Abstract
Calcium release-activated calcium (CRAC) channels are unique among ion channels that are activated in response to depletion of intracellular calcium stores and are highly permeable to Ca2+ compared to other cations. CRAC channels mediate an important calcium signal for a wide variety of cell types and are well studied in the immune system. They have been implicated in a number of disorders such as immunodeficiency, musculosketal disorders and cancer. There is growing evidence showing that CRAC channels are expressed in the nervous system and are involved in pathological conditions including pain. This review summarizes the expression, distribution, and function of the CRAC channel family in the dorsal root ganglion, spinal cord and some brain regions, and discusses their functional significance in neurons and glial cells and involvement in nociception and chronic pain. Although further studies are needed to understand how these channels are activated under physiological conditions, the recent findings indicate that the CRAC channel Orai1 is an important player in pain modulation and could represent a new target for pathological pain.
1. Introduction
Acute pain serves a warning or protective function and only lasts a few days or weeks. On the other hand, chronic pain serves no useful purpose and is often associated with an altered sensitivity to stimuli. Although the exact molecular and cellular mechanisms underlying chronic pain remain to be determined and may indeed vary depending on the type of pain and initiating events, evidence has accumulated for a role of intracellular Ca2+ in the development of persistent pain. Calcium-permeable ion channels and receptors have been implicated in pain as well as in the neuroplasticity associated with chronic pain states. Neurons express a variety of voltage-gated and ligand-gated Ca2+ channels [1-4]; however, recent studies have shown that newly discovered calcium release-activated calcium (CRAC) channels are also important in mediating Ca2+ influx in neurons and glial cells in the nervous system [5-8]. CRAC channels have emerged as promising therapeutic targets for the treatment of immune disorders, thrombosis and cancer [9-12]. However, the role of CRAC channels in pain and other CNS diseases is just beginning to be explored.
CRAC channels were firstly proposed by Putney to refill intracellular calcium stores after depletion by IP3 receptors [13]. It took two decades to identify molecular components of CRAC channels, but recent progress has elucidated several significant features of CRAC channels. In most cell types, CRAC channels are composed of ER calcium sensors STIM1/2 and poreforming proteins Orai1/2/3 [14-17]. When Ca2+ is released from the endoplasmic reticulum (ER) to the cytosol, STIM1 and STIM2 undergo oligomerization and translocate to ER-PM junctions, where they activate CRAC channels and induce Ca2+ entry [15, 18, 19]. It is well-established that CRAC channels are essential for immune cells to regulate their activation and maturation[20], cytokine production[21], and antigenic response[22]. However, the functional significance of CRAC channels in the nervous system, especially under pathological conditions including pain, is unclear. Recently, we and others have reported that the CRAC channel family is expressed in dorsal horn neurons [23, 24], glia [25-27] and in dorsal root ganglia (DRG) neurons [5, 28]. We have identified the CRAC channel Orai1 as a primary player in store-operated calcium entry (SOCE) in dorsal horn neurons and astrocytes [24, 25], while both Orai1 and Orai3 contribute to SOCE in DRG [28]. Using pharmacological and genetic approaches, we have demonstrated that inhibition or knockout of Orai1 reduces nociception and chronic pain [23, 29, 30]. In this review, we summarize recent work related to CRAC channels and pain, and highlight the role of the CRAC channel Orai1 in nociception and pathological pain.
2. Expression and function of CRAC channels in the nervous system
2.1. CRAC channels in DRG
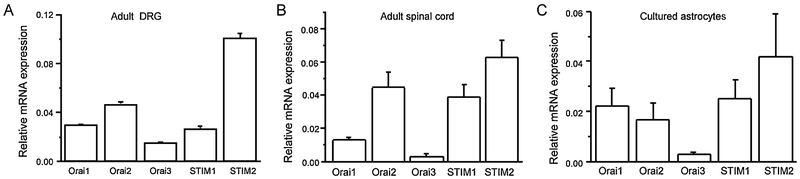
DRG neurons are primary sensory neurons innervating the skin and are responsible for conveying signals for pain sensation to the spinal cord. We and others have shown that both STIM1, STIM2 and Orai1/2/3 are expressed at both mRNA and protein levels in DRG tissue from adult mice determined by RT-qPCR and Western blotting [5, 28]. Interestingly, mRNA levels of STIM2 are approximately 3-fold greater than those of STIM1 (Figure 1A) [28]. As STIM2 is more sensitive to ER Ca2+ changes than STIM1, the high expression of STIM2 may endow DRGs with high sensitivity to fluctuations in [Ca2+]ER [19].
Figure 1. Expression of CRAC channels and STIM proteins.
mRNA levels of STIM1, STIM2, Orai1, Orai2 and Orai3 in adult DRGs (A), adult spinal cord tissue (B), and cultured astrocytes (C) by quantitative PCR (normalized to GAPDH), n=4-5. Figures are modified from Wei et al. [28], Xia et al. [24], and Gao et al. [25].
STIM1 is mainly expressed in IB4- and CGRP-positive C-fibers, which are primarily responsible for nociception, and to lesser extent, in NF-200-positive A-fibers [28]. SOCE was observed in cultured DRG neurons, especially in small-diameter sensory neurons after Ca2+ depletion by thapsigargin (TG), a non-competitive inhibitor of endoplasmic reticulum Ca2+ ATPase [28]. Interestingly, SOCE is robust in nociceptors including TRPV1-, TRPA1-, TRPM8-, and IB4-positive DRG neurons [28], indicating that CRAC channels are functional predominantly in nociceptors. Knockdown of Orai1 and Orai3, but not Orai2, by specific siRNA significantly attenuates SOCE in cultured DRG neurons [28]. Despite previous reports showing that Orai1 and Orai3 can form heteromers mediating SOCE [31, 32], it seems that they can independently contribute to SOCE in DRG neurons [28].
2.2. CRAC channels in the spinal cord
The spinal cord dorsal horn is a relay center for sensory information. Dorsal horn neurons process sensory input received from DRG neurons and transmit it to several brain regions. While voltage-gated sodium, potassium and calcium channels, as well as ionotropic glutamate receptors, are key players, G-protein coupled receptors and other ion channels also play modulatory roles in this process [33]. We have found that Orai1/2/3 and STIM1/2 are expressed in the spinal cord dorsal horn (tissue) and acutely isolated dorsal horn neurons [24]. Similar to that found in DRGs, STIM2 mRNA levels are higher than STIM1 in the dorsal horn (Figure1B) [24]. Interestingly, Orai1 mRNA level is greater in neurons than in the tissue while Orai2 mRNA level is higher in the tissue than that in neurons (Figure1B), suggesting that Orai2 mRNA expression is higher in non-neuronal cells including cells in the white matter. CRAC channels are functional in majority of dorsal horn neurons [24]. Different from DRG neurons, only Orai1 is responsible for SOCE in dorsal horn neurons and both STIM1 and STIM2 contribute to SOCE [24], indicating Orai1 and STIM1/2 are the main functional components in spinal cord dorsal horn neurons.
2.3. CRAC channels in supraspinal brain regions
Sensory information is further processed and terminated in a number of brain regions. SOCE has been reported in several supraspinal regions [34-36], however, the molecular components that mediate SOCE in these regions have remained unclear until recently. Several studies have demonstrated that STIMs and Orais are expressed in cerebral cortex, hippocampus , amygdala [7], thalamus , and cerebellum [37, 38]. Whereas, STIM2 is predominantly expressed in most brain regions [7, 39], STIM1 is highly expressed in the cerebellum [38, 40]. All three Orai isoforms are detectable in the brain, but their mRNA levels vary in different brain regions [41]. The Orai1 expression pattern in the brain and spinal cord of rodents is similar to that of humans [42]. In the cortex and hippocampus, Orai2 expression levels are much greater than Orai1 and Orai3 [39, 43]. While STIM1 and Orai1 are major components mediating SOCE in many cell types including neurons, this is not the case in cortical neurons [39], suggesting that expression levels of STIM1/2 and CRAC channels are tissue-dependent. However, expression, distribution and function of CRAC channels in pain-processing regions of brain have not been reported.
2.4. CRAC channels in glia
Glial cells play essential roles in brain homeostasis. Microglia, astrocytes and oligodendrocytes are the main types of glia in CNS. Normal activities of astrocytes and microglia are essential for maintaining many CNS functions. However, excessive activation of these cells is a hallmark of many acute and chronic neuropathologies including pain [44-46]. Reactive astrocytes and microglia release excessive proinflammatory cytokines and chemokines, which are involved in the development, maintenance and exaggeration of chronic pain [35, 47]. Cytokine production is a Ca2+-dependent process [48]. It is well-established that CRAC channels play an important role in cytokine production in immune cells [49, 50].
Previous studies have shown that STIM1/ 2 and Orai1/2/3 are expressed and functional in brain microglia [26, 27, 51]. Inhibition of CRAC channels by 2-APB, La3+ and N(p-amylcinnamoyl) anthranilic acid (ACA) dramatically blocks SOCE and CRAC currents in microglia [30-32]. Using a global knockout of STIM1, STIM2 and Orai1 mice, Michaelis et al. have demonstrated that STIM1/2 and Orai1 are primary components mediating SOCE in cultured cerebral microglia [26].
Like microglia, astrocytes in the cortex and spinal cord also express STIM1/2 and Orai1/2/3 [25, 52]. However, in hippocampal astrocytes, Orai1 is undetectable while Orai3 is the predominant isoform [53]. Calcium imaging data reveal that CRAC channels are functional and mediate a large calcium influx in cultured spinal astrocytes [25]. Using the siRNA knockdown approach, we have found that Orai1 is responsible for SOCE in spinal cord astrocytes. Although STIM2 expression levels are greater than STIM1, both almost equally contribute to SOCE in spinal cord astrocytes (Figure1C) [25]. Interestingly, an independent study has reported that both Orai1 and Orai3 contribute to the major portion of SOCE in cortical astrocytes [52], indicating that the functional components of CRAC channels in astrocytes are also tissue-dependent.
3. Functional significance of CRAC channels in the nervous system
3.1. Downstream events of CRAC channels activation in the CNS
Ca2+ serves as a second messenger and regulates diverse aspects of cellular function. Ca2+ influx through CRAC channels is essential for Ca2+-dependent cellular events such as enzymatic activity and gene expression in non-excitatory cells [51, 54-56]. SOCE-dependent signaling pathways, including nuclear factor of activated T cells (NFAT) and NF-κB, are well documented in the immune system [55, 57]. SOCE has been shown to activate NFAT signaling in neural progenitor cells and murine microglia [55, 58]. Mitogen-activated protein kinases (MAPKs) play a key role in the transduction of extracellular signals to cellular responses. SOCE induces activation of extracellular signal-regulated kinases (ERKs), members of the MAPK family in several cell types [56, 59, 60], However, downstream events of CRAC channels activation in neurons remains relatively unexplored. Increased [Ca2+]i via Orai1 can induce activation of PKC and its downstream ERKs in cultured dorsal horn neurons [23]. Orai1-dependent ERK activation was also observed in the dorsal horn under several pain conditions [23, 25]. There is substantial evidence for the importance of ERKs in chronic pain [61]. One of the important downstream targets of ERK is the potassium channel Kv4.2, which is a major component of A-type potassium channels in dorsal horn neurons [62]. Activation of ERKs is known to regulate A-type potassium channels in dorsal horn and hippocampal neurons [63, 64]. CRAC channels activation by thapsigargin significantly reduces A-type currents in dorsal horn neurons. These effects are absent in Orai1 KO neurons [23], suggesting a functional link between Orai1 and potassium channel Kv4.2.
3.2. Modulation of neuronal excitability and synaptic transmission
A-type potassium channels are important regulators of neuronal excitability. Downregulation of A-type currents has been shown increasing neuronal excitability [65, 66]. We have reported that activation of CRAC channels induces a membrane depolarization and enhances action potential firing in DRG and dorsal horn neurons by patch clamp in both cultured neurons and spinal cord slices [24, 28]. A similar result was also observed in gonadotropin-releasing hormone (GnRH) neurons [67]. A recent study has demonstrated that deletion of STIM1 reduces spontaneous and evoked firing and impairs intrinsic plasticity in Purkinje neurons [40], further indicating that SOCE is involved in the regulation of neuronal excitability. Additionally, Tobin et al have found that SOCE mediates receptor activation-induced an increase in action potential firing in supraoptic nucleus neurons [68]. In contrast, a previous study has shown that activation of CRAC channels reduces intrinsic excitability by increasing the density of functional hyperpolarization-activated cation-nonspecific (H) channels in hippocampal CA1 pyramidal neurons [69], indicating that SOCE regulates neuronal excitability in a neuron-type specific manner.
Voltage-gated Ca2+ channels are crucial for synaptic transmission. Several lines of observations indicate that CRAC signal also are implicated in synaptic plasticity. CRAC channels and STIM proteins are distributed in soma, dendrites and post-synaptic dendritic spines of cortical, hippocampal and Purkinje neurons [38, 70, 71]. Depletion of calcium stores by cyclopiazonic acid (an inhibitor of ER Ca2+/ATPases) increases spontaneous transmitter release [72]. CRAC channel inhibitors attenuate tetanus-induced dendritic Ca2+ accumulation and long-term potentiation at Schaffer collateral-CA1 synapses in hippocampal neurons [73]. SOCE appears to facilitate long-term potentiation (LTP) induced by DHPG, a group I metabotropic glutamate (mGluR) agonist [74]. In Purkinje neurons, STIM1 regulates mGluR1/TRPC3-dependent slow excitatory synaptic potentials [38]. Furthermore, activation of STIM2-mediated SOCE rescues hippocampal long-term potentiation impairment in a mouse model of Alzheimer's disease [41].
3.3. Cytokine production in astrocytes and microglia
Increasing evidence has shown that SOCE is an important calcium signal in glial cells [25, 52, 75, 76]. Both astrocytes and microglia produce proinflammatory cytokines and chemokines in response to stimulation in vitro or under pathological conditions [77-79], but the underlying mechanisms are unclear. Our recent study has found that direct activation of CRAC channels by TG increases proinflammatory cytokine production in cultured spinal astrocytes, which is significantly reduced by CRAC channels inhibitors, YM-58483 and Gd3+ [25]. SOCE is also involved in LPS-induced cytokine secretion of TNF-α and IL-6 in microglia and astrocytes [25, 51, 80]. Knockdown Orai1 or STIM1 by specific siRNA dramatically attenuates IL-6 and TNF-α secretion from microglia and astrocytes [25, 51]. However, the intracellular signaling pathways involved in this process have not be defined. Understanding how CRAC channels are involved in cytokine production under pathophysiological conditions will provide valuable insight for the development of novel therapeutics to treat disorders associated with pain and CNS inflammation.
4. CRAC channels in pain
4.1. Nociception
Nociception is the neural response to harmful or potentially harmful stimuli. Nociceptive pain occurs when nociceptors in the body detect noxious stimuli. Whether CRAC channels are directly activated by noxious stimuli is not known. We have found that inhibition of CRAC channels and the knockout of Orai1 have been shown to attenuate acute pain induced by noxious mechanical and thermal stimuli, but not non-noxious or mild stimuli [23, 25]. Capsaicin, the major components of chili peppers, activates the transient receptor potential vanilloid 1 (TRPV1) channel and mediates nociception. Intraplantar injection of capsaicin induces a robust spontaneous nociceptive response [29], which is pharmacologically blocked by YM-58483 [29]. The formalin test is commonly used as a test of nociception in rodents. Injection of formalin into the hind paw results in a typical biphasic nociceptive response. YM-58483 markedly decreases the first phase and eliminates the second phase of formalin-induced pain [29]. Although YM-58483 has relative high selectivity to CRAC channels, it also inhibits TRPC3 at the same dose [81]. Nevertheless, involvement of CRAC channels in nociception has been confirmed in Orai1 knockout mice [23].
4.2. Pathological pain
Pathological pain is caused by lesions to nerves and through tissue damage and is characterized by exaggerated pain sensitivity [82]. The underlying molecular mechanisms remain to be defined. Peripheral and central sensitization is an important mechanism underlying pathological pain [83]. It is mainly mediated by neuronal plasticity and modulated by activity of glial cells [46, 84]. The importance of Ca2+ in neuronal plasticity has been well recognized. Activated glia upregulate Ca2+ permeable ion channels as well as receptors, which lead to activate intracellular signaling pathways and increase production of cytokines, chemokines and growth factors [46]. Those glial mediators regulate synaptic contracts and neuronal plasticity [46]. As discussed above, CRAC channels are involved in cytokine production in cultured astrocytes and microglia. They may also contribute to central sensitization by enhancing cytokine release in the spinal cord under pathological conditions.
Peripheral inflammation is a response triggered by tissue damage. Inflammatory pain is associated with inflammatory mediators released at the site of inflammation or infection. Systemic administration of YM-58483 dramatically reduces paw edema and the production of proinflammatory and inflammatory mediators including TNF-α, IL-1β and Prostaglandin E2 (PGE2) induced by hind paw injection of complete Freund adjuvant (CFA), a commonly used inflammatory pain model [29]. Pretreatment with YM-58483 prevents development of collagen-induced arthritis (CIA) in DBA1 mice and decreases TNF-α, IL-1β, and IL-6 production in inflamed paws [30]. Administration of YM-58483 after onset of CIA also reduces arthritis symptoms, joint destruction and improves motor function in CIA mice [30]. As expected, inhibition of CRAC channels dramatically attenuates CFA- and collagen-induced thermal hyperalgesia and mechanical allodynia in a dose-dependent manner [29, 30]. Additionally, YM-58483 also relieves mechanical and thermal hypersensitivity induced by spared nerve injury (SNI) [29, 85], a well-established neuropathic pain model. Interestingly, SOCE and CRAC currents are increased after axonal injury by spinal nerve ligation (SNL) [5]. These findings suggest that CRAC channels may play a role in chronic pain. Recently, using Orai1 knockout mice, we have demonstrated that Orai1 deficiency greatly reduces formalin-and carrageenan-induced inflammatory pain in the ERK-dependent manner [23]. These data further confirm that the CRAC channel Orai1 is an important player in pain plasticity.
4.3. Other CNS diseases associated with pain
Pain is a common comorbid symptom associated with several CNS disorders such as Parkinson’s disease, Alzheimer's disease (AD), spinal cord injury (SCI) and depression [86-88]. Neuronal Ca2+ dyshomeostasis and loss of mushroom spines are associated with cognitive decline in AD [89]. STIM2-mediated SOCE is necessary for mushroom spines stability [90]. Recently, downregulation of STIM2 and the impaired synaptic SOCE were observed in a mouse model of AD [90, 91]. Pretreatment with NSN 21778, a CRAC channel positive modulator, rescues SOCE in hippocampal neurons and mushroom spines in both presenilin and APP knock-in AD mouse models [41], suggesting that CRAC channels may represent potential therapeutic targets for AD. Up to the present, the role of CRAC channels in other CNS diseases including SCI has not been explored. Since microglia and astrocytes are involved in the inflammatory events in SCI and CRAC channels mediate important Ca2+ signal and inflammatory responses in these cells [92], future studies of CRAC channels in diseases associated with inflammation in the CNS will help to bridge this gap.
5. Conclusion and prospects
Although CRAC channels have emerged as an important regulator of Ca2+ signaling in neurons and glial cells, their signaling pathways and regulation are largely unknown. There is limited data about the involvement of CRAC channels in murine models of CNS diseases or human specimens under pathological conditions. Our recent studies have revealed that the CRAC channel Orai1 plays an important role in nociception and chronic pain. We have begun to understand how Orai1 is involved in pain (Figure 2). STIM1 and STIM2 are important contributors to SOCE. Their specific roles in pain have not been evaluated. Neuropathic and bone cancer pain conditions are very debilitating and poorly managed. Efforts are needed to develop better analgesics to treat these types of pain. Investigation of involvement of CRAC channels in neuropathic and bone cancer pain will provide further evidence supporting the possibility that CRAC channels may be used as potential drug targets for debilitating chronic pain.
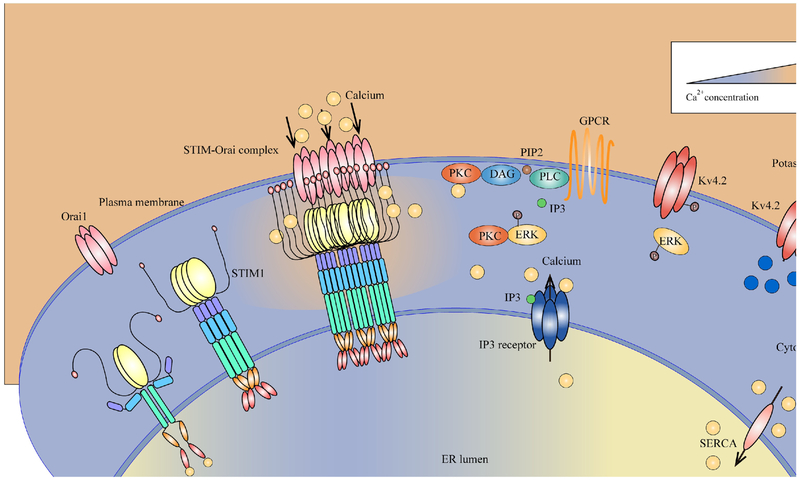
Figure 2. Current model of CRAC channels signaling in dorsal horn neurons.
Under resting condition, STIM1 (as an example) is distributed at the endoplasmic reticulum (ER) membranes in a dimeric form while Ca2+ is bound to STIM ER-luminal domains. When Ca2+ are released from (ER) by IP3 receptor activation or inhibition of SERCA, STIM1 loses Ca2+-binding, multimerizes, translocates to ER–plasma membrane (PM) junctions, where it activates Orai1 and induces Ca2+ entry. Increased cytosolic Ca2+ through CRAC channels promotes PKC activation, and then activates its downstream effector ERK. Activated ERK further phosphorylates Kv4.2 potassium channels, suppressing K+ efflux.
Highlight.
The store-operated calcium channel family is expressed in neurons and glial cells at different levels along the pain pathway
Calcium release-activated calcium channels play an important role in cytokine production in glial cells
Orai1-mediated SOCE modulates A-type potassium channels in neurons and pain
Acknowledgements:
This work was supported by NIH Grants R21NS077330 and R01NS087033 (to H.H.).
Footnotes
Conflict of Interest:
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Folander K, Bennett P, Kiss L, Expression of voltage-gated calcium channels in primate dorsal root ganglion neurons, BIOPHYSICAL JOURNAL, BIOPHYSICAL SOCIETY 9650 ROCKVILLE PIKE, BETHESDA, MD 20814–3998 USA, 2002, pp. 252A–252A. [Google Scholar]
- [2].Simms BA, Zamponi GW, Neuronal voltage-gated calcium channels: structure, function, and dysfunction, Neuron, 82 (2014) 24–45. [DOI] [PubMed] [Google Scholar]
- [3].Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo, Journal of Neuroscience, 27 (2007) 2846–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu H, Wang H, Sheng M, Jan L, Jan Y, Basbaum A, Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn, Proceedings of the National Academy of Sciences, 91 (1994) 8383–8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gemes G, Bangaru MLY, Wu H-E, Tang Q, Weihrauch D, Koopmeiners AS, Cruikshank JM, Kwok W-M, Hogan QH, Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury, Journal of Neuroscience, 31 (2011) 3536–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, Kuznicki J, Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons, PloS one, 6 (2011) e19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Skibinska-Kijek A, Wisniewska MB, Gruszczynska-Biegala J, Methner A, Kuznicki J, Immunolocalization of STIM1 in the mouse brain, Acta Neurobiol Exp (Wars), 69 (2009) 413–428. [DOI] [PubMed] [Google Scholar]
- [8].Kraft R, STIM and ORAI proteins in the nervous system, Channels, 9 (2015) 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parekh AB, Store-operated CRAC channels: function in health and disease, Nature reviews Drug discovery, 9 (2010) 399. [DOI] [PubMed] [Google Scholar]
- [10].Lacruz RS, Feske S, Diseases caused by mutations in ORAI1 and STIM1, Annals of the New York Academy of Sciences, 1356 (2015) 45–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xie J, Pan H, Yao J, Zhou Y, Han W, SOCE and cancer: recent progress and new perspectives, International journal of cancer, 138 (2016) 2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Braun A, Vogtle T, Varga-Szabo D, Nieswandt B, STIM and Orai in hemostasis and thrombosis, Frontiers in bioscience (Landmark edition), 16 (2011) 2144–2160. [DOI] [PubMed] [Google Scholar]
- [13].Putney JW Jr, A model for receptor-regulated calcium entry, Cell calcium, 7 (1986) 1–12. [DOI] [PubMed] [Google Scholar]
- [14].Vig M, Peinelt C, Beck A, Koomoa D, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry, Science, 312 (2006) 1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrell JE Jr, Meyer T, STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx, Current biology, 15 (2005) 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prakriya M, Lewis RS, Store-operated calcium channels, Physiological reviews, 95 (2015) 1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Williams RT, Manji S, Parker NJ, Hancock MS, Van Stekelenburg L, Eid J-P, Senior PV, Kazenwadel JS, Shandala T, Saint R, Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins, Biochemical Journal, 357 (2001) 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS, Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation, Nature, 454 (2008) 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brandman O, Liou J, Park WS, Meyer T, STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels, Cell, 131 (2007) 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Félix R, Crottès D, Delalande A, Fauconnier J, Lebranchu Y, Le Guennec J-Y, Velge-Roussel F, The Orai-1 and STIM-1 complex controls human dendritic cell maturation, PloS one, 8 (2013) e61595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T, Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses, Nature immunology, 9 (2008) 81. [DOI] [PubMed] [Google Scholar]
- [22].Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Hair loss and defective T-and B-cell function in mice lacking ORAI1, Molecular and cellular biology, 28 (2008) 5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dou Y, Xia J, Gao R, Gao X, Munoz FM, Wei D, Tian Y, Barrett JE, Ajit S, Meucci O, Orai1 plays a crucial role in central sensitization by modulating neuronal excitability, Journal of Neuroscience, 38 (2018) 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xia J, Pan R, Gao X, Meucci O, Hu H, Native store-operated calcium channels are functionally expressed in mouse spinal cord dorsal horn neurons and regulate resting calcium homeostasis, J Physiol, 592 (2014) 3443–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gao X, Xia J, Munoz FM, Manners MT, Pan R, Meucci O, Dai Y, Hu H, STIMs and Orai1 regulate cytokine production in spinal astrocytes, Journal of neuroinflammation, 13 (2016) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Michaelis M, Nieswandt B, Stegner D, Eilers J, Kraft R, STIM1, STIM2, and Orai1 regulate store-operated calcium entry and purinergic activation of microglia, Glia, 63 (2015) 652–663. [DOI] [PubMed] [Google Scholar]
- [27].Ohana L, Newell EW, Stanley EF, Schlichter LC, The Ca2+ release-activated Ca2+ current (ICRAC) mediates store-operated Ca2+ entry in rat microglia, Channels, 3 (2009) 129–139. [DOI] [PubMed] [Google Scholar]
- [28].Wei D, Mei Y, Xia J, Hu H, Orai1 and Orai3 mediate store-operated calcium entry contributing to neuronal excitability in dorsal root ganglion neurons, Frontiers in cellular neuroscience, 11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gao R, Gao X, Xia J, Tian Y, Barrett JE, Dai Y, Hu H, Potent analgesic effects of a store-operated calcium channel inhibitor, PAIN®, 154 (2013) 2034–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gao X, Gao R, Tian Y, McGonigle P, Barrett J, Dai Y, Hu H, A store-operated calcium channel inhibitor attenuates collagen-induced arthritis, British journal of pharmacology, 172 (2015) 2991–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schindl R, Frischauf I, Bergsmann J, Muik M, Derler I, Lackner B, Groschner K, Romanin C, Plasticity in Ca2+ selectivity of Orai1/Orai3 heteromeric channel, Proc Natl Acad Sci U S A, 106 (2009) 19623–19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gonzalez-Cobos JC, Zhang X, Zhang W, Ruhle B, Motiani RK, Schindl R, Muik M, Spinelli AM, Bisaillon JM, Shinde AV, Fahrner M, Singer HA, Matrougui K, Barroso M, Romanin C, Trebak M, Store-independent Orai1/3 channels activated by intracrine leukotriene C4: role in neointimal hyperplasia, Circ Res, 112 (2013) 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW, Calcium-permeable ion channels in pain signaling, Physiological reviews, 94 (2014) 81–140. [DOI] [PubMed] [Google Scholar]
- [34].Zufall F, Leinders-Zufall T, Greer CA, Amplification of odor-induced Ca2+ transients by store-operated Ca2+ release and its role in olfactory signal transduction, Journal of Neurophysiology, 83 (2000) 501–512. [DOI] [PubMed] [Google Scholar]
- [35].Bouron A, Activation of a capacitative Ca2+ entry pathway by store depletion in cultured hippocampal neurones, FEBS letters, 470 (2000) 269–272. [DOI] [PubMed] [Google Scholar]
- [36].Prothero L, Mathie A, Richards C, Purinergic and muscarinic receptor activation activates a common calcium entry pathway in rat neocortical neurons and glial cells, Neuropharmacology, 39 (2000) 1768–1778. [DOI] [PubMed] [Google Scholar]
- [37].Singaravelu K, Lohr C, Deitmer JW, Regulation of store-operated calcium entry by calcium-independent phospholipase A2 in rat cerebellar astrocytes, Journal of Neuroscience, 26 (2006) 9579–9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hartmann J, Karl RM, Alexander RP, Adelsberger H, Brill MS, Rühlmann C, Ansel A, Sakimura K, Baba Y, Kurosaki T, STIM1 controls neuronal Ca 2+signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior, Neuron, 82 (2014) 635–644. [DOI] [PubMed] [Google Scholar]
- [39].Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B, STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death, Sci Signal, 2 (2009) ra67. [DOI] [PubMed] [Google Scholar]
- [40].Ryu C, Jang DC, Jung D, Kim YG, Shim HG, Ryu HH, Lee YS, Linden DJ, Worley PF, Kim SJ, STIM1 Regulates Somatic Ca(2+) Signals and Intrinsic Firing Properties of Cerebellar Purkinje Neurons, J Neurosci, 37 (2017) 8876–8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang H, Sun S, Wu L, Pchitskaya E, Zakharova O, Fon Tacer K, Bezprozvanny I, Store-Operated Calcium Channel Complex in Postsynaptic Spines: A New Therapeutic Target for Alzheimer's Disease Treatment, J Neurosci, 36 (2016) 11837–11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Guzman R, Valente EG, Pretorius J, Pacheco E, Qi M, Bennett BD, Fong DH, Lin F-F, Bi V, McBride HJ, Expression of ORAI1, a plasma membrane resident subunit of the CRAC channel, in rodent and non-rodent species, Journal of Histochemistry & Cytochemistry, 62 (2014) 864–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chauvet S, Jarvis L, Chevallet M, Shrestha N, Groschner K, Bouron A, Pharmacological Characterization of the Native Store-Operated Calcium Channels of Cortical Neurons from Embryonic Mouse Brain, Frontiers in pharmacology, 7 (2016) 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Milligan ED, Watkins LR, Pathological and protective roles of glia in chronic pain, Nature reviews neuroscience, 10 (2009) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Scholz J, Woolf CJ, The neuropathic pain triad: neurons, immune cells and glia, Nature neuroscience, 10 (2007) 1361. [DOI] [PubMed] [Google Scholar]
- [46].Ji R-R, Berta T, Nedergaard M, Glia and pain: is chronic pain a gliopathy?, Pain®, 154 (2013) S10–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gerard E, Spengler RN, Bonoiu AC, Mahajan SD, Davidson BA, Ding H, Kumar R, Prasad PN, Knight PR, Ignatowski TA, Chronic constriction injury-induced nociception is relieved by nanomedicine-mediated decrease of rat hippocampal tumor necrosis factor, Pain, 156 (2015) 1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Park KS, Kim SH, Das A, Yang S-N, Jung KH, Kim MK, Berggren P-O, Lee Y, Chai JC, Kim HJ, TLR3-/4-priming differentially promotes Ca 2+ signaling and cytokine expression and Ca 2+-dependently augments cytokine release in hMSCs, Scientific reports, 6 (2016) 23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A, Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance, Nat Immunol, 9 (2008) 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Maul-Pavicic A, Chiang SC, Rensing-Ehl A, Jessen B, Fauriat C, Wood SM, Sjoqvist S, Hufnagel M, Schulze I, Bass T, Schamel WW, Fuchs S, Pircher H, McCarl CA, Mikoshiba K, Schwarz K, Feske S, Bryceson YT, Ehl S, ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis, Proc Natl Acad Sci U S A, 108 (2011) 3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Heo DK, Lim HM, Nam JH, Lee MG, Kim JY, Regulation of phagocytosis and cytokine secretion by store-operated calcium entry in primary isolated murine microglia, Cellular signalling, 27 (2015) 177–186. [DOI] [PubMed] [Google Scholar]
- [52].Kwon J, An H, Sa M, Won J, Shin JI, Lee CJ, Orai1 and Orai3 in Combination with Stim1 Mediate the Majority of Store-operated Calcium Entry in Astrocytes, Exp Neurobiol, 26 (2017) 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ronco V, Grolla AA, Glasnov TN, Canonico PL, Verkhratsky A, Genazzani AA, Lim D, Differential deregulation of astrocytic calcium signalling by amyloid-beta, TNFalpha, IL-1beta and LPS, Cell Calcium, 55 (2014) 219–229. [DOI] [PubMed] [Google Scholar]
- [54].Prakriya M, The molecular physiology of CRAC channels, Immunological reviews, 231 (2009) 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Srikanth S, Gwack Y, Orai1-NFAT signalling pathway triggered by T cell receptor stimulation, Molecules and cells, 35 (2013) 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Denys A, Aires V, Hichami A, Khan NA, Thapsigargin-stimulated MAP kinase phosphorylation via CRAC channels and PLD activation: inhibitory action of docosahexaenoic acid, FEBS letters, 564 (2004) 177–182. [DOI] [PubMed] [Google Scholar]
- [57].Liu X, Berry CT, Ruthel G, Madara JJ, MacGillivray K, Gray CM, Madge LA, McCorkell KA, Beiting DP, Hershberg U, T cell receptor-induced nuclear factor κB (NF-κB) signaling and transcriptional activation are regulated by STIM1-and Orai1-mediated calcium entry, Journal of Biological Chemistry, 291 (2016) 8440–8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Somasundaram A, Shum AK, McBride HJ, Kessler JA, Feske S, Miller RJ, Prakriya M, Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells, Journal of Neuroscience, 34 (2014) 9107–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Simo-Cheyou ER, Tan JJ, Grygorczyk R, Srivastava AK, STIM-1 and ORAI-1 channel mediate angiotensin-II-induced expression of Egr-1 in vascular smooth muscle cells, Journal of cellular physiology, 232 (2017) 3496–3509. [DOI] [PubMed] [Google Scholar]
- [60].Soltoff SP, Lannon WA, Activation of ERK1/2 by store-operated calcium entry in rat parotid acinar cells, PloS one, 8 (2013) e72881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ji R-R, Gereau IV RW, Malcangio M, Strichartz GR, MAP kinase and pain, Brain research reviews, 60 (2009) 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hu H-J, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RW, The kv4. 2 potassium channel subunit is required for pain plasticity, Neuron, 50 (2006) 89–100. [DOI] [PubMed] [Google Scholar]
- [63].Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD, The A-Type Potassium Channel Kv4.2 Is a Substrate for the Mitogen-Activated Protein Kinase ERK, Journal of neurochemistry, 75 (2000) 2277–2287. [DOI] [PubMed] [Google Scholar]
- [64].Hu H-J, Glauner KS, Gereau IV RW, ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents, Journal of neurophysiology, 90 (2003) 1671–1679. [DOI] [PubMed] [Google Scholar]
- [65].Hu HJ, R.W.t. Gereau, ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability, J Neurophysiol, 90 (2003) 1680–1688. [DOI] [PubMed] [Google Scholar]
- [66].Viatchenko-Karpinski V, Ling J, Gu JG, Down-regulation of Kv4.3 channels and a-type K(+) currents in V2 trigeminal ganglion neurons of rats following oxaliplatin treatment, Mol Pain, 14 (2018) 1744806917750995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].LeBeau AP, Van Goor F, Stojilkovic SS, Sherman A, Modeling of membrane excitability in gonadotropin-releasing hormone-secreting hypothalamic neurons regulated by Ca2+-mobilizing and adenylyl cyclase-coupled receptors, J Neurosci, 20 (2000) 9290–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tobin V, Gouty LA, Moos FC, Desarmenien MG, A store-operated current (SOC) mediates oxytocin autocontrol in the developing rat hypothalamus, Eur J Neurosci, 24 (2006) 400–404. [DOI] [PubMed] [Google Scholar]
- [69].Narayanan R, Dougherty KJ, Johnston D, Calcium store depletion induces persistent perisomatic increases in the functional density of h channels in hippocampal pyramidal neurons, Neuron, 68 (2010) 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Moccia F, Zuccolo E, Soda T, Tanzi F, Guerra G, Mapelli L, Lodola F, D'Angelo E, Stim and Orai proteins in neuronal Ca(2+) signaling and excitability, Front Cell Neurosci, 9 (2015) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ng AN, Krogh M, Toresson H, Dendritic EGFP-STIM1 activation after type I metabotropic glutamate and muscarinic acetylcholine receptor stimulation in hippocampal neuron, J Neurosci Res, 89 (2011) 1235–1244. [DOI] [PubMed] [Google Scholar]
- [72].Emptage NJ, Reid CA, Fine A, Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release, Neuron, 29 (2001) 197–208. [DOI] [PubMed] [Google Scholar]
- [73].Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y, Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity, J Neurosci, 23 (2003) 7737–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mellentin C, Jahnsen H, Abraham WC, Priming of long-term potentiation mediated by ryanodine receptor activation in rat hippocampal slices, Neuropharmacology, 52 (2007) 118–125. [DOI] [PubMed] [Google Scholar]
- [75].Papanikolaou M, Lewis A, Butt A, Store-operated calcium entry is essential for glial calcium signalling in CNS white matter, Brain Structure and Function, 222 (2017) 2993–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Moreno C, Sampieri A, Vivas O, Peña-Segura C, Vaca L, STIM1 and Orai1 mediate thrombin-induced Ca2+ influx in rat cortical astrocytes, Cell calcium, 52 (2012) 457–467. [DOI] [PubMed] [Google Scholar]
- [77].Chen J-J, Dai L, Zhao L-X, Zhu X, Cao S, Gao Y-J, Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis, Scientific reports, 5 (2015) 10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nitkiewicz J, Borjabad A, Morgello S, Murray J, Chao W, Emdad L, Fisher PB, Potash MJ, Volsky DJ, HIV induces expression of complement component C3 in astrocytes by NF-κB-dependent activation of interleukin-6 synthesis, Journal of neuroinflammation, 14 (2017) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Khaibullin T, Ivanova V, Martynova E, Cherepnev G, Khabirov F, Granatov E, Rizvanov A, Khaiboullina S, Elevated levels of proinflammatory cytokines in cerebrospinal fluid of multiple sclerosis patients, Frontiers in immunology, 8 (2017) 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li J-H, Zhao S-T, Wu C-Y, Cao X, Peng M-R, Li S-J, Liu X-A, Gao T-M, Store-operated Ca 2+ channels blockers inhibit lipopolysaccharide induced astrocyte activation, Neurochemical research, 38 (2013) 2216–2226. [DOI] [PubMed] [Google Scholar]
- [81].Qu L, Li Y, Pan X, Zhang P, LaMotte RH, Ma C, Transient receptor potential canonical 3 (TRPC3) is required for IgG immune complex-induced excitation of the rat dorsal root ganglion neurons, Journal of Neuroscience, 32 (2012) 9554–9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Woolf CJ, What is this thing called pain?, The Journal of clinical investigation, 120 (2010) 3742–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ji R-R, Kohno T, Moore KA, Woolf CJ, Central sensitization and LTP: do pain and memory share similar mechanisms?, Trends in neurosciences, 26 (2003) 696–705. [DOI] [PubMed] [Google Scholar]
- [84].Kuner R, Central mechanisms of pathological pain, Nat Med, 16 (2010) 1258–1266. [DOI] [PubMed] [Google Scholar]
- [85].Qi Z, Wang Y, Zhou H, Liang N, Yang L, Liu L, Zhang W, The central analgesic mechanism of YM-58483 in attenuating neuropathic pain in rats, Cellular and molecular neurobiology, 36 (2016) 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lee JY, Choi HY, Ju BG, Yune TY, Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation, Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, (2018). [DOI] [PubMed] [Google Scholar]
- [87].Beach PA, Huck JT, Miranda MM, Bozoki AC, Autonomic, behavioral, and subjective pain responses in Alzheimer's disease, Pain Medicine, 16 (2015) 1930–1942. [DOI] [PubMed] [Google Scholar]
- [88].Agüera-Ortiz L, Failde I, Mico J, Cervilla J, Lopez-Ibor J, Pain as a symptom of depression: prevalence and clinical correlates in patients attending psychiatric clinics, Journal of Affective Disorders, 130 (2011) 106–112. [DOI] [PubMed] [Google Scholar]
- [89].Popugaeva E, Pchitskaya E, Bezprozvanny I, Dysregulation of neuronal calcium homeostasis in Alzheimer's disease–A therapeutic opportunity?, Biochemical and biophysical research communications, 483 (2017) 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sun S, Zhang H, Liu J, Popugaeva E, Xu N-J, Feske S, White CL, Bezprozvanny I, Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice, Neuron, 82 (2014) 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T, Bezprozvanny I, Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer's disease, Journal of Neuroscience, 35 (2015) 13275–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Anwar MA, Al Shehabi TS, Eid AH, Inflammogenesis of secondary spinal cord injury, Frontiers in cellular neuroscience, 10 (2016) 98. [DOI] [PMC free article] [PubMed] [Google Scholar]