Abstract
2,5-Dimethoxyphenethylamines (2C compounds) are 5-HT2A/2C receptor agonists that induce hallucinogenic effects. N-methoxybenzylation of 2C compounds markedly increases their affinity for 5-HT2A receptors, and two such analogs, 2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25C-NBOMe) and 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe), have emerged in recreational drug markets. Here, we investigated the neuropharmacology of 25C-NBOMe and 25I-NBOMe in rats, as compared to their 2C analogs and the prototypical 5-HT2A/2C agonist 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine (DOI). Compounds were tested in vitro using 5-HT2A receptor binding and calcium mobilization assays. For in vivo experiments, 25C-NBOMe (0.01–0.3 mg/kg), 25I-NBOMe (0.01–0.3 mg/kg), 2-(4-chloro-2,5-dimethoxyphenyl)ethanamine (2C-C) (0.1–3.0 mg/kg), 2-(4-iodo-2,5-dimethoxyphenyl)ethanamine (2C-I) (0.1–3.0 mg/kg) and DOI (0.03–1.0 mg/kg) were administered subcutaneously (sc) to male rats, and 5-HT2A-mediated behaviors were assessed. NBOMes displayed higher affinity for 5-HT2A receptors than their 2C counterparts but were substantially weaker in functional assays. 25C-NBOMe and 25I-NBOMe were much more potent at inducing wet dog shakes (WDS) and back muscle contractions (BMC) when compared to 2C-C and 2C-I. Pretreatment with the selective 5-HT2A antagonist (R)-(2,3-dimethoxyphenyl){1-[2-(4-fluorophenyl)ethyl]-4-piperidinyl}methanol (M100907) reversed behaviors produced by all agonists. Interestingly, binding affinities at the 5-HT2A receptor were significantly correlated with potencies to induce BMC but not WDS. Our findings show that NBOMes are highly potent 5-HT2A agonists in rats, similar to effects in mice, and consistent with the reported hallucinogenic effects in human users.
Keywords: 5-HT2A receptor, back muscle contractions, NBOMe, wet dog shakes
Graphical abstract
1. Introduction
Serotonergic hallucinogens such as lysergic acid diethylamide (LSD) induce marked changes in sensation, perception and conscious experience, primarily through activation of 5-HT2A receptors in the brain (Nichols, 2016). One class of serotonergic hallucinogens is the phenylalkylamines, which can be further divided into phenylisopropylamines like 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine (DOI) and phenethylamines like 2-(4-iodo-2,5-dimethoxyphenyl)ethanamine (2C-I) (Halberstadt, 2015) (see Figure 1). 2C-I and its chloro analog, 2-(4-chloro-2,5-dimethoxyphenyl)ethanamine (2C-C), are phenethylamine compounds first described in the early 1990s by Shulgin and Shulgin (Shulgin and Shulgin, 1991). Subsequent investigations found that 2C-C and 2C-I bind to 5-HT2A and 5-HT2C receptors with nM affinity and engender LSD-like discriminative stimulus effects in rats (Eshleman et al. 2014). Administration of 2C-I to mice causes a dose-dependent increase in head twitch response that is blocked by pretreatment with the selective 5-HT2A antagonist (R)-(2,3-dimethoxyphenyl){1-[2-(4-fluorophenyl)ethyl]-4-piperidinyl}methanol (M100907) (Canal and Morgan, 2012; Halberstadt and Geyer, 2014). In recent times, the misuse of 2C-C and 2C-I by humans has increased, and life-threatening adverse effects have been described (Bosak et al., 2013; Dean et al., 2013; U.S. Drug Enforcement Administration, 2017). The Synthetic Drug Abuse Prevention Act of 2012 placed 2C-C, 2C-I and a number of related phenethylamines under schedule I control (Drug Enforcement Administration, 2013).
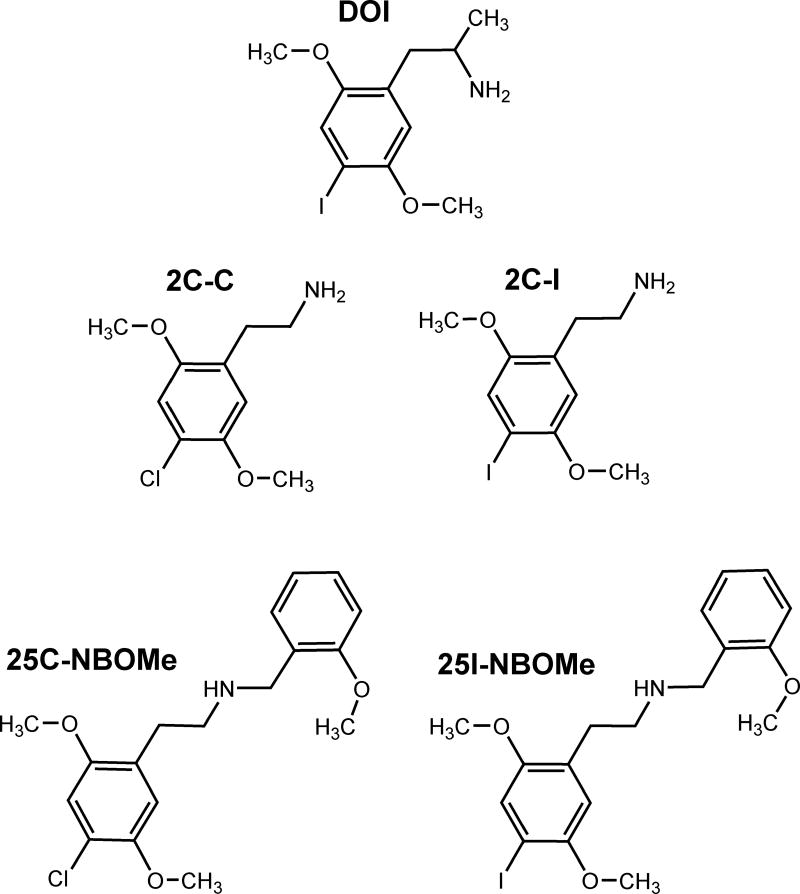
Figure 1.
Chemical structures of 1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine (DOI); 2-(4-chloro-2,5-dimethoxyphenyl)ethanamine (2C-C), and its N-methoxybenzylated derivative 2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25C-NBOMe); and 2-(4-iodo-2,5-dimethoxyphenyl)ethanamine (2C-I), and its N-methoxybenzylated derivative 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe).
In 1994, Glennon et al. first reported that N-benzylation of 2,5-dimethoxyphenethylamine compounds dramatically enhances their 5-HT2A receptor affinity and improves selectivity over other 5-HT receptor subtypes (Glennon et al., 1994). Various N-benzylated phenethylamines have been developed as radiotracers and positron emission tomography imaging agents, leading to the discovery of 2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25C-NBOMe) and 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe) (referred to collectively as NBOMes) (see Figure 1) (Heim, 2004; Braden et al., 2006; Ettrup et al., 2011). NBOMes display sub-nM affinities for the 5-HT2A receptor, with 25C-NBOMe and 25I-NBOMe exhibiting 18-fold and 6-fold greater affinities for human 5-HT2A receptors when compared to 2C-C and 2C-I, respectively (Rickli et al., 2015). In 2011, the United States (US) Drug Enforcement Administration (DEA) reported the first law enforcement encounters of 25I-NBOMe, and one year later Polish police seized blotter papers containing 25C-NBOMe, indicating these two compounds were available as new psychoactive substances (NPS) in the US and Europe (Zuba et al., 2013; U.S. Drug Enforcement Administration, 2017). Between 2011 and 2015, there were nearly 5,000 law enforcement encounters of 25I-NBOMe and 25C-NBOMe in the US alone (U.S. Drug Enforcement Administration, 2017).
The first analytically confirmed human exposure to 25I-NBOMe occurred in 2012, and many cases have been documented since that time (Kelly et al., 2012; Halberstadt, 2017). Typical adverse effects of NBOMe overdose include tachycardia, hypertension, delirium, and seizures, sometimes culminating in death (Suzuki et al., 2015; Halberstadt, 2017). NBOMes are regularly found on blotter paper, a method of drug distribution typically associated with LSD. In a study examining the contents of blotter papers seized in Brazil, 50% were found to contain NBOMes, suggesting the substances are being trafficked as counterfeit LSD (erowid.org; Coelho Neto, 2015). Patterns of drug use that are relatively safe for LSD may lead to unexpected medical complications, or even death, when NBOMe compounds are involved (Suzuki et al., 2014; Poklis et al., 2014). Insufflation and sublingual routes of powdered or liquid NBOMes are most commonly employed, since the substances are not orally active due to rapid first-pass metabolism in the liver (Leth-Peterson et al., 2014, 2016). In response to their illicit use, the DEA placed 25I-NBOMe and 25C-NBOMe into permanent schedule I control in 2016 (Drug Enforcement Administration 2013, 2016).
The preclinical pharmacology of NBOMe hallucinogens has not been well characterized, and only a few investigations have assessed the activity of 25C-NBOMe and 25I-NBOMe in vivo (Halberstadt and Geyer, 2014; Gatch et al., 2017). To this end, we sought to characterize the neuropharmacological effects of 25C-NBOMe and 25I-NBOMe in rats, as compared to their 2C counterparts and the prototypical 5-HT2A/2C agonist DOI. We first examined the effects of 25C-NBOMe, 25I-NBOMe, 2C-C, 2C-I and DOI on 5-HT2A receptor binding in native rat brain tissue, since prior studies only examined receptor binding in cells transfected with 5-HT2A receptors (Braden et al., 2006; Ettrup et al., 2011; Rickli et al., 2015). We then characterized the effects of subcutaneous (sc) administration of the compounds on traditional measures of 5-HT2A receptor function in rats, namely wet dog shakes (WDS) and back muscle contractions (BMC) (Pranzatelli, 1990; Wettstein, 1999; Higgins et al, 2001). As an additional aim, we wished to assess the utility of BMC as an in vivo read-out of 5-HT2A receptor activation in rats. Finally, pretreatment with M100907 was employed to verify 5-HT2A receptor involvement in mediating the non-contingent behaviors induced by the drugs.
2. Materials and Methods
2.1 Drugs and Reagents
[3H](R)-(2,3-dimethoxyphenyl){1-[2-(4-fluorophenyl)ethyl]-4-piperidinyl}methanol ([3H]M100907) (specific activity 80 Ci/mmol) was obtained from American Radiolabeled Chemicals (St. Louis, MO, USA) whereas (−)-1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine hydrochloride (DOI) was obtained from Sigma Aldrich (St. Louis, MO, USA). M100907 was synthesized by Dr. Sulima at the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP). 2-(4-chloro-2,5-dimethoxyphenyl)ethanamine hydrochloride (2C-C), 2-(4-iodo-2,5-dimethoxyphenyl)ethanamine hydrochloride (2C-I), 2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine hydrochloride (25C-NBOMe) and 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine hydrochloride (25I-NBOMe) were synthesized by Dr. Blough at Research Triangle Institute (RTI). M100907 was dissolved into a 1:9 mix of dimethyl sulfoxide:sterile saline. All other drugs were dissolved in sterile saline and injections were administered subcutaneously (sc) at a volume of 1.0 mL/kg.
2.2 Animals, Tissue Harvest and Surgery
Male Sprague-Dawley rats weighing 250–300 g were housed three per cage (lights on: 7:00 AM– 7:00 PM) under conditions of controlled temperature (22 ± 2°C) and humidity (45% ± 5%) with free access to food and water. Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Vivarium facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and study procedures were approved by the NIDA IRP Animal Care and Use Committee. Rats were allowed at least 2 weeks of acclimation to the vivarium facilities before being used for tissue harvest or behavioral experiments. For the radioligand binding experiments, rats were euthanized by CO2 narcosis and decapitated. Brains were excised and used immediately to prepare tissue for binding assays. For behavioral experiments, rats were briefly anesthetized using a drop jar containing a gauze pad saturated with 5 mL of isoflurane; a raised mesh floor prevented any contact between the animal and the gauze pad. Once anesthetized, each rat received a surgically-implanted IPTT-300 transponder (Bio Medic Data Systems, Seaford, DE, USA) to facilitate the non-invasive measurement of body temperature via a handheld radio frequency reader system. Transponders were 14 × 2 mm cylinders and were implanted subcutaneously (sc) posterior to the shoulder blades via a sterile guide needle. Animals were individually-housed post-operatively and allowed 7–10 days for recovery.
2.3 Radioligand Binding Assays
Each freshly-excised rat brain (minus cerebellum) was homogenized by twelve strokes of a hand-held teflon-on-glass homogenizer in 10 mL ice cold 0.25 M sucrose. The homogenate was centrifuged at 1000 × g for 10 min at 4° C. The resulting supernatant was diluted 40-fold with ice cold 50 mM Tris pH 7.4 and centrifuged at 35,000 × g for 10 min at 4° C. The pellet was resuspended in fresh ice cold 50 mM Tris pH 7.4 using a Polytron at 14,000 rpm for 10 sec, and the suspension was centrifuged at 35,000 × g for 10 min at 4° C. This pellet was resuspended by polytron in 10 mL ice cold 50 mM Tris pH 7.4 containing 0.5 mM disodium EDTA and 10 mM MgSO4 heptahydrate (assay buffer) for immediate use.
Binding assays were performed at equilibrium as described by Visser et al. with minor modifications (Visser et al., 2014). Assays were performed in triplicate in 12 × 75 mm polystyrene test tubes that contained 1 nM [3H]M100907 and test drug in 900 µL assay buffer. Incubations were initiated by the addition of 100 µL of tissue preparation to the assay tubes; incubations were carried out for 30 min at 37° C and terminated by rapid vacuum filtration using a manifold (Brandel, Gaithersburg, MD, USA) fitted with GF/B filters that were presoaked in wash buffer (ice cold 10 mM TRIS pH 7.4) containing 1% polyethylenimine. Filters were immediately rinsed with 6 mL wash buffer and were punched into 24-well flexplates (PerkinElmer, Waltham, MA, USA). Radioactivity retained on the filters was quantified by a MicroBeta2 scintillation counter (PerkinElmer) at 40% efficiency after 15 h in 500 µL Cytoscint (MP Biomedicals, Santa Ana, CA, USA) per well. Nonspecific binding was determined in the presence of 10 µM ketanserin. Under these conditions, specific binding of [3H]M100907 was proportional to the amount of rat brain membrane added (data not shown). Dose-response curves for inhibition of radioligand binding were assayed in triplicate, for N=3 separate experiments. Effects of test drugs on [3H]M100907 binding were expressed as % inhibition of specific binding versus log of drug concentration. Data were analyzed by nonlinear regression of 8-point dose-response curves using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). Dose-response values for binding inhibition were fit to the equation, Y(x) = Ymin + (Ymax − Ymin) / (1+ 10exp[(logP50 − logx)] * n), where x is the concentration of the compound tested, Y(x) is the response measured, Ymax is the maximal response, P50 is the IC50 (the concentration that yields half-maximal binding inhibition), and n is the Hill slope parameter.
2.4 Calcium Mobilization Assays
HEK293 cell lines stably expressing human 5-HT2A or 5-HT2C receptors were maintained in DMEM-HG supplemented with 10% fetal bovine serum, 100 units of penicillin/streptomycin, 15 mM HEPES, and 400 µg/mL G418. The day prior to assay, cells were plated into 96-well black-walled assay plates at 40,000 cells/well (5-HT2A) or 35,000 cells/well (5-HT2C) in 100 µL of growth medium without G418. The cells were incubated overnight at 37°C in 5% CO2. On the following day (test day), Calcium 5 dye (Molecular Devices, Sunnyvale, CA, USA) was reconstituted per the manufacturer instructions and diluted 1:40 in pre-warmed 37°C assay buffer (1× HBSS, 20 mM HEPES, 2.5 mM probenecid, pH 7.4 at 37°C). Growth medium was removed from the assay plates and the cells were gently washed with 100 µL of pre-warmed assay buffer. The cells were incubated for 45 minutes at 37°C, 5% CO2 in 200 µL of the diluted dye. For agonist (EC50) assays, serial dilutions of the test compounds were prepared at 10× the desired final concentration in 1% DMSO/assay buffer and aliquoted into 96-well polypropylene V-bottom plates. After the dye-loading incubation period, the cells were pretreated with 25 µL of 9% DMSO/assay buffer and incubated for 15 min at 37°C along with the compound plates. After the pretreatment incubation period, each plate was read with a FlexStation II (Molecular Devices). Calcium-mediated changes in fluorescence were monitored every 1.52 seconds over a 60 second time period, with the FlexStation II adding 25 µL of material from the compound plate at the 19 second time point (excitation at 485 nm, detection at 525 nm). Dose-response curves for calcium mobilization were assayed in duplicate, for at least N=3 separate experiments. Peak kinetic reduction (SoftMax, Molecular Devices) relative fluorescent units (RFU) were plotted against the log of drug concentration, and nonlinear regression analysis was used to generate EC50 values (GraphPad Software).
2.5 Dose-Response Effects of 5-HT2A/2C Agonists on Behavior
Separate cohorts of 12 rats were used to determine dose-response effects for DOI, 25C-NBOMe, 25I-NBOMe, 2C-C and 2C-I (60 rats total). Rats in each cohort were tested once per week for 4 consecutive weeks, until the 5 dose groups for the drug of interest (vehicle plus 4 drug doses) reached a completed N=9 rats/group (i.e., total N=45). At each test session, rats received a single sc injection of drug or vehicle, and doses were randomly assigned, with the exception that rats receiving the highest drug dose always received vehicle treatment the following week. On test days, groups of rats were moved to the testing room in their home cages and given 1 h to acclimate. Feeding trays were removed, and wire lids were placed atop the cages. For the DOI experiments, rats received 0 (saline), 0.03, 0.1, 0.3 or 1.0 mg/kg doses; for the 25C-NBOMe and 25I-NBOMe experiments, rats received 0 (saline), 0.01, 0.03, 0.1 or 0.3 mg/kg doses; and for the 2C-C and 2C-I experiments, rats received 0 (saline), 0.1, 0.3, 1.0, or 3.0 mg/kg doses. Immediately before vehicle or drug injection and at 15, 30, 45, 60, 75, 90, 105 and 120 min post-injection, animals were observed for 90 sec and body temperatures were measured with the handheld reader. For behavioral scoring, the number of WDS and BMC were counted during the 90-sec observation period by an experienced rater who was not blind to the treatments. WDS were defined as rapid shaking of the head, neck and trunk from one side to the other, analogous to a wet dog shaking to dry itself, whereas BMC were defined as brief contractions of the paraspinal back muscles which elicit movement of the skin in a tail to head direction (Pranzatelli, 1990; Canal and Morgan, 2012). Rats were given summed scores for WDS and BMC which were totaled from all eight observation periods, as previously described (Baumann and Rothman, 1996).
2.6 Antagonism of Agonist-Induced Behavioral Effects
Separate cohorts of 12 rats were used to determine the effects of pretreatment with the 5-HT2A receptor antagonist M100907 on behaviors induced by DOI, 25C-NBOMe, 25I-NBOMe, 2C-C and 2C-I (60 rats total). Rats in each cohort were tested once per week for 3 or 4 consecutive weeks, until the treatment groups reached a completed N=9/group. For antagonism experiments using DOI, rats were pretreated with vehicle or M100907 (0.01, 0.03, or 0.1 mg/kg) 30 min before receiving 1.0 mg/kg DOI or saline. For antagonism experiments with all other agonists, rats were pretreated with either vehicle or a fixed dose of M100907 (0.1 mg/kg) 30 min before receiving 0.03 mg/kg 25C-NBOMe, 0.1 mg/kg 25I-NBOMe, 0.3 mg/kg 2C-C, 1.0 mg/kg 2C-I or saline vehicle. Behavioral scoring and body temperature measurements were carried out in the same manner as described above in Section 2.5.
2.7 Data Analysis and Statistics
The data from in vivo experiments were tabulated, analyzed, and graphically depicted using GraphPad Prism (GraphPad Software). Temperature data were analyzed by two-way (dose × time) analysis of variance (ANOVA) followed by Bonferroni post-hoc tests to establish significant differences between dose groups at specific timepoints. Summed behavioral scores for WDS and BMC from dose-response experiments with DOI, NBOMes and 2C compounds were evaluated by one-way (dose) ANOVA, followed by Bonferroni post-hoc tests to determine significance between doses. ED50 values were calculated from the rising phase of the dose-effect function for each drug using non-linear regression analysis of behavioral score versus log of the dose administered. Summed behavioral scores from the M100907 antagonism experiments were analyzed by one-way (treatment group) ANOVA followed by Bonferroni post-hoc tests. Relationships between in vitro and in vivo endpoints were investigated using Pearson’s correlation analysis. p<0.05 was considered the minimal criterion for statistical significance.
3. Results
3.1 Radioligand Binding and Functional Assays
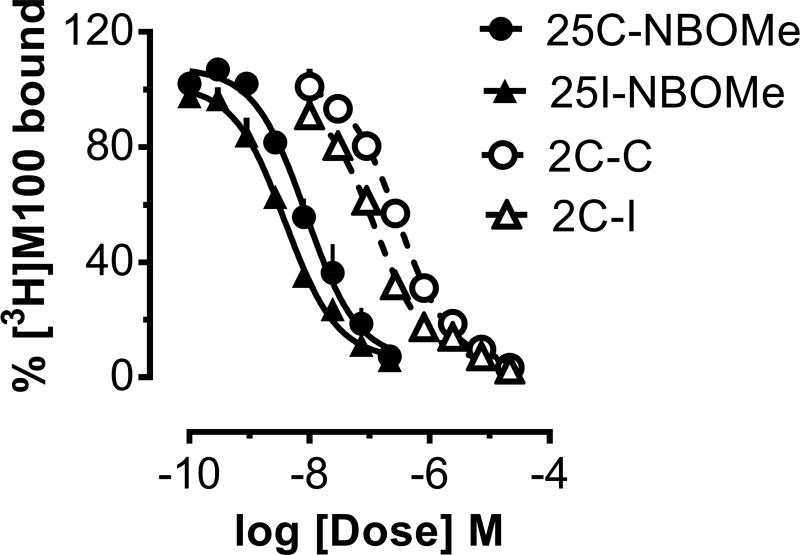
Figure 2 depicts the dose-response curves for NBOMes and their 2C counterparts in the [3H]M100907 binding assay in rat brain tissue. Table 1 summarizes the potencies of test compounds in assays of 5-HT2A receptor binding in rat brain (IC50 values) and calcium mobilization in cells expressing human 5-HT2A and 5-HT2C receptors (EC50 values). The NBOMe compounds showed much greater potency to inhibit binding at 5-HT2A receptors (IC50 range 4–9 nM) when compared to the 2C compounds (IC50 range 125–307 nM). Specifically, 25C-NBOMe displayed 35-fold higher affinity than 2C-C at the 5-HT2A receptor, whereas 25I-NBOMe displayed 32-fold higher affinity than 2C-I at this site. DOI had a 5-HT2A receptor affinity intermediate between that of the NBOMes and 2C compounds. In contrast to the 5-HT2A binding data, the 2C compounds showed greater potency than NBOMes in the 5-HT2A functional assay. For example, 2C-C was 13-fold more potent than 25C-NBOMe in the 5-HT2A-mediated calcium mobilization assay, whereas 2C-I was 8-fold more potent than 25I-NBOMe. All compounds acted as fully efficacious 5-HT2A agonists. In the 5-HT2C-mediated calcium mobilization assay, all compounds displayed roughly similar potency ranging from 47.2–178.0 nM, but the NBOMes were more efficacious (60–70% Emax) than their 2C counterparts (~30% Emax).
Figure 2.
Dose-response effects of NBOMe compounds and their 2C analogs to inhibit binding of [3H]M100907 at 5-HT2A receptors in rat brain tissue. Various concentrations of test compounds were incubated with 1 nM [3H]M100907, and assays were terminated using rapid filtration. Non-specific binding was determined in the presence of 10 µM ketanserin. Data are % inhibition of specific binding in the absence of added drug and are expressed as mean ± SEM for N=3 experiments performed in triplicate.
Table 1.
Effects of DOI, 25C-NBOMe, 25I-NBOMe, 2C-C and 2C-I on 5-HT2A receptor binding, calcium mobilization mediated by 5-HT2A and 5-HT2C receptors, and behaviors.
| Compound | 5-HT2A Inhibition of Binding IC50 (nM) |
5-HT2A Mobilization of Calcium++ EC50 (nM) [%Emax] |
5-HT2C Mobilization of Calcium++ EC50 (nM) [% Emax] |
Rat WDS ED50 (mg/kg, sc) |
Rat BMC ED50 (mg/kg, sc) |
|---|---|---|---|---|---|
| DOI | 56.1 ± 4.9 | 1.83 ± 0.3 [109] | 47.7 ± 3.0 [50] | 0.065 ± 0.024 | 0.180 ± 0.066 |
| 25C-NBOMe | 8.7 ± 1.0 | 61.7 ± 3.5 [104] | 178 ± 25 [60] | 0.010 ± 0.005 | 0.021 ± 0.008 |
| 25I-NBOMe | 3.9 ± 0.4 | 34.7 ± 4.2 [108] | 88.9 ± 11 [70] | 0.062 ± 0.026 | 0.037 ± 0.011 |
| 2C-C | 307.5 ± 21.6 | 4.57 ± 0.5 [95] | 77.6 ± 5.5 [29] | 0.173 ± 0.066 | 0.550 ± 0.190 |
| 2C-I | 124.9 ± 13.0 | 3.97 ± 0.7 [89] | 47.2 ± 6.7 [28] | 0.690 ± 0.268 | 0.192 ± 0.060 |
3.2 Behavioral Effects of DOI
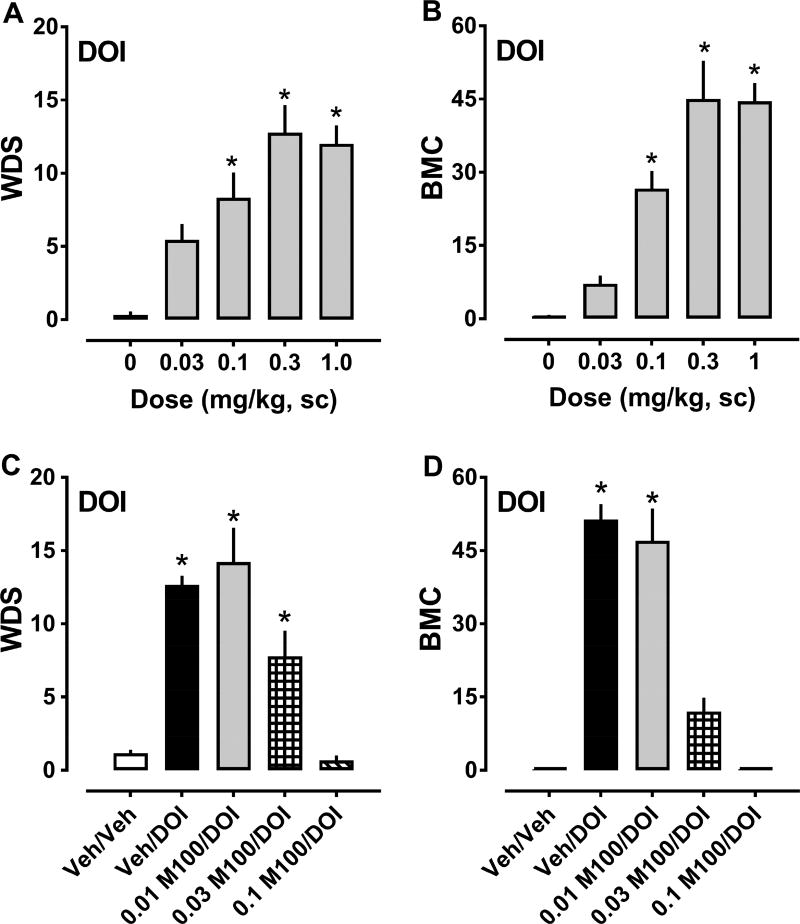
As a first step in examining the in vivo effects of drugs for this study, we opted to characterize non-contingent behavioral responses produced by the prototypical 5-HT2A/2C agonist DOI as a standard for comparison. Our previous work identified WDS and BMC as robust indicators of 5-HT2A receptor activation in rats treated with intravenous doses of DOI (Baumann and Rothman, 1996). Here, the effects of sc administered DOI on behaviors are depicted in Figure 3, while ED50 estimates for inducing WDS and BMC are summarized in Table 1. DOI administration markedly increased the frequency of WDS (F5,48 = 14.80, p<0.0001) and BMC (F5,48 = 27.34, p<0.0001), with significant increases in both behaviors occurring after 0.1, 0.3, and 1.0 mg/kg as compared to vehicle (Fig. 3, panels A and B). DOI exhibited ED50 values of 0.065 and 0.180 mg/kg for producing WDS and BMC, respectively. DOI had no significant effects on body temperature at any dose tested. Pretreatment with various doses of selective 5-HT2A antagonist M100907, 30 min before 1.0 mg/kg DOI, dose-dependently decreased WDS (F4,40 = 21.87, p<0.0001) and BMC (F4,40 = 50.94, p<0.0001). In particular, 0.1 mg/kg M100907 significantly decreased shakes and contractions as compared to vehicle-pretreated rats (Fig. 3, panels C and D). Behaviors observed in rats treated with 0.1 mg/kg M100907/DOI did not differ significantly from those treated with vehicle/vehicle, in contrast to robust behaviors elicited in the vehicle/DOI group.
Figure 3.
Dose-response effects of DOI (0.03–1.0 mg/kg, sc) on summed WDS and BMC in rats (panels A and B), and effects of 30 min pretreatment with 0.01–0.1 mg/kg M100907 (M100) or its vehicle (Veh) on behaviors induced by 1.0 mg/kg DOI (panels C and D). Rats were observed for 90 s every 15 min for 2 h post-injection, and the number of WDS and BMC was totaled. Data are mean ± SEM for N = 9 rats per group. * represents significant effects on behavior when compared to the corresponding vehicle (0 dose) or vehicle/vehicle (Veh/Veh) groups (Bonferroni, p<0.05).
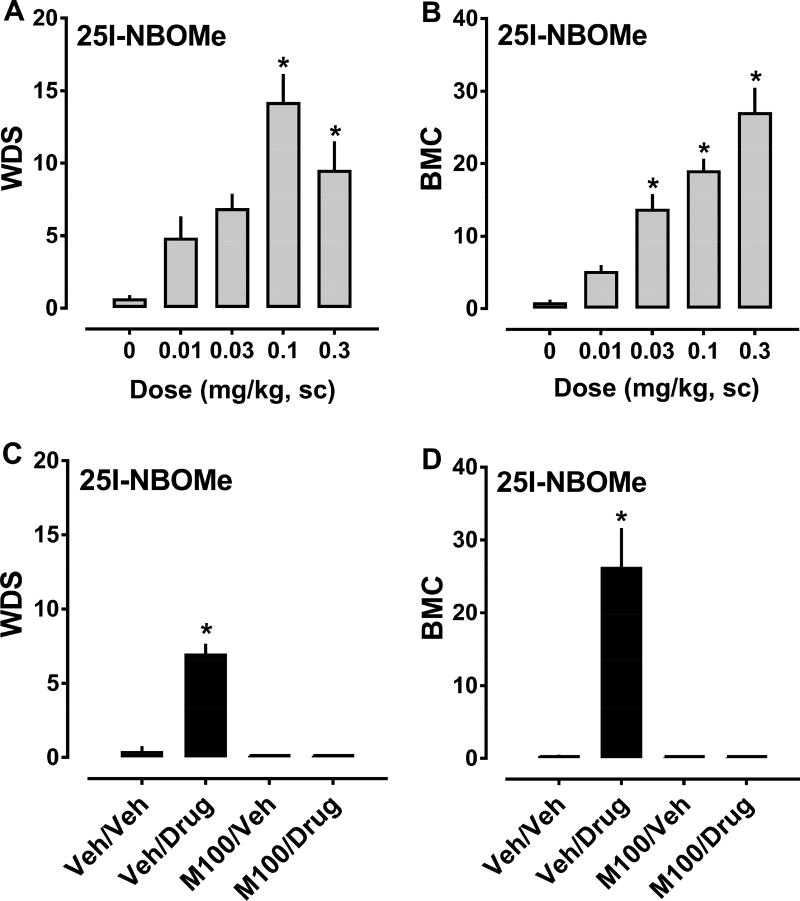
3.3 Behavioral Effects of NBOMe Compounds
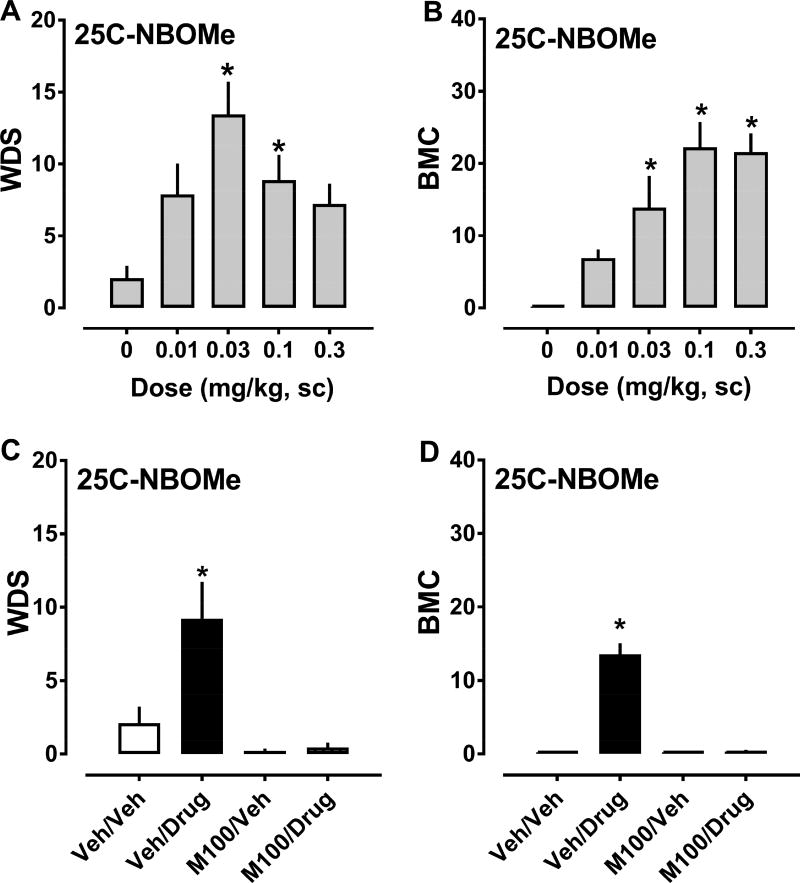
The effects of 25C-NBOMe on behaviors are depicted in Figure 4, and ED50 estimates for inducing WDS and BMC are summarized in Table 1. 25C-NBOMe increased the frequency of WDS (F4,43 = 6.343, p<0.0004) and BMC (F4,43 = 72.44, p<0.0001) compared to vehicle-treated rats. Significant stimulation of shaking behavior occured after 0.03 and 0.1 mg/kg doses, while stimulation of contractions occured after 0.03, 0.1 and 0.3 mg/kg doses (Fig. 4, panels A and B). It is noteworthy that effects of 25C-NBOMe on WDS generated a hyperbolic dose-response curve (i.e., inverted U-shaped curve), whereas effects of the drug on BMC generated a more sigmoidal curve. 25C-NBOMe displayed ED50 values of 0.010 and 0.021 mg/kg for producing WDS and BMC, respectively. 25C-NBOMe did not significantly alter body temperature at any dose tested. Pretreatment with 0.1 mg/kg M100907, 30 min before treatment with 0.03 mg/kg 25C-NBOMe, significantly decreased WDS (F3,32 = 9.345, p<0.0001) and BMC (F3,32 = 73.26, p<0.0001) produced by the agonist. Behaviors observed in the M100907/vehicle and M100907/25C-NBOMe groups did not differ significantly from vehicle/vehicle animals, in contrast to vehicle/25C-NBOMe treated animals which displayed marked increases in shakes and contractions (Fig. 4, panels C and D).
Figure 4.
Dose-response effects of 25C-NBOMe (0.01–0.3 mg/kg, sc) on summed WDS and BMC in rats (panels A and B), and effects of 30 min pretreatment with 0.1 mg/kg M100907 (M100) or its vehicle (Veh) on WDS and BMC induced by 0.03 mg/kg 25C-NBOMe (panels C and D). Rats were observed for 90 s every 15 min for 2 h post-injection, and the number of WDS and BMC was totaled. Data are mean ± SEM for N = 9 rats per group. * represents significant effects on behavior when compared to the corresponding vehicle (0 dose) or vehicle/vehicle (Veh/Veh) groups (Bonferroni, p<0.05).
The effects of 25I-NBOMe on behaviors are shown in Figure 5, and the ED50 values for inducing WDS and BMC are summarized in Table 1. 25I-NBOMe increased WDS (F4,39 = 12.62, p<0.0001) and BMC (F4,40 = 27.94, p<0.0001) compared to vehicle-treated rats. Significant increases in shakes occured after the 0.1 and 0.3 mg/kg doses, while increases in contractions occurred after the 0.03, 0.1, and 0.3 mg/kg doses (Fig 5., panels A and B). As noted for the 25C-NBOMe experiments, the effects of 25I-NBOMe on WDS generated a hyperbolic dose-response curve but effects on BMC generated a more sigmoidal curve. 25I-NBOMe exhibited ED50 values of 0.062 and 0.037 mg/kg for producing WDS and BMC, respectively. 25I-NBOMe did not significantly alter body temperature at any dose tested. Pretreatment with 0.1 mg/kg M100907, 30 min before treatment with 0.1 mg/kg 25I-NBOMe, decreased WDS (F3,32 = 78.92, p<0.0001) and BMC (F3,32 = 30.41, p<0.0001) produced by the agonist. Behaviors observed in the M100907/vehicle and M100907/25I-NBOMe groups did not differ significantly from vehicle/vehicle rats, in contrast to vehicle/25I-NBOMe rats which displayed large increases in WDS and BMC (Fig. 5, panels C and D).
Figure 5.
Dose-response effects of 25I-NBOMe (0.01–0.3 mg/kg, sc) on summed WDS and BMC in rats (panels A and B), and effects of 30 min pretreatment with 0.1 mg/kg M100907 (M100) or its vehicle (Veh) on the WDS and BMC induced by 0.1 mg/kg 25I-NBOMe (panels C and D). Rats were observed for 90 s every 15 min for 2 h post-injection, and the number of WDS and BMC was totaled. Data are mean ± SEM for N = 9 rats per group. * represents significant effects on behavior when compared to the corresponding vehicle (0 dose) or vehicle/vehicle (Veh/Veh) groups (Bonferroni, p<0.05).
3.4 Behavioral Effects of 2C compounds
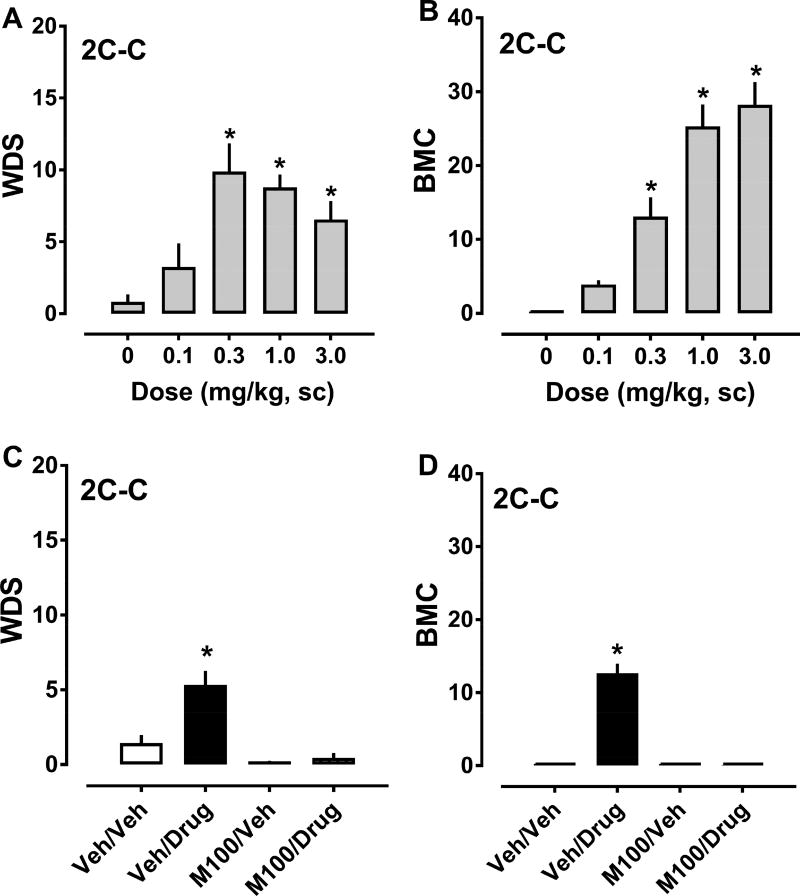
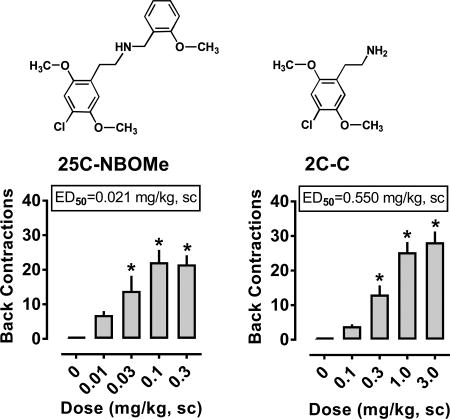
The effects of 2C-C on behaviors are depicted in Figure 6, and ED50 estimates for inducing WDS and BMC are summarized in Table 1. 2C-C increased the frequency of WDS (F4,42 = 9.633, p<0.0001) and BMC (F4,43 = 36.2, p<0.0001) when compared to vehicle-treated rats (Fig. 6, panels A and B). Significant stimulation of both behaviors occurred after administration of 0.3, 1.0, and 3.0 mg/kg doses. Effects of 2C-C on WDS generated a hyperbolic curve but effects on BMC generated a more sigmoidal curve. 2C-C displayed ED50 values of 0.173 and 0.550 mg/kg for producing WDS and BMC, respectively. 2C-C failed to significantly alter body temperature at any dose tested. Pretreatment with 0.1 mg/kg M100907, 30 min before treatment with 0.3 mg/kg 2C-C, decreased WDS (F3,32 = 18.77, p<0.0001) and BMC (F3,32 = 87.64, p<0.0001) produced by the agonist. Behaviors observed in the M100907/vehicle and M100907/2C-C groups did not differ significantly from vehicle/vehicle animals, in contrast to vehicle/2C-C treated animals which displayed robust increases in shakes and contractions (Figure 6, panels C and D).
Figure 6.
Dose-response effects of 2C-C (0.1–3.0 mg/kg, sc) on summed WDS and BMC in rats (panels A and B), and effects of 30 min pretreatment with 0.1 mg/kg M100907 (M100) or its vehicle (Veh) on the WDS and BMC induced by 0.3 mg/kg 2C-C. Rats were observed for 90 s every 15 min for 2 h post-injection, and the number of WDS and BMC was totaled. Data are mean ± SEM for N = 9 rats per group. * represents significant effects on behavior when compared to the corresponding vehicle (0 dose) or vehicle/vehicle (Veh/Veh) groups (Bonferroni, p<0.05).
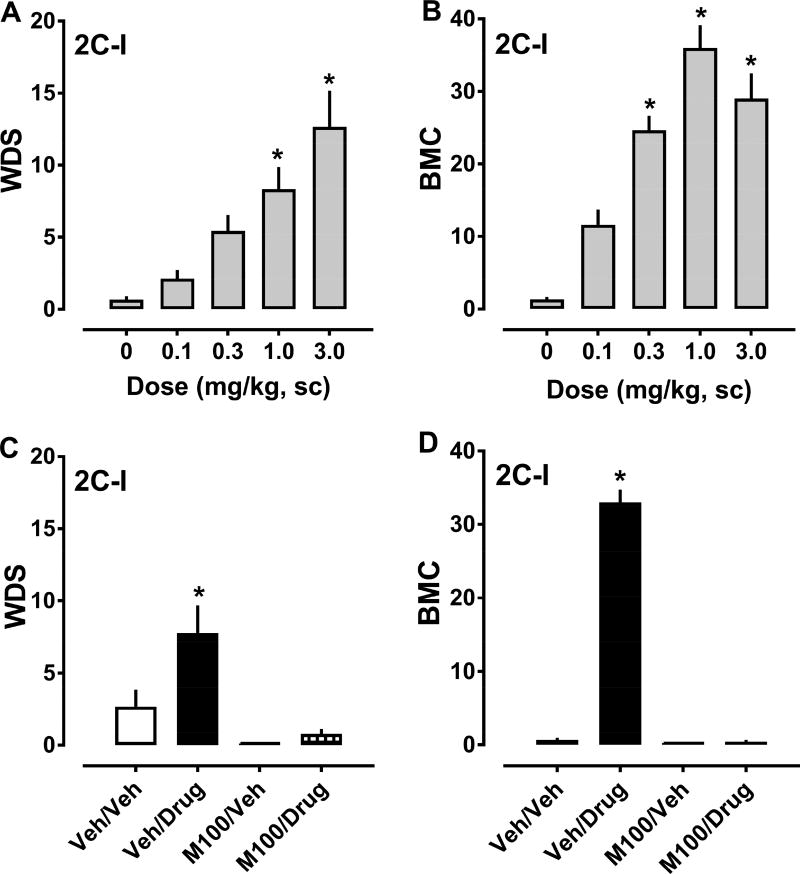
The effects of 2C-I on behaviors are shown in Figure 7, and the ED50 values for inducing WDS and BMC are summarized in Table 1. 2C-I increased WDS (F4,39 = 11.04, p<0.0001) and BMC (F4,39 = 28.05, p<0.0001) with respect to vehicle-treated rats. Significant stimulation of shaking behavior ocurred after 1.0 and 3.0 mg/kg doses, while increases in BMC occurred after 0.3, 1.0, and 3.0 mg/kg doses (Fig. 7, panels A and B). In contrast to the behavioral effects produced by 2C-C and the other agonists, the effects of 2C-I on WDS generated a sigmoidal curve, while effects of the drug on BMC generated a hyperbolic curve. 2C-I exhibited ED50 values of 0.690 and 0.192 mg/kg for producing WDS and BMC, respectively. 2C-I failed to alter body temperature at any dose tested. Pretreatment with 0.1 mg/kg M100907, 30 min before treatment with 1.0 mg/kg 2C-I, decreased WDS (F3,32 = 9.369, p<0.0001) and BMC (F3,32 = 330.2, p<0.0001) produced by the agonist. Behaviors observed in the M100907/vehicle and M100907/2C-I groups did not differ significantly from vehicle/vehicle rats, while animals in the vehicle/2C-I group displayed large increases in WDS and BMC (Fig. 7, panels C and D).
Figure 7.
Dose-response effects of 2C-I (0.1–3.0 mg/kg, sc) on summed WDS and BMC in rats (panels A and B), and effects of 30 min pretreatment with 0.1 mg/kg M100907 (M100) or its vehicle (Veh) on the WDS and BMC induced by 1.0 mg/kg 2C-I. Rats were observed for 90 s every 15 min for 2 h post-injection, and the number of WDS and BMC was totaled. Data are mean ± SEM for N = 9 rats per group. * represents significant effects on behavior when compared to the corresponding vehicle (0 dose) or vehicle/vehicle (Veh/Veh) groups (Bonferroni, p<0.05).
3.5 Correlations Between In Vitro and In Vivo Endpoints
To examine the potential relationships between in vitro receptor findings and in vivo behavioral effects of test drugs, the results shown in Table 1 were subjected to Pearson correlation analysis. Several significant correlations were noted. There was a significant positive correlation between EC50 values for 5-HT2A and 5-HT2C receptor activation in the calcium mobilization assay (r = 0.942, p<0.017), yet no correlation between EC50s for 5-HT2A receptor activation versus IC50s for 5-HT2A receptor binding. Interestingly, 5-HT2A receptor binding across the 5 test compounds was highly correlated with ED50 values for in vivo induction of BMC (r=0.984, p<0.003) but not WDS (r= 0.192, p<0.492).
4. Discussion
A major aim of the present study was to characterize the neuropharmacology of the hallucinogenic drugs, 25C-NBOMe and 25I-NBOMe, as compared to their 2C counterparts and the prototypical 5-HT2A receptor agonist DOI. NBOMe compounds have emerged in the recreational drug marketplace in recent years, and serious medical complications have been reported (Suzuki et al., 2015; Halberstadt, 2017). Data from our in vitro assays demonstrate that 25C-NBOMe and 25I-NBOMe display at least 30-fold higher affinity for rat 5-HT2A receptors when compared to their affinity for 2C-C and 2C-I, respectively. Interestingly, NBOMes were much less potent in functional assays measuring calcium mobilization in cell lines expressing human 5-HT2A receptors. Regardless of potency differences among the compounds, all test drugs acted as fully efficacious agonists at 5-HT2A receptors but partial agonists at 5-HT2C receptors. Data from our in vivo experiments reveal that 25C-NBOMe and 25I-NBOMe are 5- to 20-fold more potent than 2C-C and 2C-I in their ability to induce WDS and BMC. Pretreatment with the selective 5-HT2A antagonist M100907 effectively eradicates both behaviors from all drug groups, demonstrating that the behaviors are dependent upon 5-HT2A receptor activation. Overall, our findings show that 25C-NBOMe and 25I-NBOMe are potent 5-HT2A receptor agonists in rats, consistent with previous findings from rats and mice (Halberstadt and Geyer, 2014; Gatch et al., 2017).
The present study examined the effects of test compounds on inhibition of [3H]M100907 binding to 5-HT2A receptors in rat brain tissue. We utilized this antagonist as a radioligand due to its superior selectivity for 5-HT2A receptors (Marek and Aghajanian, 1994; Visser et al., 2014); such selectivity is advantageous when performing assays in brain tissue, where other 5-HT receptor subtypes can interfere with binding signal. Additionally, we favor the use of radiolabeled antagonists for binding studies because these agents simplify data interpretation by labelling only a single site. In agreement with previous findings, our data show that 25C-NBOMe and 25I-NBOMe are at least 30-fold more potent at inhibiting 5-HT2A receptor binding when compared to 2C-C and 2C-I (Braden et al., 2006; Rickli et al., 2015). The IC50 values for 25C-NBOMe (8.7 nM) and 25I-NBOMe (3.9 nM) that we measured at 5-HT2A receptors in rat brain are consistent with the Ki values for these compounds (i.e., 1.5–3.0 nM) reported by Ettrup et al. who examined [3H]M100907 binding in NIH-3T3 cells stably expressing rat 5-HT2A receptors (Ettrup et al., 2011). By contrast, the IC50 values for NBOMes at 5-HT2A receptors in our work are much higher than the sub-nM Ki values for inhibition of radiolabeled DOI binding in cells expressing rat or human 5-HT2A receptor subtypes (Braden et al., 2006; Nichols et al., 2015). Importantly, Braden et al. (2006) demonstrated that various NBOMe compounds inhibit [125I]DOI binding at rat and human 5-HT2A receptors with nearly identical potency, suggesting no species differences in terms of binding affinity for these compounds. One obvious difference between the present binding results and most previous studies is that we used a radiolabeled antagonist. Indeed, others have reported weaker potency for 25I-NBOMe at inhibiting [3H]ketanserin binding when compared to [125I]DOI binding (Braden et al., 2006). Despite the differences in absolute in vitro potencies for NBOMes reported across several studies, all data agree that N-methoxybenzyl analogs display at least 10-fold greater affinity for 5-HT2A receptors when compared to their 2C counterparts.
An intriguing observation from the present work is that 25C-NBOMe and 25I-NBOMe were less potent than 2C-C and 2C-I in functional assays measuring 5-HT2A-mediated calcium mobilization. As a specific example, 25I-NBOMe had an EC50 of 34.70 nM in the calcium mobilization assay whereas 2C-I had an EC50 of 3.97 nM. Accordingly, our correlation analyses found no relationship between 5-HT2A binding and function in the present study. One complicating factor with interpretation of our results is that binding assays were carried out in rat brain tissue while functional assays were carried out in cells transfected with human receptors. Nevertheless, our in vitro findings with 25C- and 25I-NBOMe agree with the results of Rickli et al. (2015) who examined the effects of various NBOMe compounds on receptor binding and functional activity in cells expressing human 5-HT2A receptors. These investigators found that NBOMe compounds are much less potent in calcium mobilization assays when compared their effects in receptor binding assays, and 25I-NBOMe is weaker (EC50 = 240 nM) in the calcium assay than 2C-I (EC50 = 60 nM) (Rickli et al, 2015). Braden et al. (2006) used cells transfected with rat 5-HT2A receptors to demonstrate that NBOMe compounds are at least 20-fold less potent in functional assays measuring accumulation of inositol phosphate (IP) when compared to their effects in binding assays. However, in the Braden et al. study, NBOMe compounds displayed greater potency than 2C compounds in the IP assay. Future studies are warranted to further explore the relationships between 5-HT2A receptor binding affinity and functional activity for NBOMe compounds, especially in native brain tissue preparations. Finally, it is important to note that all of the compounds tested in our work acted as efficacious agonists at 5-HT2A receptors but partial agonists at 5-HT2C receptors, with 2C compounds exhibiting only 30% efficacy at 5-HT2C sites.
Few investigations have examined the effects of NBOMe compounds in traditional animal models of hallucinogenic drug effects. Corne and Pickering (1967) first described the use of head twitch behavior in mice as a marker of hallucinogenic drug actions, while others subsequently demonstrated that WDS represent a behavioral equivalent in rats (Bedard and Pycock, 1977; Leysen et al., 1982). Here we show that DOI administration to rats increases WDS in a dose-related manner, consistent with many prior studies (Pranzatelli, 1990; Baumann and Rothman, 1996; Wettstein, 1999; Canal and Morgan, 2012). In a recent investigation using mice, Halberstadt and Geyer found that 25I-NBOMe is about 10-fold more potent than 2C-I at eliciting head twitch behavior after sc administration (Halberstadt and Geyer, 2014). These investigators reported ED50 values of 0.08 and 0.83 mg/kg in the head twitch assay for 25I-NBOMe and 2C-I, respectively. Consistent with the findings in mice, we show 25I-NBOMe is 10-fold more potent than 2C-I at inducing WDS in rats. We found ED50 values of 0.06 and 0.69 mg/kg for inducing WDS after sc administration of 25I-NBOMe and 2C-I, respectively. Thus, our potency values in rats are very close to those reported by Halberstadt and Geyer in mice, even though they used a magnetometer coil to measure head shakes continuously, while we used time-sampling methods to observe rats for 90 s epochs every 15 min. Similar to our results with 25I-NBOMe, we found 25C-NBOMe was much more potent than 2C-C in the wet dog shake assay.
An important observation from the present work is that NBOMe and 2C compounds produce inverted U-shaped dose-response curves in the wet dog shake assay. It is tempting to speculate that activation of non-5-HT2A receptors (e.g., 5-HT2C receptors) after administration of high drug doses may serve to counteract shaking behavior (Higgins et al., 2001; Vickers et al., 2001). Indeed, Vickers et al. (2001) provided convincing evidence that 5-HT2C receptor stimulation can inhibit 5-HT2A-mediated WDS in rats. As noted already, all of the compounds studied here display efficacious agonist activity at 5-HT2A receptors, and partial agonist activity at 5-HT2C receptors, suggesting the possibility of interaction between these two sites in vivo. In a recent study, Fantegrossi et al. (2015) investigated the behavioral effects of the NBOMe analog 2-([2-(4-cyano-2,5-dimethoxyphenyl)ethylamino]methyl)phenol (25CN-NBOH) in mice, and showed this compound induces head twitch behavior that displays a bell-shaped dose-response curve very similar to the effects reported here for 25C- and 25I-NBOMe in rats (Fantegrossi et al., 2015). Halberstadt and Geyer (2014) failed to observe an inverted U-shaped dose-response for head twitches induced by 25I-NBOMe, but it seems possible that testing of higher drug doses in their study might have uncovered 5-HT2C-mediated suppression of behavior. Collectively, our behavioral findings demonstrate that NBOMe compounds display similar potency to induce shaking behaviors in rats and mice, with the NBOMes exhibiting at least 10-fold greater potency in vivo when compared to their 2C counterparts.
Back muscle contractions, or BMC, are an established 5-HT2A-mediated behavior in rats but are not typically used to assess the in vivo effects of hallucinogens, likely due to the apparent absence of any comparable behavior in mice (Baumann and Rothman, 1996; Wettstein et al., 1999; Higgins et al, 2001). An additional goal of the current study was to assess the validity and utility of BMC as a behavioral read-out of 5-HT2A receptor activation in rats. We demonstrate that NBOMes are more potent at eliciting BMC when compared to their 2C counterparts. For example, the ED50 values for induction of BMC were 0.04 and 0.19 mg/kg for 25I-NBOMe and 2C-I, respectively. Within the dose ranges given, the dose-response curves for BMC were more classically sigmoidal in shape for all drugs except 2C-I. Thus, 2C-I seems to display subtle in vivo pharmacological differences when compared to the other drugs tested. In this regard, Eshleman et al. (2014) found that 2C-C is a full agonist in the IP and [H3]arachidonic acid ([3H]AA) assays, tests for functional activity of the 5-HT2A receptor, while 2C-I is an agonist in the IP assay, and an antagonist in the [H3]AA assay (Eshleman et al., 2014). An important observation from the current study is that 5-HT2A receptor binding potency across the test drugs is positively correlated with BMC (r = 0.984, p<0.003) but not WDS (r= 0.306, p<0.616). These results suggest that BMC represent a valid index of 5-HT2A receptor activation in rats, at least for the small group of compounds tested. Despite the lack of correlation between receptor binding and WDS, we believe that shaking behavior is a useful measure of 5-HT2A activity in rats due to its similarity to the head twitch response in mice, which allows cross-species comparison.
Gatch et al. recently published an investigation which examined the behavioral effects of NBOMe hallucinogens in mice and rats (Gatch et al., 2017). In their study, 25C- and 25I-NBOMe dose-dependently reduced locomotor activity in mice, an effect often associated with 5-HT2A agonism in mice (Halberstadt, 2015). Gatch et al. also found that 25C-NBOMe fully substituted for the 5-HT2A agonist 1-(2,5-dimethoxy-4-methylphenyl)propan-2-amine (DOM) in rat drug discrimination experiments, while only partially substituting for 3,4-methylenedioxymethamphetamine (MDMA). By contrast, 25I-NBOMe engendered roughly equivalent discrimination for DOM and MDMA (~74–78%) but fell shy of full substitution. The effects of NBOMes in drug discrimination studies are slightly different from the effects of 2C-C and 2C-I reported by Eshleman et al. (2014) who found 2C-C fully substitutes for MDMA while 2C-I fully substitutes for prototypical hallucinogens like LSD (Eshleman et al., 2014; Gatch et al., 2017). Importantly, the efficacious doses of NBOMes producing robust non-contingent behaviors in our experiments (i.e., 0.03–0.10 mg/kg) are somewhat lower than the doses producing discriminative stimulus effects in rats reported by Gatch and colleagues (2017). As a specific example, 25C-NBOMe produced significant increases in both WDS and BMC in our experiments at a dose of 0.03 mg/kg, but this dose engendered <20% DOM-appropriate responding in discrimination studies. One possible explanation for the apparent higher potency of NBOMes in our study is that we utilized the sc route of administration while Gatch et al. utilized the ip route. Anecdotal evidence suggests that NBOMe compounds are inactive after oral administration due to extensive first-pass metabolism, and NBOMe compounds have faster metabolic clearance compared to their 2C analogs (Leth-Peterson et al., 2014). Therefore, differences in the efficacious in vivo doses between our experiments and those of Gatch et al. could be due to longer drug half-life after sc versus ip routes of drug administration.
M100907 is a highly potent 5-HT2A antagonist, with more than 100-fold selectivity over 5-HT2C and other receptor subtypes (Marek and Aghajanian, 1994). In rats pre-treated with 0.1 mg/kg M100907, we found the effects of DOI, 25C-NBOMe, 25I-NBOMe, 2C-C and 2C-I were never significantly different from vehicle/vehicle treated animals, and were always significantly reduced compared to vehicle/agonist treated animals. From these findings, we conclude that WDS and BMC induced by NBOMes and 2C compounds are 5-HT2A-dependent behaviors, in agreement with the existing literature (Higgins et al, 2001; Canal and Morgan, 2012; Halberstadt and Geyer, 2014).
In summary, we show that 25C-NBOMe and 25I-NBOMe dose-dependently induce WDS and BMC in rats, with both behaviors being blocked by the selective 5-HT2A antagonist M100907. Our study is only the second to assess the behavioral effects of NBOMe compounds in rats, and the first to assess the effects of the drugs on WDS and BMC. Consistent with the existing literature, both NBOMe drugs are very potent 5-HT2A agonists, as 25I-NBOMe is 10 times more potent than 2C-I in producing WDS and five times more potent in producing BMC. Similarly, 25C-NBOMe is 17 times more potent than 2C-C in producing WDS and 27 times more potent in producing BMC. Our correlation findings indicate that BMC are a reliable endpoint for determining the 5-HT2A activity of hallucinogenic drugs in rats, but further research is required to assess the relationship between BMC and 5-HT2A agonism. Finally, our data from rats are consistent with the high potency of NBOMe compounds reported in human users, and may explain the increased propensity for these drugs to induce serious side-effects.
HIGHLIGHTS.
25C-NBOMe and 25I-NBOMe exhibit 30-fold greater affinity for rat 5-HT2A receptors when compared to 2C-C and 2C-I
NBOMe compounds are much more potent than their 2-C counterparts at inducing wet dog shakes (WDS) and back muscle contractions (BMC) in male rats
WDS and BMC produced by NBOMe administration are reversed by the selective 5-HT2A antagonist M100907
In rats, NBOMe compounds are highly potent 5-HT2A agonists in vitro and in vivo, consistent with their powerful hallucinogenic effects in human users
Acknowledgments
This research was generously supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), United States of America (DA00523 to MHB).
Abbreviations
- BMC
back muscle contractions
- 2C-C
2-(4-chloro-2,5-dimethoxyphenyl)ethanamine
- 2C-I
2-(4-iodo-2,5-dimethoxyphenyl)ethanamine
- 25C-NBOMe
2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine
- 25CN-NBOH
2-([2-(4-cyano-2,5-dimethoxyphenyl)ethylamino]methyl)phenol
- 25I-NBOMe
2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine
- DOI
1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine
- LSD
lysergic acid diethylamide
- M100907
(R)-(2,3-dimethoxyphenyl){1-[2-(4-fluorophenyl)ethyl]-4-piperidinyl}methanol
- NPS
new psychoactive substances
- WDS
wet dog shakes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumann MH, Rothman RB. Chronic cocaine exposure potentiates prolactin and head shake responses to 5-HT2 receptor stimulation in rats. Neuropharmacology. 1996;35:295–301. doi: 10.1016/0028-3908(95)00166-2. [DOI] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. "Wet-dog" shake behaviour in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology. 1977;16:663–670. doi: 10.1016/0028-3908(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Bosak A, LoVecchio F, Levine M. Recurrent seizures and serotonin syndrome following "2C-I" ingestion. J Med Toxicol. 2013;9:196–198. doi: 10.1007/s13181-013-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012;4:556–576. doi: 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho Neto J. Rapid detection of NBOME's and other NPS on blotter papers by direct ATR-FTIR spectrometry. Forensic Sci Int. 2015;252:87–92. doi: 10.1016/j.forsciint.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Dean BV, Stellpflug SJ, Burnett AM, Engebretsen KM. 2C or not 2C: phenethylamine designer drug review. J Med Toxicol. 2013;9:172–178. doi: 10.1007/s13181-013-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, D. o. J. Establishment of drug codes for 26 substances. Final rule. Fed Regist. 2013;78:664–666. [PubMed] [Google Scholar]
- Drug Enforcement Administration. D. o. J. Schedules of controlled substances: temporary placement of three synthetic phenethylamines into Schedule I. Final order. Fed Regist. 2013;78:68716–68719. [PubMed] [Google Scholar]
- Drug Enforcement Administration, D. o. J. Schedules of Controlled Substances: Placement of Three Synthetic Phenethylamines Into Schedule I. Final rule. Fed Regist. 2016;81:66181–66184. [PubMed] [Google Scholar]
- Erowid.org. [Last accessed 12/10/2017];2C-C-NBOMe dose. https://erowid.org/chemicals/2cc_nbome/2cc_nbome_dose.shtml.
- Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology (Berl) 2014;231:875–888. doi: 10.1007/s00213-013-3303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers. Eur J Nucl Med Mol Imaging. 2011;38:681–693. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gray BW, Bailey JM, Smith DA, Hansen M, Kristensen JL. Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylamino]methyl)phenol (25CN-NBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT2A receptors, in mice. Psychopharmacology (Berl) 2015;232:1039–1047. doi: 10.1007/s00213-014-3739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. Locomotor and discriminative stimulus effects of four novel hallucinogens in rodents. Behav Pharmacol. 2017;28:375–385. doi: 10.1097/FBP.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Dukat M, el-Bermawy M, Law H, De los Angeles J, Teitler M, King A, Herrick-Davis K. Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines. J Med Chem. 1994;37:1929–1935. doi: 10.1021/jm00039a004. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL. Pharmacology and toxicology of N-benzylphenethylamine ("NBOMe") hallucinogens. Curr Top Behav Neurosci. 2017;32:283–311. doi: 10.1007/7854_2016_64. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology. 2014;77:200–207. doi: 10.1016/j.neuropharm.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R. [Last accessed 12/10/2017];Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur (Thesis) 2004 http://www.diss.fu-berlin.de/diss/receive/FUDISS_thesis_000000001221?lang=en.
- Higgins GA, Ouagazzal AM, Grottick AJ. Influence of the 5-HT(2C) receptor antagonist SB242,084 on behaviour produced by the 5-HT(2) agonist Ro60-0175 and the indirect 5-HT agonist dexfenfluramine. Br J Pharmacol. 2001;133:459–466. doi: 10.1038/sj.bjp.0704082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Eisenga B, Riley B, Judge B. Case series of 25I-NBOMe exposures with laboratory confirmation (Abstract); Annual Meeting of the North American Congress of Clinical Toxicology (NACCT); October 1–6, 2012; Las Vegas, NV, USA. 2012. entry 284. [Google Scholar]
- Leth-Petersen S, Bundgaard C, Hansen M, Carnerup MA, Kehler J, Kristensen JL. Correlating the metabolic stability of psychedelic 5-HT(2)A agonists with anecdotal reports of human oral bioavailability. Neurochem Res. 2014;39:2018–2023. doi: 10.1007/s11064-014-1253-y. [DOI] [PubMed] [Google Scholar]
- Leth-Petersen S, Gabel-Jensen C, Gillings N, Lehel S, Hansen HD, Knudsen GM, Kristensen JL. Metabolic fate of hallucinogenic NBOMes. Chem Res Toxicol. 2016;29:96–100. doi: 10.1021/acs.chemrestox.5b00450. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM. [3H]Ketanserin, a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982;21:301–314. [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. Excitation of interneurons in piriform cortex by 5-hydroxytryptamine: blockade by MDL 100,907, a highly selective 5-HT2A receptor antagonist. Eur J Pharmacol. 1994;259:137–141. doi: 10.1016/0014-2999(94)90502-9. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Psychedelics. Pharmacol Rev. 2016;68:264–355. doi: 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ. N-Benzyl-5-methoxytryptamines as potent serotonin 5-HT2 receptor family agonists and comparison with a series of phenethylamine analogues. ACS Chem Neurosci. 2015;6:1165–1175. doi: 10.1021/cn500292d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis JL, Devers KG, Arbefeville EF, Pearson JM, Houston E, Poklis A. Postmortem detection of 25I-NBOMe [2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine] in fluids and tissues determined by high performance liquid chromatography with tandem mass spectrometry from a traumatic death. Forensic Sci Int. 2014;234:e14–20. doi: 10.1016/j.forsciint.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzatelli MR. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioral effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl aminopropane) 2 (DOI) Neurosci Lett. 1990;115:74–80. doi: 10.1016/0304-3940(90)90520-j. [DOI] [PubMed] [Google Scholar]
- Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs) Neuropharmacology. 2015;99:546–553. doi: 10.1016/j.neuropharm.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Transform Press; California: 1991. [Google Scholar]
- Suzuki J, Dekker MA, Valenti ES, Arbelo Cruz FA, Correa AM, Poklis JL, Poklis A. Toxicities associated with NBOMe ingestion-a novel class of potent hallucinogens: a review of the literature. Psychosomatics. 2015;56:129–139. doi: 10.1016/j.psym.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Poklis JL, Poklis A. "My friend said it was good LSD": a suicide attempt following analytically confirmed 25I-NBOMe ingestion. J Psychoactive Drugs. 2014;46:379–382. doi: 10.1080/02791072.2014.960111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Drug Enforcement Administration, Diversion Control Division. 2C-Phenethylamines, Piperazines, and Tryptamines Reported in NFLIS, 2011–2015. Springfield, VA: U.S. Drug Enforcement Administration; 2017. [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Visser AK, Meerlo P, Ettrup A, Knudsen GM, Bosker FJ, den Boer JA, Dierck RA, van Waarde A. Acute social defeat does not alter cerebral 5-HT2A receptor binding in male Wistar rats. Synapse. 2014;68:379–386. doi: 10.1002/syn.21750. [DOI] [PubMed] [Google Scholar]
- Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:533–544. doi: 10.1016/s0278-5846(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Zuba D, Sekula K, Buczek A. 25C-NBOMe--new potent hallucinogenic substance identified on the drug market. Forensic Sci Int. 2013;227:7–14. doi: 10.1016/j.forsciint.2012.08.027. [DOI] [PubMed] [Google Scholar]