Abstract
Background:
To evaluate the short- and long-term outcomes of adult patients with hematologic malignancies receiving chemotherapy in the intensive care unit (ICU).
Methods:
Retrospective single center study comparing the outcomes of patients with hematologic malignancies who received chemotherapy in the ICU with a matched cohort of ICU patients who did not receive chemotherapy. Conditional logistic regression and shared frailty Cox regression were used to assess short-term (ICU and hospital) mortality and death by 12-months post-hospital discharge, respectively.
Results:
One hundred eighty one patients with hematologic malignancies received chemotherapy in the ICU. ICU and hospital mortality rates were 25% and 42%, respectively. Higher severity of illness score on ICU admission were significantly associated with higher ICU (OR 1.07, p<.001) and hospital mortality (OR 1.05, p=<.001). Six and 12-month survival estimates post-hospital discharge were 58% and 50%, respectively. When compared to the matched cohort of patients who did not receive chemotherapy, patients who received chemotherapy had a significantly longer length of stay in the ICU (median 6 vs. 3 days, p<0.001) and hospital (median 22 vs. 14 days, p=0.024). The relationship between mortality by 12-months and receiving chemotherapy approached, but did not reach statistical significance in multivariable analyses (HR 1.45, p=0.08).
Conclusions:
Short-term mortality was similar among patients with hematologic malignancies who did or did not receive chemotherapy in the ICU although patients who received chemotherapy had increased resource utilization. These results may inform ICU triage and goals of care discussions with patients and their families regarding the outcomes of patients receiving chemotherapy in the ICU.
Keywords: chemotherapy, cancer, hematologic, intensive care unit, outcomes
Condensed Abstract
Our study is the first and largest study to date of patients with hematologic malignancies who received chemotherapy in the ICU at a US tertiary cancer center. Our data can assist oncology and critical care teams when discussing goals of care and prognosticating outcomes of patients who receive chemotherapy in the ICU and in dealing with triaging scarce ICU resources.
INTRODUCTION
A complex host of factors influences the decision to administer oncologic therapies in the intensive care unit (ICU) for patients with hematologic malignancies. These include the improved short-term (ICU and hospital) and long-term (post-hospital discharge) survival rates associated with this patient cohort,1-3 the type and status of the underlying malignancy, concern for potential for adverse effects due to oncologic therapies and the presence of life-threatening critical illness syndromes. Other important considerations include the capabilities of the ICU to support active treatment of oncologic patients and the negative biases of the ICU towards patients with a perceived poor outcome due to their cancer or need for oncologic therapies.
While the prognostic factors and outcomes in patients with hematological malignancies admitted to oncologic ICUs have been extensively examined,4-14 only four studies (three retrospective and one prospective) specifically address the outcomes of patients receiving chemotherapy in the ICU.4-7 All four studies were based in oncologic ICUs at single centers outside the United States (US), retrospective in design, had relatively small number of patients (<100 patients with hematologic malignancies), and except for one study7, reported only short-term outcomes (<6 months). Notably, these studies 4-7 focused on patients receiving initiation chemotherapy for newly diagnosed hematologic malignancies and did not address the clinical factors and outcomes of patients receiving continuation chemotherapy.
The purpose of this study was to improve our understanding of the characteristics and outcomes of patients with hematologic malignancies receiving chemotherapy in the ICU. Our goals were to describe reasons for ICU admission, the timing and types of chemotherapy regimens given, whether the regimens were initial or part of ongoing oncologic treatment, and both short-term (ICU and hospital mortality) and long-term (death by 12 months) outcomes. We also compared the outcomes of these patients with a cohort of matched patients with similar hematologic malignancies who were admitted to the ICU during the same time period, but did not receive chemotherapy.
METHODS
This retrospective study was conducted between January 1, 2010 - December 31, 2015 in the 20-bed, adult ICU of Memorial Sloan Kettering Cancer Center. The ICU is staffed by intensivist-led multidisciplinary teams and all patients are cared for in collaboration with the patient’s primary oncologist or surgeon. Consultations for ICU admission are all triaged through the admitting intensivist attending physicians. Patients are admitted primarily with one or more organ dysfunctions and for life extending therapeutic options associated with potentially severe adverse effects.
The decision to administer chemotherapy in the ICU is jointly agreed upon by oncologists and intensivists after discussions with the patients and/or their authorized representatives. These medications are solely prescribed by oncologists, and prepared by specialty pharmacists. Intravenous agents are administered by chemotherapy nurses; in contrast, oral agents are administered by ICU nurses. In this study, we define chemotherapy as any combination of traditional cytotoxic chemotherapy, biologic or immune therapy directed at a hematologic malignancy. Radiation therapy alone was not considered. A waiver of authorization for this study was obtained through our Institutional Review Board.
Data Sources
Using hospital-wide and ICU databases, we identified all ICU patients with hematologic malignancies who received chemotherapy in the ICU. We included for analysis only the first ICU admission during which the patient received chemotherapy; subsequent ICU admissions for chemotherapy were excluded. We also excluded patients receiving chemotherapeutic agents as part of the conditioning regimen for autologous or allogeneic hematopoietic stem cell transplantation (HSCT); concomitantly administered with major surgical procedures; instilled in body cavities to facilitate sclerosis (i.e., pericardial and pleural spaces); or given for non-cancerous conditions (e.g., autoimmune or viral diseases). Similarly, patients receiving corticosteroids only were also excluded due to the extensive use of many types of corticosteroids for a myriad of conditions in our ICU.
Demographics and outcomes
Demographic and clinical data included age, gender, severity of illness score using the Mortality Probability Model at ICU admission version 2 (MPM0-II), history of HSCT, primary ICU admission diagnosis as noted in the admission day critical care attending physician note, the Charlson comorbidity index15, dates of hospital and ICU admissions, Sequential Organ Failure Assessment (SOFA) score at the start of chemotherapy, use of mechanical ventilation, vasopressor medications, and renal replacement therapies (hemodialysis or continuous renal replacement therapy) on admission and during ICU stay, and ICU and hospital length of stay (LOS). Primary outcomes were ICU and hospital mortality and survival by 6 and 12 months post-hospital discharge.
Cancer diagnoses and chemotherapy
Hematologic malignancies were classified as newly diagnosed (within 30 days of ICU admission) or older diagnosed (>30 days; includes relapse/progression). We coded chemotherapy as initiation (initial administration) or continuation (part of an ongoing regimen). Patients who were treated with their first chemotherapy within 5 days prior to ICU admission with carry-over into the ICU were also included in the initiation category.
Chemotherapy data collected included the ICU start date, type (cytotoxic, biologic or immune), number (single or multiple), and route of administration (parenteral, oral, intrathecal, or subcutaneous). We also determined the dates of the original cancer diagnosis, and the last chemotherapy administered prior to ICU admission for the continuation group.
Statistical Analysis
Patients who received chemotherapy were 1:1 individually matched to non-chemotherapy patients on age, gender (male vs. female) and disease type (leukemia, lymphoma, and other). Exact matches were used for gender and disease type, while a 0.25 caliper match was used for age and a greedy match algorithm was employed. Patients who were not matched were dropped from the analyses. Characteristics between chemotherapy and non-chemotherapy patients were compared with McNemar’s Test and the Wilcoxon Signed Rank test where appropriate.
We assessed three outcomes: death in ICU, death in hospital, and death by 12-months post-hospital discharge. Death in ICU and death in hospital were treated as binary covariates.16 The relationships between covariates and short-term outcomes were assessed with univariable and multivariable conditional logistic regression with matched pair serving as strata. All factors were adjusted for in multivariable models regardless of significance; however, as the MPM II score on ICU admission incorporates the need for mechanical ventilation and vasopressor agents, only the MPM II score was included in multivariable models. Death by 12-months was assessed from the time of hospital discharge until death. Patients alive at 12 months were censored. Only patients who were alive at hospital discharge were included in this analysis. Kaplan Meier methods were used to estimate survival, and univariable and multivariable shared frailty Cox regression were used to assess the relationship between covariates and death by 12-months. The proportional hazards assumption was assessed through residual plots.
To analyze the effect of initiation versus continuation chemotherapy on ICU and hospital mortality and survival by 12-months in the chemotherapy cohort, standard logistic regression and Cox proportional hazards regression were used. Fisher’s Exact test and Wilcoxon Rank Sum test were used to check for differences between the initiation and continuation chemotherapy groups. Two-sided p-values less than or equal to 0.05 were considered statistically significant. All analyses were performed with SAS 9.4 (The SAS Institute, Cary, NC).
RESULTS
During the study period, 4973 patients were admitted to the ICU. Of these, 186 patients (3.7%) with hematologic malignancies received chemotherapy in the ICU (Figure 1). Of the 186 patients, 181 were successfully matched to ICU patients who did not receive chemotherapy (Table 1). Cancer type and gender were identically matched. The cohorts did not differ in age (p>0.95). The five patients who were not matched were female leukemia patients aged 30 or younger. Median Charlson comorbidity index was similar in both groups (p=0.55). Median MPM-II score on ICU admission was 39 (range, 7-95) in the chemotherapy cohort and 46 (range, 8-94) in the non-chemotherapy cohort (p=0.57).
Figure 1.
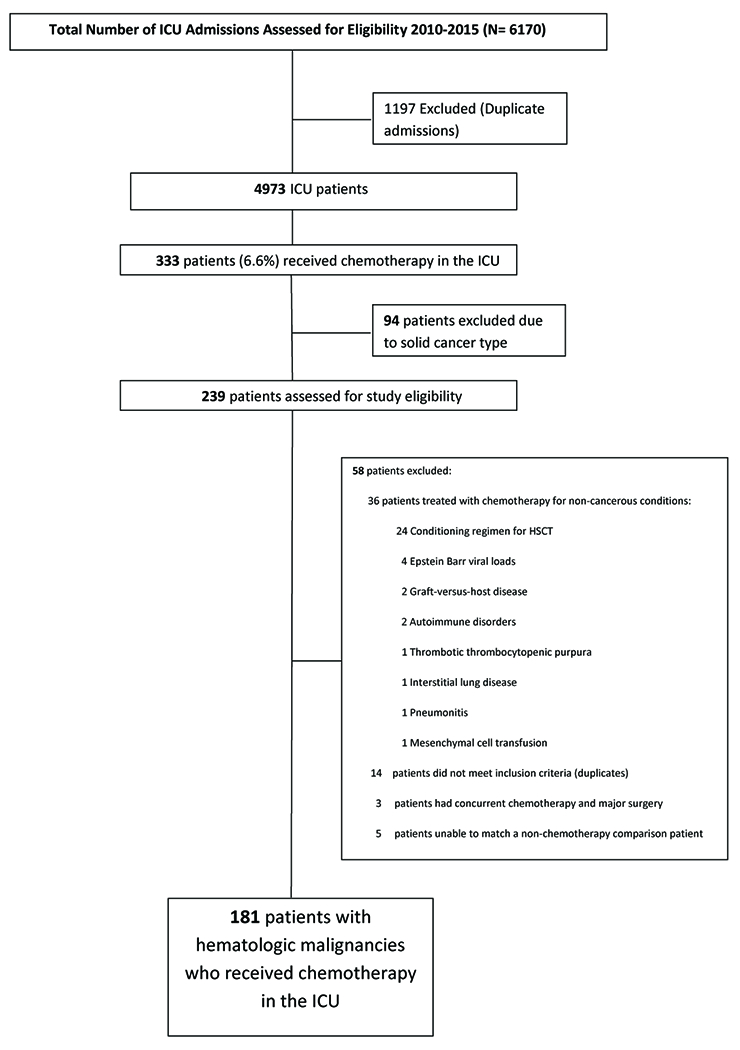
CONSORT Diagram – Flowchart of ICU Admissions
Table 1.
Patient Characteristics and Cohort Comparisons
| Chemotherapy in ICU | |||||
|---|---|---|---|---|---|
| All N (%) | Chemotherapy N (%) | No Chemotherapy N (%) | p-value | ||
| Sample Size | 362 | 181 | 181 | ||
|
| |||||
| Age at Admission, years | Median (range) | 62 (18-87) | 62 (18-86) | 62 (19-87) | 0.81 |
| Gender | Male | 230 (63.5) | 115 (63.5) | 115 (63.5) | |
| Female | 132 (36.5) | 66 (36.5) | 66 (36.5) | ||
| MPM II Score | Median (range) | 42.3 (7.40-94.7) | 39.1 (7.40-94.7) | 45.6 (8.28-94.0) | 0.57 |
| Cancer Type | Lymphoma | 206 (56.9) | 103 (56.9) | 103 (56.9) | |
| Leukemia | 156 (43.1) | 78 (43.1) | 78 (43.1) | ||
| Primary Admit Diagnosis | Bleeding | 25 (7.6) | 11 (6.2) | 14 (9.1) | |
| Cardiac | 19 (5.7) | 7 (4) | 12 (7.8) | ||
| Chemotherapy Admin | 45 (13.6) | 45 (25.4) | 0 (0) | ||
| Hematologic | 4 (1.2) | 4 (2.3) | 0 (0) | ||
| Neurological | 9 (2.7) | 6 (3.4) | 3 (1.9) | ||
| Renal | 32 (9.7) | 23 (13) | 9 (5.8) | ||
| Respiratory | 59 (17.8) | 33 (18.6) | 26 (16.9) | ||
| Sepsis | 128 (38.7) | 47 (26.6) | 81 (52.6) | ||
| Other | 10 (3) | 1 (0.6) | 9 (5.8) | ||
| Charlson Comorbidity Index | Median (range) | 6.0 (2.0-13.0) | 6.0 (2.0-11.0) | 6.0 (2.0-13.0) | 0.55 |
| Mechanical Ventilation, on Admit | Yes | 42 (11.6) | 17 (9.4) | 25 (13.8) | 0.26 |
| No | 320 (88.4) | 164 (90.6) | 156 (86.2) | ||
| Mechanical Ventilation, During Stay | Yes | 137 (37.8) | 75 (41.4) | 62 (34.3) | 0.15 |
| No | 225 (62.2) | 106 (58.6) | 119 (65.7) | ||
| Vasopressors, on Admit | Yes | 41 (11.3) | 16 (8.8) | 25 (13.8) | 0.19 |
| No | 321 (88.7) | 165 (91.2) | 156 (86.2) | ||
| Vasopressors, During Stay | Yes | 123 (34) | 59 (32.6) | 64 (35.4) | 0.63 |
| No | 239 (66) | 122 (67.4) | 117 (64.6) | ||
| CRRT/HD | Yes | 25 (6.9) | 17 (9.4) | 8 (4.4) | 0.11 |
| No | 337 (93.1) | 164 (90.6) | 173 (95.6) | ||
| Same Day Hospital/ICU Admit | Yes | 86 (23.8) | 34 (18.8) | 52 (28.7) | 0.036 |
| No | 276 (76.2) | 147 (81.2) | 129 (71.3) | ||
| ICU LOS, days | Median (range) | 5 (0-47) | 6 (1-47) | 3 (0-28) | <.001 |
| Hospital LOS, days | Median (range) | 18 (0-126) | 22 (2-121) | 14 (0-126) | 0.024 |
| Hx of Transplant | Yes | 33 (9.1) | 20 (11) | 13 (7.2) | 0.30 |
| No | 329 (90.9) | 161 (89) | 168 (92.8) | ||
| Newly Diagnosed | Yes | 89 (24.6) | 69 (38.1) | 20 (11) | <.001 |
| No | 273 (75.4) | 112 (61.9) | 161 (89) | ||
| Radiation during ICU stay | Yes | 2 (0.6) | 2 (1.1) | 0 (0) | |
| No | 360 (99.4) | 179 (98.9) | 181 (100) | ||
| # Deaths by ICU Discharge | Yes | 86 (23.8) | 46 (25.4) | 40 (22.1) | |
| No | 276 (76.2) | 135 (74.6) | 141 (77.9) | ||
| # Deaths by Hospital Discharge | Yes | 135 (37.3) | 76 (42) | 59 (32.6) | |
| No | 227 (62.7) | 105 (58) | 122 (67.4) | ||
No comparison was done for gender or cancer type as these were perfectly matched.
Patient Characteristics: Chemotherapy Patients (n=181)
Patients who received chemotherapy in the ICU were primarily male (n=115 [64%]) with a median age of 62 years (18-86) (Table 1). One hundred three patients (57%) were treated for lymphoma and 78 (43%) for leukemia. Sixty-nine (38%) patients were treated for newly diagnosed hematologic malignancies. Twenty patients (11%) had prior HSCT and two patients (1%) received radiation therapy during their ICU stay. Primary ICU admitting diagnoses were sepsis/septic shock (n=47 [27%]), chemotherapy administration (n=45 [25%]), respiratory insufficiency/failure (n=33 [19%]), renal insufficiency/failure (n=23 [13%]), and bleeding (n=11 [6%]) (Table 1). Median MPM II score on ICU admission was 39 (7-95). Median SOFA score at start of chemotherapy was 6.0 (range: 0-15). During ICU stay, 38% of patients (137/181) needed mechanical ventilation, 34% (123/181) required vasopressor therapy and 7% (25/181) underwent renal replacement therapy. Median ICU LOS was 6 days (range: 1-47) and median hospital LOS was 22 days (range: 2-121).
Treatment Characteristics: Chemotherapy Patients
Cytotoxic chemotherapies were given to 135 patients (75%), immunotherapy agents to 21 patients (12%) and combined regimens to 25 patients (14%). One hundred three patients (57%) received multiple agents. Median time between the diagnosis of cancer and ICU admission was 5 months (range: 0-318). Sixty eight patients (38%) were diagnosed with cancer within 30 days of ICU admission.
Fifty six patients (31%) received chemotherapy within 5 days of ICU admission, 33 patients (18%) received chemotherapy greater than 5 days before admission, and 92 patients (51%) did not receive chemotherapy prior to ICU admission. Seventy two patients (40%) received initiation and 109 patients (60%) received continuation therapies in the ICU (Table 2). Of the 56 patients who received chemotherapy within 5 days, only 8 (14%) were from the initiation cohort. In those patients who received chemotherapy after ICU admission, the median time was 1.5 days overall (range: 1-17 days) with a median of 1 day (range: 1-16) for patients in the continuation cohort and 2 days (range: 1-17) in the initiation cohort.
Table 2.
Conditional Logistic Regression for ICU Mortality and Hospital Mortality (N=362)
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Death in: | N(#D) | OR [95% CI] | p-value | OR [95% CI] | p-value | ||
| ICU | Chemotherapy in ICU | Chemo | 181 (46) | 1.20 [0.74-1.95] | 0.46 | 0.82 [0.39-1.75] | 0.61 |
| No-Chemo | 181 (40) | REF | REF | ||||
| MPM II Score | 1.03 [1.02-1.04] | <.001 | 1.07 [1.03-1.10] | <.001 | |||
| Charlson Comorbidity Index | 0.99 [0.89-1.11] | 0.88 | 0.80 [0.56-1.15] | 0.22 | |||
| Mechanical Ventilation, on Admit | Yes | 42 (18) | 5.50 [1.22-24.81] | 0.027 | ---- | ||
| No | 320 (68) | REF | |||||
| Vasopressors, on Admit | Yes | 41 (17) | 3.67 [1.02-13.14] | 0.046 | ---- | ||
| No | 321 (69) | REF | |||||
| Same Day Hospital/ICU Admit | Yes | 86 (14) | 0.64 [0.28-1.49] | 0.30 | 0.27 [0.08-0.91] | 0.035 | |
| No | 276 (72) | REF | REF | ||||
| CRRT/HD | Yes | 25 (14) | 2.75 [0.88-8.64] | 0.08 | 6.16 [1.02-37.26] | 0.048 | |
| No | 337 (72) | REF | REF | ||||
| Hx of Transplant | Yes | 33 (10) | 2.00 [0.60-6.64] | 0.26 | 1.17 [0.19-7.24] | 0.86 | |
| No | 329 (76) | REF | REF | ||||
|
| |||||||
| Hospital | Chemotherapy in ICU | Chemo | 181 (76) | 1.52 [0.98-2.35] | 0.06 | 1.58 [0.85-2.92] | 0.14 |
| No-Chemo | 181 (59) | REF | REF | ||||
| MPM II Score | 1.03 [1.02-1.04] | <.001 | 1.05 [1.03-1.07] | <.001 | |||
| Charlson Comorbidity Index | 0.98 [0.89-1.08] | 0.74 | 0.90 [0.70-1.14] | 0.37 | |||
| Mechanical Ventilation, on Admit | Yes | 42 (26) | 4.33 [1.23-15.21] | 0.022 | ---- | ||
| No | 320 (109) | REF | |||||
| Vasopressors, on Admit | Yes | 41 (22) | 3.25 [1.06-9.97] | 0.039 | ---- | ||
| No | 321 (113) | REF | |||||
| Same Day Hospital/ICU Admit | Yes | 86 (22) | 0.48 [0.22-1.01] | 0.053 | 0.27 [0.10-0.74] | 0.011 | |
| No | 276 (113) | REF | REF | ||||
| CRRT/HD | Yes | 25 (17) | 1.67 [0.61-4.59] | 0.32 | 1.06 [0.31-3.64] | 0.92 | |
| No | 337 (118) | REF | REF | ||||
| Hx of Transplant | Yes | 33 (17) | 3.33 [0.92-12.11] | 0.07 | 2.03 [0.37-11.03] | 0.41 | |
| No | 329 (118) | REF | REF | ||||
N = total number, #D = number of deaths, OR = Odds Ratio, REF = Reference Level (OR=1), 95% CI=95% Confidence Interval
Chemotherapy Patients Compared to Non-Chemotherapy Patients (N=362)
ICU and Hospital Mortality
ICU mortality was 25% (46/181) in the chemotherapy cohort and 22% (40/181) in the non-chemotherapy cohort. Receiving chemotherapy in the ICU was not significantly associated with worse ICU mortality (p=0.61) after adjustment for known mortality confounding factors (Table 3). Overall hospital mortality was 42% (76/181) in the chemotherapy cohort and 33% (59/181) in the non-chemotherapy cohort (Table 1). Although as a univariable factor, patients who received chemotherapy in the ICU had higher odds of dying in the hospital that approached statistical significance (OR: 1.52, 95% CI: 0.98-2.35, p=0.06), after adjusting for known mortality predictors, there was not enough evidence to suggest that patients who received chemotherapy had significantly worse hospital mortality (OR: 1.58, 95% CI: 0.85-2.92, p=0.14) (Table 3). Additionally, higher MPM II scores on ICU admission were associated with higher risk of ICU (OR: 1.07, 95% CI: 1.03-1.10, p<0.001) and hospital mortality (OR:1.05, 95%CI: 1.03-1.07, p<0.001) (Table 2).
Table 3.
Shared Frailty Cox Regression for 12 Month Survival from Discharge (N=227)
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Death by: | N(#D) | HR [95% CI] | p-value | HR [95% CI] | p-value | ||
| 12 month | Chemotherapy in ICU | Chemo | 105 (53) | 1.37 [0.93-2.04] | 0.11 | 1.45 [0.95-2.19] | 0.08 |
| No-Chemo | 122 (50) | REF | REF | ||||
| MPM II Score | 227 (103) | 1.01 [1.00-1.02] | 0.013 | 1.01 [1.00-1.02] | 0.14 | ||
| Charlson Comorbidity Index | 227 (103) | 1.12 [1.03-1.22] | 0.011 | 1.09 [0.98-1.20] | 0.11 | ||
| Mechanical Ventilation, on Admit | Yes | 16 (7) | 0.94 [0.42-2.07] | 0.87 | ---- | ||
| No | 211 (96) | REF | |||||
| Vasopressors, on Admit | Yes | 19 (11) | 1.37 [0.72-2.63] | 0.34 | ---- | ||
| No | 208 (92) | REF | |||||
| Same Day Hospital/ICU Admit | Yes | 64 (31) | 1.04 [0.67-1.62] | 0.85 | 1.16 [0.74-1.82] | 0.53 | |
| No | 163 (72) | REF | REF | ||||
| CRRT/HD | Yes | 8 (5) | 1.44 [0.56-3.68] | 0.45 | 1.14 [0.43-3.05] | 0.79 | |
| No | 219 (98) | REF | REF | ||||
| Hx of Transplant | Yes | 16 (9) | 1.45 [0.71-2.98] | 0.31 | 1.48 [0.71-3.08] | 0.29 | |
| No | 211 (94) | REF | REF | ||||
N = total number, #D = number of deaths, HR = Hazard Ratio, 95% CI=95% Confidence Interval
Post-hospital Survival Outcomes
Of the 362 patients, 227 patients (63%) were discharged alive: 105 from the chemotherapy cohort and 122 from the non-chemotherapy cohort. At 6-months, the estimated survival was 58% (95% CI: 48-67%) for chemotherapy patients and 73% (95% CI: 64-80%) for non-chemotherapy patients. At 12-months, the estimated survival was 50% (95% CI: 40-59%) for chemotherapy patients and 59% (95% CI: 50-67%) for non-chemotherapy patients (Figure 2). Patients who received chemotherapy demonstrated a lower survival distribution compared with non-chemotherapy patients (Supplemental Figure 1). Further, in multivariable analyses, patients who received chemotherapy in the ICU had a trend of higher risk of dying by 12 months (OR: 1.45, 95% CI: 0.95-2.19, p=0.08) (Table 4). In contrast to ICU and hospital mortality, MPM II score on ICU admission was not significantly associated with death by 12 months (p=0.14) (Table 3).
Figure 2.
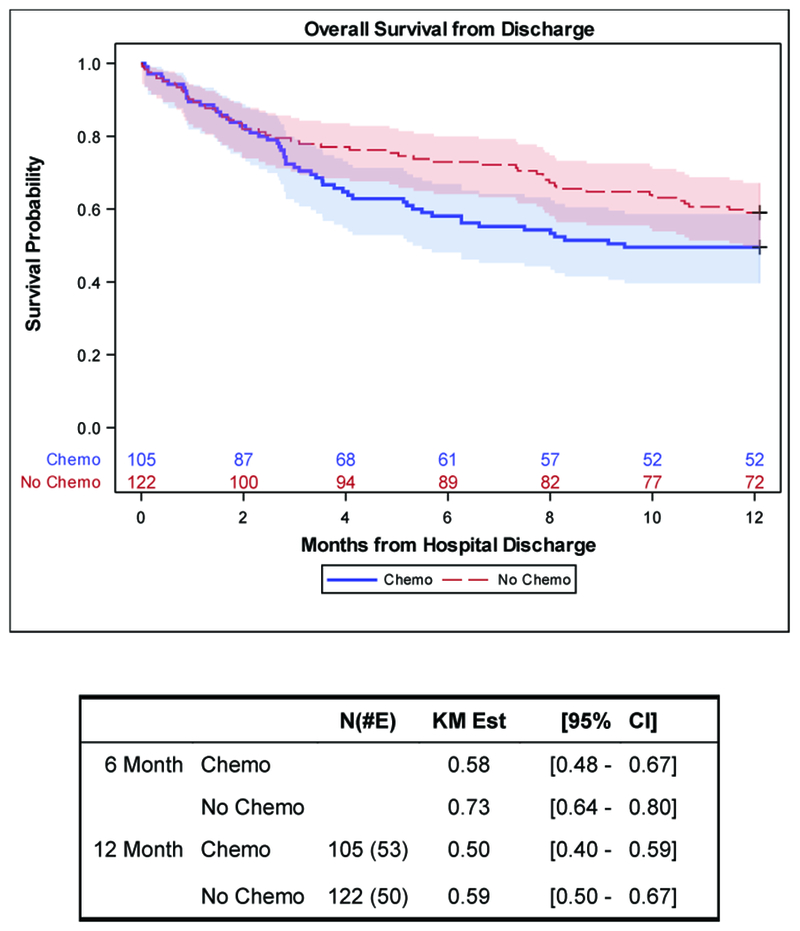
Overall Survival from Hospital Discharge Stratified by Chemotherapy Status (N=227)
Table 4. Patient Characteristics for Initiation versus Continuation Chemotherapy Patients.
Chemotherapy Cohort Only (N=181)
| Chemotherapy in ICU | |||||
|---|---|---|---|---|---|
| All 181 (100%) | Continuation 109 (60%) | Initiation 72 (40%) | p-value | ||
| Age at Admission, years | Median (range) | 62 (18-86) | 61 (18-86) | 65 (22-86) | 0.14 |
| Gender | Male | 115 (63.5) | 68 (62.4) | 47 (65.3) | 0.75 |
| Female | 66 (36.5) | 41 (37.6) | 25 (34.7) | ||
| MPM II Score | Median (range) | 39.1 (7.40-94.7) | 42.7 (7.40-94.7) | 34.2 (8.30-94.1) | 0.19 |
| SOFA Score | Median (range) | 6.0 (0.0-15.0) | 6.0 (0.0-15.0) | 5.5 (0.0-14.0) | 0.27 |
| Cancer Type | Lymphoma | 103 (56.9) | 58 (53.2) | 45 (62.5) | 0.22 |
| Leukemia | 78 (43.1) | 51 (46.8) | 27 (37.5) | ||
| Primary Admit Diagnosis | Bleeding | 11 (6.2) | 8 (7.5) | 3 (4.2) | |
| Cardiac | 7 (4) | 3 (2.8) | 4 (5.6) | ||
| Chemotherapy Admin | 45 (25.4) | 20 (18.9) | 25 (35.2) | ||
| Hematologic | 4 (2.3) | 3 (2.8) | 1 (1.4) | ||
| Neurological | 6 (3.4) | 5 (4.7) | 1 (1.4) | ||
| Renal | 23 (13) | 14 (13.2) | 9 (12.7) | ||
| Respiratory | 33 (18.6) | 22 (20.8) | 11 (15.5) | ||
| Sepsis | 47 (26.6) | 30 (28.3) | 17 (23.9) | ||
| Other | 1 (0.6) | 1 (0.9) | 0 (0) | ||
| Charlson Comorbidity Index | Median (range) | 6.0 (2.0-11.0) | 5.0 (2.0-11.0) | 6.0 (2.0-9.0) | 0.75 |
| Mechanical Ventilation, on Admit | Yes | 17 (9.4) | 12 (11) | 5 (6.9) | |
| No | 164 (90.6) | 97 (89) | 67 (93.1) | 0.44 | |
| Mechanical Ventilation, During Stay | Yes | 75 (41.4) | 46 (42.2) | 29 (40.3) | |
| No | 106 (58.6) | 63 (57.8) | 43 (59.7) | 0.88 | |
| Vasopressors, on Admit | Yes | 16 (8.8) | 12 (11) | 4 (5.6) | |
| No | 165 (91.2) | 97 (89) | 68 (94.4) | 0.29 | |
| Vasopressors, During Stay | Yes | 59 (32.6) | 37 (33.9) | 22 (30.6) | |
| No | 122 (67.4) | 72 (66.1) | 50 (69.4) | 0.75 | |
| CRRT/HD | No | 164 (90.6) | 102 (93.6) | 62 (86.1) | 0.12 |
| Yes | 17 (9.4) | 7 (6.4) | 10 (13.9) | ||
| Same Day Hospital/ICU Admit | Yes | 34 (18.8) | 21 (19.3) | 13 (18.1) | |
| No | 147 (81.2) | 88 (80.7) | 59 (81.9) | >0.95 | |
| ICU LOS, days | Median (range) | 6 (1-47) | 5 (1-44) | 7 (1-47) | 0.13 |
| Hospital LOS, days | Median (range) | 22 (2-121) | 21 (2-92) | 23 (2-121) | 0.64 |
| Hx of Transplant | Yes | 20 (11) | 18 (16.5) | 2 (2.8) | 0.003 |
| No | 161 (89) | 91 (83.5) | 70 (97.2) | ||
| Newly Diagnosed | Yes | 69 (38.1) | 2 (1.8) | 67 (93.1) | <.001 |
| No | 112 (61.9) | 107 (98.2) | 5 (6.9) | ||
| Prior Radiation | Yes | 2 (1.1) | 2 (1.8) | 0 (0) | |
| No | 179 (98.9) | 107 (98.2) | 72 (100) | ||
| # Deaths by ICU Discharge | Yes | 46 (25.4) | 31 (28.4) | 15 (20.8) | |
| No | 135 (74.6) | 78 (71.6) | 57 (79.2) | ||
| # Deaths by Hospital Discharge | Yes | 76 (42) | 52 (47.7) | 24 (33.3) | |
| No | 105 (58) | 57 (52.3) | 48 (66.7) | ||
Initiation (n=72) versus Continuation (n=109) Chemotherapy Patients
The proportion of patients with a history of HSCT was higher (18/109 [17%]) for the continuation chemotherapy patients compared to the initiation patients (2/72 [3%]) (p=0.003). Additionally, initiation chemotherapy patients were more likely to be newly diagnosed (67/72 [93%]) compared with continuation patients (2/109 [2%]) (p<0.001) (Table 2). No other differences were found between the initiation and continuation groups (p=0.12->0.95).
ICU and Hospital Mortality
ICU mortality was 28% (31/109) in the continuation cohort and 21% (15/172) in the initiation cohort. In multivariable analyses, no significant differences in ICU mortality were found between continuation and initiation chemotherapy patients (p=0.27) (Supplemental Table 1). Overall hospital mortality was 48% (52/109) in the continuation cohort and 33% (24/72) in the initiation cohort. Although patients who received continuation chemotherapy had a trend of higher odds of hospital mortality than initiation patients in univariable analyses (OR: 1.82, 95% CI: 0.98-3.38, p=0.056), no significant difference in hospital mortality was found between continuation therapy patients after adjusting for known mortality predictors (OR: 1.80, 95% CI: 0.89-3.64, p=0.10).
Post-hospital Survival Outcomes
At 12 months, estimated survival was 42% (95% CI: 29-54%) in the continuation cohort and 58% (95% CI: 43-71%) in the initiation cohort (Supplemental Figure 2). In multivariable analyses, continuation chemotherapy patients had a significantly higher risk of death by 12 months (HR: 1.77, 95% CI: 1.21-2.60, p=0.003) than initiation patients.
DISCUSSION
Our study is the first and largest study to date of patients with hematologic malignancies who received chemotherapy in the ICU at a US tertiary cancer center. Moreover, to our knowledge, we are the first to describe the short- and long-term outcomes of patients receiving initiation or continuation chemotherapy in the ICU.
The main findings of our study are that patients with hematologic malignancies who receive chemotherapy in the ICU were not found to have differences in short term mortality outcomes, but had a significantly longer ICU and hospital LOS and higher risk of death by 12 months than matched patients not on chemotherapy. There were no significant differences in ICU mortality between the initiation and continuation chemotherapy patients. The trend towards higher odds of hospital mortality among patients who received continuation chemotherapy may reflect their underlying disease severity.
Our ICU and hospital mortality rates of 25% and 42% respectively, for the chemotherapy cohort are comparable to the 25%-40% and 41%-43% ICU and hospital mortality rates, respectively, reported in four previous studies of patients undergoing chemotherapy in the ICU.4-7 We also found that the severity of illness score (MPM-II) on ICU admission was significantly associated with worse short-term outcomes. Three of the four prior studies reported the severity of illness score (i.e., Simplified Acute Physiology Score [SAPS] II) as strongly associated with short-term outcomes.4, 6, 7 Studies of critically ill cancer patients have consistently shown that short-term survival is primarily determined by the extent of organ failures at ICU admission, particularly the need for mechanical ventilation and vasopressors.17-28 In our chemotherapy cohort, 38% and 34% required mechanical ventilation and vasopressors, respectively. We were unable to provide a precise reason for why despite a lower MPM score on ICU admission the patients in the chemotherapy group had a higher in-hospital mortality (albeit non-statistically significant). We can only speculate that some of the patients who received chemotherapy in the ICU were already on third or fourth line regimens and although they survived the ICU admission, they were more likely to have been designated as DNR on ICU discharge, not return to the ICU, and because of the nature of their malignancy, had a higher hospital mortality. We also included for analysis only the first ICU admission during which the patient received chemotherapy. Readmissions of patients who had earlier received chemotherapy in the ICU were not analyzed.
We also found that patients who had the same day hospital and ICU admission had lower odds of ICU and hospital mortality, possibly related to their pre-emptive ICU admission in anticipation of possible deterioration. A small retrospective singe center study showed that direct admission to the ICU of patients with high-risk acute myelogenous leukemia receiving chemotherapy and with physiological disturbances but no organ dysfunction was associated with improved outcomes.29
Only one previous study analyzed long-term survival of cancer patients receiving chemotherapy in the ICU.7 That analysis involved 56 cancer patients (87.5% with hematologic malignancies) and reported survival rates of 49% and 41% at 6 and 12 months, respectively, which is lower than the 66% and 55% survival rates in our chemotherapy cohort (Supplemental Figure 1). Their survival may have been lower for a few reasons related to sample selection and treatment. First, our patients may have been less sick on ICU admission thus positively impacting their long term survival.30 Second, it is possible that our cancer center offers superior post-hospital oncologic care and therapies. Lastly, there were underlying differences in study sample selection (i.e., inclusion of solid and hematologic malignancies in the earlier study).
Our study findings may offer guidance for triage for oncologic patients being considered for ICU admission. Recent literature demonstrates that oncologic patients with critical illness have far better outcomes than previously1-3 thus encouraging their admission to the ICU. However, recent national society guidelines31 do not specifically address the triage question of admitting cancer patients for therapeutic processes such as chemotherapy. In our study, we found that this specific group of oncological patients admitted for chemotherapy had equal outcomes to the matched group. This suggests that outcomes alone should not affect triage or the administration of chemotherapy. However, we also found that the patients who received chemotherapy had a significantly longer ICU and hospital LOS than patients who did not. The correlation between chemotherapy treatment duration and ICU LOS was weak to moderate (rho=0.28, p=0.001), so treatment alone did not fully explain the difference in LOS. This increase in resource utilization may certainly play a role in the triage and therapeutic decisions when ICU beds are severely limited or within non-cancer hospitals which do not specialize in oncologic therapeutic administration in the ICU.
A major strength of our study is the comparison with a matched cohort of ICU patients with similar malignancies who did not receive chemotherapy. This allowed us to control for the influence of three possible confounding factors (age, gender and type of malignancy) on the outcomes of hematologic cancer patients receiving chemotherapy in the ICU. First, we demonstrated that in contrast to the patients who did not receive chemotherapy in the ICU, the patients who received chemotherapy were more likely to have a longer ICU and hospital LOS, greater need for mechanical ventilation (during ICU stay), and higher risk of death by 12 months. Our study also reinforces the crucial role of understanding the capabilities and the triage approaches of the ICU to support these seriously ill patients.
Our study has several limitations. First, despite the large number of patients and analysis of prospectively collected clinical data, the study was retrospective and observational in nature and conducted at a single center with an ICU staff that is dedicated to supporting oncological therapies. This is a similar limitation to the previous studies 4-7 that were also performed in specialized oncologic ICUs. Second, it is possible that there was selection bias in the triage of patients admitted to the ICU for chemotherapy. However, even among oncologists and intensivists, there is variability in ICU triage of cancer patients with favorable and unfavorable prognosis32 that may compensate for this limitation. Third, while it was obvious why chemotherapy were given in the initiation group, it was not possible for us to determine the reasons why the chemotherapy were administered (compassionate/end-of-life, heroic, others) in the continuation group. Finally, we did not collect quality of life outcomes, including functional status and ability to receive continued oncologic treatment after ICU discharge.
In conclusion, our study showed that there were no differences in short-term mortality among patients with hematologic malignancies who did or did not receive chemotherapy in the ICU. However, patients who received chemotherapy had increased ICU and hospital resource utilization and a marginally higher risk of death by 12-months. The severity of illness score on ICU admission was significantly associated with ICU and hospital mortality and death at 12 months. Our data can assist oncology and critical care teams when discussing goals of care and prognosticating outcomes of patients who receive chemotherapy in the ICU and in dealing with triaging scarce ICU resources.
Supplementary Material
ACKNOWLEDGEMENTS:
We acknowledge the assistance of Gleb Kirnicinii, Senior Application Analyst, Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY for data management and pre-analysis, and Richard F Tizon, PharmD, Department of Pharmacy, Memorial Sloan Kettering Cancer Center, New York, NY for additional pharmacy data collection.
This study was supported, in part, by the Core Grant (P30 CA008748) and the Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article.
References
- 1.Azoulay E, Soares M, Darmon M, Benoit D, Pastores S, Afessa B. Intensive care of the cancer patient: recent achievements and remaining challenges. Ann Intensive Care. 2011;23;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schellongowski P, Sperr WR, Wohlfarth P, Knoebl P, Rabitsch W, Watzke HH, Staudinger T. Critically ill patients with cancer: chances and limitations of intensive care medicine-a narrative review. ESMO Open. 2016;1(5):e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mokart D, Pastores SM, Darmon M. Has survival increased in cancer patients admitted to the ICU? Yes. Intensive Care Med. 2014;40(10):1570–2 [DOI] [PubMed] [Google Scholar]
- 4.Darmon M, Thiery G, Ciroldi M, et al. Intensive care in patients with newly diagnosed malignancies and a need for cancer chemotherapy. Crit Care Med 2005;33(11):2488–2493 [DOI] [PubMed] [Google Scholar]
- 5.Benoit DD, Depuydt PO, Vandewoude KH, et al. Outcome in severely ill patients with hematological malignancies who received intravenous chemotherapy in the intensive care unit. Intensive Care Med 2006;32(1):93–99. [DOI] [PubMed] [Google Scholar]
- 6.Song JU, Suh GY, Chung MP, et al. Risk factors to predict outcome in critically ill cancer patients receiving chemotherapy in the intensive care unit. Support Care Cancer 2011;19(4):491–495 [DOI] [PubMed] [Google Scholar]
- 7.Wohlfarth P, Staudinger T, Sperr WR, et al. Prognostic factors, long-term survival, and outcome of cancer patients receiving chemotherapy in the intensive care unit. Ann Hematol. 2014;93(10):1629–36. [DOI] [PubMed] [Google Scholar]
- 8.Azoulay E, Mokart D, Pène F, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium--a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol. 2013;31(22):2810–8. [DOI] [PubMed] [Google Scholar]
- 9.Jackson K, Mollee P, Morris K, Butler J, Jackson D, Kruger P, Klein K, Kennedy G. Outcomes and prognostic factors for patients with acute myeloid leukemia admitted to the intensive care unit. Leuk Lymphoma. 2014;55(1):97–104 [DOI] [PubMed] [Google Scholar]
- 10.Lengliné E, Raffoux E, Lemiale V, et al. Intensive care unit management of patients with newly diagnosed acute myeloid leukemia with no organ failure. Leuk Lymphoma. 2012;53(7):1352–9. [DOI] [PubMed] [Google Scholar]
- 11.Bird GT, Farquhar-Smith P, Wigmore T, Potter M, Gruber PC. Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: a 5 year study. Br J Anaesth. 2012;108(3):452–9. [DOI] [PubMed] [Google Scholar]
- 12.Schellongowski P, Staudinger T, Kundi M, et al. Prognostic factors for intensive care unit admission, intensive care outcome, and post-intensive care survival in patients with de novo acute myeloid leukemia: a single center experience. Haematologica. 2011;96(2):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roze des Ordons AL, Chan K, Mirza I, Townsend DR, Bagshaw SM. Clinical characteristics and outcomes of patients with acute myelogenous leukemia admitted to intensive care: a case-control study. BMC Cancer. 2010;10:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azoulay E, Fieux F, Moreau D, et al. Acute monocytic leukemia presenting as acute respiratory failure. Am J Respir Crit Care Med. 2003;167(10):1329–33. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 16.Schoenfeld D Survival methods, including those using competing risks analysis, are not appropriate for intensive care unit outcome studies. Crit Care 2006;10(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kress JP, Christenson J, Pohlman AS, Linkin DR, Hall JB. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med. 1999;160:1957–1961. [DOI] [PubMed] [Google Scholar]
- 18.Aygencel G, Turkoglu M, Turkoz Sucak G, Benekli M. Prognostic factors in critically ill cancer patients admitted to the intensive care unit. J Crit Care 2014;29:618–626. [DOI] [PubMed] [Google Scholar]
- 19.Guiguet M, Blot F, Escudier B, Antoun S, Leclercq B, Nitenberg G. Severity-of-illness scores for neutropenic cancer patients in an intensive care unit. Which is the best predictor? Do multiple assessment times improve the predictive value? Crit Care Med. 1998;26:488–493. [DOI] [PubMed] [Google Scholar]
- 20.Groeger JS, Lemeshow S, Price K, et al. Multicenter outcome study of cancer patients admitted to the intensive care unit: a probability of mortality model. J Clin Oncol. 1998;16:761–770. [DOI] [PubMed] [Google Scholar]
- 21.Groeger JS, White P Jr, Nierman DM, Glassman J, Shi W, Horak D, Price K Outcome for cancer patients requiring mechanical ventilation. J Clin Oncol. 1999;17:991–997. [DOI] [PubMed] [Google Scholar]
- 22.Staudinger T, Stoiser B, Mullner M, et al. Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med. 2000;28:1322–1328. [DOI] [PubMed] [Google Scholar]
- 23.Benoit DD, Wandewoude KH, Decruyenaere JM, Hoste EA, Colardyn FA. Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the intensive care unit for a life-threatening complication. Crit Care Med. 2003;31:104–112. [DOI] [PubMed] [Google Scholar]
- 24.Maschmeyer G, Bertschat FL, Moesta KT, et al. Outcome analysis of 189 consecutive cancer patients referred to the intensive care unit as emergencies during a 2-year period. Eur J Cancer. 2003;39:783–792. [DOI] [PubMed] [Google Scholar]
- 25.Sculier J- P, Paesmans M, Markiewicz E, Berghmans T. Scoring systems in cancer patients admitted for an acute complication in a medical intensive care unit. Crit Care Med. 2000;28:2786–2792. [DOI] [PubMed] [Google Scholar]
- 26.Schellongowski P, Benesch M, Lang T, et al. Comparison of three severity scores for critically ill cancer patients. Intensive Care Med. 2004;30:430–436. [DOI] [PubMed] [Google Scholar]
- 27.Soares M, Fontes E, Dantas J, et al. Performance of six severity-of-illness scores in cancer patients requiring admission to the intensive care unit: a prospective observational study. Crit Care 2004;8(4): R194–R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moors I, Pene F, Lengline E, Benoit D. Urgent chemotherapy in hematological patients in the ICU. Curr Opin Crit Care 2015;21:559–568. [DOI] [PubMed] [Google Scholar]
- 29.Lengline E, Raffoux E, Lemiale V, et al. Intensive care unit management of patients with newly diagnosed acute myeloid leukemia with no organ failure. Leuk Lymphoma 2012;53(7):1352–59. [DOI] [PubMed] [Google Scholar]
- 30.Level C, Tellier E, Dezou P, et al. Outcome of older persons admitted to intensive care, mortality, prognosis factors, dependency scores and ability trajectory within 1 year: a prospective cohort study. Aging Clin Exp Res 2017. December 6 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Nates JL, Nunnally M, Kleinpell R, et al. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med 2016;44:1553–1602. [DOI] [PubMed] [Google Scholar]
- 32.Von Bergwelt-Baildon M, Hallek MJ, Shimabukuro-Vornhagen AA, Kochanek M. CCC meets ICU: redefining the role of critical care of cancer patients. BMC Cancer 2010;10:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


