Abstract
The formation of the cryopyrin inflammasome in the heart induces an intense inflammatory response during acute myocardial infarction (AMI), which mediates further damage and promotes adverse cardiac remodeling. Active interleukin-1β (IL-1β) is a key product of the inflammasome, being cleaved by active caspase-1. The aim of this study was to dissect the role of IL-1β from that of the inflammasome by using a neutralizing monoclonal antibody directed against IL-1β and measuring the intensity of the inflammatory response, the activity of caspase-1 in the inflammasome, cardiomyocyte apoptosis, and cardiac remodeling in a mouse model of non-reperfused AMI. A mouse monoclonal IgG2a antibody directed against IL-1β (10 mg/Kg [IL-1β-AB]) was given i.p. immediately after surgery and then repeated 1 week later. Cardiac tissue was analyzed at 72 hours after surgery in a subgroup of mice for inflammasome aggregates and caspase-1 activity (inflammasome) and for DNA fragmentation and caspase-3 activity (apoptosis). All sham-operated mice were alive at 10 weeks, whereas 40% of the control-AB-treated mice and 30% of the IL-1β-AB-treated mice died during the 4 weeks after surgery. When compared with vehicle, treatment with the IL-1β-AB did not affect inflammasome formation or caspase-1 activation in the heart tissue at 72 hours after AMI nor circulating plasma IL-6 levels, but did inhibit cardiomyocyte apoptosis, limit left ventricular enlargement by 40% (P<0.01) and improve systolic dysfunction by 17% (P<0.01) after AMI. These findings suggest that IL-1β mediates the deleterious effects on the heart during sterile inflammatory response.
Keywords: Inflammasome, heart failure, remodeling, apoptosis, myocardial infarction
INTRODUCTION
Myocardial necrosis triggers a sterile inflammatory response in the heart during acute myocardial infarction (AMI).(Mezzaroma et al., 2011; Frangogiannis, 2012) Tissue debris induces chemotactic recruitment of infiltrating cells and subsequent formation of the inflammasome, a macromolecular structure responsible for the amplification of the inflammatory response.(Franchi et al., 2009) Caspase-1 is the effector enzyme of the inflammasome and is primarily responsible for the processing and release of IL-1β (as well as IL-18 and IL-33).(Franchi et al., 2009) Formation of the inflammasome, increased caspase-1 and IL-1β activity during AMI ultimately promote cell death, cardiac remodeling, and heart failure.(Frantz et al., 2003; Merkle et al., 2007; Mezzaroma et al., 2011)
IL-1β is the prototypical inflammatory cytokine that induces the synthesis and release of a cascade of secondary inflammatory mediators and of IL-1β itself.(Dinarello, 2011) This principle of autoinduction, known as autoinflammation, suggests that IL-1β may be both a product and a determinant of the sterile inflammatory response.(Dinarello et al., 1987; Dinarello, 2011)
Multiple studies based on genetic or pharmacologic IL-1β blockade also confirm the benefit of IL-1β blockade showing decreased apoptosis, improved cardiac function, and more favorable cardiac remodeling after AMI.(Abbate et al., 2008b; Van Tassell et al., 2010b) Cardiomyocytes exposed to IL-1β in vitro undergo classic apoptosis,(Ing et al., 1999) and IL-1β blockade has been shown to inhibit apoptosis in vitro and in vivo.(Abbate et al., 2008b; Van Tassell et al., 2010b)
It remains unclear in AMI, however, whether blockade of IL-1β prevents the effects of the inflammasome by antagonizing the binding of IL-1β to its receptor or if IL-1β blockade inhibits the formation of the inflammasome through prevention of IL-1β autoinduction or autoinflammation.
In the current study we used a monoclonal antibody (IL-1β-AB) to elucidate the specific role of IL-1β in the sterile inflammatory response to myocardial infarction at the level of inflammatory infiltration, inflammasome formation, caspase-1 and caspase-3 activities, cardiomyocyte apoptosis, infarct size, cardiac remodeling, and overall survival during a 10-week course after surgical coronary artery ligation.
METHODS
Ethical approval
The experiments were conducted under the guidelines of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996). The study protocol was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Experimental AMI model
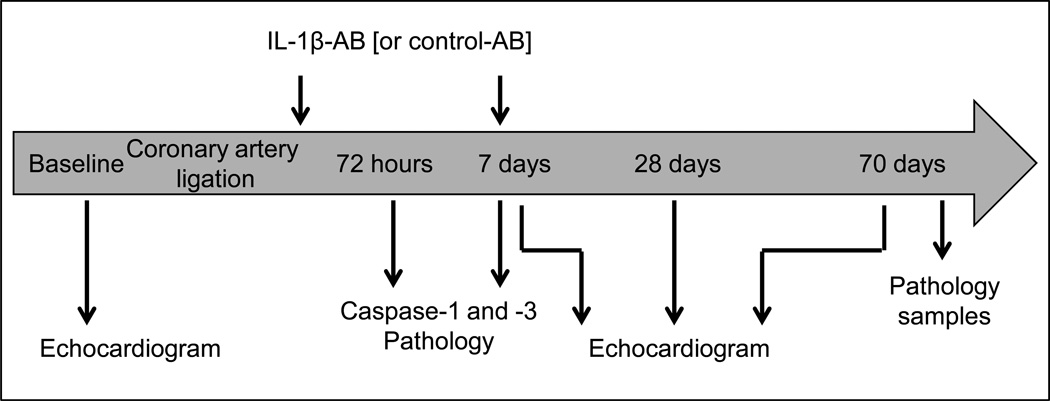
Adult out-bred male CD1 mice (8–12 weeks of age) were supplied by Harlan Sprague Dawley (Indianapolis, IN). Experimental AMI was induced by permanent coronary artery ligation to induce a large non-reperfused infarct involving approximately 30% of the left ventricle and leading to an ischemic dilated cardiomyopathy.(Abbate et al., 2008b; Seropian et al., 2010; Van Tassell et al., 2010a; Van Tassell et al., 2010b) Briefly, mice were orotracheally intubated under anesthesia (pentobarbital 50 to 70 mg/kg), placed in the right lateral decubitus position, then subjected to left thoracotomy, pericardiectomy, and ligation of the proximal left coronary artery, before closure of the thorax. The mice surviving surgery were randomly assigned to the different groups of treatment (N=10–12 per group). Sham operations were performed wherein animals underwent the same surgical procedure without coronary artery ligation (N=6–8 per group). A timeline of the study is shown in Figure 1.
Figure 1.
Timeline of the study protocol.
Treatment
A mouse monoclonal IgG2a antibody directed against IL-1β (derived from the 1400.24.17 antibody,(Osborn et al., 2008) 10 mg/Kg [IL-1β-AB]) was given i.p. immediately after surgery and then repeated 1 week later. We tested the neutralizing effect of the IL-1β antibody by measuring Interleukin-6 (IL-6) plasma levels after challenge with recombinant murine IL-1β (rm-IL-1β, 3 µg/Kg) intraperitoneally, as described below. A mouse monoclonal IgG2a antibody directed against cyclosporine was used as control antibody (10 mg/Kg, control-AB). Novartis Pharmaceuticals (Basel, Switzerland) provided both antibodies. Mice were randomly assigned to receive IL-1β-AB or control-AB (final volume 0.2 ml). Another group of mice was randomly assigned to receive a single dose of the IL-1β-AB to explore a duration-response relationship. An additional group of mice was treated with 0.2 ml of NaCl 0.9% as an additional control, however, since the data of the control-AB treatment were not significantly different to those of NaCl treatment, only results of control-AB treatment are reported throughout the manuscript. The concentration of the IL-1β-AB in the heart tissue was measured using a specific ELISA. In brief, samples of hearts harvested 3 and 7 days after AMI were homogenized as described earlier, and antibody concentration was assessed by a competitive ELISA based on a specific anti-idiotypic antibody.(Clausen et al., 2009)
Myocardial inflammatory infiltrate and systemic levels of Interleukin-6
We measured CD45 expression (a leukocyte marker) using Western Blot to quantify the inflammatory infiltrate in the heart during AMI. The hearts collected at 72 h after AMI were homogenized in Ripa Buffer (Sigma Aldrich, St Louis, MO) supplemented with a protease inhibitor cocktail (Sigma Aldrich) and centrifuged at 16,200×g for 20 minutes. Thirty micrograms of each sample were diluted in Laemmli Buffer, denatured for 10 minutes at 96°C and resolved with SDS/PAGE using an 8% acrylamide gel to allow protein separation. The proteins were transferred onto a nitrocellulose membrane. Following saturation with 5% milk in phosphate buffered saline the membrane was incubated with a rat anti-mouse antibody raised against CD45 (R&D systems, Minneapolis, MN). To normalize the protein loading a monoclonal antibody for β-actin (Sigma Aldrich) was used. The enhanced-chemiluminescence (ECL) assay and autoradiography were used to detect the bands corresponding to CD45 and beta-actin. The band intensity was measured by densitometric analysis using the Scion Image software and the results were expressed as percentage increase in intensity compared to the control sham samples. IL-6 plasma levels were measured in samples collected 4 hours after challenge with rm-IL-1β or 72 hours after coronary artery ligation surgery. IL-6 was measured using the mouse IL-6 Quantikine ELISA kit (R&D systems, Minneapolis, MN) following the manufacturer directions.
Formation of the inflammasome in the heart during AMI
Formalin fixed paraffin-embedded heart tissue slides were used. Heart sections were deparaffinized and rehydrated. After antigen retrieval with 0.01 M citrate buffer (pH 6.0) for 20 minutes, slides were blocked with 1% normal swine serum in TBS for 15 minutes. For characterization of cell type-specific expression of the inflammasome, double immunofluorescence technique was used. After antigen retrieval, slides were incubated with primary antibody #1 for a structural component of the inflammasome called apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC; Sigma-Aldrich) (Mezzaroma et al. 2011) overnight at 4°C. Anti-rabbit Alexa Fluor 594-conjugated secondary antibody #1 (1:100) was applied for 4 hours at room temperature, then slides were incubated with primary antibody #2 for cardiac Actin (1:200, Sigma- Aldrich) overnight at 4°C. Then, Alexa Fluor 488-conjugated secondary antibody (1:100, Invitrogen) was applied for 4 hours at room temperature.(Mezzaroma et al., 2011) Counterstaining was performed with 4',6-diamidino-2-phenylindole (DAPI) 1:20,000 for 5 minutes and the slides were coverslipped with SlowFade® Antifade (both Invitrogen). Negative controls with nonspecific IgG were run in parallel. Images were acquired with an IX70 microscope and MagnaFire 1.1 software (both Olympus) using a 40× objective (400× magnification). Color composite images were generated with ImageJ software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2011).
Expression of the components of the ASC inflammasome in the infarct area was quantified using a semiquantitative scale ranging from 0 (no expression) to 1+ (minimal expression meaning either few aggregates [<1 per high power field] or mild diffuse stain without aggregates), 2+ (moderate expression meaning either 1–5 aggregates per high power field or diffuse stain with few aggregates), 3+ (diffuse intense staining with many cytoplasmic aggregates [>5 per high power field]). Measurements were performed by two investigators who were blinded to treatment allocation.
Caspase-1 activity in the heart
An additional subset of mice was sacrificed 72 hours after surgery (N=4–6 per treatment group). The heart was removed as described above. Caspase-1 activity was measured by 2 independent means: measuring tissue activity using a fluorogenic substrate, and determining the amount of cleaved vs uncleaved caspase-1 using Western blot. The tissue activity of caspase-1 was measured by cleavage of a fluorogenic substrate (Ac-YVAD-AMC) specific for caspase-1 (CaspACE, Promega, Madison, WI).(Mezzaroma et al., 2011) After homogenization using RIPA buffer containing a cocktail of protease inhibitors and centrifugation at 16,000 rpm for 20 minutes, 75 µg of protein from each sample were used for the assay according to the supplier’s instructions. Fluorescence was measured after 60 minutes and was expressed as arbitrary fluorescence units produced by one microgram of sample per minute (fluorescence/µg/min) and calculated as fold change compared to the caspase-1 activity in homogenates of the hearts of sham-operated mice.
Caspase-1 protein levels were measured in clarified homogenates of the whole hearts explanted 3 days after surgery (N=4–6 per group) and immediately frozen in liquid nitrogen. The samples were homogenized using RIPA buffer (Sigma Aldrich, St Louis, MO) containing a cocktail of protease inhibitors (Sigma Aldrich) and were centrifuged at 16,000 rpm for 20 minutes. The supernatants were collected and the protein contents were quantified using the Bradford assay. Fifty µg of proteins for each sample were analyzed by Western blot. Proteins were denatured for 10 min at 97°C, and subjected to SDS-PAGE in 15% acrylamide gels to allow for separation of the bands. The proteins were then transferred onto a nitrocellulose membrane and incubated with a rabbit polyclonal antibody (C4851, Sigma-Aldrich) that hybridizes with both pro- and cleaved caspase-1 (p20). A mouse anti-β-actin monoclonal antibody (clone C-2, Sigma-Aldrich) was used for the normalization following enhanced-chemiluminescence (ECL) analysis and autoradiography. The protein bands were compared by densitometric analysis (Scion-Image, Scion Image Corporation) and the results were adjusted to the β-actin quantity in the samples.
Caspase-3 activity in the heart and nuclear DNA-fragmentation in cardiomyocytes
The activity of Caspase-3, a central mediator in apoptosis, was measured using a fluorogenic substrate (Ac-DEVD-AMC). After processing of the samples as described above, 75 µg of protein from each sample were used for the assay according to the supplier’s instructions (CaspACE, Promega, Madison, WI). Fluorescence was measured after 60 minutes and was expressed as arbitrary fluorescence units produced by one microgram of sample per minute (fluorescence/µg/min) and calculated as fold change compared to the caspase-3 activity in homogenates of the hearts of sham-operated mice.
In situ end-labeling of DNA fragmentation (TUNEL) was used to measure the rate of apoptotic cardiomyocytes in the heart.(Abbate et al., 2008b) Cardiomyocytes were identified at immunofluorescence using immunostaining for cardiac actin (as mentioned above). DNA fragmentation was identified using the In Situ Cell Death Detection Kit-Fluorescein, according to the supplier’s instructions (Roche Diagnostic, Indianapolis, IN). DAPI counterstaining was used to identify nuclei. The number of TUNEL+ cardiomyocytes was counted as % of all positive nuclei in the area of the heart bordering the infarct where cardiomyocytes were prevalent and the granulation tissue was scarce.(Abbate et al., 2005) Measurements were performed by two investigators who were blinded to treatment group allocation.
Longitudinal analysis and post-mortem examination
After surgery, the mice were allowed to recover for up to 10 weeks in cages of 2–4 mice. The cages were examined daily by the employees of the Department of Animal Resources. Three to five times weekly an investigator from the team examined the cages, noted the survival, and when available performed a gross post-mortem examination to determine the apparent cause of death (cardiac rupture, severe cardiac enlargement, or unknown).
Echocardiography
All mice underwent transthoracic echocardiography at baseline (before surgery), and at 7, 28 and 70 days after surgery (prior to sacrifice). Echocardiography was performed with the Vevo770 imaging system (VisualSonics Inc, Toronto, Ontario, Canada) and a 30-MHz probe.(Abbate et al., 2008b) The heart was visualized in B-mode from parasternal short axis and apical views. We measured the left ventricular (LV) end-diastolic and end-systolic areas at B-Mode and the LV end-diastolic diameter (LVEDD), LV end-systolic diameters (LVESD), LV anterior wall diastolic thickness (LVAWDT), and LV posterior wall diastolic thickness (LVPWDT) at M-Mode, as previously described(Abbate et al., 2008b) and according to the American Society of Echocardiography recommendations.(Gardin et al., 2002) LV fractional shortening (FS), LV ejection fraction (EF), LV mass and eccentricity (LVEDD/LVPWDT ratio) were calculated. The transmitral and left ventricular out flow tract Doppler spectra were recorded from an apical 4-chamber vies, and the myocardial performance index (MPI or Tei index) was calculated as the ratio of the isovolumetric contraction and relaxation time divided by the ejection time.(Broberg et al., 2003; Syed et al., 2005) Right ventricular (RV) enlargement was assessed measuring the RV end-diastolic area in the parasternal short-axis view mid-ventricular section and RV systolic function was estimated using M-Mode and measuring the tricuspidal annular plane systolic excursion (TAPSE).(Toldo et al., 2011) The investigators performing and reading the echocardiogram were blinded to the treatment allocation.
Infarct Size Assessment
After the 70-day echocardiogram, all mice were sacrificed using pentobarbital overdose and/or cervical dislocation. The hearts were explanted and fixed in formalin 10% for at least 48 hours. A transverse section of the median third of the heart was dissected, included in paraffin, cut into 5 µm slides, and stained with Masson’s trichrome (Sigma-Aldrich).(Abbate et al., 2008b; Seropian et al., 2010) The areas of fibrosis and the whole left ventricle were determined by computer morphometry using the Image Pro Plus 6.0 software.
Statistics
Differences between the groups were analyzed using the one-way ANOVA followed by Student’s t-test to compare 2 groups at a time and applying Bonferroni’s correction for multiple comparisons. Changes in repeated measures of echocardiographic data were analyzed using the random effects ANOVA for repeated-measures to determine the main effect of time, group, and time-by-group interaction. Survival analysis was performed by generating a Kaplan-Meyer survival curve and using logistic regression analysis. Plasma IL-6 levels were skewed and therefore logarithmically transformed before analysis. Calculations were completed using the SPSS 15.0 package for Windows (SPSS, Chicago, IL).
RESULTS
IL-1β-AB does not limit the intensity of the sterile inflammatory response
Tissue concentrations of IL-1β-AB were 40±6 and 30±5 ng/mg of protein at 3 and 7 days respectively in the hearts of IL-1β-AB treated mice, while they were undetectable in all other groups. This concentration is deemed sufficient to completely neutralize the bioactivity of IL-1β present in tissue.(Osborn et al., 2008; Clausen et al., 2009) In the healthy mouse pretreatment with the IL-1β-AB significantly prevented the increase in IL-6 4 hours after challenge with rm-IL-1β (29.3±5.1 pg/ml vs 266.1±95.5 pg/ml respectively; p=0.026).
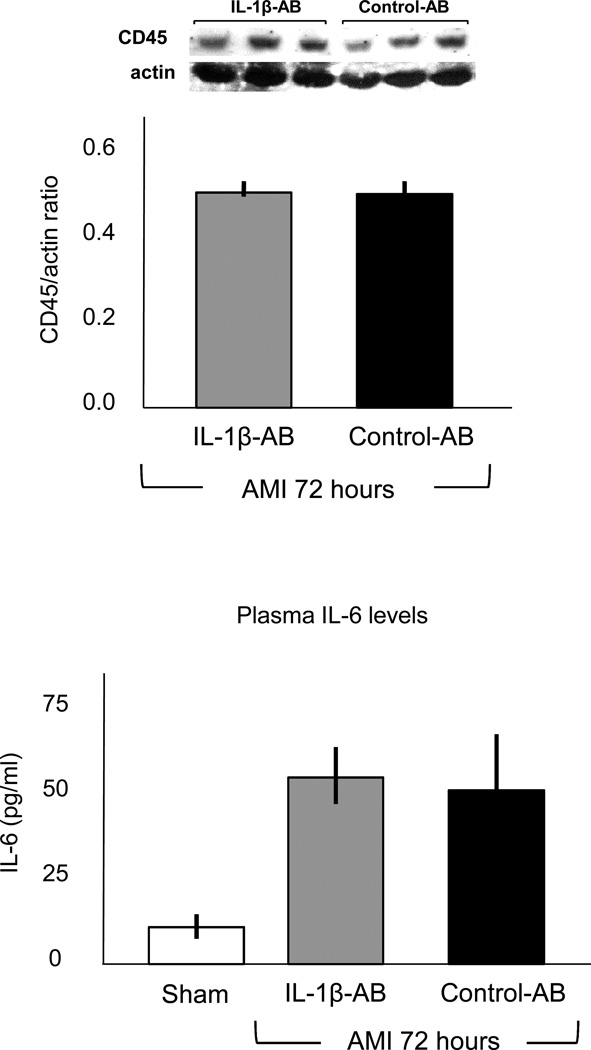
We measured CD45 expression to quantify the intensity of the inflammatory infiltration during infarction. CD45 expression was significantly increased after AMI, without significant differences between the group receiving the IL-1β-AB and the group treated with the control-AB (Figure 2), indicating that IL-1β may not be essential for the recruitment and chemotaxis of infiltrating leukocytes during AMI in the mouse. Systemic levels of IL-6 were significantly increased 72 hours after AMI, without significant differences between the group receiving the IL-1β-AB and the group treated with the control-AB (Figure 2), indicating that IL-1β may not be essential for the systemic inflammatory response during AMI in the mouse.
Figure 2.
Administration of the IL-1β-AB after coronary ligation (10 mg/kg i.p.) had no significant effects on the leukocyte infiltrate measured using Western Blot for CD45 nor on systemic levels of IL-6 as measured in the plasma 72 hours after surgery. N=4–7 per group.
Abbreviations: AB=antibody; IL-1β-AB=antibody directed towards Interleukin-1β.
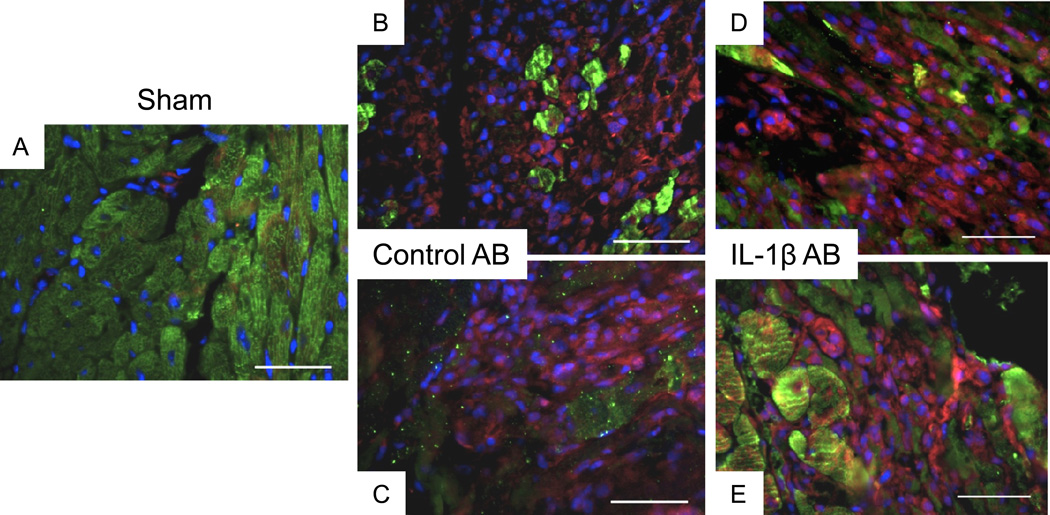
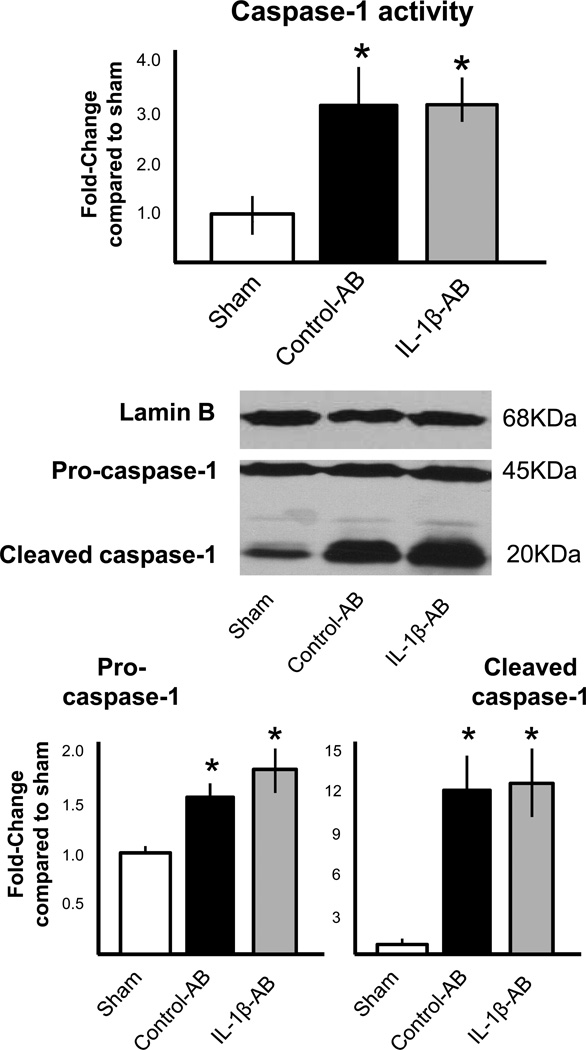
Treatment with the IL-1β-AB also had no significant effects on the inflammasome formation (Figure 3) or caspase-1 activity (measured as enzymatic activity and cleaved caspase-1) in the heart during AMI (Figure 4).
Figure 3.
Administration of the IL-1β-AB immediately after coronary ligation (10 mg/kg i.p.) had no significant effects on the formation of the ASC-containing inflammasomes 72 hours after surgery when compared with the control AB (* P<0.001 vs sham). N=4–6 per group. Representative image of the myocardium of a sham-operated mouse is shown (panel A). Two representative images of the peri-infarct myocardium of a mouse with acute myocardial infarction treated with a control AB (panels B–C) and of a mouse treated with a IL-1β-AB (panels D–E) are shown. Green represents staining for cardiac actin; red represents staining for ASC infllammasome; blue represents DAPI nuclear staining.
Figure 4.
Administration of the IL-1β-AB immediately after coronary ligation (10 mg/kg i.p.) had no significant effects on caspase-1 activity in the heart tissue measured 72 hours after surgery. *P<0.001 vs sham; N=4–6 per group.
IL-1β-AB inhibits cardiomyocyte apoptosis
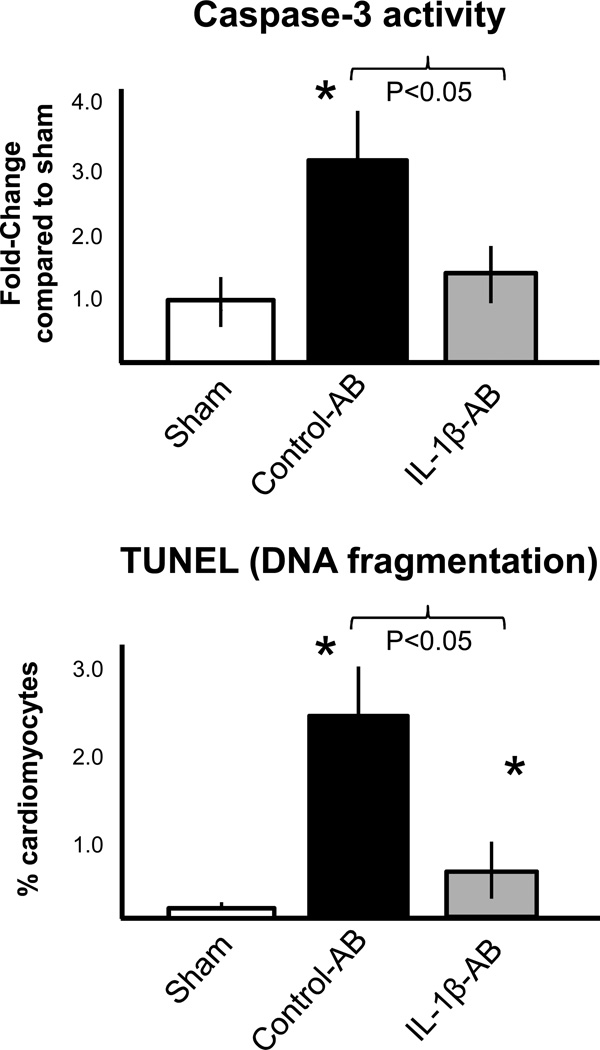
We measured the activity of caspase-3, a key mediator of apoptosis, in the heart 3 days after AMI and then measured the number of cardiomyocytes displaying nuclear DNA fragmentation as seen in apoptosis.(Abbate et al., 2002a) Caspase-3 activity and DNA fragmentation was significantly increased after AMI compared with sham (Figure 5). Treatment with the IL-1β-AB significantly inhibited caspase-3 activity and DNA fragmentation when compared with the control-AB (Figure 5).
Figure 5.
Administration of the IL-1β-AB immediately after coronary ligation (10 mg/kg i.p.) significantly inhibited caspase-3 activity in the heart tissue measured 72 hours after surgery, and inhibited cardiomyocyte apoptosis measured as nuclear DNA fragmentation using the TUNEL technique. *P<0.001 vs sham; N=4–6 per group. Abbreviations: AB=antibody; IL-1β-AB=antibody directed towards Interleukin-1β.
Effects of the IL-1β-AB on survival after coronary artery ligation surgery
None of the sham operated mice died. Fifty-nine percent of the control-AB-treated mice survived to 70 days after coronary artery ligation surgery (10/17 [59%] vs 10/10 [100%], P<0.001 vs sham), whereas 71% of mice treated with the IL-1β-AB were alive (10/14 [71%], P=NS versus control-AB). Most of deaths were sudden and cardiac rupture was rare in both groups (1 case in the control-AB group and 1 case in the IL-1β-AB group).
Effects of the IL-1β-AB on cardiac remodeling
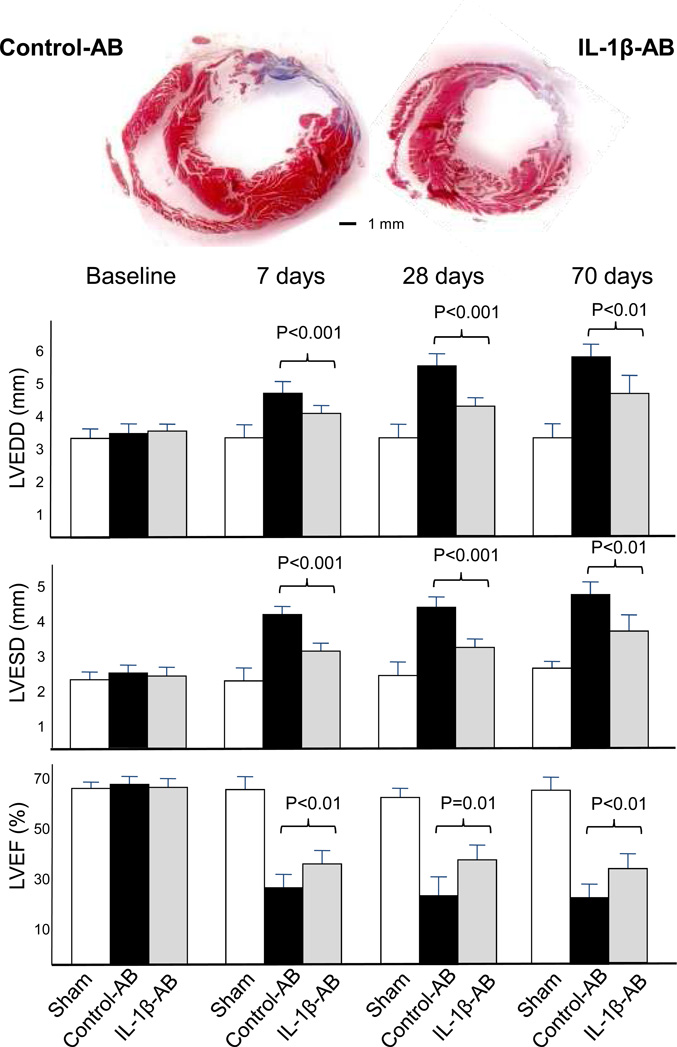
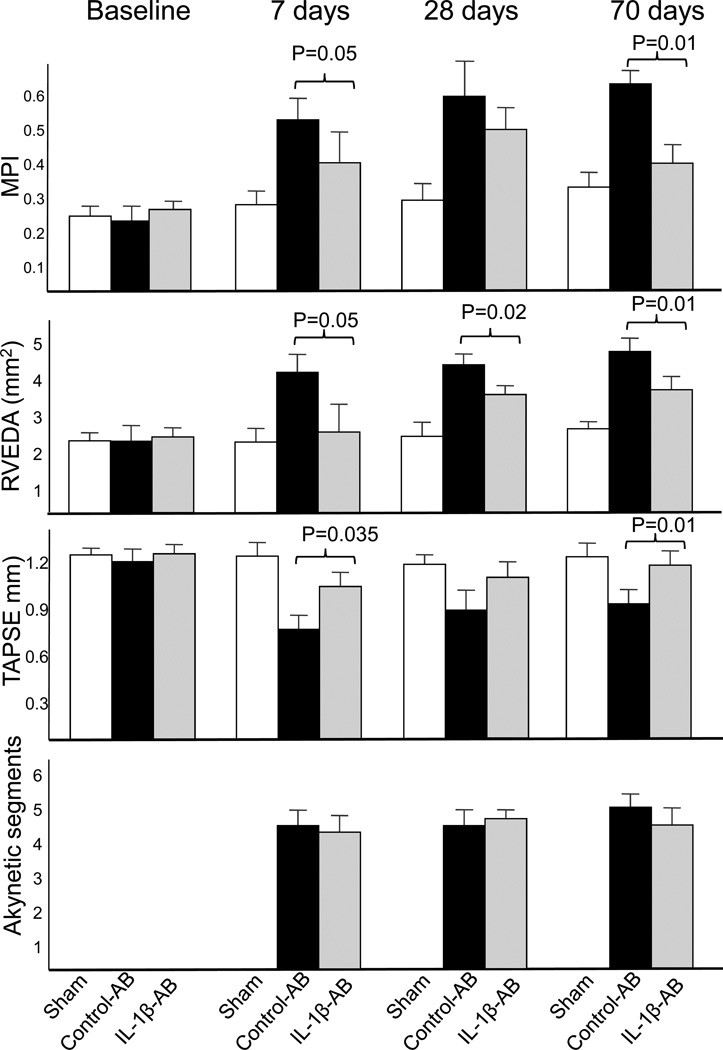
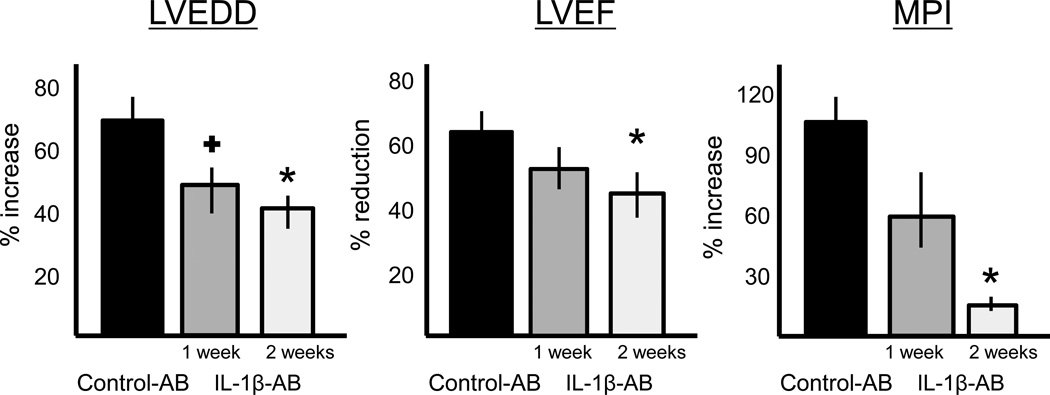
Cardiac remodeling was measured non-invasively using transthoracic echocardiography. Treatment with the IL-1β-AB led to a significant attenuation of left and right ventricular enlargement and contractile dysfunction at 1, 4 and 10 weeks (Figure 6). At 10 weeks, 9 weeks after the last dose of drug had been given, the IL-1β-AB had prevented LV enlargement after AMI by >40% in comparison to control-AB. LVEF was also significantly greater in the IL-1β-AB (absolute difference in mean LVEF of 7%). The attenuation in cardiac remodeling was paralleled by preservation of myocardial diastolic/systolic performance (myocardial performance index [MPI], Figure 6), while no differences were seen in the extent of the infarct measured at echocardiography or at post-mortem examination (Figure 6). A second dose of IL-1β-AB at 1 week after surgery produced additional improvements on cardiac remodeling (compared to a single treatment), suggesting that continued treatment beyond 1 week may be advantageous (Figure 7).
Figure 6.
Following coronary artery ligation surgery, mice treated with the control antibody (AB) had a significant enlargement of the left and right ventricles (left ventricular end-diastolic diameter [LVEDD], left ventricular end-systolic diameter [LVESD] and right ventricular end-diastolic area [RVEDA]) and a significant reduction in left and right ventricular function (left ventricular ejection fraction [LVEF], myocardial performance index [MPI] and tricuspidal annular plane systolic exercusion [TAPSE]). Administration of the antibody directed towards Interleukin-1β (IL-1β-AB) immediately after coronary ligation (10 mg/kg i.p.) significantly limited the cardiac enlargement and dysfunction without affecting the extent of infarct size measured as akynetic segments or as % area fibrosis at pathology. N=10 for sham; N=17 for control-AB; N=14 for IL-1β-AB.
Figure 7.
Treatment with the IL-1β-AB for 1 week (N=6) or 2 weeks (N=14) caused a reduction of the left ventricular enlargement (left ventricular end-diastolic diameter [LVEDD])and preservation of left ventricular function (left ventricular ejection fraction [LVEF] and myocardial performance index [MPI]) 10 weeks after acute myocardial infarction surgery that is greater for the 2-week vs the 1-week treatment. + P<0.05 vs control-AB; *P<0.01 vs control-AB. Abbreviations: AB=antibody; IL-1β-AB=antibody directed towards Interleukin-1β.
DISCUSSION
The sterile inflammatory response associated with myocardial ischemic injury has been identified as a target for intervention in order to prevent adverse cardiac remodeling in patients with AMI.(Mezzaroma et al., 2011; Frangogiannis, 2012) Cellular debris triggers the formation of the inflammasome that functions as scaffold for activation and secretion of pro-inflammatory cytokines, primarily IL-1β.(Stutz et al., 2009; Abbate, 2012; Strowig et al., 2012) Inhibition of the components of the inflammasome in experimental AMI (cryopyrin,(Mezzaroma et al., 2011) ASC,(Kawaguchi et al., 2011) or caspase-1(Frantz et al., 2003; Merkle et al., 2007)) protects the heart from adverse remodeling. Whether the benefits of inhibiting the inflammasome are related to a reduced production of active IL-1β or to other functions of the inflammasome is unclear.
The results of the current study confirm that IL-1β blockade limits apoptosis in the heart and ameliorates cardiac remodeling after AMI (Abbate et al., 2008b; Van Tassell et al., 2010b), and further expand our knowledge of the role of IL-1β and the inflammasome in the heart during AMI by showing that (1) selective IL-1β blockade does not affect the formation of the active inflammasome in the heart or the systemic inflammatory response in the mouse and (2) IL-1β blockade prevents adverse cardiac remodeling independent of caspase-1 activity. These findings confirm that the release of active IL-1β following inflammasome activation promotes adverse remodeling in AMI through induction of cardiomyocyte apoptosis. The role of apoptosis in promoting adverse cardiac remodeling after AMI is indeed well established.(Abbate et al., 2002b; Abbate et al., 2003; Abbate et al., 2005)
Within the limitations of the experimental mouse model used, the results also show safety and efficacy of selective IL-1β blockade using a monoclonal antibody. This is of particular interest because a large clinical trial of IL-1β blockade in patients with prior AMI is ongoing.(Ridker et al., 2011; Abbate et al., 2012) These results are in line with many observations using genetic models,(Bujak et al., 2008; Abbate et al., 2011) recombinant IL-1 receptor antagonist,(Abbate et al., 2008b) IL-1 Trap,(Van Tassell et al., 2010b) a different monoclonal antibody,(Abbate et al., 2010b) and a pilot clinical trial in patients,(Abbate et al., 2010a) while being in apparent contrast with the results of another study using a hamster-anti-mouse IL-1β antibody (which was developed for immunohistochemistry and not as a therapeutic).(Hwang et al., 2001) Equally important, these findings suggest that IL-1β blockade may be sufficient to prevent adverse cardiac remodeling in AMI whereas other inhibition of other functions of the inflammasome or of caspase-1 may be less important for apoptosis and remodeling. This is important because it is foreseeable that inhibition of the inflammasome may affect other functions potentially involved in tissue healing during injury. Indeed one of the concerns of anti-inflammatory therapies in AMI have been related to impaired healing and perceived risk of cardiac rupture. The data presented show that the IL-1β blockade protects the heart from apoptosis and remodeling without inhibiting the tissue inflammatory response (i.e. infiltration) and without signs of impaired infarct healing. This does not imply, however, that other functions of the inflammasome (i.e. processing of IL-18, secretion of other proteins across the membrane, promotion of pyroptosis) are not involved in cardiac healing and heart failure. The results seen with the IL-1βAB are consistent with those seen with the recombinant IL-1 receptor antagonist, Anakinra, in the mouse in which treatment reduced apoptosis and ameliorated remodeling without affecting the intensity of the leukocyte infiltrate.(Abbate et al., 2008b) The reasons by which blockade of IL-1β, a key pro-inflammatory mediator, fails to reduce local and systemic inflammatory responses in AMI in the mouse are not immediately clear. The pleiotropic inflammatory response to tissue injury involving release of multiple different cytokines (i.e. IL-1α, IL-18, TNF-α, and others) may explain why blocking one single cytokine does not block the inflammatory cascade. IL-1α, in example, may be rapidly released outside the cell during the early events of ischemic cell necrosis and serve as an ‘alarmin’ thus promoting leukocyte infiltration and secondary cytokine generation (Dinarello, 2011). Nevertheless this study and others show that blocking IL-1β is sufficient to ameliorate cardiac remodeling making it a promising target for the prevention of post-AMI heart failure.
Of note, in a non-ischemic model of cardiomyopathy Bracey and coll. (Bracey et al., 2012), also show that the inflammasome occupies a central role in the cardiac remodeling also in a model of non-ischemic cardiomyopathy due to genetic manipulation of the mouse, showing that the response to tissue injury is rather stereotyped and not directly related to the inciting stimulus (being ischemia or other), and also showing that, independent of the stimulus, the detrimental effects of the formation of the inflammasome are largely mediated by active IL-1β (Abbate, 2012; Bracey et al., 2012).
From a safety standpoint the finding that IL-1β blockade does not inhibit the formation of the inflammasome in response to tissue injury may be relevant as it suggests that blockade of IL-1β will not impair the tissue response in other conditions such as microbial infections. Our data also suggest that the induction of the inflammasome in the heart during AMI is largely independent of IL-1β, consistent with the concept that multiple stimuli can trigger formation of the inflammasome.(Stutz et al., 2009; Strowig et al., 2012)
We chose the model of non-reperfused AMI, although most of the patients with AMI receive some form of intervention aimed at obtaining reperfusion, because incomplete tissue level reperfusion (no reflow) occurs in a relatively large number of patients, which negates the benefit of reperfusion and is associated with a greater risk of subsequent heart failure.(Abbate et al., 2008a) This model of non-reperfused myocardial infarction produces global cardiac remodeling that involves the infarct and border zones, the remote left ventricle, and also the right ventricle.(Pfeffer & Braunwald, 1990; Anversa et al., 1991; Patten et al., 1998; Toldo et al., 2011) In the current study, IL-1β blockade provided a greater preservation in both left and right ventricular dimensions and improved function. This is in line with the benefits seen with numerous anti-inflammatory strategies that target caspase-1, the inflammasome and interleukin-1.(Frantz et al., 2003; Merkle et al., 2007; Abbate et al., 2008b; Bujak et al., 2008; Abbate et al., 2010a; Abbate et al., 2010b; Van Tassell et al., 2010b; Abbate et al., 2011)
From a translational point of view, we used a clinically relevant treatment plan in which the animals were not pretreated and the neutralizing antibody was administered after the onset of ischemia. Moreover, the effects of treatment were measured using clinically relevant endpoints such as left ventricular enlargement and function. Finally, we treated the animals for a short period (2 weeks) and followed them for an additional period showing no evidence of rebound after cessation of treatment. The superiority of the 2 doses 1 week apart vs a single dose supports the notion that an extended period of IL-1β neutralization might be necessary to achieve full benefit.
In conclusion, IL-1β neutralization using a monoclonal antibody improves cardiac remodeling after AMI in the mouse without affecting the sterile inflammatory response in the heart. These findings suggest that IL-1β mediates the deleterious effects on the heart during sterile inflammatory response.
New Findings.
-
What is the central question of this study?
The formation of the cryopyrin inflammasome in the heart induces an intense inflammatory response during acute myocardial infarction (AMI), which mediates further damage, and promoted adverse cardiac remodeling. The current study investigates the role of IL-1β in mediating the pathologic effects of the inflammasome in the heart.
-
What is the main finding and its importance?
IL-1β blockade improves cardiac remodeling after AMI in the mouse by inhibiting apoptosis without affecting the formation or the activity of the inflammasome in the heart. These findings suggest that IL-1βmediates the deleterious effects on the heart during sterile inflammatory response
ACKNOWLEDGEMENTS
The data presented were generated in the context of an investigator-initiated study to Dr. Antonio Abbate supported by Novartis Pharmaceuticals (Basel, Switzerland). Dr. Abbate is also supported by an American Heart Association Scientist Development Grant, Dr. Van Tassell is supported by an institutional National Institute of Health K12 award, and Dr. Mezzaroma is supported by an American Heart Association Post-Doctoral Award.
CONFLICT OF INTEREST
Dr. Abbate has received research support from Gilead, Novartis, and XOMA; he has served on advisory boards for Gilead, Janssen, and XOMA; he has given lectures for Novartis and XOMA. Dr. Van Tassell has received research support from Gilead and has served on an advisory board for Novartis. Dr. Voelkel has received research support from Actelion.
REFERENCES
- Abbate A. The heart on fire: inflammasome and cardiomyopathy. Exp Physiol. 2012 doi: 10.1113/expphysiol.2012.069021. [DOI] [PubMed] [Google Scholar]
- Abbate A, Biondi-Zoccai GG, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol. 2002a;193:145–153. doi: 10.1002/jcp.10174. [DOI] [PubMed] [Google Scholar]
- Abbate A, Biondi-Zoccai GG, Bussani R, Dobrina A, Camilot D, Feroce F, Rossiello R, Baldi F, Silvestri F, Biasucci LM, Baldi A. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41:753–760. doi: 10.1016/s0735-1097(02)02959-5. [DOI] [PubMed] [Google Scholar]
- Abbate A, Bussani R, Biondi-Zoccai GG, Rossiello R, Silvestri F, Baldi F, Biasucci LM, Baldi A. Persistent infarct-related artery occlusion is associated with an increased myocardial apoptosis at postmortem examination in humans late after an acute myocardial infarction. Circulation. 2002b;106:1051–1054. doi: 10.1161/01.cir.0000030936.97158.c4. [DOI] [PubMed] [Google Scholar]
- Abbate A, Bussani R, Biondi-Zoccai GG, Santini D, Petrolini A, De Giorgio F, Vasaturo F, Scarpa S, Severino A, Liuzzo G, Leone AM, Baldi F, Sinagra G, Silvestri F, Vetrovec GW, Crea F, Biasucci LM, Baldi A. Infarct-related artery occlusion, tissue markers of ischaemia, and increased apoptosis in the peri-infarct viable myocardium. Eur Heart J. 2005;26:2039–2045. doi: 10.1093/eurheartj/ehi419. [DOI] [PubMed] [Google Scholar]
- Abbate A, Kontos MC, Biondi-Zoccai GG. No-reflow: the next challenge in treatment of ST-elevation acute myocardial infarction. Eur Heart J. 2008a;29:1795–1797. doi: 10.1093/eurheartj/ehn281. [DOI] [PubMed] [Google Scholar]
- Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010a;105:1371–1377. e1371. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- Abbate A, Salloum FN, Van Tassell BW, Vecile E, Toldo S, Seropian I, Mezzaroma E, Dobrina A. Alterations in the interleukin-1/interleukin-1 receptor antagonist balance modulate cardiac remodeling following myocardial infarction in the mouse. PLoS One. 2011;6:e27923. doi: 10.1371/journal.pone.0027923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, Gustini E, Biasucci LM, Severino A, Capogrossi MC, Vetrovec GW, Crea F, Baldi A, Kukreja RC, Dobrina A. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008b;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- Abbate A, Van Tassell BW, Biondi-Zoccai GG. Blocking interleukin-1 as a novel therapeutic strategy for secondary prevention of cardiovascular events. Biodrugs. 2012;26:217–233. doi: 10.1007/BF03261881. [DOI] [PubMed] [Google Scholar]
- Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, Varma A, Salloum FN, Smithson L, Dinarello CA. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010b;12:319–322. doi: 10.1093/eurjhf/hfq017. [DOI] [PubMed] [Google Scholar]
- Anversa P, Olivetti G, Capasso JM. Cellular basis of ventricular remodeling after myocardial infarction. Am J Cardiol. 1991;68:7D–16D. doi: 10.1016/0002-9149(91)90256-k. [DOI] [PubMed] [Google Scholar]
- Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, Wright JR, Macdonald JA, Lees-Miller JP, Roach D, Semeniuk LM, Duff HJ. The Nlrp3 Inflammasome promotes myocardial dysfunction in structural cardiomyopathy through IL-1beta. Exp Physiol. 2012 doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- Broberg CS, Pantely GA, Barber BJ, Mack GK, Lee K, Thigpen T, Davis LE, Sahn D, Hohimer AR. Validation of the myocardial performance index by echocardiography in mice: a noninvasive measure of left ventricular function. J Am Soc Echocardiogr. 2003;16:814–823. doi: 10.1067/S0894-7317(03)00399-7. [DOI] [PubMed] [Google Scholar]
- Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen F, Hanell A, Bjork M, Hillered L, Mir AK, Gram H, Marklund N. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2009;30:385–396. doi: 10.1111/j.1460-9568.2009.06820.x. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz S, Ducharme A, Sawyer D, Rohde LE, Kobzik L, Fukazawa R, Tracey D, Allen H, Lee RT, Kelly RA. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35:685–694. doi: 10.1016/s0022-2828(03)00113-5. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Adams DB, Douglas PS, Feigenbaum H, Forst DH, Fraser AG, Grayburn PA, Katz AS, Keller AM, Kerber RE, Khandheria BK, Klein AL, Lang RM, Pierard LA, Quinones MA, Schnittger I. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of Echocardiography's Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J Am Soc Echocardiogr. 2002;15:275–290. doi: 10.1067/mje.2002.121536. [DOI] [PubMed] [Google Scholar]
- Hwang MW, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, Hara M, Miyamoto T, Touma M, Sasayama S. Neutralization of interleukin-1beta in the acute phase of myocardial infarction promotes the progression of left ventricular remodeling. J Am Coll Cardiol. 2001;38:1546–1553. doi: 10.1016/s0735-1097(01)01591-1. [DOI] [PubMed] [Google Scholar]
- Ing DJ, Zang J, Dzau VJ, Webster KA, Bishopric NH. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, Bcl-x. Circ Res. 1999;84:21–33. doi: 10.1161/01.res.84.1.21. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- Merkle S, Frantz S, Schon MP, Bauersachs J, Buitrago M, Frost RJ, Schmitteckert EM, Lohse MJ, Engelhardt S. A role for caspase-1 in heart failure. Circ Res. 2007;100:645–653. doi: 10.1161/01.RES.0000260203.55077.61. [DOI] [PubMed] [Google Scholar]
- Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an Interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine. 2008;44:141–148. doi: 10.1016/j.cyto.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten RD, Aronovitz MJ, Deras-Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME, Konstam MA. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol. 1998;274:H1812–H1820. doi: 10.1152/ajpheart.1998.274.5.H1812. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Seropian IM, Abbate A, Toldo S, Harrington J, Smithson L, Ockaili R, Mezzaroma E, Damilano F, Hirsch E, Van Tassell BW. Pharmacologic inhibition of phosphoinositide 3-kinase gamma (PI3Kgamma) promotes infarct resorption and prevents adverse cardiac remodeling after myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;56:651–658. doi: 10.1097/FJC.0b013e3181f9a905. [DOI] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed F, Diwan A, Hahn HS. Murine echocardiography: a practical approach for phenotyping genetically manipulated and surgically modeled mice. J Am Soc Echocardiogr. 2005;18:982–990. doi: 10.1016/j.echo.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Toldo S, Bogaard HJ, Van Tassell BW, Mezzaroma E, Seropian IM, Robati R, Salloum FN, Voelkel NF, Abbate A. Right ventricular dysfunction following acute myocardial infarction in the absence of pulmonary hypertension in the mouse. PLoS One. 2011;6:e18102. doi: 10.1371/journal.pone.0018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tassell BW, Seropian IM, Toldo S, Salloum FN, Smithson L, Varma A, Hoke NN, Gelwix C, Chau V, Abbate A. Pharmacologic inhibition of myeloid differentiation factor 88 (MyD88) prevents left ventricular dilation and hypertrophy after experimental acute myocardial infarction in the mouse. J Cardiovasc Pharmacol. 2010a;55:385–390. doi: 10.1097/FJC.0b013e3181d3da24. [DOI] [PubMed] [Google Scholar]
- Van Tassell BW, Varma A, Salloum FN, Das A, Seropian IM, Toldo S, Smithson L, Hoke NN, Chau VQ, Robati R, Abbate A. Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010b;55:117–122. doi: 10.1097/FJC.0b013e3181c87e53. [DOI] [PubMed] [Google Scholar]