Abstract
Hyperoxia-induced oxidative stress contributes to the pathogenesis of bronchopulmonary dysplasia (BPD), the most common respiratory morbidity of preterm infants. Importantly, the disease lack specific therapies and is associated with long-term cardiopulmonary and neurodevelopmental morbidities, signifying the need to discover novel therapies and decrease the disease burden. We and others have demonstrated that leflunomide, a food and drug administration approved drug to treat humans with rheumatoid arthritis, increases the expression of the anti-oxidant enzymes, NAD(P)H quinone dehydrogenase 1 (NQO1), catalase, and superoxide dismutase (SOD). However, whether this drug can decrease oxidative stress in fetal human pulmonary arterial endothelial cells (HPAECs) is unknown. Therefore, we tested the hypothesis that leflunomide will decrease hyperoxia-induced oxidative stress by upregulating these anti-oxidant enzymes in HPAECs. Leflunomide decreased hydrogen peroxide (H2O2) levels and increased the mRNA and protein levels of catalase, NQO1, and SOD2 in HPAECs at basal conditions. Further, leflunomide-treated cells continued to have decreased H2O2 and increased SOD2 levels upon hyperoxia exposure. Leflunomide did not affect the expression of other anti-oxidant enzymes, including hemoxygenase-1 and SOD1. AhR-knockdown experiments suggested that leflunomide regulated NQO1 levels via AhR-dependent mechanisms and H2O2, catalase, and SOD2 levels via AhR-independent mechanisms. Collectively, the results support the hypothesis that leflunomide decreases oxidative stress in HPAECs via SOD2- and catalase-dependent, but AhR- and NQO1-independent mechanisms. Our findings indicate that leflunomide is a potential drug for the management of BPD in preterm infants.
Keywords: Bronchopulmonary dysplasia, Hyperoxia, Leflunomide, Catalase, NAD(P)H quinone dehydrogenase 1, Superoxide dismutase 2
Introduction
Bronchopulmonary dysplasia (BPD) is the most common respiratory morbidity of preterm infants that is characterized by interrupted lung development [1, 2]. Hyperoxia-mediated increase in lung oxidative stress contributes to the pathogenesis of BPD and its sequelae [3]. Infants with BPD have long-term cardiopulmonary and neurodevelopmental morbidities causing a significant impact on the economy and quality of life [1, 4, 5]. Importantly, the disease lacks specific therapies. Hence, there is an urgent need for improved therapies to prevent and treat BPD.
Oxidative stress, an imbalance favoring a pro-oxidant over an anti-oxidant state, is one of the major risk factors implicated in the development of BPD. Supplemental oxygen is frequently used as a life-saving therapy in preterm infants with hypoxic respiratory failure; however, excessive or prolonged oxygen exposure results in oxidative stress characterized by increased reactive oxygen species (ROS) generation. Preterm infants are at high risk of developing oxidative stress due to their immature antioxidant defenses, increased susceptibility to infection and inflammation, and exposure to free iron [6]. Oxidative stress leads to interrupted lung development by mechanisms entailing disruption of growth factor signaling, extracellular matrix assembly, cell proliferation, apoptosis, and vasculogenesis [7].
Leflunomide or N- (4-trifluoromethylphenyl) – 5 – methylisoxazol - 4 – carboxamide) is an US Food and Drug Administration approved immunomodulatory drug that is used to treat rheumatoid arthritis in humans [8]. Several studies, including ours indicate that leflunomide increases the expression of anti-oxidant enzymes such as NAD(P)H quinone dehydrogenase 1 (NQO1), catalase, and superoxide dismutase (SOD) [9–12]. These observations suggest that leflunomide treatment may compensate for the immature antioxidant defenses of preterm infants and increase their resilience against hyperoxia-mediated lung injury. Thus, the goal of this study was to investigate if leflunomide modulates hyperoxia-induced oxidative stress in fetal human pulmonary arterial endothelial cells (HPAECs). We chose HPAECs for our study because lung angiogenesis actively contributes to lung development in infants and oxidative-stress mediated endothelial cell dysfunction is a major risk factor for the development of BPD [13, 14]. Using these cells, we tested the hypothesis that leflunomide decreases oxidative stress by upregulating the anti-oxidant enzymes, catalase, NQO1, and SOD2 in HPAECs.
Materials and Methods
Cell culture, treatment, and hyperoxia exposure
The fetal human pulmonary artery endothelial cells (HPAECs) were obtained from ScienCell research laboratories (San Diego, CA; 3100) and grown according to the manufacturer’s protocol. Cells were treated with either 0.01% v/v dimethyl sulfoxide (DMSO) (Sigma Aldrich, St. Louis, MO; 276855) or up to 10 μM leflunomide (Sigma Aldrich; L5025). Hyperoxia experiments were conducted in a plexiglass sealed chamber into which a mixture of 95% O2 and 5% CO2 was circulated continuously [15].
Cell viability assay
Cell viability was determined by a colorimetric assay based on the ability of viable cells to reduce the tetrazolium salt, MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide), to formazan [16].
Measurement of hydrogen peroxide generation
Hydrogen peroxide (H2O2) levels were quantified by the ROS-Glo™ H2O2 Assay (Promega, Madison, WI; G8820). Briefly, cells grown to 60–70% confluence on 96-well plates were treated with DMSO or leflunomide, and exposed to normoxia (21% O2 and 5% CO2) or hyperoxia (95% O2 and 5% CO2) for up to 24 h, following which the H2O2 levels were determined [17].
Real-time RT-PCR assays
Total mRNA was isolated and reverse transcribed to cDNA [16]. Real-time RT-PCR analysis was performed using iTaq Universal SYBR Green Supermix (Biorad, Hercules, CA; 1725121). The sequences of the primer pairs were aryl hydrocarbon receptor (AhR): 5'-CACCGATGGGAAATGATACTATCC-3' and 5'-GGTGACCTCCAGCAAATGAGTT -3'; catalase: 5'-TGT GCA TGC TAA AGG AGC AG -3' and 5'-CGA TGG GAG TCT TCT TTC CA -3'; NQO1: 5'-ACGCCC-GAATTCAAATCCT-3’ and 5'-CCTGCCTGGAAGTTTAGGTCAA-3'; heme oxygenase-1 (HO-1): 5'-AGGCCAAGACTGCGTTCC-3’ and 5'-GCAGAATCTTGCACTTTGTTGCT-3’; superoxide dismutase (SOD) 1: 5'-TGG CCG ATG TGT CTA TTG AA -3' and 5'-ACC TTT GCC CAA GTC ATC TG -3'; SOD2: 5'-TTC AAT AAG GAA CGG GGA CA -3' and 5'-ACA CAT CAA TCC CCA GCA GT -3'; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH): 5'-TGG AAG GAC TCA TGA CCA CA -3' and 5'-GAG GCA GGG ATG ATG TTC TG -3'.
Western blotting
Whole-cell protein extracts obtained by using radio immunoprecipitation assay lysis buffer system (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-24948) were subjected to western blotting using antibodies against: β-actin (Santa Cruz Biotechnologies; sc-47778, dilution 1:4000), catalase (Santa Cruz Biotechnologies; sc-271803, dilution 1:500), HO-1 (Enzo Life Sciences, Farmingdale, NY; ADI-SPA-896F, dilution 1:1000), NQO1 (Santa Cruz Biotechnologies; sc-16464, dilution 1:500), SOD1 (Santa Cruz Biotechnologies; sc-8637, dilution 1:300), and SOD2 (Santa Cruz Biotechnologies; sc-137254, dilution 1:500).
Small interfering RNA (siRNA) transfections
HPAECs were transfected with either 50 nM control siRNA (Dharmacon, Lafayette, CO; d-001810) or AhR specific siRNA (Dharmacon; L-004990) using LipofectamineRNAiMAX (Life Technologies, Grand Island, NY; 13778030). siRNA mediated knockdown of AhR was validated by determining AhR mRNA expression by real time RT-PCR analysis. Following transfection, whole-cell protein extracts of cells treated with leflunomide or DMSO were obtained to quantify catalase, NQO1, and SOD2 protein expression. Additionally, the transfected cells were treated with leflunomide and DMSO and exposed to normoxia or hyperoxia for 24 h, after which cells were harvested to quantify the H2O2 levels.
Analyses of data
The results were analyzed by GraphPad Prism 5 software. The data are expressed as mean ± SD. One-way ANOVA was used to determine the effect of leflunomide on cell viability, H2O2 levels and expression of anti-oxidant enzymes, while two-way ANOVA was used to determine the effects of leflunomide and AhR gene, and their associated interactions for the outcome variables, H2O2 levels and expression of anti-oxidant enzymes. Post hoc multiple t-tests with Bonferroni corrections were performed if statistical significance of either variable or interaction was noted by ANOVA. A p value of <0.05 was considered significant.
Results and Discussion
In this study, we investigated the effects and mechanisms by which leflunomide modulates oxidative stress in HPAECs. In wild-type HPAECs exposed to normoxia or hyperoxia in vitro, leflunomide increased catalase, NQO1, and SOD2 expression and decreased H2O2 levels compared with controls. siRNA-mediated knockdown of the AhR gene abrogated the effects of leflunomide on NQO1, but not on catalase, SOD2 or H2O2, implying that leflunomide increases NQO1 expression via AhR-dependent mechanisms and decreases H2O2 generation and upregulates catalase and SOD2 expression via AhR-independent mechanisms.
Hyperoxia-induced ROS generation can injure and disrupt the reparative processes in the developing lungs and contribute to BPD pathogenesis [18]. Therefore, we investigated whether leflunomide modulates the level of a reliably measured ROS such as H2O2 using HPAECs. Leflunomide in concentrations greater than 30 μM causes mitochondrial dysfunction and cytotoxicity [19, 20]. Further, drug-induced toxicity can induce oxidative stress and affect the expression of several anti-oxidant enzymes. Hence, we used lower leflunomide concentrations and tested for its cytotoxicity by MTT assay. Leflunomide in concentrations up to 10 μM did not affect HPAEC viability (Fig. 1A), indicating that concentrations up to 10 μM can be used to investigate its effects on the levels of H2O2 and anti-oxidant enzymes. To determine whether leflunomide modulates oxidative stress, H2O2 levels were measured by ROS-Glo™ H2O2 assay. Leflunomide at concentrations of 5 or 10 μM decreased H2O2 levels at basal conditions (Fig. 1B). To determine the mechanims associated with this effect, we quantified the levels of the antioxidant enzymes, including catalase, HO-1, NQO1, SOD1 and SOD2. While leflunomide increased the mRNA and protein levels of catalase (Figs. 2A, F, and G), NQO1 (Figs. 2C, F, and I), and SOD2 (Figs. 2E, F, and K), it did not affect the expression of HO-1 (Figs. 2B, F, and H) and SOD1 (Figs. 2D, F, and J). Having seen that leflunomide modulates H2O2 levels and anti- oxidant enzymes at basal conditions, our logical next step was to elucidate whether it exerts similar effects in hyperoxic conditions. Hyperoxia exposure increased H2O2 levels in HPAECs; however, leflunomide attenuated the effects of hyperoxia on H2O2 generation (Fig. 3A). Consistently we observed that leflunomide increased the mRNA and protein levels of catalase (Figs. 3B, G, and H), NQO1 (Figs. 3D, G, and J), and SOD2 (Figs. 3F, G, and L), without affecting the expression of HO-1 (Figs. 3C, G, and I) and SOD1 (Figs. 3E, G, and K) in normoxic conditions. Hyperoxia exposure increased the expression of HO-1 (Figs. 3C, G, and I) and NQO1 (Figs. 3D, G, and J). Interestingly, the leflunomide treated cells had increased levels of NQO1 (Figs. 3G and J) and SOD2 (Figs. 3G and L) protein compared with vehicle-treated cells upon hyperoxia exposure. Although, the HO-1 mRNA levels (Fig. 3C) were increased in leflunomide treated cells exposed to hyperoxia, there were no corresponding changes in HO-1 protein levels (Figs. 3G and I).
Figure 1. Leflunomide decreases H2O2 levels in HPAECs.
HPAECs were treated with dimethylsulfoxide (DMSO) or leflunomide (L) at concentrations of 1 (L1), 5 (L5) or 10 (L10) μM for up to 24 h, after which cell viability was assessed by MTT (3-(4, 5-dimethylthiazolyl-2)- 2, 5-diphenyltetrazolium bromide) assay (A) and H2O2 level was measured by ROS-Glo™ H2O2 assay (B). Data are representative of at least three independent experiments. Values are presented as mean ± SD (n=4). One-way ANOVA showed an effect of leflunomide for the dependent variable, H2O2 level. Significant differences between DMSO- and leflunomide –treated cells are indicated by *, p < 0.05.
Figure 2. Leflunomide-treated HPAECs display increased catalase, NQO1, and SOD2 expression.
HPAECs were treated with leflunomide (L) at doses of 5 (L5) or 10 (L10) μM or DMSO for 24 h, and the cells were harvested for real-time RT-PCR and immunoblot analyses. A–E. Real-time RT-PCR analyses-based determination of catalase (A), HO-1 (B), NQO1 (C), SOD1 (D), and SOD2 (E) mRNA-expression levels. F. Determination of catalase, HO-1, SOD1, SOD2, and NQO1 protein levels by immunoblotting. G-K. Quantification and normalization of catalase (G), HO-1 (H), NQO1 (I), SOD1 (J), and SOD2 (K) band intensities to those of β-actin. The data shown are representative of three independent experiments. Values are presented as the mean ± SD (n = 3/group). One-way ANOVA showed an effect of L5 and L10 for catalase, NQO1, and SOD2 mRNA and protein expression. Significant differences between DMSO-, L5- and L10-treated cells are indicated by *, p < 0.05.
Figure 3. Leflunomide treated HPAECs display decreased H2O2 levels and increased NQO1 and SOD2 protein expression upon exposure to hyperoxia.
HPAECs were treated with leflunomide (L) at doses of 5 (L5) or 10 (L10) μM or DMSO and exposed to normoxia or hyperoxia for 24 h, and the cells were harvested for H2O2 estimation (A), and real-time RT-PCR and immunoblot analyses. B–F. Real-time RT-PCR analyses-based determination of catalase (B), HO-1 (C), NQO1 (D), SOD1 (E), and SOD2 (F) mRNA-expression levels. G. Determination of catalase, HO-1, NQO1, SOD1 and SOD2 protein levels by immunoblotting. H-L. Quantification and normalization of catalase (H), HO-1 (I), NQO1 (J), SOD1 (K), and SOD2 (L) band intensities to those of β-actin. The data shown are representative of three independent experiments. Values are presented as the mean ± SD (n = 3/group). Two-way ANOVA showed an effect of leflunomide and hyperoxia on the dependent variables, H2O2 level, and HO-1 and NQO1 expression, and an interaction between leflunomide and hyperoxia for the dependent variables, H2O2 level, and NQO1 and SOD2 expression. Significant differences between vehicle and leflunomide treated cells exposed to normoxia are indicated by p, p < 0.05. Significant differences between normoxia and hyperoxia-exposed cells are indicated by *, p < 0.05. Significant differences between vehicle and leflunomide treated cells exposed to hyperoxia are indicated by †, p < 0.05.
The biological effects of leflunomide are concentration- or dose-dependent. It exerts toxic effects, including mitochondrial dysfunction, cytotoxicity, and disrupted angiogenesis when used in high concentrations [19–21]. On the other hand, leflunomide also exerts beneficial anti-inflammatory and anti-oxidant properties in several pathological states [11, 22, 23]. Our study supports the hypothesis that low concentrations of leflunomide has beneficial anti-oxidant properties without any cytotoxic effects in hyperoxic conditions. This conclusion is supported by recent studies, which have demonstrated that leflunomide protects lungs against several insults by upregulating the expression of catalase and SOD [10, 24, 25].
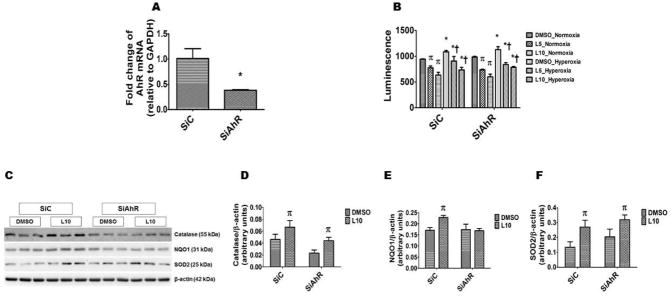
The aryl hydrocarbon receptor (AhR), a member of basic - helix – loop – helix family of transcriptional regulators , modulates oxidant stress by inducing detoxifying enzymes or via “cross-talk” with other signal transduction pathways [26–29]. We and others have shown that leflunomide is an AhR agonist [9, 30]. Therefore, the question we asked was that whether leflunomide attenuates oxidative stress via AhR-dependent mechanisms. To answer this question, we knocked down AhR by siRNA-mediated transfections and determined H2O2 generation and anti-oxidant enzyme expression in the transfected cells treated with DMSO or leflunomide. As expected, AhR siRNA significantly decreased the AhR mRNA expression (Fig. 4A). Leflunomide decreased H2O2 generation in normoxic and hyperoxic conditions in both AhR-sufficient and –deficient cells (Fig. 4B). Likewise, leflunomide increased catalase (Figs. 4C and D) and SOD2 (Figs. 4C and F) protein levels in both AhR-sufficient and –deficient cells exposed to normoxia. However, leflunomide-induced NQO1 protein (Figs. 4C and E) expression was significantly attenuated in AhR-deficient cells, indicating that leflunomide induces NQO1 via AhR-dependent mechanisms, a finding consistent with our previous study [9]. Our AhR- knock down experiments suggest that leflunomide decreases H2O2 levels via catalase- and SOD2-dependent, but AhR- and NQO1-independent mechanisms. It is possible that leflunomide modulates oxidative stress via nuclear factor erythroid 2–related factor 2-dependent pathways; further studies are necessary to address this speculation.
Figure 4. Leflunomide decreases H2O2 levels and increases catalase and SOD2 expression in HPAECs via an AhR-independent mechanism.
Twenty-four hours after HPAECs were transfected with either 50 nM control (SiC) or AhR (SiAhR) siRNA, the RNA was extracted to determine AhR (A) mRNA expression. Further, 24 h after transfection, the cells were treated with leflunomide (L) at doses of 5 (L5) or 10 (L10) μM or a vehicle control (DMSO) and exposed to normoxia or hyperoxia for 24 h and the H2O2 level was estimated by ROS-Glo™ H2O2 assay (B). In a separate set of experiments, the transfected cells were treated with L10 or DMSO as described above in normoxic conditions, and the cells were harvested for immunoblot analyses. C. Determination of catalase, HO-1, NQO1, and SOD2 protein levels by immunoblotting. D-F. Quantification and normalization of catalase (D), NQO1 (E), and SOD2 (F) band intensities to those of β-actin. The data shown are representative of two independent experiments. Values are presented as the mean ± SD (n = 3/group). Two-way ANOVA showed an effect of leflunomide, but not the AhR gene for the dependent variables, H2O2 level, catalase and SOD2 protein expression. However, there was an effect of both leflunomide and the AhR gene for the dependent variable, NQO1 protein expression. Significant difference in the AhR mRNA expression between control and AhR siRNA-transfected cells are indicated by *, p < 0.05. Significant differences between vehicle and leflunomide treated cells exposed to normoxia are indicated by p, p < 0.05. Significant differences between normoxia and hyperoxia-exposed cells are indicated by *, p < 0.05. Significant differences between vehicle and leflunomide treated cells exposed to hyperoxia are indicated by †, p < 0.05.
In summary, we demonstrate for the first time that leflunomide attenuates H2O2 generation by inducing multiple anti-oxidant enzymes in HPAECs. Thus, our results suggest that leflunomide can be beneficial in the prevention and treatment of oxidative stress-induced disorders like BPD, retinopathy of prematurity, and necrotizing enterocolitis in preterm infants.
Supplementary Material
Highlights.
Leflunomide decreases oxidative stress in fetal human lung endothelial cells.
Leflunomide induces catalase, NQO1, and SOD2 enzymes in fetal human lung cells.
Leflunomide effects on oxidative stress, catalase, and SOD2 are AhR-independent.
AhR deficiency abrogates leflunomide-mediated induction of NQO1 enzyme.
Acknowledgments
This work was supported by grants from National Institutes of Health [K08 HD073323]; American Heart Association [BGIA 20190008]; and American Lung Association [RG 349917] to B.S.
Abbreviations
- AhR
aryl hydrocarbon receptor
- BPD
bronchopulmonary dysplasia
- DMSO
dimethylsulfoxide
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- HPAECs
human pulmonary artery endothelial cells
- L
leflunomide (PubChem CID: 3899)
- L 1
leflunomide 1 μM
- L 5
leflunomide 5 μM
- L 10
leflunomide 10 μM
- MTT
3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide
- NQO1
NAD(P)H quinone dehydrogenase 1
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Seminars in fetal & neonatal medicine. 2009;14:358–366. doi: 10.1016/j.siny.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8:39–49. doi: 10.1016/s1084-2756(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 4.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, Baley J, Singer LT. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. The Journal of pediatrics. 2013;162:243–249e241. doi: 10.1016/j.jpeds.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrone S, Tataranno ML, Buonocore G. Oxidative stress and bronchopulmonary dysplasia. Journal of clinical neonatology. 2012;1:109–114. doi: 10.4103/2249-4847.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madurga A, Mizikova I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. American journal of physiology. Lung cellular and molecular physiology. 2013;305:L893–905. doi: 10.1152/ajplung.00267.2013. [DOI] [PubMed] [Google Scholar]
- 8.Pinto P, Dougados M. Leflunomide in clinical practice. Acta reumatologica portuguesa. 2006;31:215–224. [PubMed] [Google Scholar]
- 9.Shrestha AK, Patel A, Menon RT, Jiang W, Wang L, Moorthy B, Shivanna B. Leflunomide induces NAD(P)H quinone dehydrogenase 1 enzyme via the aryl hydrocarbon receptor in neonatal mice. Biochemical and biophysical research communications. 2017;485:195–200. doi: 10.1016/j.bbrc.2017.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozturk E, Demirbilek S, Begec Z, Surucu M, Fadillioglu E, Kirimlioglu H, Ersoy MO. Does leflunomide attenuate the sepsis-induced acute lung injury? Pediatric surgery international. 2008;24:899–905. doi: 10.1007/s00383-008-2184-y. [DOI] [PubMed] [Google Scholar]
- 11.Ozturk E, Surucu M, Karaman A, Samdanci E, Fadillioglu E. Protective effect of leflunomide against oxidative intestinal injury in a rodent model of sepsis. The Journal of surgical research. 2014;187:610–615. doi: 10.1016/j.jss.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz Y, Kose H, Cecen S, Ergin K, Demir EM, Serter M. Protective effects of leflunomide on intestinal ischemia-reperfusion injury: leflunomide against intestinal ischemia-reperfusion. Digestive diseases and sciences. 2010;55:245–252. doi: 10.1007/s10620-009-0737-0. [DOI] [PubMed] [Google Scholar]
- 13.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. American journal of respiratory and critical care medicine. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath-Morrow SA, Cho C, Cho C, Zhen L, Hicklin DJ, Tuder RM. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. American journal of respiratory cell and molecular biology. 2005;32:420–427. doi: 10.1165/rcmb.2004-0287OC. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Patel A, Chu C, Jiang W, Wang L, Welty SE, Moorthy B, Shivanna B. Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: Mechanistic roles of antioxidant enzymes and RelB. Toxicology and applied pharmacology. 2015 doi: 10.1016/j.taap.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Patel A, Moorthy B, Shivanna B. Omeprazole induces NAD(P)H quinone oxidoreductase 1 via aryl hydrocarbon receptor-independent mechanisms: Role of the transcription factor nuclear factor erythroid 2-related factor 2. Biochemical and biophysical research communications. 2015;467:282–287. doi: 10.1016/j.bbrc.2015.09.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, Zhang S, Shrestha AK, Maturu P, Moorthy B, Shivanna B. Omeprazole induces heme oxygenase-1 in fetal human pulmonary microvascular endothelial cells via hydrogen peroxide-independent Nrf2 signaling pathway. Toxicology and applied pharmacology. 2016;311:26–33. doi: 10.1016/j.taap.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free radical biology & medicine. 2006;41:4–18. doi: 10.1016/j.freeradbiomed.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Xuan J, Ren Z, Qing T, Couch L, Shi L, Tolleson WH, Guo L. Mitochondrial dysfunction induced by leflunomide and its active metabolite. Toxicology. 2018;396–397:33–45. doi: 10.1016/j.tox.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu M, Zhang C. Inhibition of angiogenesis by leflunomide via targeting the soluble ephrin-A1/EphA2 system in bladder cancer. Scientific reports. 2018;8:1539. doi: 10.1038/s41598-018-19788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Huang Q, Zhou H, Wang Y, Hu X, Li T. Inhibition of canonical WNT/beta-catenin signaling is involved in leflunomide (LEF)-mediated cytotoxic effects on renal carcinoma cells. Oncotarget. 2016;7:50401–50416. doi: 10.18632/oncotarget.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y, Ding CZ, Fang Y. Combination of MTX and LEF attenuates inflammatory bone erosion by down-regulation of receptor activator of NF-kB ligand and interleukin-17 in type II collagen-induced arthritis rats. Rheumatology international. 2013;33:1845–1853. doi: 10.1007/s00296-013-2674-7. [DOI] [PubMed] [Google Scholar]
- 23.Huang JL, Wu SY, Xie XJ, Wang MX, Zhu S, Gu JR. Inhibiting effects of Leflunomide metabolite on overexpression of CD147, MMP-2 and MMP-9 in PMA differentiated THP-1 cells. European journal of pharmacology. 2011;670:304–310. doi: 10.1016/j.ejphar.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 24.He C, Larson-Casey JL, Gu L, Ryan AJ, Murthy S, Carter AB. Cu,Zn-Superoxide Dismutase-Mediated Redox Regulation of Jumonji Domain Containing 3 Modulates Macrophage Polarization and Pulmonary Fibrosis. American journal of respiratory cell and molecular biology. 2016;55:58–71. doi: 10.1165/rcmb.2015-0183OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayhan S, Guzel A, Duran L, Tutuncu S, Guzel A, Gunaydin M, Salis O, Okuyucu A, Selcuk MY. Effects of leflunomide on inflamation and fibrosis in bleomycine induced pulmonary fibrosis in wistar albino rats. Journal of thoracic disease. 2013;5:641–649. doi: 10.3978/j.issn.2072-1439.2013.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. The Journal of pharmacology and experimental therapeutics. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- 27.Shivanna B, Zhang W, Jiang W, Welty SE, Couroucli XI, Wang L, Moorthy B. Functional deficiency of aryl hydrocarbon receptor augments oxygen toxicity-induced alveolar simplification in newborn mice. Toxicology and applied pharmacology. 2013;267:209–217. doi: 10.1016/j.taap.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. The Journal of biological chemistry. 2008;283:28944–28957. doi: 10.1074/jbc.M800685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. The American journal of pathology. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell EF, Saili KS, Koch DC, Kopparapu PR, Farrer D, Bisson WH, Mathew LK, Sengupta S, Kerkvliet NI, Tanguay RL, Kolluri SK. The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PloS one. 2010;5 doi: 10.1371/journal.pone.0013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.