Summary
Background
Interleukin-31 (IL-31) is implicated in pruritus associated with pruritic skin diseases like atopic dermatitis. Although pruritus is a prominent feature in dermatomyositis (DM), few studies have evaluated the pathogenesis of DM-associated itch.
Objectives
Our goals were to establish the prevalence of itch in DM, and to investigate the role of IL-31 in DM-related itch.
Methods
Pruritus and disease activity of DM were evaluated by a visual analog scale (VAS) and the Cutaneous Disease and Activity Severity Index (CDASI), respectively. Expression of IL-31 and IL-31 receptor alpha (IL-31RA) in lesional DM, non-lesional DM and healthy control (HC) skin was evaluated by qRT-PCR and immunofluorescence. Flow cytometry was performed on skin cells isolated from lesional DM skin to identify cellular sources of IL-31 in DM.
Results
Among 191 DM patients, 50.8% had moderate to severe itch, and itch was correlated with increased cutaneous severity (r= 0.34). In itchy DM patients, gene expression of IL-31 and IL-31RA in lesional skin was upregulated compared to non-lesional skin and HC skin. IL-31 mRNA expression positively correlated with VAS itch score (r= 0.67). On immunofluorescence, immunoreactivity for IL-31 and IL31RA was stronger in lesional skin. Flow cytometry showed lesional DM skin contained significantly more IL-31-producing cells and CD4+ cells were the most common cell type. Lenabasum, an emerging treatment for DM, significantly downregulated IL-31 from CpG-stimulated PBMCs.
Conclusion
Increased skin IL-31 may play a role in DM-associated itch, and ongoing trials will evaluate the effects of systemic treatment on IL-31 and itch in DM.
Keywords: Dermatomyositis, Interleukin-31, Pruritus
Introduction
Dermatomyositis (DM) is an autoimmune disease that frequently involves skin, muscle, and/or lung. Pruritus is one of the prominent cutaneous features in DM, and can help distinguish patients with DM from those with lupus erythematosus (LE) clinically.1,2 Although one case review studied 20 patients with juvenile DM and found that 38% complained of pruritus,3 there is a paucity of quantitative studies of pruritus in DM. Shirani Z et al.4 observed a significant itch sensation in a majority of their patient population, but the number of patients studied was limited and the questionnaire was anonymous, preventing the analysis of correlations with other disease characteristics. Dermatologic aspects of DM, especially pruritus, have a significant impact on quality of life (QOL), rendering management of pruritus important in DM patients.1,5 However, neither the extent of itch in this population nor the pathogenesis of the itch associated with DM has been clearly understood.
Interleukin-31 (IL-31) is a short-chain 4-helix bundle cytokine that is important for both innate and adaptive immunity in the skin. IL-31 is mostly expressed by activated CD4+ T cells, and preferentially by T cells skewed toward a Th2 phenotype.6,7 Mast cells and a subset of the monocyte/macrophage population also produce IL-31.8–10 IL-31 signals through a heterodimeric receptor composed of IL-31 receptor alpha (IL-31RA) and oncostatin M receptor beta (OSMRβ).6 A variety of cell types like activated monocytes, macrophages, eosinophils, basophils, dorsal root ganglion (DRG), and keratinocytes express the IL-31 receptor complex.11 IL-31 has been incriminated in pruritus in humans and mice. When overexpressed in transgenic mice or cutaneously injected, IL-31 causes dermatitis, alopecia, and pruritus.6,12 IL-31 mRNA and protein are increased in pruritic human skin diseases, including atopic dermatitis (AD), allergic contact dermatitis, and prurigo nodularis.13 IL-31, IL-31RA, and OSMRβ were elevated in the epidermis of affected cutaneous T cell lymphoma (CTCL) skin and that IL-31 expression was significantly correlated with the severity of itch.14
Recently, lenabasum (JBT-101) has been studied as a new potential treatment option for DM. Lenabasum is a nonpsychoactive analog of tetrahydrocannabinol that selectively binds pro-resolving receptor on immune cells. Lenabasum suppressed inflammatory cytokines, including TNFα, IFN-α, and IFN-β, which are important in the pathogenesis of DM.15 We hypothesized that lenabasum could also inhibit IL-31 secretion, contributing to its therapeutic effect.
Our goals were to establish the prevalence and severity of itch in patients with DM, and to determine any differences in itch related to gender or DM subtype. We further investigated the presence of IL-31 in the skin of DM patients, its relationship with itch severity, and the cellular source of IL-31, to explain the pathogenic mechanism of itch in these patients. We also evaluated the suppressive effect of lenabasum on IL-31 secretion in peripheral blood mononuclear cells (PBMCs) from DM patients.
Patients and Methods
Patients
A total of 191 subjects with DM seen at the dermatology clinic of the Hospital of the University of Pennsylvania were included in the study, regardless of whether or not they were currently undergoing treatment. Information regarding age, sex, and subtype of DM (classic DM, CDM; clinically amyopathic DM, CADM) was recorded. When the patient had characteristic DM skin lesions together with muscle symptoms such as muscle weakness and difficulty standing from a sitting position or walking upstairs, along with EMG or MRI documentation of muscle involvement, the patient was diagnosed with classic DM. If the patient had only skin symptoms without muscle symptoms, even if muscle enzymes such as creatinine kinase and aldolase were elevated, the patient was diagnosed as having clinically amyopathic DM. Skin symptoms characteristic of DM included gottron’s papules and sign, heliotrope rash, violaceous erythema including shawl sign, V sign, and holster sign, and mechanic’s hands. Skin tissue samples were collected from 15 DM patients and 4 healthy controls (HC). In DM patients, lesional skin was erythematous patches. Exclusion criteria for skin sampling and information about healthy controls is detailed in the supplementary data. The experimental protocol was approved by the Ethics Committee of the University of Pennsylvania and conformed to the principles outlined in the Declaration of Helsinki. A written informed consent was given by every subject involved in the study.
Measurement of pruritus and dermatomyositis cutaneous disease severity
The severity of pruritus was measured using a 10.0 cm Visual Analogue Scale (VAS), with one end representing no itch and the other expressing maximum itch.16 The VAS scores were divided into four groups for analysis: no pruritus with a score of 0 on the VAS; mild pruritus with VAS scores of 1.0–3.3; moderate pruritus with VAS scores of 3.4–6.6; and severe pruritus with VAS scores of 6.7–10.0.4
The severity of cutaneous disease was evaluated using the Cutaneous Disease and Activity Severity Index (CDASI), which is a validated disease severity score of DM. CDASI Activity score ranges 0-100 and Damage score 0-32. We considered scores lower than 14 as mild disease.17
RNA isolation and quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR)
Methods are available in supplementary data.
Immunofluorescence
Methods are available in supplementary data.
Skin cell isolation and flow cytometry
To analyze the cellular source of IL-31 in skin, fresh skin tissues were obtained by 6-mm punch biopsy from lesional skin of 3 itchy DM patients and 4 healthy controls, and cells were isolated as described previously.18 To assess IL-31 production, the isolated cells were cultured in the presence of phorbol 12-myristate 13-acetate (PMA)/ionomycin, and brefeldin A for a total of 5 h. Single-cell suspensions underwent flow cytometric analysis on a LSR-II flow cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar, Ashland, OR, USA). 50,000 events were collected for analysis. Events were gated on single, live cell populations, and IL-31 production was assessed in CD3+ CD4+, CD3+ CD8+, CD11b+, CD11c+, and CD68+ cells. Detailed methods are described in supplementary data.
Lenabasum
An ultrapure powder formulation of lenabasum was used in this study (Corbus Pharmaceuticals Holdings, Norwood, MA, USA). The lenabasum was dissolved in 100% dimethyl sulfoxide.
PBMC isolation, lenabasum treatment, and quantification of IL-31, IL-4, TGFβ secretion
To see if lenabasum can inhibit IL-31 secretion in DM patients, PBMCs were isolated from heparinized venous blood of 8 DM patients. Details are available in supplementary data. The PBMCs were treated with 0, 3, 10 and 15 μM concentrations of lenabasum for 18 hours at 37°C and 5% CO2. Three hours after lenabasum treatment, the PBMCs were stimulated with CpG (Sigma-Aldrich, St. Louis, MO, USA) to ensure sufficient production of IL-31, to evaluate the suppressive effects of lenabasum. The concentration of IL-31, IL-4, and TGF-β secreted into the supernatants was quantified in triplicate via enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Human IL-31 ELISA Kit, abcam, Cambridge, MA, USA; Human IL-4 ELISA Kit, abcam, Cambridge, MA, USA; Human Latent TGF-β ELISA Kit, BioLegend, San Diego, USA).
Statistical analysis
The Mann-Whitney test, the Spearman rank test, and the Kruskal-Wallis test with Dunn’s post hoc test was used. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). A two-tailed P-value of < 0.05 was considered significant in all statistical analyses. Details are available in supplementary data.
Results
Patient characteristics
Among 191 subjects (28 male, 163 females, age 50.9 ± 13.3 years), 73 had CDM with active muscle involvement, and 118 had CADM. Of the patients, 9.4% had no itch, 39.8 % had mild itch, 28.8% had moderate itch, and 22.0% severe itch. Although the finding was not statistically significant, female patients tended to be itchier than male patients (median VAS: females 4.0 vs male 1.9, p=0.52) (Fig. 1A). Of females, 52.1% had moderate to severe pruritus, compared to 42.9% of males. The median VAS itch score between subtypes of DM was not statistically different (CDM 3.3 vs CADM 3.95, p=0.77) (Fig. 1B).
Figure 1. Epidemiologic characteristics of itch in dermatomyositis (DM).
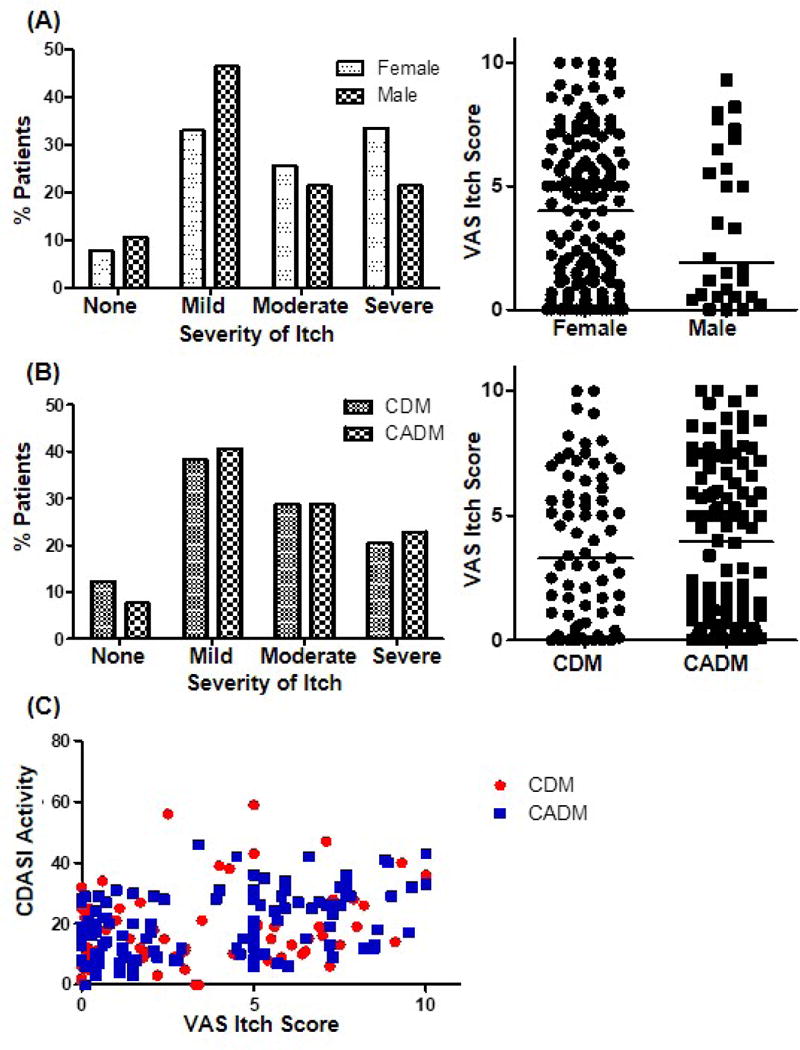
(A-B) Prevalence (%) and distribution of itch in dermatomyositis (n=191) according to (A) gender (28 male, 163 female) and (B) subtype of DM (73 CDM, 118 CADM). (C) Correlation between VAS itch score and CDASI activity score (CDM r=0.318, p=0.0065; CADM r=0.3742, p<0.0001). CDM: Classic type dermatomyositis, CADM: Clinically amyopathic dermatomyositis
Correlation between pruritus and skin severity of DM
Pruritus scores in all patients significantly correlated with CDASI activity scores (correlation coefficient r=0.35, p<0.0001), suggesting that itch is correlated with increased cutaneous severity. CDASI damage score did not show a correlation with itch (r=0.10, p=0.16). When evaluated according to the subtype of DM, itch scores in both CDM and CADM groups showed a significant correlation with the CDASI activity score (CDM r=0.32, p=0.0065; CADM r=0.37, p<0.0001), but not with the CDASI damage score (CDM r=0.17, p=0.16; CADM r=0.05, p=0.60) (Fig. 1C, Fig. S1).
Upregulated gene expression of IL-31 and IL-31RA in lesional skin of DM and its correlation with VAS itch score
In 12 DM patients with pruritus, gene expressions of IL-31 and IL-31RA in lesional skin were compared to their non-lesional skin and skin samples from HC. The mRNA levels of IL-31 were significantly higher in lesional DM skin than non-lesional DM skin and HC skin (Fig. 2A). IL-31RA gene expression was enhanced in lesional DM skin in comparison to HC skin (Fig. 2B). Lesional skin IL-31 expression was positively correlated with VAS itch score (r= 0.5884, p=0.044), while correlation between IL-31RA expression and itch score was not statistically significant (r= 0.2006, p=0.5837) (Fig. 2C). To rule out the effects of other cytokines dominant in DM, we evaluated gene expression of IL-4 and its correlation with IL-31. As previously noted,19,20 IL-4 expression was significantly up-regulated in lesional DM skin compared to healthy control (Fig. 2D). However, it did not correlate with VAS itch score (Fig. 2E).
Figure 2. Upregulated gene expression of IL-31 and IL-31RA in lesional skin of dermatomyositis (DM) and its correlation with the severity of itch.
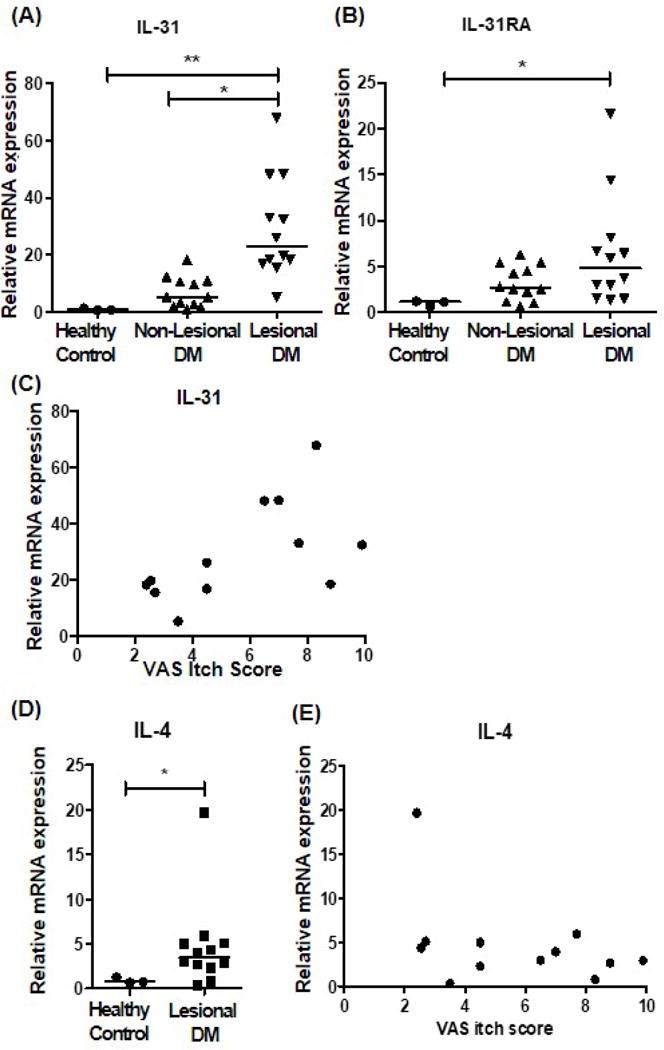
(A-B) In 12 DM patients with pruritus, gene expression of (A) IL-31 and (B) IL-31RA in lesional skin were compared to their non-lesional skin and the skin samples from 3 healthy controls (HC). (C) Correlation between VAS itch score and lesional gene expression of IL-31 (correlation coefficient= 0.5884, p=0.044). (D) mRNA expression of IL-4 in lesional DM and healthy control skin. (E) Correlation between VAS itch score and lesional gene expression of IL-4 (correlation coefficient= −0.4165, p=0.658). For A, B, and D, * p<0.05, ** p<0.01
IL-31 expression is increased in lesional DM skin compared to non-lesional DM and HC skin
Immunofluorescence revealed lesional DM skin has a higher area fraction and MFI of IL-31 than non-lesional DM skin and HC skin (Figs. 3A, 3C, 3E, 3G). Double staining of CD4 and IL-31 showed co-localization, suggesting that at least some of the IL-31 producing cells were CD4+ cells in DM patients (Fig. 3A). The area fraction and MFI of IL-31RA were increased in lesional skin when compared to HC skin (Figs. 3B, 3D, 3F, 3H).
Figure 3. Increased expression of IL-31 and IL-31RA in lesional dermatomyositis (DM) skin in immunofluorescence.
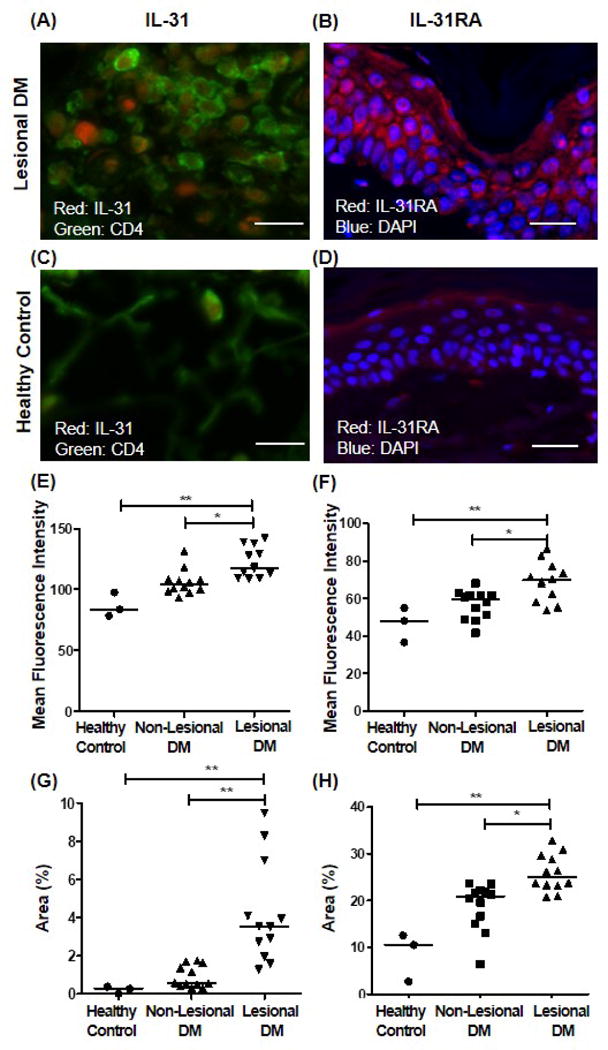
(A-D) Representative immunofluorescence microphotographs of (A) lesional skin, IL-31; (B) lesional skin, IL-31RA; (C) healthy control, IL-31; and (D) healthy control, IL-31RA. IL-31-stained sections were double-stained to identify co-localization with CD4. Bar=100μm. (E-H) Mean fluorescence intensity (MFI) and area fraction of IL-31- and IL-31RA- expressing area were evaluated. n=12 for DM patients, and n=3 for healthy control. (E) MFI of IL-31, (F) MFI of IL-31RA, (G) area fraction of IL-31, and (H) area fraction of IL-31RA. *p<0.05, **p<0.01
Flow cytometry indicates increased population of IL-31-producing cells in lesional dermatomyositis skin, with CD4+ T cells being the most common inflammatory cell type
Although many IL-31-expressing cells co-localized with CD4 on immunofluorescence staining, some IL-31-expressing cells were not CD4+ cells (Fig. 3B). To identify other cellular sources of IL-31 in DM, we performed flow cytometry on single cell suspensions of lesional DM skin using CD4, CD8, CD11b, CD11c, and CD68 as cell population markers. Our results showed that the inflammatory cell population in lesional DM skin contained significantly increased percentages of IL-31-producing cells compared to HC skin (Fig. 4A, B). CD4+ cells were the most common cell types to produce IL-31 in DM, but other cell types that express CD8, CD68, CD11b, or CD11c also secreted IL-31 in DM. The proportion of each inflammatory cell type, a percentage of which were found to produce IL-31 in DM patients, was similar in lesional DM and HC skin. Although CD4+ cells and CD8+ cells were approximately two times more in lesional DM skin compared to healthy control skin, the increase was much more prominent in the IL-31-producing population (Fig. 4C). The percentage increase in CD68+, CD11b+, and CD11c+ cells in lesional DM skin were not as much as in the CD4+ and CD8+ cell population. In addition, the MFI of IL-31-producing cells was also higher in lesional skin compared to HC skin in all cell types studied (Fig. 4D).
Figure 4. IL-31-producing cells are increased in lesional dermatomyositis (DM) skin compared to healthy control (HC) skin, with CD4+ T cells being the most common.
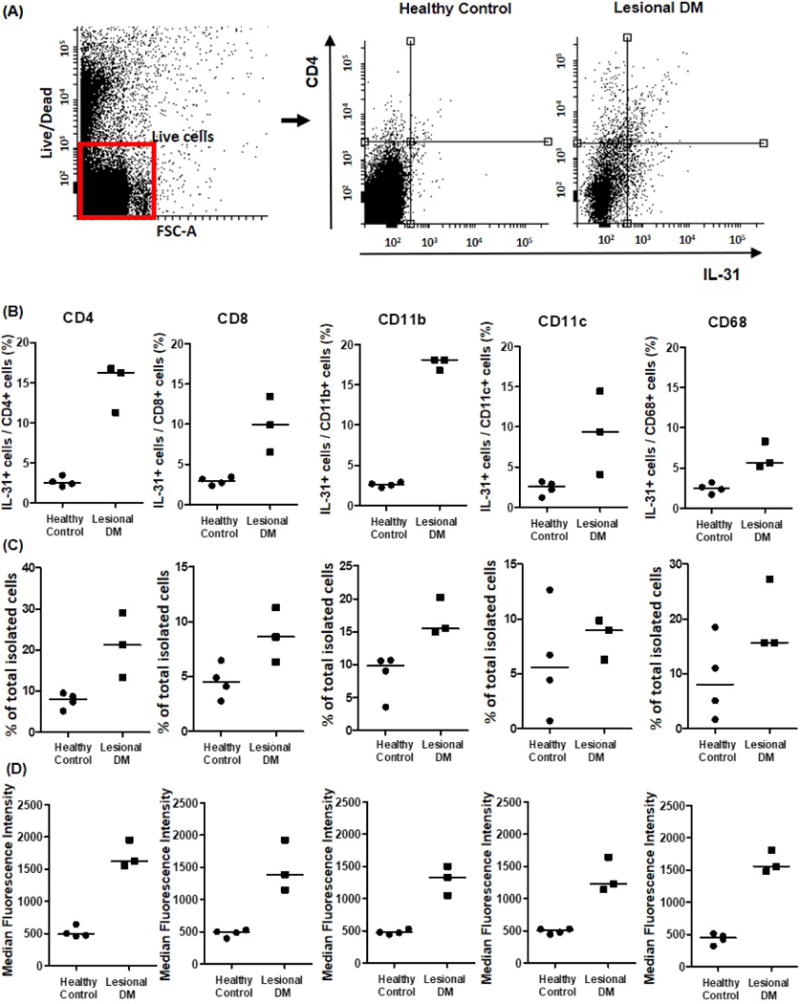
To identify cellular sources of IL-31 in DM, skin samples from 3 itchy DM patients and 4 HC were stimulated with phorbol 12-myristate 13-acetate/ionomycin and assessed by flow cytometry. (A) Representative flow cytometric plot of live cell gating. (B) Lesional DM skin contained significantly increased percentages of IL-31-producing cells compared to HC skin. (C) The proportion of each cell type relative to total isolated skin cells. (D) Median fluorescence intensity of IL-31-producing cells was also higher in lesional skin than HC skin.
Moderate and high lenabasum concentrations suppressed secretion of IL-31 and IL-4 by CpG-stimulated PBMCs
To evaluate the suppressive effect of lenabasum on the production of cytokines important in DM, PBMCs were isolated from 8 DM patients and 8 HC, and stimulated by CpG with or without lenabasum treatment. Levels of IL-31, IL-4, and tumor growth factor (TGF)-β were subsequently evaluated by ELISA. CpG-mediated induction of IL-31 in PBMCs was significantly higher in DM patients than HC (Figure 5A). Lenabasum significantly suppressed the production of IL-4 and IL-31 by CpG-stimulated PBMCs isolated from DM patients at concentrations of 10 and 15 μM (p<0.05) (Fig. 5B, C). Lenabasum did not inhibit TGF-β secretion by CpG-stimulated PBMCs at any concentrations (Fig. 5D).
Figure 5. Suppression of pro-inflammatory cytokines in PBMCs isolated from dermatomyositis (DM) patients after in vitro lenabasum treatment.
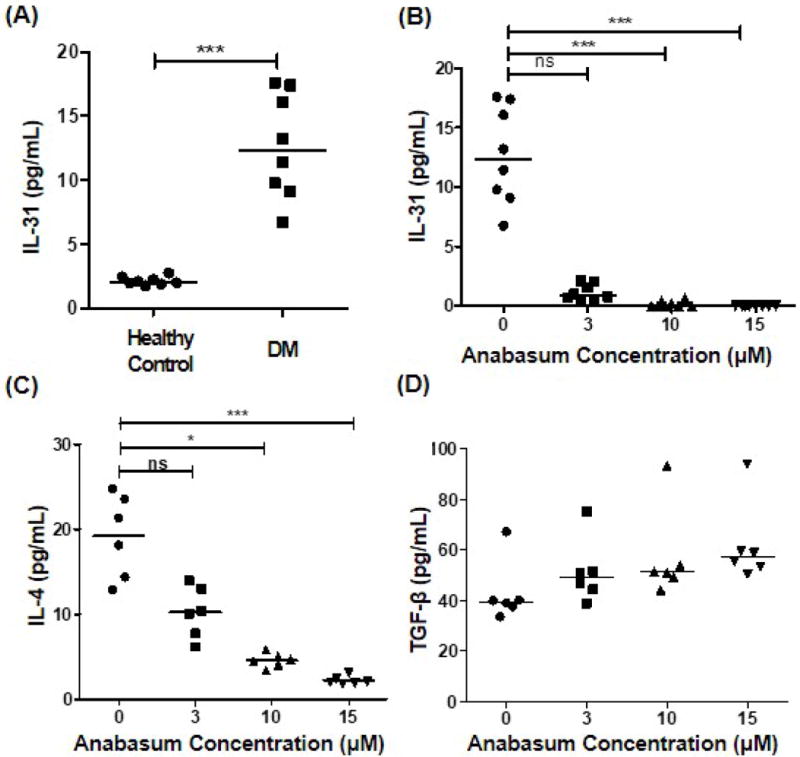
To evaluate the inhibitory effect of lenabasum on various inflammatory cytokines in DM patients, PBMCs were isolated from 8 DM patients and 8 healthy controls (HC), stimulated with CpG with or without lenabasum treatment. (A) CpG-mediated induction of IL-31 in PBMCs was significantly higher in DM patients compared to HC. (B) Lenabasum significantly downregulated the production of IL-31 from CpG-stimulated PBMCs at concentrations of 10 and 15 μM. (C) IL-4 production was significantly inhibited by treatment with higher concentration of lenabasum. (D) Lenabasum did not suppress TGF-β production from CpG-stimulated PBMCs at any concentrations. *p<0.05, **p<0.01, ***p<0.001
Discussion
Common cutaneous symptoms of DM include pruritus and photosensitivity. In one study, the mean VAS itch score was consistently over 5.0, placing the majority of the study population in the moderate to severe itch categories (VAS score of > 3.4).4 In this study, more than 90% of DM patients had pruritus, with just over half experiencing moderate to severe itch. The severity of itch correlated with disease activity, but not with disease damage. The extensive prevalence of itch and the strong correlation of itch severity with disease activity in DM patients indicate that management of itch is an important component of overall DM treatment.1,4 However, the pathogenesis of DM-related itch remains largely unknown.
IL-31 is an IL-6 family cytokine, and works by binding to the IL-31 receptor complex. Subsequent signaling is through the Janus kinase (Jak)/signal transducer and activator of transcription (STAT) pathway, the phosphoinositide-3-kinase (PI3K)/Akt pathway, and the mitogen-activated protein (MAP) kinase pathway.8,21 IL-31 has a wide range of biological functions, including the regulation of immune responses, induction of chemokines, signal proteins, and proinflammatory cytokines, regulation of cell proliferation and, most prominently, induction of itch.11,22 IL-31 has been incriminated in pruritus in humans and mice. Scratching behavior was increased in transgenic mice overexpressing IL-31, and in IL-31-injected mice and dogs.6,23 IL-31expression is increased in the lesional skin of patients with itchy skin disorders, such as AD.7,24 Studies report a correlation of serum IL-31 levels with the disease activity in AD.13,25,26 Serum IL-31 levels were increased in chronic spontaneous urticaria compared to healthy controls, but lower than in AD patients.27 IL-31 was also increased in the serum of CTCL patients, and the levels increased in advanced CTCL disease.28–30 Patients with uremic pruritus also had higher serum IL-31 than those without pruritus.31 As a therapeutic option for pruritus, a monoclonal anti-IL-31 antibody was reported to reduce scratching behavior and dermatitis in a mouse model of AD.32 Recently, humanized antihuman IL-31RA antibody successfully reduced pruritus in AD patients, suggesting that IL-31 could potentially be a good target for pruritus in AD and other pruritic diseases.33
The exact mechanism of how IL-31 mediates itch is still not clear. IL-31 receptors are present on DRG in humans and mice, and cutaneously injected IL-31-induced itching was enhanced by DRG IL-31RA expression in mice.6,12,34 Despite the evidence that IL-31 activates a functional IL-31RA on DRG neurons and induces chronic pruritic skin disease,6,12,13,35 IL-31 did not induce immediate itch in AD patients and HC after skin challenge. The late onset of IL-31-induced itch suggests that IL-31 might exert its pruritic effect indirectly via secondary pruritogenic mediators, rather than through its receptors on cutaneous nerves.36
IL-31 is mainly produced by activated CD4+ T cells, particularly of the Th2 phenotype, and IL-31 expression by Th2 cells are induced by their autocrine IL-4 expression.22,37 Th2 cells predominate in the peripheral blood of active DM compared to HC with significantly higher Th2/Th1 and Th2/Th17 ratios.19,20 Our epidemiologic data confirming the predominance of itch in DM, along with the increased Th2 cells in DM, led us to examine the possible role of IL-31 in DM itch. In this study, we found that skin IL-31 gene and protein expression was higher in DM patients than in HC, with a correlation between the itch intensity and skin IL-31 levels. CpG-induced IL-31 levels in PBMCs were also higher in DM patients than in HC. Immunohistochemical studies of lesional DM skin demonstrated CD4 and IL-31 co-localization on cells. Flow cytometry showed that lesional DM skin had a significantly larger population of IL-31-producing cells compared to HC skin. While CD4+ cells were identifed as the most common cell types to produce IL-31 in DM, other cell types expressing CD8, CD68, CD11b, or CD11c were also able to secrete IL-31. How IL-31 production is regulated in these cells in DM is not known. Since monocyte/macrophage are known to produce IL-31,9,10 and they express IL-4 receptors,38,39 stimulation by IL-4 or a different pathway that stimulates IL-31 could be responsible for IL-31 production by these cells.
Recently, lenabasum (JBT-101), an investigational, nonpsychoactive and anti-inflammatory cannabinoid was found to suppress secretion of TNFα, IFN-α, and IFN-β from immune cells of DM patients in vitro.15 These cytokines are thought to be key immunostimulatory cytokines contributing to the inflammation seen in cutaneous DM. Prior data from animal studies and phase I clinical trials has led to an ongoing phase II study of lenabasum in DM patients with significant skin disease. In the present study, lenabasum significantly suppressed the secretion of IL-4 and IL-31 from CpG-stimulated PBMCs in vitro, indicating its potential as a new therapy for itch in DM. Because Th2 cells are a major source of IL-31, and because IL-31 production is dependent on IL-4 secretion by Th2 cells,37 suppression of IL-4 and IL-31 by lenabasum could be the mechanism by which lenabasum reduces clinical disease activity in DM. The TGF-β1/IL-31 pathway is known to play an important role in cell injury and inflammation in hepatitis B virus (HBV)-related liver injury.40,41 Although TGF-β was significantly upregulated in DM compared with controls,20,42 and lenabasum successfully inhibited TGF-β in scleroderma,43,44 lenabasum did not suppress TGF-β production by PBMCs isolated from DM patients in the present study, implying that its suppressive effect on IL-31 are independent of the TGF-β/IL-31 pathway.
In conclusion, we confirmed that itch is refractory prevalent and problematic symptom in many patients with DM involving the skin. Skin IL-31 is significantly higher in itchy DM patients than in HC, implicating its possible role in itch associated with DM.
Supplementary Material
What’s already known about this topic?
IL-31 has been implicated in pruritus associated with various pruritic skin diseases, including atopic dermatitis and cutaneous T cell lymphoma.
Pruritus is a prominent feature in dermatomyositis.
What does this study add?
The severity of itch correlates with the disease activity of dermatomyositis.
Skin IL-31 is significantly upregulated in dermatomyositis, and CD4+ T cells are the most common cell type to produce IL-31.
Flow cytometry indicates not only CD4+ T cells, but also other cell types expressing CD8, CD68, CD11b, or CD11c also secrete IL-31 in dermatomyositis.
What is the translational message?
This study suggests that IL-31 may play a role in the pathogenesis of dermatomyositis-related pruritus.
Lenabasum, a new emerging treatment for dermatomyositis, significantly downregulated IL-31 as well as IL-4 from CpG-stimulated PBMCs. Ongoing trials will evaluate the effects of systemic treatment of IL-31 and itch in dermatomyositis.
Acknowledgments
We appreciate Corbus Pharmaceuticals Holdings, Inc for providing lenabasum used in the study.
Funding
This work was supported by National Institutes of Health [R21 AR066286] and Veterans Affairs Merit Review [I01BX000706] to Dr. Victoria P Werth.
Footnotes
Conflict of Interest
Dr. Victoria P Werth is doing a NIH-funded study with the medication examined as part of this study. The other authors have no conflict of interest to declare.
HEE JOO KIM (Orcid ID : 0000-0003-1585-8184)
DR JANICE TIAO (Orcid ID : 0000-0001-8793-9456)
The senior author (VPW) was involved in the development of the CDASI. The copyright for this instrument is owned by the University of Pennsylvania.
References
- 1.Goreshi R, Chock M, Foering K, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011;65:1107–16. doi: 10.1016/j.jaad.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. 2002;20:387–408. doi: 10.1016/s0733-8635(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 3.Peloro TM, Miller OF, 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28–34. doi: 10.1067/mjd.2001.113686. [DOI] [PubMed] [Google Scholar]
- 4.Shirani Z, Kucenic MJ, Carroll CL, et al. Pruritus in adult dermatomyositis. Clin Exp Dermatol. 2004;29:273–6. doi: 10.1111/j.1365-2230.2004.01510.x. [DOI] [PubMed] [Google Scholar]
- 5.Hundley JL, Carroll CL, Lang W, et al. Cutaneous symptoms of dermatomyositis significantly impact patients’ quality of life. J Am Acad Dermatol. 2006;54:217–20. doi: 10.1016/j.jaad.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 7.Szegedi K, Kremer AE, Kezic S, et al. Increased frequencies of IL-31-producing T cells are found in chronic atopic dermatitis skin. Exp Dermatol. 2012;21:431–6. doi: 10.1111/j.1600-0625.2012.01487.x. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann K, Wagner N, Rabenhorst A, et al. Serum IL-31 levels are increased in a subset of patients with mastocytosis and correlate with disease severity in adult patients. J Allergy Clin Immunol. 2013;132:232–5. doi: 10.1016/j.jaci.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Kato A, Fujii E, Watanabe T, et al. Distribution of IL-31 and its receptor expressing cells in skin of atopic dermatitis. J Dermatol Sci. 2014;74:229–35. doi: 10.1016/j.jdermsci.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen C, Brans R, Czaja K, et al. Ultraviolet B radiation and reactive oxygen species modulate interleukin-31 expression in T lymphocytes, monocytes and dendritic cells. Br J Dermatol. 2011;165:966–75. doi: 10.1111/j.1365-2133.2011.10487.x. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen C, Marquardt Y, Czaja K, et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol. 2012;129:426–33. 33 e1–8. doi: 10.1016/j.jaci.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Arai I, Tsuji M, Miyagawa K, et al. Repeated administration of IL-31 upregulates IL-31 receptor A (IL-31RA) in dorsal root ganglia and causes severe itch-associated scratching behaviour in mice. Exp Dermatol. 2015;24:75–8. doi: 10.1111/exd.12587. [DOI] [PubMed] [Google Scholar]
- 13.Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–7. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Nattkemper LA, Martinez-Escala ME, Gelman AB, et al. Cutaneous T-Cell Lymphoma and Pruritus: the Expression of IL-31 and its Receptors in the Skin. Acta Derm Venereol. 2016 doi: 10.2340/00015555-2417. [DOI] [PubMed] [Google Scholar]
- 15.Robinson ES, Alves P, Bashir MM, et al. Cannabinoid Reduces Inflammatory Cytokines, Tumor Necrosis Factor-alpha, and Type I Interferons in Dermatomyositis In Vitro. The Journal of investigative dermatology. 2017;137:2445–7. doi: 10.1016/j.jid.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langner MD, Maibach HI. Pruritus measurement and treatment. Clin Exp Dermatol. 2009;34:285–8. doi: 10.1111/j.1365-2230.2009.03218.x. [DOI] [PubMed] [Google Scholar]
- 17.Klein RQ, Bangert CA, Costner M, et al. Comparison of the reliability and validity of outcome instruments for cutaneous dermatomyositis. Br J Dermatol. 2008;159:887–94. doi: 10.1111/j.1365-2133.2008.08711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii W, Matsuda M, Shimojima Y, et al. Flow cytometric analysis of lymphocyte subpopulations and TH1/TH2 balance in patients with polymyositis and dermatomyositis. Intern Med. 2008;47:1593–9. doi: 10.2169/internalmedicine.47.0967. [DOI] [PubMed] [Google Scholar]
- 20.Shimojima Y, Ishii W, Matsuda M, et al. Phenotypes of Peripheral Blood Lymphocytes and Cytokine Expression in Polymyositis and Dermatomyositis before Treatment and after Clinical Remission. Clinical medicine insights Arthritis and musculoskeletal disorders. 2012;5:77–87. doi: 10.4137/CMAMD.S10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasraie S, Niebuhr M, Baumert K, et al. Functional effects of interleukin 31 in human primary keratinocytes. Allergy. 2011;66:845–52. doi: 10.1111/j.1398-9995.2011.02545.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Putheti P, Zhou Q, et al. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347–56. doi: 10.1016/j.cytogfr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzales AJ, Humphrey WR, Messamore JE, et al. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013;24:48–53. e11, 2. doi: 10.1111/j.1365-3164.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 24.Nobbe S, Dziunycz P, Muhleisen B, et al. IL-31 expression by inflammatory cells is preferentially elevated in atopic dermatitis. Acta Derm Venereol. 2012;92:24–8. doi: 10.2340/00015555-1191. [DOI] [PubMed] [Google Scholar]
- 25.Raap U, Wichmann K, Bruder M, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;122:421–3. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 26.Ezzat MH, Hasan ZE, Shaheen KY. Serum measurement of interleukin-31 (IL-31) in paediatric atopic dermatitis: elevated levels correlate with severity scoring. J Eur Acad Dermatol Venereol. 2011;25:334–9. doi: 10.1111/j.1468-3083.2010.03794.x. [DOI] [PubMed] [Google Scholar]
- 27.Raap U, Wieczorek D, Gehring M, et al. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp Dermatol. 2010;19:464–6. doi: 10.1111/j.1600-0625.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 28.Miyagaki T, Sugaya M, Suga H, et al. Increased CCL18 expression in patients with cutaneous T-cell lymphoma: association with disease severity and prognosis. J Eur Acad Dermatol Venereol. 2013;27:e60–7. doi: 10.1111/j.1468-3083.2012.04495.x. [DOI] [PubMed] [Google Scholar]
- 29.Ohmatsu H, Sugaya M, Suga H, et al. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm Venereol. 2012;92:282–3. doi: 10.2340/00015555-1345. [DOI] [PubMed] [Google Scholar]
- 30.Singer EM, Shin DB, Nattkemper LA, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. The Journal of investigative dermatology. 2013;133:2783–5. doi: 10.1038/jid.2013.227. [DOI] [PubMed] [Google Scholar]
- 31.Ko MJ, Peng YS, Chen HY, et al. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol. 2014;71:1151–9 e1. doi: 10.1016/j.jaad.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Grimstad O, Sawanobori Y, Vestergaard C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 33.Nemoto O, Furue M, Nakagawa H, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2016;174:296–304. doi: 10.1111/bjd.14207. [DOI] [PubMed] [Google Scholar]
- 34.Bando T, Morikawa Y, Komori T, et al. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 2006;142:1263–71. doi: 10.1016/j.neuroscience.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Bilsborough J, Leung DY, Maurer M, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–25. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 36.Hawro T, Saluja R, Weller K, et al. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy. 2014;69:113–7. doi: 10.1111/all.12316. [DOI] [PubMed] [Google Scholar]
- 37.Stott B, Lavender P, Lehmann S, et al. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol. 2013;132:446–54 e5. doi: 10.1016/j.jaci.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 38.Feldman GM, Finbloom DS. Induction and regulation of IL-4 receptor expression on murine macrophage cell lines and bone marrow-derived macrophages by IFN-gamma. J Immunol. 1990;145:854–9. [PubMed] [Google Scholar]
- 39.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Yu X, Guo R, Ming D, et al. The Transforming Growth Factor beta1/Interleukin-31 Pathway Is Upregulated in Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure and Is Associated with Disease Severity and Survival. Clin Vaccine Immunol. 2015;22:484–92. doi: 10.1128/CVI.00649-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ming D, Yu X, Guo R, et al. Elevated TGF-beta1/IL-31 Pathway Is Associated with the Disease Severity of Hepatitis B Virus-Related Liver Cirrhosis. Viral Immunol. 2015;28:209–16. doi: 10.1089/vim.2014.0142. [DOI] [PubMed] [Google Scholar]
- 42.Confalonieri P, Bernasconi P, Cornelio F, et al. Transforming growth factor-beta 1 in polymyositis and dermatomyositis correlates with fibrosis but not with mononuclear cell infiltrate. J Neuropathol Exp Neurol. 1997;56:479–84. doi: 10.1097/00005072-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Lucattelli M, Fineschi S, Selvi E, et al. Ajulemic acid exerts potent anti-fibrotic effect during the fibrogenic phase of bleomycin lung. Respir Res. 2016;17:49. doi: 10.1186/s12931-016-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez EG, Selvi E, Balistreri E, et al. Synthetic cannabinoid ajulemic acid exerts potent antifibrotic effects in experimental models of systemic sclerosis. Ann Rheum Dis. 2012;71:1545–51. doi: 10.1136/annrheumdis-2011-200314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


