffusion coefficient mapping of hyperpolarized (13) C metabolites. Magn. Reson. Med. 74, 622–633. 10.1002/mrm.25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch B. L.; Sriram R.; Keshari K. R.; Leon Swisher C.; Van Criekinge M.; Sukumar S.; Vigneron D. B.; Wang Z. J.; Larson P. E. Z.; Kurhanewicz J. (2016) Separation of extra- and intracellular metabolites using hyperpolarized (13)C diffusion weighted MR. J. Magn. Reson. 270, 115–123. 10.1016/j.jmr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comment A.; van den Brandt B.; Uffmann K.; Kurdzesau F.; Jannin S.; Konter J. A.; Hautle P.; Wenckebach W. T.; Gruetter R.; van der Klink J. J. (2007) Design and performance of a DNP prepolarizer coupled to a rodent MRI scanner. Concepts Magn. Reson., Part B 31B, 255–269. 10.1002/cmr.b.20099. [DOI] [Google Scholar]
- Jannin S.; Comment A.; Kurdzesau F.; Konter J. A.; Hautle P.; van den Brandt B.; van der Klink J. J. (2008) A 140 GHz prepolarizer for dissolution dynamic nuclear polarization. J. Chem. Phys. 128, 241102. 10.1063/1.2951994. [DOI] [PubMed] [Google Scholar]
- Gruetter R.; Tkac I. (2000) Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 43, 319–323. . [DOI] [PubMed] [Google Scholar]
- Cheng T.; Mishkovsky M.; Bastiaansen J. A. M.; Ouari O.; Hautle P.; Tordo P.; van den Brandt B.; Comment A. (2013) Automated transfer and injection of hyperpolarized molecules with polarization measurement prior to in vivo NMR. NMR Biomed. 26, 1582–1588. 10.1002/nbm.2993. [DOI] [PubMed] [Google Scholar]
- Vandesompele J.; De Preter K.; Pattyn F.; Poppe B.; Van Roy N.; De Paepe A.; Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034.1. 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howles G. P.; Bing K. F.; Qi Y.; Rosenzweig S. J.; Nightingale K. R.; Johnson G. A. (2010) Contrast-Enhanced In Vivo Magnetic Resonance Microscopy of the Mouse Brain Enabled by Noninvasive Opening of the Blood-Brain Barrier With Ultrasound. Magn. Reson. Med. 64, 995–1004. 10.1002/mrm.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konofagou E. E., Choi J. J., and Small S. A. (2006) Optimization of Blood-Brain Barrier Opening in Mice using Focused Ultrasound. In 2006 IEEE Ultrasonics Symposium, pp 540–543, IEEE, New York.
- Howles G. P.; Qi Y.; Johnson G. A. (2010) Ultrasonic disruption of the blood-brain barrier enables in vivo functional mapping of the mouse barrel field cortex with manganese-enhanced MRI. NeuroImage 50, 1464–1471. 10.1016/j.neuroimage.2010.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naressi A.; Couturier C.; Devos J. M.; Janssen M.; Mangeat C.; de Beer R.; Graveron-Demilly D. (2001) Java-based graphical user interface for the MRUI quantitation package. MAGMA 12, 141–152. 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Hill D. K.; Orton M. R.; Mariotti E.; Boult J. K. R.; Panek R.; Jafar M.; Parkes H. G.; Jamin Y.; Miniotis M. F.; Al-Saffar N. M. S.; Beloueche-Babari M.; Robinson S. P.; Leach M. O.; Chung Y.-L.; Eykyn T. R. (2013) Model Free Approach to Kinetic Analysis of Real-Time Hyperpolarized 13C Magnetic Resonance Spectroscopy Data. PLoS One 8, e71996 10.1371/journal.pone.0071996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.