Abstract
The Fragile X-related disorders (FXDs) are members of a large group of human neurological or neurodevelopmental conditions known as the Repeat Expansion Diseases. The mutation responsible for all of these diseases is an expansion in the size of a disease-specific tandem repeat tract. However, the underlying cause of this unusual mutation is unknown. Genome-wide association studies have identified single nucleotide polymorphisms (SNPs) in the vicinity of the FAN1 (MIM 613534) gene that are associated with variations in the age at onset of a number of Repeat Expansion Diseases. FAN1 is a nuclease that has both 5’-3’ exonuclease and 5’ flap endonuclease activities. Here we show in a model for the FXDs that Fan1−/− mice have expansions that, in some tissues including brain, are 2-3 times as extensive as they are in Fan1+/+ mice. However, no effect of the loss of FAN1 was apparent for germ line expansions. Thus, FAN1 plays an important role in protecting against somatic expansions but is either not involved in protecting against intergenerational repeat expansions or is redundant with other related enzymes. However, since loss of FAN1 results in increased expansions in brain and other somatic tissue, FAN1 polymorphisms may be important disease modifiers in those Repeat Expansion Diseases in which somatic expansion contributes to age at onset or disease severity.
Keywords: FMR1-related disorders (FMR1 disorders), FX-associated tremor and ataxia syndrome (FXTAS), FX-associated primary ovarian insufficiency (FXPOI), Fragile X syndrome (FXS), Repeat Expansion, Mismatch repair, 5’ flap endonuclease activity, 5’-3’ exonuclease activity
1. Introduction
The Fragile X related disorders (FXDs) result from expansion of a CGG-repeat tract located at the 5’ end of the transcript of the FMR1 gene. These disorders, which include Fragile X syndrome (MIM# 300624), Fragile X-associated tremor/ataxia syndrome (MIM# 300623) and Fragile X-associated primary ovarian insufficiency (MIM# 311360), belong to a larger group of genetic disorders known as the Repeat Expansion Diseases, that includes Huntington Disease (HD) and many of the Spinocerebellar ataxias (SCAs). The mechanism of expansion in these disorders is not well understood nor are the factors that protect against such expansions.
Genome-wide association studies (GWAS) have identified SNPs in the vicinity of FANCD2 and FANCI Associated Nuclease 1 (FAN1) gene (MIM* 613534) as being associated with variation in the age at onset (AAO) of a number of the CAG-repeat expansion diseases [1-3]. FAN1 is an evolutionarily conserved nuclease whose biological roles are not fully understood. It has a C-terminal virus-type replication repair nuclease (VVR_NUC) domain and, in complex eukaryotes, an N-terminal ubiquitin-binding zinc finger (UBZ) domain. The UBZ domain is important for interaction with components of the Fanconi anemia (FA) pathway, while the VRR_NUC domain contains a catalytic PD-(D/E)XK nuclease motif characteristic of many endonucleases. FAN1 cleaves 5’ flaps endonucleolytically and acts exonucleolytically on both 5’ and 3’ flaps, as well as double-stranded DNA substrates [4]. FAN1 was first identified based on its ability to repair interstrand crosslinks (ICLs) [5]. However, despite its name, FAN1 is not epistatic with the Fanconi anemia (FA) pathway [6-8] and a loss of FAN1 is associated with Karyomegalic Interstitial Nephritis (MIM# 614817) in humans [9] and mice [10-12] rather than FA. In addition to a role in the repair of ICLs, FAN1 is also recruited to stalled replication forks independently of ICLs [8, 13]. FAN1 also interacts with the mismatch repair protein MLH1 [14, 15] and is required for efficient homologous recombination but not for double-strand break resection [15].
Here we show that FAN1 also protects against repeat expansion in a FXD mouse model. Our previous data suggests that expansion occurs via a replication-independent process triggered by oxidative damage [16], that involves the interaction between the base excision repair (BER) machinery [17] and components of the mismatch repair (MMR) machinery [18-20]. Thus, the effects of FAN1 on repeat expansion suggests yet another way that this protein contributes to the maintenance of genome integrity.
2. Materials and methods
2.1. Reagents and services
All reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Primers were from Life Technologies (Grand Island, NY). Capillary electrophoresis of fluorescently labeled PCR products was carried out by the Roy J Carver Biotechnology Center, University of Illinois (Urbana, IL).
2.2. Mouse breeding and maintenance
The generation of the FXD mice was described previously [21]. Fan1 mice were produced from cells generated by the National Institutes of Health (NIH) Knock-Out Mouse Program (KOMP) at University of California, Davis, and generously provided to us by Dr. Rannar Airik of the University of Pittsburgh [10]. All mice were on a C57BL/6 background. Mice were maintained in accordance with the guidelines of the NIDDK Animal Care and Use Committee, who approved this research (ASP-K021-LMCB-15) and consistent with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996).
2.3. DNA isolation
DNA from mouse tails at 3-week-old was extracted for genotyping using KAPA Mouse Genotyping Kit (KAPA Biosystems, Wilmington, MA). DNA was isolated from different organs of 3-month and 6-month old mice using a Maxwell®16 Mouse Tail DNA Purification kit (Promega, Madison, WI) according to the manufacturer’s instructions.
2.4. Genotyping and analysis of repeat number
Fan1 genotyping was carried out as previously described [10]. Fmr1 PM allele genotyping and repeat size analysis was carried out as described previously [20]. The PCR products were resolved by capillary electrophoresis on an ABI Genetic Analyzer [22]. The resultant fsa file was then displayed using a custom R script [23] that is available on request. This allows the repeat number to be determined in the tail DNA taken at weaning from the progeny of Fan1+/+ and Fan1−/− parents and compared to the tail DNA taken at weaning of the parents. The extent of somatic expansion was assessed in two ways. The number of repeats added to the original allele was determined by comparison of the repeat number in each organ, as determined by capillary electrophoresis, to the repeat number present in the allele in heart. The heart shows little/no postnatal expansion and thus reflects the number of repeats present in the original allele. The previously described Somatic Instability Index (SII) was also determined as previously described [24] with minor modifications [17]. Fisher’s exact test and Student’s t test were carried out using the GraphPad QuickCalcs website (http://www.graphpad.com/quickcalcs). Mann-Whitney U tests were carried out using the Vassarstats website (vassarstats.net). The t test and U test were always concordant and so for simplicity sake only the t test p values are reported.
2.5. Fan1 mRNA quantitation
Mouse tissues were homogenized using Precellys lysing kits (Bertin Technologies, Rockville, MD). Total RNA was isolated using Maxwell® 16 LEV simplyRNA tissue Kits (Promega) according to the manufacturer’s instructions. The RNA was quantitated using a Nanodrop Spectrophotometer (Denovix). Reverse transcription of RNA was done using a SuperScript™ VILO™ cDNA synthesis kit (Life Technologies). Real-time PCR was done in triplicate using TaqMan® Fast universal PCR master mix and the Taqman probe-primer pairs Mm00625959_m1 (Life Technologies). For comparison of mRNA expression in different tissue, equal amounts of RNA were used for each determination since there is no good endogenous control for different tissues. Normalization was carried out by comparing the Ct value for the mRNA in different organs to the Ct value obtained from heart.
3. Results
3.1. FAN1 protects against repeat expansion in somatic tissues of the FXD mouse.
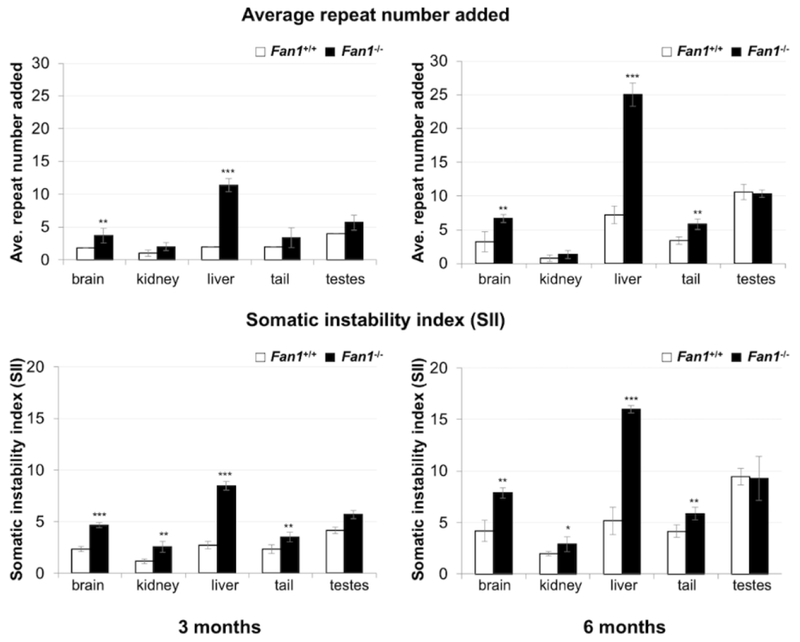
Comparison of the repeat PCR profiles in the tissues of 3-month old and 6-month old male Fan1+/+ and Fan1−/− mice shows a larger increase in the extent of expansion in the livers and brains of the Fan1−/− mice at both ages (Fig. 1). In particular, the repeat PCR profiles from the liver of the 3-month old Fan1−/− mouse already shows a bimodal distribution of repeat sizes that is only beginning to become apparent in the 6-month old Fan1+/+ mouse. A bimodal profile arises in tissues like liver where some cell types expand whilst others do not. Over time, as repeat tract continues to increase in size in the expansion-prone cells, there is a decrease in the extent of overlap between the PCR products from the expansion-prone cell population and those from the cells that do not expand. At 3 months of age expanded alleles in the livers of Fan1−/− mice show the addition of an average of 11.3 repeats compared to just 2 in Fan1+/+ mice (top panel of Fig. 2), while at 6 months of age, the expanded alleles seen in the livers of Fan1+/+ mice are still smaller than the alleles seen in 3-month old Fan1−/− mice. In brain which shows less expansion than liver in wildtype animals, a unimodal distribution is seen for the PCR products in both Fan1+/+ and Fan1−/− mice even at 6 months of age. However, the average repeat number added in Fan1−/− mice was about twice that of Fan1+/+ mice at both 3 and 6 months of age. While these are the only tissues that show a significantly larger increase in the number of repeats added, a more sensitive measure of the extent of expansion, the somatic instability index (SII), showed that expansions were also more extensive in tails and kidneys of Fan1−/− mice than Fan1+/+ mice in both 3 and 6-month old cohorts (bottom panel of Fig. 2). Thus, FAN1 shows a significant protective effect against expansion in a number of somatic tissues in this mouse model.
Fig. 1. The effect of the loss of FAN1 on expansion in the FXD mouse model.
Representative repeat PCR profiles from different organs of Fan1+/+ and Fan1−/− males at 3-months (A) and 6-month of age (B). The number shown beside each profile is the difference in the number of repeats present on expanded alleles relative to the repeat number on the original allele, i.e., the number of repeats added. The dotted line marks the size of the original allele as assessed by the allele size in heart, an organ that shows little, if any, postnatal expansion [22]. The original allele sizes are 150 and 148 for the 3-month old Fan1+/+ and Fan1−/− males and 155 and 153 repeats for the 6-month old Fan1+/+ and Fan1−/− males respectively.
Fig. 2. Quantification of the effect of FAN1 on repeat expansion.
Top panels: The average of the repeats added to expanded alleles in different tissues of 3 Fan1+/+ and 3 Fan1−/− mice at 3 months of age and 5 Fan1+/+ and 3 Fan1−/− male mice at 6 months of age. Bottom panel: Somatic instability index (SII) from the animals shown in the top panels. Both the 3-month old Fan1+/+ mice and the 3-month old Fan1−/− mice had 148-150 repeats. The 6-month old Fan1+/+ mice had 152-159 repeats with an average of 156.2. The 6-month old Fan1−/− mice had repeats of 153-154 repeats with an average of 153.3 repeats. *: p<0.05; **: p<0.01 ***: p<0.001 (t test). Error bars represent the standard deviation.
3.2. Loss of FAN1 does not increase intergenerational repeat expansions.
Three-month old Fan1−/− mice showed a slightly larger increase in the numbers of repeats added in testes relative to age-matched Fan1+/+ mice (Fig. 1). However, this did not reach statistical significance. This trend was also seen when the SII was used, but again this did not reach statistical significance (Fig. 2). At 6 months of age, the testes repeat PCR profiles, the repeat number added and SII in Fan1+/+ and Fan1−/− mice were indistinguishable. Since gametes are the most abundant cells in the testis and are the only cell type to expand [22], any effect on expansion in this cell type should have been apparent.
The proportion of expansions were also similar in the progeny of male Fan1+/+ and age-matched Fan1−/− fathers with similar repeat numbers (Fig. 3). Furthermore, distribution of transmitted allele sizes was similar in Fan1+/+ and Fan1−/− mice. When the repeat size was monitored on maternal transmission of the PM allele, the proportion of the progeny of Fan1+/+ mice with expanded alleles was also not significantly different from the proportion seen in the progeny of Fan1−/− mice, nor was the distribution of transmitted allele sizes (Fig. 3). Thus, the loss of FAN1 does not have a significant effect on intergenerational expansions.
Fig. 3. The effect of the loss of FAN1 on the frequency of intergenerational expansions.
Left panel: the number of alleles bigger than, smaller than and the same size as the parental allele seen on paternal (top) and maternal (bottom) transmission from Fan1+/+ and Fan1−/− parents that were 2-6 months old. At least 5 breeding pairs were used for each condition. For paternal transmission, 31 pups from Fan1+/+ parents and 34 pups from Fan1−/− parents were tested. For maternal transmissions, 37 pups from Fan1+/+ parents and 57 pups from Fan1−/− parents were tested. Right panel: The distribution of allele sizes in the progeny of Fan1+/+ and Fan1−/− male (top) and female (bottom) mice. The p values are from the t test of the distribution of repeat numbers added. While some repeat change classes show large differences between Fan1+/+ and Fan1−/− mice, these are likely statistical anomalies that do not affect the distribution of the repeat changes observed. Error bars represent the 95% confidence interval.
3.3. The protective effect of FAN1 in different organs does not correlate with Fan1 gene expression.
FAN1 protein expression in humans is high in kidney and gonads but relatively low in liver [9]. Since no comparable data is available for mice and a number of commercially available anti-mouse FAN1 antibodies produce no FAN1-specific bands on western blots (data not shown), we quantified the levels of Fan1 mRNA in different mouse tissues using quantitative real-time PCR. As with FAN1 protein levels in humans, Fan1 mRNA was high in mouse kidney and testis, but relatively low in liver (Fig. 4). High levels of Fan1 mRNA were also seen in brain. Therefore, there does not seem to be a good correlation between the tissues that show a significant protective effect of FAN1 and the level of FAN1 expression. This may reflect the contribution of other FAN1-like enzymes that are able to compensate for the loss of FAN1 in some tissues.
Fig. 4. Fan1 mRNA expression in different mouse tissues.
The amount of Fan1 mRNA in different tissues of three 3 month old male mice was evaluated by real-time qPCR as a function of total RNA and the values expressed relative to the levels of Fan1 transcript in heart. Error bars represent the standard deviation.
4. Discussion
FAN1 protects the genome against expansion of the CGG-repeat tract in the Fmr1 gene of our FXD model as evidenced by an increase in the extent of expansion that was seen in Fan1−/− mice (Fig. 1 and 2). However, while this effect was seen in a number of somatic cells tested including those of the brain and liver, Fan1−/− mice did not show significantly more extensive expansions in testis, an organ that is primarily comprised of germ line cells (Fig. 1 and 2). Furthermore, no effect of the loss of FAN1 was seen on intergenerational transmission from either males or females (Fig. 3). Thus, our data suggest that FAN1 protects against somatic but either does not protect against intergenerational expansions or is redundant with other enzymes that may act in a similar way.
FAN1 expression did not correlate well with those tissues that show the greatest protective effect. For example, high levels of expression were seen in testis, an organ that does not show a significant effect of the loss of FAN1 on repeat expansion (Fig. 4). In contrast, low levels of FAN1 expression were seen in liver, despite the significant protective effect of FAN1 that was seen. This suggests that it is not the absolute level of the protein that determines whether its loss is felt in a given tissue. Rather, its protective effect may depend on the relative levels of the proteins that promote expansions or the expression of one of the many other 5’-3’ exonucleases/5’ Flap endonucleases that may have a similar protective effect. For example, we have shown that EXO1 (MIM* 606063), another enzyme with 5’-3’ exonuclease and 5’ flap nuclease activity, also protects against expansion and does so primarily in the germ line (Zhao and Usdin, manuscript in revision). EXO1 is also highly expressed in testis [25] and it may be that the EXO1 activity in the testis of Fan1−/− mice is able to compensate for the loss of FAN1.
EXO1 plays an important role in MMR by excising the strand nicked by the MMR protein, MutLα, and it has been suggested that FAN1 may contribute to some of the EXO1-independent MMR that is seen in mammalian cells [26]. Since FAN1, like EXO1 is part of the MutLα interactome [14] and EXO1 can also participate in ICL removal [27], the two nucleases may act similarly to prevent expansions. One current model for repeat expansion involves strand-slippage and strand-displacement during repair synthesis by Polβ (MIM* 17460) [17, 28]. The loop-outs formed by strand-slippage in turn recruit MutSβ [20, 29-31] and sometimes MutSα [20, 32]. FAN1 may act like EXO1 to repair the repeat tract in an error-free way via a MMR-like process. Since both EXO1 and FAN1 also cleave 5’ flaps, they may both also protect against repeat expansion via the removal of the 5’ flap generated by strand-displacement in a manner analogous to the effect of the yeast FEN1 homolog, rad27p in yeast models of repeat expansion [33, 34]. Since FAN1’s flap cleavage activity is higher than its exonuclease activity, at least in vitro [15], this may be the major way that FAN1 protects against repeat expansion. While no effect of the loss of FEN1 (MIM* 600393) was seen in early embryos of a mouse model of a CTG-expansion disorder, myotonic dystrophy type 1 (MIM# 160900) [35], it may be that FEN1 is redundant with EXO1 and/or FAN1.
Suppression of somatic expansion delays the onset of pathophysiology in a mouse model of HD [36], suggesting that in some Repeat Expansion Diseases modulation of the extent of somatic expansion could also affect the AAO. Since the brain is an organ in which FAN1’s protective effect is seen in mice, FAN1 polymorphisms could be clinically relevant in those Repeat Expansion Diseases in which somatic expansion is an important contributor to AAO and disease severity.
Highlights.
FAN1 protects against repeat expansions in a mouse model of the FXDs
Protection is seen in somatic tissues, including brain, but is not seen for intergenerational expansions
As GWAS suggest, FAN1 mutations may affect the age at onset of some Repeat Expansion Diseases.
Acknowledgements
The work described in this manuscript was funded by a grant from the Intramural Program of the NIDDK to KU (DK057808-07).
Grant Sponsor: Intramural program of the NIDDK, NIH (DK057808)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- [1].Bettencourt C, Hensman-Moss D, Flower M, Wiethoff S, Brice A, Goizet C, Stevanin G, Koutsis G, Karadima G, Panas M, Yescas-Gomez P, Garcia-Velazquez LE, Alonso-Vilatela ME, Lima M, Raposo M, Traynor B, Sweeney M, Wood N, Giunti P, Network S, Durr A, Holmans P, Houlden H, Tabrizi SJ, Jones L, DNA repair pathways underlie a common genetic mechanism modulating onset in polyglutamine diseases, Ann. Neurol, 79 (2016) 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keum JW, Shin A, Gillis T, Mysore JS, Abu Elneel K, Lucente D, Hadzi T, Holmans P, Jones L, Orth M, Kwak S, MacDonald ME, Gusella JF, Lee JM, The HTT CAG-Expansion Mutation Determines Age at Death but Not Disease Duration in Huntington Disease, Am. J. Hum. Genet, 98 (2016) 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Genetic Modifers of Huntington’s Disease Consortium, Identification of Genetic Factors that Modify Clinical Onset of Huntington’s Disease, Cell, 162 (2015) 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhao Q, Xue X, Longerich S, Sung P, Xiong Y, Structural insights into 5’ flap DNA unwinding and incision by the human FAN1 dimer, Nat Commun, 5 (2014) 5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smogorzewska A, Desetty R, Saito TT, Schlabach M, Lach FP, Sowa ME, Clark AB, Kunkel TA, Harper JW, Colaiacovo MP, Elledge SJ, A genetic screen identifies FAN1, a Fanconi anemia-associated nuclease necessary for DNA interstrand crosslink repair, Mol. Cell, 39 (2010) 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yoshikiyo K, Kratz K, Hirota K, Nishihara K, Takata M, Kurumizaka H, Horimoto S, Takeda S, Jiricny J, KIAA1018/FAN1 nuclease protects cells against genomic instability induced by interstrand cross-linking agents, Proc. Natl. Acad. Sci. U. S. A, 107 (2010) 21553–21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Trujillo JP, Mina LB, Pujol R, Bogliolo M, Andrieux J, Holder M, Schuster B, Schindler D, Surralles J, On the role of FAN1 in Fanconi anemia, Blood, 120 (2012) 86–89. [DOI] [PubMed] [Google Scholar]
- [8].Lachaud C, Moreno A, Marchesi F, Toth R, Blow JJ, Rouse J, Ubiquitinated Fancd2 recruits Fan1 to stalled replication forks to prevent genome instability, Science, 351 (2016) 846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou W, Otto EA, Cluckey A, Airik R, Hurd TW, Chaki M, Diaz K, Lach FP, Bennett GR, Gee HY, Ghosh AK, Natarajan S, Thongthip S, Veturi U, Allen SJ, Janssen S, Ramaswami G, Dixon J, Burkhalter F, Spoendlin M, Moch H, Mihatsch MJ, Verine J, Reade R, Soliman H, Godin M, Kiss D, Monga G, Mazzucco G, Amann K, Artunc F, Newland RC, Wiech T, Zschiedrich S, Huber TB, Friedl A, Slaats GG, Joles JA, Goldschmeding R, Washburn J, Giles RH, Levy S, Smogorzewska A, Hildebrandt F, FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair, Nat. Genet, 44 (2012) 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Airik R, Schueler M, Airik M, Cho J, Porath JD, Mukherjee E, Sims-Lucas S, Hildebrandt F, A FANCD2/FANCI-Associated Nuclease 1-Knockout Model Develops Karyomegalic Interstitial Nephritis, J. Am. Soc. Nephrol, 27 (2016) 3552–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thongthip S, Bellani M, Gregg SQ, Sridhar S, Conti BA, Chen Y, Seidman MM, Smogorzewska A, Fan1 deficiency results in DNA interstrand cross-link repair defects, enhanced tissue karyomegaly, and organ dysfunction, Genes Dev., 30 (2016) 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lachaud C, Slean M, Marchesi F, Lock C, Odell E, Castor D, Toth R, Rouse J, Karyomegalic interstitial nephritis and DNA damage-induced polyploidy in Fan1 nuclease-defective knock-in mice, Genes Dev., 30 (2016) 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Porro A, Berti M, Pizzolato J, Bologna S, Kaden S, Saxer A, Ma Y, Nagasawa K, Sartori AA, Jiricny J, FAN1 interaction with ubiquitylated PCNA alleviates replication stress and preserves genomic integrity independently of BRCA2, Nat Commun, 8 (2017) 1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cannavo E, Gerrits B, Marra G, Schlapbach R, Jiricny J, Characterization of the interactome of the human MutL homologues MLH1, PMS1, and PMS2, J. Biol. Chem, 282 (2007) 2976–2986. [DOI] [PubMed] [Google Scholar]
- [15].MacKay C, Declais AC, Lundin C, Agostinho A, Deans AJ, MacArtney TJ, Hofmann K, Gartner A, West SC, Helleday T, Lilley DM, Rouse J, Identification of KIAA1018/FAN1, a DNA repair nuclease recruited to DNA damage by monoubiquitinated FANCD2, Cell, 142 (2010) 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Entezam A, Lokanga RA, Le W, Hoffman G, Usdin K, Potassium bromate, a potent DNA oxidizing agent, exacerbates germline repeat expansion in a fragile X premutation mouse model, Hum. Mutat, 31 (2010) 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lokanga RA, Senejani AG, Sweasy JB, Usdin K, Heterozygosity for a hypomorphic polbeta mutation reduces the expansion frequency in a mouse model of the fragile x-related disorders, PLoS Genet, 11 (2015) e1005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lokanga RA, Zhao XN, Usdin K, The mismatch repair protein MSH2 is rate limiting for repeat expansion in a fragile X premutation mouse model, Hum. Mutat, 35 (2014) 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhao XN, Kumari D, Gupta S, Wu D, Evanitsky M, Yang W, Usdin K, Mutsbeta generates both expansions and contractions in a mouse model of the Fragile X-associated disorders, Hum. Mol. Genet, 24 (2015) 7087–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhao XN, Lokanga R, Allette K, Gazy I, Wu D, Usdin K, A MutSbeta-Dependent Contribution of MutSalpha to Repeat Expansions in Fragile X Premutation Mice?, PLoS Genet, 12 (2016) e1006190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K, Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model, Gene, 395 (2007) 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lokanga RA, Entezam A, Kumari D, Yudkin D, Qin M, Smith CB, Usdin K, Somatic expansion in mouse and human carriers of fragile X premutation alleles, Hum. Mutat, 34 (2013) 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayward BE, Zhou Y, Kumari D, Usdin K, A Set of Assays for the Comprehensive Analysis of FMR1 Alleles in the Fragile X-Related Disorders, J. Mol. Diagn, 18 (2016) 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Møllersen L, Rowe AD, Larsen E, Rognes T, Klungland A, Continuous and periodic expansion of CAG repeats in Huntington’s disease R6/1 mice, PLoS Genet, 6 (2010) e1001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee BI, Shannon M, Stubbs L, Wilson DM 3rd, Expression specificity of the mouse exonuclease 1 (mExo1) gene, Nucleic Acids Res., 27 (1999) 4114–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Desai A, Gerson S, Exo1 independent DNA mismatch repair involves multiple compensatory nucleases, DNA Repair (Amst), 21 (2014) 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kato N, Kawasoe Y, Williams H, Coates E, Roy U, Shi Y, Beese LS, Scharer OD, Yan H, Gottesman ME, Takahashi TS, Gautier J, Sensing and Processing of DNA Interstrand Crosslinks by the Mismatch Repair Pathway, Cell Rep, 21 (2017) 1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McMurray CT, Mechanisms of trinucleotide repeat instability during human development, Nat Rev Genet, 11 (2010) 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guo J, Gu L, Leffak M, Li GM, MutSbeta promotes trinucleotide repeat expansion by recruiting DNA polymerase beta to nascent (CAG) or (CTG) hairpins for error-prone DNA synthesis, Cell Res., (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lai Y, Budworth H, Beaver JM, Chan NL, Zhang Z, McMurray CT, Liu Y, Crosstalk between MSH2-MSH3 and polbeta promotes trinucleotide repeat expansion during base excision repair, Nat Commun, 7 (2016) 12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Y, Prasad R, Beard WA, Hou EW, Horton JK, McMurray CT, Wilson SH, Coordination between polymerase beta and FEN1 can modulate CAG repeat expansion, J. Biol. Chem, 284 (2009) 28352–28366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Du J, Campau E, Soragni E, Ku S, Puckett JW, Dervan PB, Gottesfeld JM, Role of mismatch repair enzymes in GAA.TTC triplet-repeat expansion in Friedreich ataxia induced pluripotent stem cells, J. Biol. Chem, 287 (2012) 29861–29872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang J, Freudenreich CH, Haploinsufficiency of yeast FEN1 causes instability of expanded CAG/CTG tracts in a length-dependent manner, Gene, 393 (2007) 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Freudenreich CH, Kantrow SM, Zakian VA, Expansion and length-dependent fragility of CTG repeats in yeast, Science, 279 (1998) 853–856. [DOI] [PubMed] [Google Scholar]
- [35].van den Broek WJ, Nelen MR, van der Heijden GW, Wansink DG, Wieringa B, Fen1 does not control somatic hypermutability of the (CTG)(n)*(CAG)(n) repeat in a knock-in mouse model for DM1, FEBS Lett, 580 (2006) 5208–5214. [DOI] [PubMed] [Google Scholar]
- [36].Budworth H, Harris FR, Williams P, Lee D-Y, Holt A, Pahnke J, Szczesny B, Acevedo-Torres K, Ayala-Pena S, McMurray CT, Suppression of somatic expansion delays the onset of pathophysiology in a mouse model of Huntington’s disease, PLoS Genet, In Press (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]