Abstract
Background
Spiropyrrolidine tethered piperidone heterocyclic hybrids were synthesized with complete regio- and stereoselectively in excellent yield via a tandem three-component 1,3-dipolar cycloaddition and subsequent enamine reaction in [bmim]Br. The synthesized compounds were evaluated for their anticancer activity against FaDu hypopharyngeal tumor cells.
Findings
Interestingly, most compounds displayed cytotoxicities similar to the standard anticancer agent bleomycin, with two of them (5a and 5g) being slightly more active than the reference drug.
Conclusion
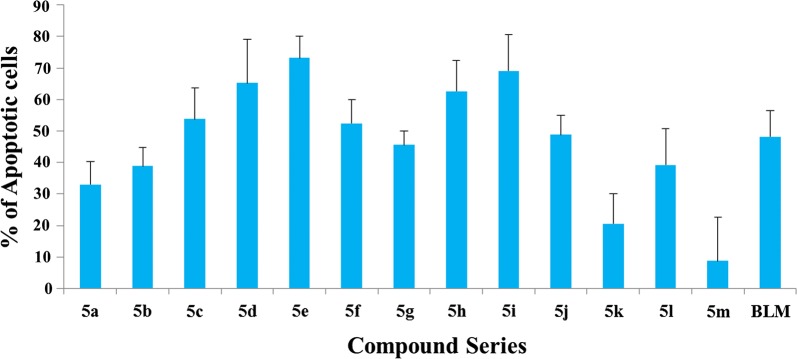
Synthesized compounds have also been evaluated for their apoptosis-inducing properties in a cancer cell model, finding that treatment with compounds 5a–e led to apoptotic cell death.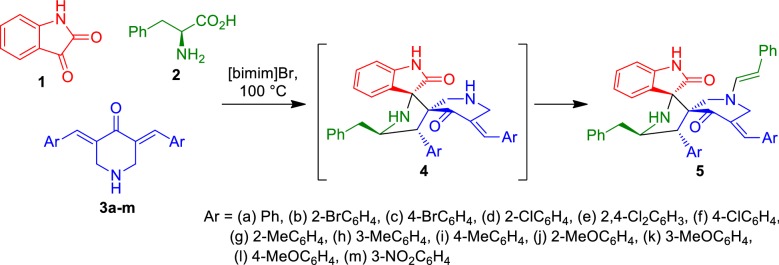
Electronic supplementary material
The online version of this article (10.1186/s13065-018-0462-x) contains supplementary material, which is available to authorized users.
Keywords: Spiropyrrolidine, Piperidone, Domino reactions, Chemo divergent multicomponent reactions, Antiproliferative activity, Apoptosis induction
Background
Cancer can be viewed as a group of related diseases that arise from abnormal cell growth and the loss of regulation of processes associated to programmed cell death via apoptosis [1]. Although cancer chemotherapy has progressed in major strides in recent years, there is still an unmet need for new anti-cancer agents with good potency, diminished toxicity and able to treat tumors that are resistant to currently known drugs [2].
Medicinal chemistry faces major challenges in designing new synthetic compounds with therapeutic importance. In particular, the therapy of complex and multifactorial diseases such as cancer may benefit from molecular design based on the multitarget ligand paradigm, i.e., by incorporation of various biologically active heterocyclic pharmacophores into a single molecule. The hybrid compounds thus generated, carrying more than one pharmacophoric entity and wherein each individual active unit may exert diverse modes of action, offer a new hope in the treatment of cancer.
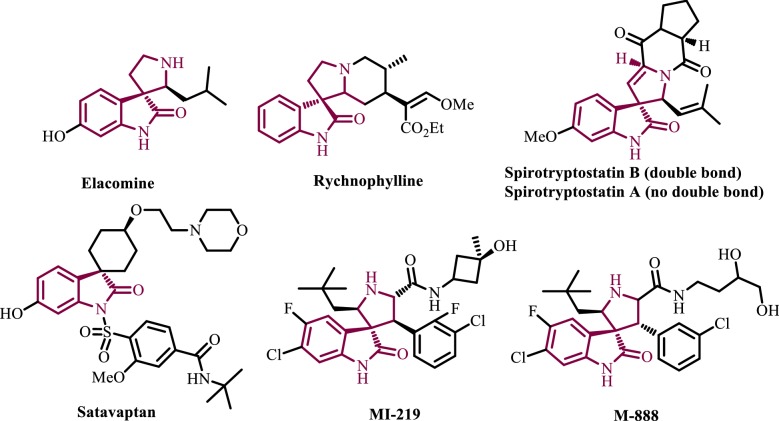
One of the current trends in the discovery of lead compounds for drug discovery programs, that has been described as “escape from flatland”, is an increased three-dimensionality, involving the move from planar aromatic or heteroaromatic systems to others with a higher level of saturation. Such compounds are expected to interact more efficiently with binding pockets in proteins, which are three-dimensional in nature, and have better solubility, a crucial property in the process of drug development [3]. Spiro compounds are very attractive in this connection, since they are intrinsically three-dimensional and many bioactive natural products contain spirocyclic cores that can be assumed to have arisen in the course of evolution to allow better interaction with proteins [4]. In particular, spiro-oxindolepyrrolidine cores can be found in a variety of alkaloids [5], including elacomine, rhynchophylline and the spirotryprostatins, among many others. These compounds and many additional synthetic spirooxindolo-pyrrolidine derivatives (Fig. 1) have shown anticancer [6–8] and other important pharmacological activities [9–13]. 3-Arylmethylene-4-piperidone is another important heterocyclic scaffold present in several families of tumor-specific cytotoxins that display excellent apoptotic-inducing properties against a number of human cancer cell lines, being especially effective against colon cancers and leukemic cells [14].
Fig. 1.
Representative biologically relevant natural spiro(oxindole-pyrrolidine) derivatives
In recent years, our research group has been involved in the synthesis [15–18] and biological evaluation [19–22] of spiroheterocyclic hybrids containing piperidin-4-one units, which were obtained through domino reaction sequences comprising a multicomponent 1,3-dipolar cycloaddition step. In continuation of our research interest in this area, we reasoned that the combination of the spirooxindole framework with pyrrolidine and piperidone motifs in a single molecule would be of interest in the context of anticancer drug discovery.
Results and discussion
Chemistry
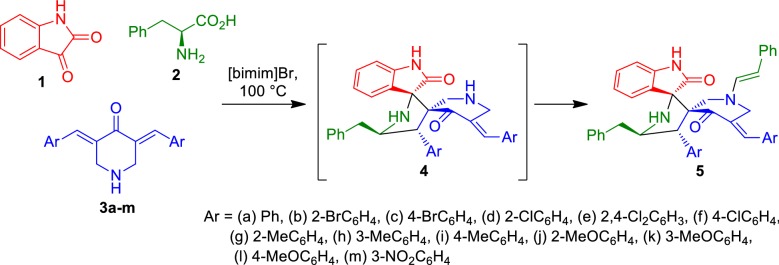
Synthetic methodology employed in the present work was based on the multicomponent 1,3-dipolar cycloaddition reaction strategy as summarized in Scheme 1, and involved a tandem process comprising the 1,3-dipolar cycloaddition reaction between bis-benzylidenepiperidinone and azomethine ylide 6, generated in situ from isatin 1 and l-phenylalanine 2, to afford spiroheterocycle 5. This intermediate subsequently reacts with 2-phenylacetaldehyde, generated in situ in the course of the reaction mechanism (see Scheme 2 below) to afford the final N-substituted arylmethylidene piperidone tethered dispiropyrrolidines 5 through formation of an enamine reaction. Since l-phenylalanine, one of the reaction components, takes part at two different stages of the mechanism and with two different roles, this reaction can be regarded as an example of a rare chemo-differentiating ABCC′ multicomponent reaction [23].
Scheme 1.
Synthesis of N-arylidenepiperidone tethered dispiropyrrolidine 5
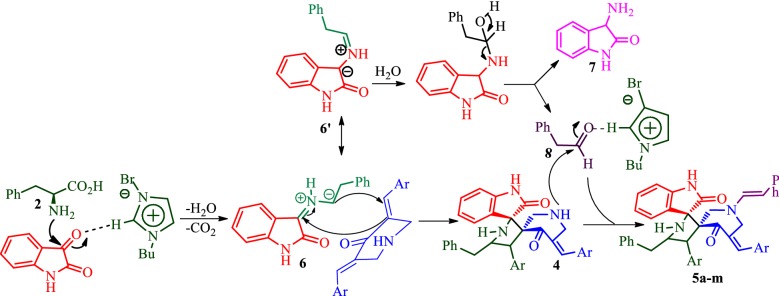
Scheme 2.
Plausible mechanism for the regio- and stereo selective product formation through a domino sequence
Regarding solvent optimization, the reaction was initially performed with an equimolar mixture of (3E,5E)-3,5-bis(4-methylbenzylidene)piperidin-4-one, isatin and l-phenylalanine in methanol, which afforded the product 5a in only 25% yield even after 10 h under reflux. The starting material 3 was still present in the reaction mixture, as evidenced by TLC. After verifying the participation of two molecules of phenylalanine, the same reaction was performed in 1:2:2.05 molar ratio and was found to be complete in 2 h (TLC), affording the product in good yield. The reaction was also attempted under reflux in different solvents or solvent mixtures, viz, dioxane, acetonitrile, dioxane/methanol (1:1 v/v) and toluene. In all these cases, compound 5a was formed only in moderate yields even after long reaction times. As part of our interest in the use of ionic liquids to promote 1,3-dipolar cycloadditions [20, 22], we also examined the use of [bmim]Br as the reaction medium for the present reaction. An excellent yield of the product was obtained in a short reaction time, as shown in Table 1, all successive reactions were accomplished under these optimized reaction conditions (Table 2).
Table 1.
Optimization of solvent for synthesis of spiroheterocyclic hybrids 5a
| Entry | Solvents | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | Methanol | 2 | 90 |
| 2 | Dioxane | 2 | 62 |
| 3 | Acetonitrile | 2 | 65 |
| 4 | Dioxane: methanol | 2 | 75 |
| 5 | Toluene | 2 | 55 |
| 6 | [bmim]Br | 1 | 95 |
Table 2.
Structures, yields and melting points of compounds 5a–m
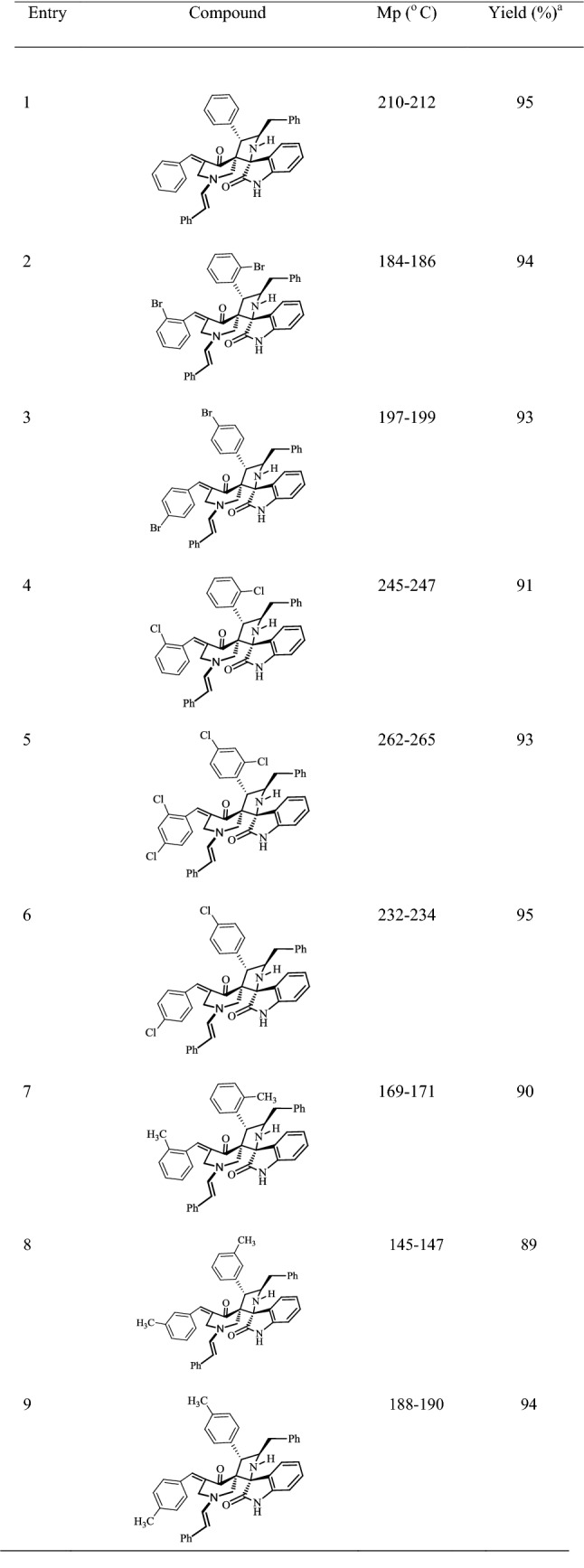
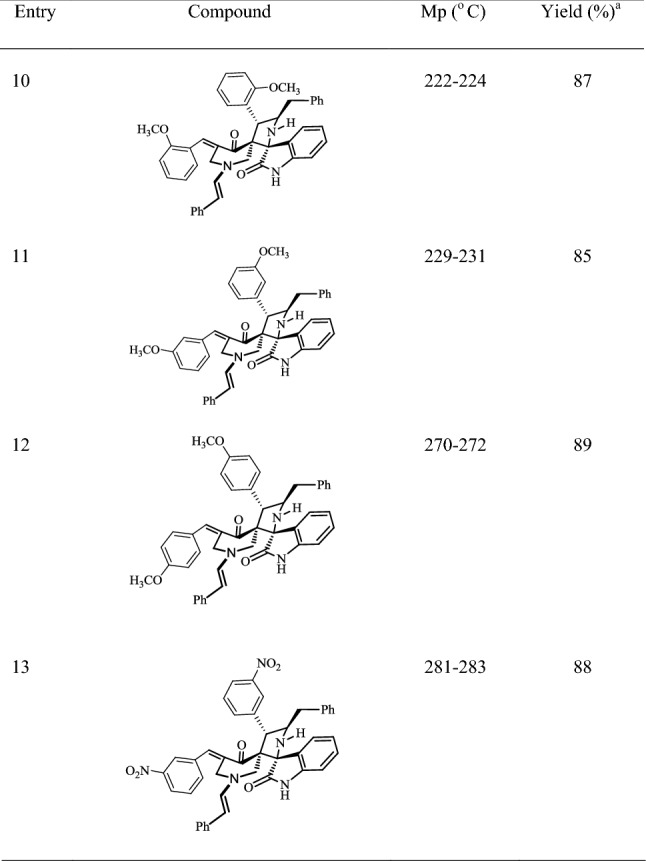
aIsolated yield after column chromatography
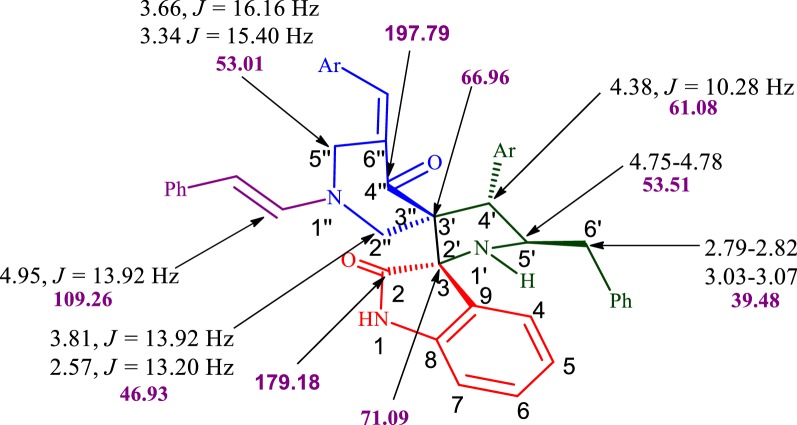
The spiropyrrolidine derivatives 5a–m thus obtained were characterized by one- and two-dimensional NMR experiments, as shown in Fig. 2 below for the representative case of 5a. Its 1H NMR spectrum shows a doublet at 4.38 ppm (J = 10.3 Hz) for 4′-CH, i.e., the benzylic proton belonging to the pyrrolidine ring. This coupling constant value establishes the regiochemistry of the cycloaddition, since 4′-CH should give a singlet for the other possible regiomers arising from the cycloaddition. The multiplet found at 4.75–4.78 ppm, which was shown to be coupled to 4′-CH in the H,H-COSY experiment, was assigned to 5′-CH. The multiplets at 2.79–2.82 and 3.03–3.07 ppm were assigned to 6′-CH2 because they are coupled with 5′-CH. Again from COSY data, the doublets at 3.81 ppm (J = 13.5 Hz) and 2.57 ppm (J = 13.5 Hz) can be assigned to the 2″-CH2 protons, which were also correlated with the carbonyl group of piperidone moiety at 197.79 ppm, as shown by the HMQC experiment. The 7″-arylmethylene proton was observed as a doublet at δ 4.95 ppm (J =13.9 Hz). The signals at 71.09 and 66.96 ppm in the 13C-NMR spectrum of 5a were attributed to C-3′ and C-2′, respectively, while those at 39.48, 46.93 and 53.01 ppm were assigned to the three methylene carbons (C-6′, C-2″ and C-6″) using DEPT-135 data (Additional file 1). Finally in the mass spectrum of 5a, the presence of molecular ion peak at m/z = 627 (M+) and a comparison with a similar analogue reported by us earlier [24] confirms the proposed structure.
Fig. 2.
Assignments of selected signals of compound 5a
A feasible mechanism for the formation of compounds 5 is illustrated in Scheme 2. Initially, the azomethine ylide 6 generated in situ by the reaction of indoline-2,3-dione and l-phenylalanine via decarboxylative condensation. The intermediate 6 then adds regioselectively to one of the C=C bonds of arylidinepiperidone 3 furnish cycloadduct 4. Simultaneously, the azomethine ylide 6′ would be attacked by a molecule of water to furnish 2-phenylacetaldehyde 8 and 3-aminoindolin-2-one 7 as a by-product. Finally, the condensation of this aldehyde with the free secondary amino group in 4 would form the enamine group in compounds 5.
Cytotoxicity analysis
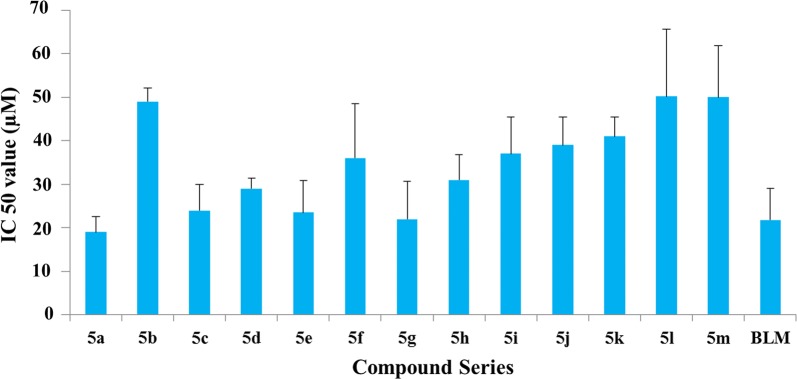
The cytotoxicity of compounds 5a–m was assessed on FaDu hypopharyngeal tumor cells after their exposure to the compounds for 48 h, in comparison with the commercial anti-cancer drug bleomycin under identical conditions (Fig. 3). While 5l and 5m were inactive, the other compounds showed IC50 values in the 19–41 μM range (Additional file 1: Table S1), which are comparable to the one found for the standard anticancer drug bleomycin (IC50 = 21.8 ± 7.3). While the similar activities found for most compounds make it difficult to extract meaningful structure–activity relationships, the data obtained suggest that, with the exception of the Br derivatives, the most favourable position for substitution in the variable aryl ring is ortho- (e.g., 5g vs 5i, 5d vs 5f, 5h vs 5i). There does not seem to be any connection between activity and the electron-releasing or electron-withdrawing nature of the substituents, and in fact the three best compounds, which were comparable in terms of activity to the bleomycin positive control, are the parent unsubstituted system 5a, the 2-methyl derivative 5g and the 2,4-dichloro derivative 5e.
Fig. 3.
In vitro cytotoxicity analysis of synthesized compounds 5(a–m) and bleomycin against FaDu hypopharyngeal cancer cells after 48 h incubation. The data are presented as the mean ± SD of four replicates each. The graph shows the IC50 values of the synthesized compounds and bleomycin
Quantitation of apoptotic cell percentage
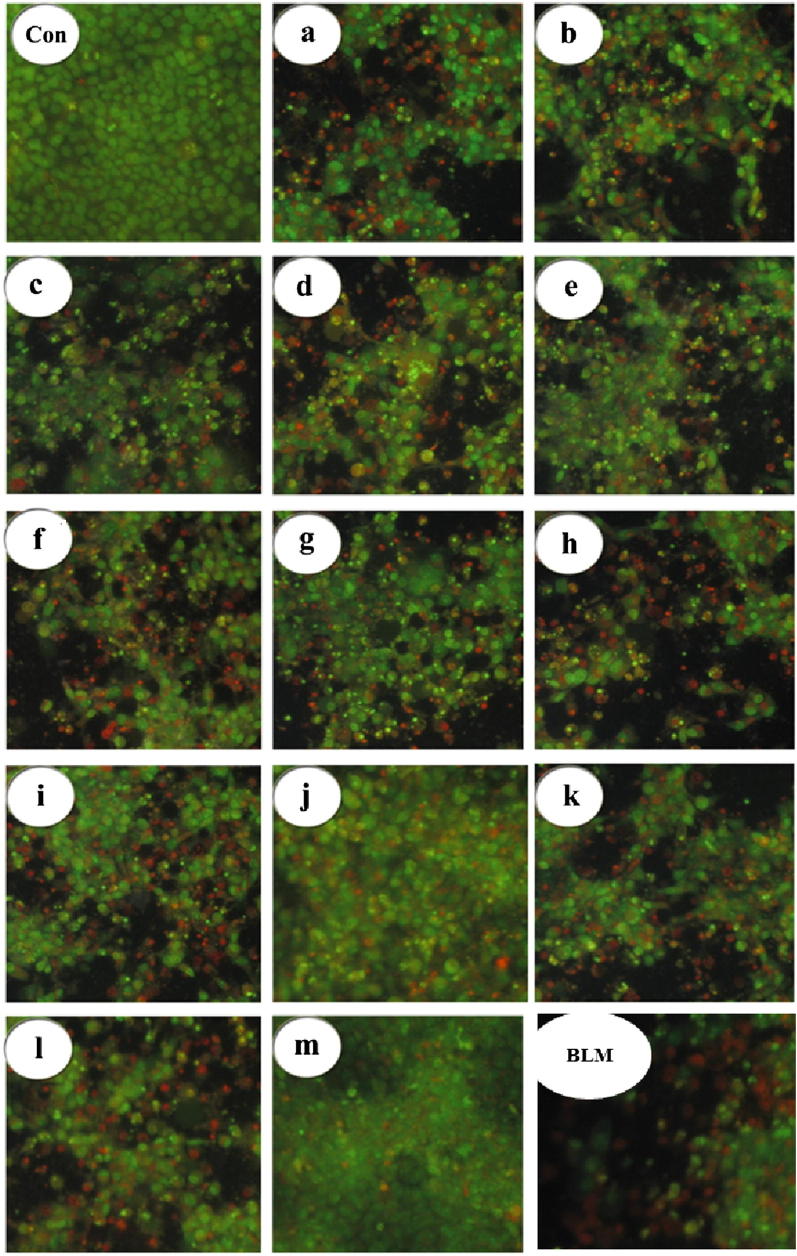
We next studied the changes that our compounds induced in cell morphology. In order to study both cytoplasmic and nuclear morphological changes, we carried out a dual staining with acridine orange and ethidium bromide. The cancer cells were treated with our compounds at their IC50 concentrations for 48 h. The observed morphological changes could be classified as follows: (i) viable cells had shining nuclei, evenly green in color, and displayed a highly organized structure; (ii) early apoptotic cells showed shining nuclei, yellow-green in color, with nuclear chromatin that was crescent-shaped and condensed or fragmented (iii) late apoptotic cells displayed shining nuclei, orange to red in color, with chromatin condensation and fragmentation; and (iv) necrotic cells had orange to red shining nuclei and their volume was increased (Fig. 4). The early response observed following treatment with our compounds 5 was death by apoptosis, and the surviving cells succumbed to necrosis on prolonged treatment. Our findings indicate that the ability of compounds 5a–m to induce apoptosis in FaDu cells had a good correlation to their cytotoxicity values, as shown in Fig. 5.
Fig. 4.
AO/EB dual staining data showing the response of FaDu hypopharyngeal cancer cells exposed to synthesized compounds 5(a–m) and bleomycin at 48 h, in terms of apoptosis. The percentage of apoptotic cells is indicated by the histograms. The data shown are the means from triplicates
Fig. 5.
Cytological features of synthesized compounds 5(a–m) and bleomycin-treated FaDu hypopharyngeal cancer cells (48 h). Magnification: ×400
Conclusion
A one-pot protocol has been developed in [bmim]Br for the construction of spiropyrrolidine tethered piperidone heterocyclic hybrids 5 from simple starting materials that involves the generation of novel structurally interesting N-arylidenepiperidone tethered spirooxindolopyrrolidine 5a–m in good to excellent yields by creation of four new bonds and four adjacent stereocenters in a single operation. This four-component process involves the generation of an azomethine ylide, a regio- and diastereoselective 1,3-dipolar cycloaddition an enamine-formation reaction. These compounds were evaluated for their cytotoxicity against FaDu hypopharyngeal tumor cells and it was observed that most of them exhibited a cytotoxicity similar to the one found for the standard anticancer drug bleomycin, with two compounds (5a and 5g) being slightly more potent than the reference drug. In addition, the compounds were shown to induce apoptosis in the same cancer cell model.
Experimental
General methods
The melting points were measured using open capillary tubes and are uncorrected. 1H, 13C and 2D NMR spectra were recorded on a JEOL 400 MHz instrument. Elemental analyses were carried out on a Perkin Elmer 2400 Series II Elemental CHNS analyser. Mass spectra were performed on JEOL-DX303 HF mass spectrometer.
General procedure for synthesis of dispiropyrrolidines fused piperidinone heterocyclic hybrids, 5(a–m)
The suitable arylimethylenepiperidin-4-one (1.36 mmol), isatin (2.72 mmol) and l-Phenylalanine (2.72 mmol) in [bmim]Br (3 mL) was heated while stirred for 1 h at 100 °C. After the reaction completion, EtOAc (2 × 5 mL) was added and the resulting mixture was stirred for an additional time of 10 min. The EtOAc layer was separated and the solvent was evaporated. The residue was recrystallized from ethanol to furnish compounds 5.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5a)
1H NMR: δ/ppm 4.95 (1H, d, J = 4.92 Hz), 4.75–4.78 (1H, m), 4.38 (1H, d, J = 10.28 Hz), 3.81 (1H, d, J = 13.92 Hz), 3.66 (1H, d, J = 16.16 Hz), 3.34 (1H, d, J = 15.40 Hz), 3.03–3.07 (1H, m), 2.79–2.82 (1H, m), 2.57 (1H, d, J = 13.20 Hz), 6.61–6.64 (2H, m), 6.89–7.48 (24H, m, Ar), 7.68 (1H, s, NH); 13C NMR: δC/ppm 39.48, 46.93, 53.01, 53.51, 61.24, 66.96, 71.09, 100.03, 109.26, 122.30, 124.09, 124.18, 126.37, 126.69, 127.23, 127.85, 128.46, 128.62, 128.64, 128.72, 129.27, 129.35, 129.42, 130.30, 130.69, 134.62, 137.26, 138.53, 138.64, 138.78, 139.15, 141.01, 179.18, 197.79. MS: m/z 627 (M+). Anal.Calcd for C43H37N3O2: C, 82.27; H, 5.94; N, 6.69. Found: C, 82.39; H, 5.81; N, 6.57.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5b)
1H NMR: δ/ppm 4.88–4.91 (1H, m), 4.57–4.62 (2H, m), 3.58 (1H, d, J = 16.16 Hz), 3.21 (1H, d, J = 13.92 Hz), 3.00–3.08 (1H, m), 2.97–2.99 (1H, m), 2.74 (1H, d, J = 15.4 Hz), 6.35 (1H, d, J = 13.92 Hz), 6.65 (1H, d, J = 8.04 Hz), 6.92–7.69 (22H, m, Ar), 7.95 (1H, s, NH); 13C NMR: δ/ppm 39.58, 46.92, 53.24, 53.57, 61.29, 65.45, 73.57, 100.13, 109.16, 122.39, 123.75, 125.18, 126.14, 126.26, 126.74, 127.28, 127.89, 128.30, 128.40, 129.26, 129.34, 129.41, 129.44, 130.38, 130.78, 130.86, 132.82, 132.83, 135.07, 135.35, 136.28, 137.35, 138.59, 138.69, 138.81, 139.21 141.17, 179.24, 197.81. MS: m/z 785 (M+). Anal.Calcd for H35Br2N3O2: C, 65.74; H, 4.49; N, 5.35; Found: C, 65.86; H, 4.61; N, 5.47.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5c)
1H NMR: δ/ppm 4.94 (1H, d, J = 13.92 Hz), 4.66–4.70 (1H, m), 4.30 (1H, d, J = 10.24 Hz), 3.74 (1H, d, J = 13.96 Hz), 3.65 (1H, d, J = 15.4 Hz), 3.27 (1H, d, J = 15.76 Hz), 2.98–3.03 (1H, dd, J = 13.92, 2.96 Hz), 2.77–2.82 (1H, m, 13.92, 8.08 Hz), 2.56 (1H, d, J = 13.2 Hz), 6.59–6.64 (2H, m), 6.89–7.49 (22H, m, Ar); 13C NMR: δ/ppm 39.52, 46.96, 52.94, 53.06, 61.53, 66.73, 71.14, 100.57, 109.37, 121.28, 122.31, 124.17, 124.40, 126.48, 126.55, 126.56, 128.24, 128.50, 128.64, 129.28, 129.41, 131.13, 131.51, 131.62, 131.84, 131.95, 133.35, 136.30, 137.91, 138.21, 138.29, 138.48, 141.01, 179.22, 197.94. MS: m/z 785 (M+). Anal.Calcd for C43H35Br2N3O2: C, 65.74; H, 4.49; N, 5.35;. Found: C, 65.86; H, 4.62; N, 5.47.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5d)
1H NMR: δ/ppm 4.88–4.92 (1H, m), 4.62–4.65 (2H, m), 3.59 (1H, d, J = 16.16 Hz), 3.26 (1H, d, J = 13.92 Hz), 2.96–3.10 (2H, m), 2.77–2.86 (2H, m), 6.38 (1H, d, J = 13.92 Hz), 6.64 (1H, d, J = 7.32 Hz), 6.89–7.42 (22H, m, Ar), 7.76 (1H, s); 13C NMR: δ/ppm 40.53, 46.15, 51.89, 52.62, 62.90, 65.46, 73.28, 98.25, 109.78, 122.55, 123.84, 126.18, 126.25, 126.31, 126.52, 127.52, 127.09, 127.69, 128.09, 128.36, 128.61, 129.18, 129.29, 130.03, 130.36, 130.50, 130.57, 132.94, 133.11, 135.07, 135.80, 135.92, 136.20, 137.94, 138.51, 139.08, 141.16, 177.48, 200.01. MS: m/z 696 (M+). Anal. Calcd for C43H35Cl2N3O2: C, 74.13; H, 5.06; N, 6.03; Found: C, 74.24; H, 5.17; N, 6.15.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5e)
1H NMR: δ/ppm 4.82–4.87 (1H, m), 4.58–4.67 (2H, m), 3.55–60 (1H, d, J = 16.16 Hz), 3.23 (1H, d, J = 13.92 Hz), 2.96–3.01 (2H, m), 2.77–2.81 (2H, m), 6.33 (1H, d, J = 13.92 Hz), 6.66 (1H, d, J = 7.36 Hz) 6.90–7.50 (20H, m, Ar), 7.58 (1H, s, NH); 13C NMR: δ/ppm 39.54, 46.92, 53.17, 53.65, 61.36, 67.01, 71.18, 100.12, 109.30, 122.31, 123.90, 124.18, 124.20, 126.04, 126.42, 126.70, 127.05, 127.91, 128.27, 128.39, 128.63, 129.09, 129.58, 129.90, 129.96, 130.66, 131.52, 133.27, 133.46, 134.91, 135.75, 135.81, 137.57, 138.11, 138.69, 139.24, 141.11, 179.26, 197.64. MS: m/z 765 (M+). Anal.Calcd for C43H33Cl4N3O2: C, 67.46; H, 4.34; N, 5.49. Found: C, 67.58; H, 4.47; N, 5.62.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5f)
1H NMR: δ/ppm 4.94 (1H, d, J = 13.96 Hz), 4.67–4.68 (1H, m), 4.32 (1H, d, J = 10.4 Hz), 3.75 (1H, d, J = 13.16 Hz), 3.62 (1H, d, J = 16.12 Hz), 3.29 (1H, d, J = 16.16 Hz), 2.97–3.00 (m, 1H), 2.77–2.80 (1H, m), 2.55 (1H, d, J = 13.92 Hz), 6.56–6.64 (2H, m), 6.88–7.39 (22H, m, Ar), 7.80 (1H, s, NH); 13C NMR: δ/ppm 39.55, 46.98, 52.88, 52.94, 61.47, 66.73, 71.12, 100.55, 109.40, 122.29, 124.14, 124.37, 126.47, 126.63, 127.68, 128.47, 128.62, 128.87, 128.99, 129.33, 129.38, 129.41, 131.05, 131.46, 132.95, 133.10, 135.57, 135.80, 137.83, 138.21, 138.32, 138.51, 141.07, 179.24, 197.50. MS: m/z 696 (M+). Anal.Calcd for C43H35Cl2N3O2: C, 74.13; H, 5.06; N, 6.03 Found: C, 74.24; H, 5.18; N, 6.15.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5g)
1H NMR: δ/ppm 4.91–4.93 (1H, m), 4.70 (1H, d, J = 13.92 Hz), 4.49 (1H, d, J = 10.28 Hz), 3.53–3.59 (2H, m), 2.97–3.09 (1H, m), 2.83–2.89 (2H, m), 2.63 (1H, d, J = 13.96 Hz), 2.23 (3H, s), 2.21 (3H, s), 6.48 (1H, d, J = 14.64 Hz), 6.65 (1H, d, J = 7.32 Hz), 6.76 (1H, d, J = 8.08 Hz), 6.92–7.76 (21H, m, Ar), 7.65 (1H, s, NH); 13C NMR: δ/ppm 20.06, 20.99, 40.12, 46.22, 50.46, 53.21, 63.16, 65.67, 72.53, 98.54, 109.58, 122.38, 123.87, 125.75, 125.81, 126.30, 126.38, 126.40, 126.51, 126.87, 128.43, 128.60, 128.65, 128.81, 129.15, 129.29, 130.41, 131.54, 133.67, 136.04, 137.86, 138.01, 138.24, 138.36, 138.43, 138.95, 139.08, 141.22, 177.91, 199.89. MS: m/z 655 (M+). Anal.Calcd for C45H41N3O2; C, 82.41; H, 6.30; N, 6.41; Found: C, 82.54; H, 6.42; N, 6.53.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5h)
1H NMR: δ/ppm 4.95 (1H, d, J = 13.96 Hz), 4.72–4.76 (m, 1H), 4.34 (1H, d, J = 11.0 Hz), 3.82 (1H, d, J = 13.92 Hz), 3.66 (1H, d, J = 17.6 Hz), 3.36 (d, J = 16.12 Hz, 1H), 3.04–3.07 (1H, m), 2.75–2.81 (1H, m), 2.58 (1H, d, J = 13.2 Hz), 2.36 (3H, s), 2.34 (3H, s), 6.60–6.64 (2H, m), 6.74–7.34 (22H, m, Ar), 7.72 (1H, s, NH); 13C NMR: δ/ppm 20.03, 20.98, 40.16, 46.29, 50.42, 53.19, 63.20, 65.73, 72.56, 98.58, 109.63, 122.41, 123.91, 125.79, 126.32, 126.45, 126.54, 126.89, 126.92, 128.41, 128.64, 128.86, 129.18, 129.32, 129.41, 129.44, 129.58, 130.47, 131.55, 133.68, 136.09, 137.88, 138.03, 138.25, 138.41, 138.99, 139.08, 141.23, 177.96, 199.91. MS: m/z 655 (M+). Anal.Calcd for C45H41N3O2; C, 82.41; H, 6.30; N, 6.41; Found: C, 82.54; H, 6.42; N, 6.51.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5i)
1H NMR: δ/ppm 4.96 (1H, d, J = 13.96 Hz), 4.71–4.75 (1H, m), 4.35 (1H, d, J = 10.28 Hz), 3.79 (1H, d, J = 13.96 Hz), 3.67 (1H, d, J = 15.4 Hz), 3.35 (d, J = 16.12 Hz), 3.03–3.06 (1H, m), 2.76–2.78 (1H, m), 2.60 (1H, d, J = 13.92 Hz), 2.36 (3H, s), 2.34 (3H, s), 6.60 (2H, m), 6.64 (1H, s), 6.84–7.35 (21H, m, Ar), 7.64 (1H, s, NH); 13C NMR: δ/ppm 21.20, 21.55, 39.40, 47.09, 53.03, 53.16, 61.22, 66.83, 71.12, 99.94, 109.19, 122.24, 124.06, 124.11, 126.33, 126.69, 127.96, 128.44, 128.60, 129.04, 129.19, 129.37, 129.41, 129.91, 130.12, 130.50, 131.87, 134.17, 136.81, 138.62, 138.76, 138.85, 139.14, 139.85, 141.02, 179.33, 197.84. MS: m/z 655 (M+). Anal.Calcd for C45H41N3O2; C, 82.41; H, 6.30; N, 6.41; Found: C, 82.53; H, 6.44; N, 6.52.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5j)
1H NMR: δ/ppm 5.00–5.02 (1H, m), 4.66 (1H, d, J = 13.96 Hz), 4.47 (1H, d, J = 10.28 Hz), 3.86 (3H, s), 3.82–3.74 (4H, m), 3.67 (1H, d, J = 16.12 Hz), 3.26 (1H, d, J = 13.96 Hz), 3.06–3.08 (1H, m), 2.87–2.93 (1H, m), 2.80 (1H, d, J = 13.92 Hz), 6.45 (1H, m, J = 13.92 Hz), 6.61 (1H, d, J = 8.08 Hz), 6.76–7.34 (22H, m, Ar), 7.73 (1H, s, NH); 13C NMR: δ/ppm 40.48, 46.40, 48.37, 52.25, 55.01, 55.45, 60.49, 65.14, 72.71, 97.48, 109.58, 110.71, 120.20, 120.81, 122.24, 123.55, 123.68, 126.38, 126.43, 126.56, 126.88, 126.95, 127.79, 128.37, 128.56, 128.62, 128.76, 128.98, 129.12, 130.24, 130.92, 131.58, 134,11, 138.27, 139.26, 139.38, 139.47, 141.27, 179.92, 200.45. MS: m/z 687 (M+). Anal.Calcd for C45H41N3O4; C, 78.58; H, 6.01; N, 6.11; Found: C, 78.70; H, 6.13; N, 6.21.40.48, 46.40, 48.37, 52.25, 55.01, 55.45, 60.49, 65.14, 72.71, 97.48,
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5k)
1H NMR: δ/ppm 5.01–5.04 (1H, m), 4.68 (1H, d, J = 13.96 Hz), 4.49 (1H, d, J = 10.28 Hz), 3.84 (3H, s), 3.80–3.82 (1H, m), 3.79 (3H, s), 3.65 (1H, d, J = 16.12 Hz), 3.28 (1H, d, J = 13.96 Hz), 3.05–3.07 (1H, m), 2.86–2.92 (1H, m), 2.82 (1H, d, J = 13.92 Hz), 6.44 (1H, m, J = 13.92 Hz), 6.62 (1H, d, J = 8.08 Hz), 6.77–7.38 (22H, m, Ar),7.71 (1H, s, NH); 13C NMR: δ/ppm 40.45, 46.40, 48.39, 52.21, 55.09, 60.52, 65.17, 72.73, 97.50, 109.59, 110.74, 120.23, 120.78, 122.21, 123.50, 123.61, 126.39, 126.41, 126.44, 126.54, 126.58, 126.80, 126.92, 126.92, 127.80, 128.41, 128.61, 128.91, 129.14, 130.27, 130.93, 131.55, 134,12, 138.29, 139.30, 139.39, 139.46, 141.32, 179.89, 199.82. MS: m/z 687 (M+). Anal.Calcd for C45H41N3O4; C, 78.58; H, 6.01; N, 6.11; Found: C, 78.71; H, 6.12; N, 6.23.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5l)
1H NMR: δ/ppm 4.99–5.02 (1H, m), 4.66 (1H, d, J = 13.96 Hz), 4.46 (1H, d, J = 10.28 Hz), 3.83 (3H, s), 3.80–3.82 (1H, m), 3.79 (3H, s), 3.64 (1H, d, J = 16.12 Hz), 3.29 (1H, d, J = 13.96 Hz), 3.04–3.08 (1H, m), 2.87–2.93 (1H, m), 2.83 (1H, d, J = 13.92 Hz), 6.46 (1H, m, J = 13.92 Hz), 6.61 (1H, d, J = 8.08 Hz), 6.78–7.39 (22H, m, Ar), 7.73 (1H, s, NH); 13C NMR: δ/ppm 40.42, 46.41, 48.40, 52.29, 55.11, 60.46, 65.22, 72.78, 97.80, 109.60, 110.76, 120.27, 120.79, 122.44, 123.93, 124.25, 124.50, 126.43, 126.58, 128.22, 128.31, 128.61, 129.28, 129.43, 129.80, 132.84, 134.67, 135.59, 136.41, 137.62, 138.07, 139.62, 140.81, 141.24, 148.30, 179.85, 199.94. MS: m/z 687 (M+). Anal.Calcd for C45H41N3O4; C, 78.58; H, 6.01; N, 6.11; Found: C, 78.72; H, 6.11; N, 6.22.
Dispiropyrrolidine tethered piperidinone heterocyclic hybrid (5m)
1H NMR: δ/ppm 1H NMR: δ/ppm 4.95 (1H, d, J = 14.68 Hz), 4.74–4.78 (1H, m), 4.42 (1H, d, J = 10.28 Hz), 3.71–3.76 (1H, m), 3.62 (1H, d, J = 16.12 Hz), 3.44–3.48 (1H, m), 3.32 (1H, d, J = 16.16 Hz), 2.84–2.88 (m, 1H), 2.52 (1H, d, J = 13.2 Hz), 6.71 (1H, d, J = 8.08 Hz), 6.93–7.48 (22H, m, Ar), 7.87 (1H, s, NH); 13C NMR: δ/ppm 40.42, 46.91, 53.45, 53.54, 55.12, 61.28, 66.97, 71.18, 100.23, 109.28, 122.35, 124.11, 124.21, 126.40, 126.74, 127.29, 127.37, 127.51, 127.68, 127.89, 128.51, 128.68, 128. 69, 128.71, 128.77, 129.29, 129.33, 129.39, 130.32, 130.71, 134.66, 137.31, 138.67, 138.74, 138.78, 139.19, 141.11, 179.19, 197.81. MS: m/z 717 (M+). Anal.Calcd for C43H35N5O6: 71.95; H, 4.91; N, 9.76; Found: 71.87; H, 4.99; N, 9.88.
Additional file
Additional file 1. Experiment details and NMR spectra. Table S1. IC50 values of spiropyrrolidines 5 against FaDu hypopharyngeal cancer cells. Figure S1. 1H NMR spectrum of 5a. Figure S2. Expanded 1H NMR spectrum of 5a. Figure S3. 13C NMR spectrum of 5a. Figure S4. DEPT-135 spectrum of 5a. Figure S5. 1H, 1H-COSY spectrum of 5a.
Authors’ contributions
Design and synthesis of all compounds by NA, AIA, RSK, GP and JCM. The biological assays were done by VSP, JA and AAA. Structural elucidation of compounds was done by NA, JCM and SM. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the Deanship of Scientific Research at King Saud University for funding this work through Research Group No. RG-1438-052.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- [bmim]Br
1-butyl 3-methylimidazolium bromide
- TLC
thin layer Chromatography
- EtOAc
ethyl acetate
- NMR
nuclear magnetic resonance
- COSY
correlated spectroscopy
- DEPT
distortion less enhancement by polarization transfer
- EI-MS
electron ionization mass spectrometry
- m/z
mass–charge ratio
Contributor Information
Natarajan Arumugam, Email: anatarajan@ksu.edu.sa, Email: aruorgchem@gmail.com.
Abdulrahman I. Almansour, Email: almansor@ksu.edu.sa
Raju Suresh Kumar, Email: sraju@ksu.edu.sa.
Dhaifallah M. Al-thamili, Email: daife54321@hotmail.com
Govindasami Periyasami, Email: pkandhan@ksu.edu.sa.
V. S. Periasamy, Email: psubbarayan@ksu.edu.sa
Jegan Athinarayanan, Email: jegan.dna@gmail.com.
Ali A. Alshatwi, Email: alshatwi@ksu.edu.sa
S. M. Mahalingam, Email: mahalingam.sm@manipal.edu
J. Carlos Menéndez, Email: josecm@farm.ucm.es.
References
- 1.Koff JL, Ramachandiran S, Bernal-Mizrachi L. A time to kill: targeting apoptosis in cancer. Int J Mol Sci. 2015;16:2942–2955. doi: 10.3390/ijms16022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui JJ. Targeting receptor tyrosine kinase MET in cancer: small molecule inhibitors and clinical progress. J Med Chem. 2014;57:4427–4453. doi: 10.1021/jm401427c. [DOI] [PubMed] [Google Scholar]
- 3.Lovering F, Bikker J, Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J Med Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 4.Zheng YJ, Tice CM. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin Drug Discov. 2016;11:831–834. doi: 10.1080/17460441.2016.1195367. [DOI] [PubMed] [Google Scholar]
- 5.Saraswat P, Jeyabalan G, Hassan MZ, Rahman MU, Nyola NK. Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moities. Synth Commun. 2016;46:1643–1664. doi: 10.1080/00397911.2016.1211704. [DOI] [Google Scholar]
- 6.Yu B, Yu DQ, Liu HM. Spirooxindoles: promising scaffolds for anticancer agents. Eur J Med Chem. 2015;97:673–698. doi: 10.1016/j.ejmech.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 7.Kathirvelan D, Haribabu J, Reddy BSR, Balachandran C, Duraipandiyan V. Facile and diastereoselective synthesis of 3,2′-spiropyrrolidineoxindole derivatives, their molecular docking and antiproliferative activities. Bioorg Med Chem Lett. 2015;25:389–399. doi: 10.1016/j.bmcl.2014.10.099. [DOI] [PubMed] [Google Scholar]
- 8.Arun Y, Saranraj K, Balachandran C, Perumal PT. Novel spirooxindole-pyrrolidine compounds: synthesis, anticancer and molecular docking studies. Eur J Med Chem. 2014;74:50–64. doi: 10.1016/j.ejmech.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Bhaskar G, Arun Y, Balachandran C, Saikumar C, Perumal PT. Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur J Med Chem. 2012;51:79–91. doi: 10.1016/j.ejmech.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Rajesh SM, Perumal S, Menéndez JC, Yogeeswari P, Sriram D. Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med Chem Commun. 2011;2:626–630. doi: 10.1039/c0md00239a. [DOI] [Google Scholar]
- 11.Rajanarendar E, Ramakrishna S, Reddy KG, Nagaraju D, Reddy YN. A facile synthesis, anti-inflammatory and analgesic activity of isoxazolyl-2,3-dihydrospiro[benzo[f]isoindole-1,3′-indoline]-2′,4,9-triones. Bioorg Med Chem Lett. 2013;23:3954–3958. doi: 10.1016/j.bmcl.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 12.Kia Y, Osman H, Kumar RS, Basiri A, Murugaiyah V. Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg Med Chem Lett. 2014;24:1815–1819. doi: 10.1016/j.bmcl.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Kia Y, Osman H, Kumar RS, Murugaiyah V, Basiri A, Perumal S, Razak IA. A facile chemo-, regio- and stereoselective synthesis and cholinesterase inhibitory activity of spirooxindole-pyrrolizine-piperidine hybrids. Bioorg Med Chem Lett. 2013;23:2979–2983. doi: 10.1016/j.bmcl.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Cheng D, Valente S, Castellano S, Sbardella G, Santo RD, Costi R, Bedford MT, Mai A. Novel 3,5-bis(bromohydroxybenzylidene)piperidin-4-ones as coactivator-associated arginine methyltransferase 1 inhibitors: enzyme selectivity and cellular activity. J Med Chem. 2011;54:4928–4932. doi: 10.1021/jm200453n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumugam N, Almansour AI, Kumar RS, Perumal S, Ghabbour HA, Fun HK. A 1,3 dipolar cycloaddition–annulation protocol for the expedient regio-, stereo- and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres. Tetrahedron Lett. 2013;54:2515–2519. doi: 10.1016/j.tetlet.2013.03.021. [DOI] [Google Scholar]
- 16.Arumugam N, Raghunathan R, Almansour AI, Karama U. An efficient synthesis of highly functionalized novel chromeno[4,3-b]pyrroles and indolizino[6,7-b]indoles as potent antimicrobial and antioxidant agents. Bioorg Med Chem Lett. 2012;22:1375–1379. doi: 10.1016/j.bmcl.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 17.Almansour AI, Arumugam N, Kumar RS, Mahalingam SM, Sau S, Bianchini G, Menéndez JC, Altaf M, Ghabbour HA. Design, synthesis and antiproliferative activity of decarbonyl luotonin analogues. Eur J Med Chem. 2017;138:932–941. doi: 10.1016/j.ejmech.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Arumugam N, Almansour AI, Kumar RS, Menéndez JC, Sultan MA, Karama U, Ghabbour HA, Fun HK. An expedient regio- and diastereoselective synthesis of hybrid frameworks with embedded spiro[9, 10]dihydroanthracene [9,3′]-pyrrolidine and spiro[oxindole-3,2′-pyrrolidine] motifs via an ionic liquid-mediated multicomponent reaction. Molecules. 2015;20:16142–16153. doi: 10.3390/molecules200916142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arumugam A, Almansour AI, Kumar RS, Periasamy VS, Athinarayanan J, Alshatwi AA, Govindasami P, Altaf M, Menéndez JC. Regio- and diastereoselective synthesis of anticancer spirooxindoles derived from tryptophan and histidine via three-component 1,3-dipolar cycloadditions in an ionic liquid. Tetrahedron. 2018 [Google Scholar]
- 20.Arumugam N, Periyasami G, Raghunathan R, Kamalraj S, Muthumary J. Synthesis and antimicrobial activity of highly functionalised novel β-lactam grafted spiropyrrolidines and pyrrolizidines. Eur J Med Chem. 2011;46:600–607. doi: 10.1016/j.ejmech.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 21.Arumugam N, Almansour AI, Kumar RS, Altaf M, Padmanaban R, Sureshbabu P, Angamuthu G, Kotresha D, Manohar TS, Venketesh S. Spiropyrrolidine/spiroindolizino[6,7-b]indole heterocyclic hybrids: stereoselective synthesis, cholinesterase inhibitory activity and their molecular docking study. Bioorg Chem. 2018;79:64–71. doi: 10.1016/j.bioorg.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Arumugam N, Raghunathan R, Shanmugaiah V, Mathivanan N. Synthesis of novel β-lactam fused spiroisoxazolidine chromanones and tetralones as potent antimicrobial agent for human and plant pathogens. Bioorg Med Chem Lett. 2010;20:3698–3702. doi: 10.1016/j.bmcl.2010.04.084. [DOI] [PubMed] [Google Scholar]
- 23.Tejedor D, García-Tellado F. Chemo-differentiating ABB′ multicomponent reactions. Privileged building blocks. Chem Soc Rev. 2007;36:484–491. doi: 10.1039/B608164A. [DOI] [PubMed] [Google Scholar]
- 24.Almansour AI, Arumugam N, Suresh Kumar R, Subbarayan PV, Alshatwi AA, Ghabbour HA (2016) Anticancer Compound. US Patent 9486444 B1, 8 November
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Experiment details and NMR spectra. Table S1. IC50 values of spiropyrrolidines 5 against FaDu hypopharyngeal cancer cells. Figure S1. 1H NMR spectrum of 5a. Figure S2. Expanded 1H NMR spectrum of 5a. Figure S3. 13C NMR spectrum of 5a. Figure S4. DEPT-135 spectrum of 5a. Figure S5. 1H, 1H-COSY spectrum of 5a.