Abstract
Background
This retrospectively study was conducted to assess the efficiency and safety of computed tomography (CT)‐guided hook wire localization of pulmonary ground‐glass nodules (GGNs) prior to video‐assisted thoracoscopic surgery (VATS).
Methods
From 2015 to 2018, a total of 86 patients with 86 pulmonary GGNs underwent preoperative CT‐guided hook wire localization before VATS. The technical details and clinicopathological findings were analyzed.
Results
All 86 pulmonary GGNs (25 pure GGNs and 61 part‐solid GGNs) were successfully located and resected. The mean diameter of the GGNs was 1.4 ± 0.4 cm (range 0.6–2.2) and the mean lesion distance to the pleural surface was 7.3 ± 4.3 mm (range 2–19). Complications of hook wire marking included asymptomatic minor pneumothorax in 21 patients (24%) and focal pulmonary hemorrhage in 18 (21%). The median hook wire localization time was 19.1 minutes (range 10–30) and the median VATS time was 49 minutes (range 28–89). Pathology revealed 72 precancerous lesions or primary lung adenocarcinomas, 5 metastatic tumors, and 9 benign lesions.
Conclusions
Preoperative localization of small pulmonary GGNs using CT‐guided hook wire was efficient and safe prior to VATS resection.
Keywords: Ground‐glass nodule, hook wire, localization, video‐assisted thoracoscopic surgery
Introduction
Low‐dose spiral computed tomography (LDCT) has become available for screening lung cancer, and as a result more ground‐glass nodules (GGNs) are incidentally detected. GGNs fall into two categories on thin‐section chest computed tomography (CT): pure GGN (pGGN) and part‐solid GGN (psGGN).1 A pure GGN is defined as a focal density‐increased area of pulmonary parenchyma through which the pulmonary vessels or bronchial structures can be seen. A part‐solid GGN presents with both ground glass and solid components. Pathologically, GGNs often correspond to precancerous lesions, early‐stage adenocarcinoma, or even metastasized tumors.2, 3 Researchers have reported a GGN malignancy rate of 59%–73%.4 However, not all GGNs are malignant. Some are benign lesions, such as focal interstitial fibrosis, focal hemorrhage, or focal inflammation.5 Early and accurate diagnosis of these small GGNs is crucial to develop an appropriate clinical treatment plan. Unfortunately, it is still challenging to obtain a definitive pathological diagnosis with dedicated CT, (F18) fluorodeoxyglucose positron emission tomography/CT, or even image‐guided percutaneous biopsy.
Video‐assisted thoracoscopic surgery (VATS) offers a minimally invasive method to diagnose and treat small pulmonary GGNs.6 However, it is often difficult to localize GGNs during VATS because of their small size, deep location, or subsolid nature. Thus, preoperative marking of these invisible and unpalpable nodules can accurately guide surgeons to localize and resect them during VATS.
In this study, we retrospectively assess the efficiency and safety of utilizing a CT‐guided hook wire to locate GGNs prior to VATS in our hospital.
Methods
Patients
The Ethics Committee of Qilu Hospital of Shandong University approved this study, and written informed consent was obtained from all patients.
Eighty‐six consecutive patients underwent preoperative CT‐guided hook wire placement from 2015 to 2018. The indications for CT‐guided hook wire localizations in patients with suspected malignant GGNs were: persistent GGN after a six‐month follow‐up, psGGN, or newly presented GGN with extrathoracic malignant tumor history. Exclusion criteria were: severe emphysema, advanced interstitial pulmonary disease, refractory coagulation dysfunction, positive‐pressure ventilation, cardiac insufficiency, pleuritis, and a lesion located close to the mediastinal great vessels.
A total of 86 pulmonary GGNs were diagnosed by CT examination (maximum diameter < 3 cm at lung window). The characteristics of each nodule were recorded, including maximum diameter, location, density, and nodule distance from viscera pleural. Each nodule was classified according to its density as pGGO or psGGO. Two senior radiologists performed radiological assessments in consensus.
Computed tomography‐guided hook wire placement
A 256‐slice multidetector CT scanner (Philips Brilliance ICT, Philips Healthcare, Cleveland, OH, USA) was used in this study. The scan area was limited to the lesion area using the same breath position. The following parameters were used: 3 or 5 mm section thickness, 30 mAs, and 120 kV.
A senior radiologist and a thoracic surgeon carefully reviewed recent chest CT images to determine the appropriate puncture route and body position for hook wire placement. A 20‐G hook wire system (Argon Medical Devices, Frisco, TX, USA) was employed to locate all GGN lesions under CT guidance. The 1 cm stainless steel folded hook wire was enclosed within the introducer. The hook wire is commonly used for breast surgery, and has also been successfully applied for pulmonary nodule localization.
During the procedure, the first chest CT scan was used to determine the optimal puncture site, angle, and depth. The puncture route should avoid traversing important vessels, including the internal mammary, axillary, subclavian, intercostal vessel, and pulmonary vessels. Bulla and interlobar fissures should also be avoided to prevent pneumothorax. After local anesthesia of the puncture route with 2% lidocaine, the introducer of the hook wire system was inserted into the chest wall with the tip close to the parietal pleura. The introducer was oriented almost perpendicular to the pleural surface.
After verification of the plane and angulation, the introducer was advanced into the lung parenchyma to reach the lesion, but not into the lesion to avoid tumor seeding via a puncture tract. The hook wire was then released via the introducer. A final chest CT scan was performed to confirm the position of the hook wire relative to the nodule and identify any postprocedural complications (Fig 1). Complications during hook wire localization were recorded. The tail of the hook wire was cut near the skin surface and allowed to withdraw into the chest when the lung collapsed during VATS surgery.
Figure 1.
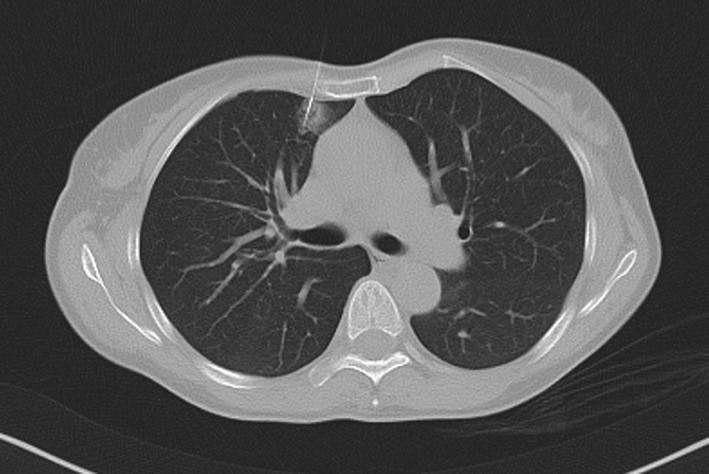
A computed tomography scan shows that the hook wire is positioned in the ground‐glass nodule of a 52‐year old woman.
The surgeon participated in the procedure so that he knew the relationship between the hook wire and the target nodule on the post‐localization CT image. Patients were then immediately transferred to the operating room for VATS.
Video‐assisted thoracoscopic surgery (VATS) procedure
In all cases, VATS was performed within an hour of hook wire placement. According to standard surgical procedures, wedge resection of the lesion area was performed using a stapler device according to the localization by the hook wire. Finger palpation of each GGN was also performed before resection. After resection, an experienced pathologist performed intraoperative frozen section examinations. When a frozen specimen revealed lung cancer, if physiologically feasible, lobectomy with mediastinal lymph node dissection was performed via VATS. When a lesion was diagnosed as benign or as a metastatic carcinoma, no further surgery was necessary. The specimens were also prepared for postoperative histological examination and the results were classified according to the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASL/ATS/ERS) guidelines for pulmonary adenocarcinoma.7 The VATS procedural duration was recorded.
Statistical analysis
All data were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation. A Student's t‐test was used to analyze continuous variables. The χ2 test was used to compare categorical variables between groups. P < 0.05 was considered significant.
Results
Patient and ground‐glass nodule characteristics
Eighty‐six patients (49 women, mean age 58.4 ± 9.2, range 39–75) with 86 GGNs (25 pGGNs, 61 psGGNs) were included in the study. Patient and GGN characteristics are summarized in Table 1. The mean diameter of the GGNs was 1.4 ± 0.4 cm (range: 0.6–2.2 cm) and the mean lesion distance to the pleural surface was 7.3 ± 4.3 mm (range: 2–19 mm). There were 31 nodules located in the right upper lobe, 9 in the right middle lobe, 13 in the right lower lobe, 21 in the left upper lobe, and 12 in the left lower lobe.
Table 1.
Patient and nodule characteristics
| Characteristics | Number |
|---|---|
| Male/Female | 37/49 |
| Age (years) | Mean 58.4 (range: 39–75) |
| Smoking history | 38 (44%) |
| Mild‐moderate emphysema | 24 (28%) |
| Tumor history | 12 |
| Nodule size (cm) | Mean 1.4 (range: 0.6–2.2) |
| < 1 | 12 (13.9%) |
| 1–2 | 64 (74.4%) |
| > 2 | 10 (11.6%) |
| Nodule location | |
| RUL | 31 |
| RML | 9 |
| RLL | 13 |
| LUL | 21 |
| LLL | 12 |
| Nodule feature | |
| pGGN | 25 (29%) |
| psGGN | 61 (71%) |
| Nodule distance to pleural surface (mm) | Mean 7.3 (range: 2–19) |
| Nodule distance to hook wire (mm) | |
| Intratumoral | 69 (80.3%) |
| 2–10 mm | 17 (19.7%) |
| Duration of hook wire placement | Mean 19.1 minutes (range: 10–30) |
| Duration of VATS procedure | Mean 49 minutes (range: 28–89) |
| Complication of hook wire | |
| Pneumothorax | 21 (24%) |
| Pulmonary hemorrhage | 18 (21%) |
| Hookwire dislodgement | 1 (1.6%) |
LLL, left lower lobe; LUL, left upper lobe; pGGN, pure ground glass nodule; psGGN, part‐solid GGN; RML, right middle lobe; RUL, right upper lobe; VATS, video‐assisted thoracoscopic surgery.
Hook wire localization
The hook wire was positioned successfully in all 86 pulmonary nodules within a mean duration of 19.1 minutes (range: 10–30). In 69 cases, the hook wire directly penetrated the nodule. In the other 17 cases, the hook wire passed alongside the nodule less than 1.0 cm from the GGN (Table 1). The duration of hook wire localization of lower lobular GGNs was significantly longer than of upper lobular GGNs (P < 0.05).
In terms of complications, asymptomatic minor pneumothorax was observed in 21 patients (24%), but further intervention was not required (Fig 2). Pneumothorax frequently occurs in patients with emphysema (P < 0.05). Focal pulmonary hemorrhage along the puncture tract was observed in 18 cases (21%), but was limited without clinical consequence. No hemothorax or pulmonary air embolism were reported.
Figure 2.
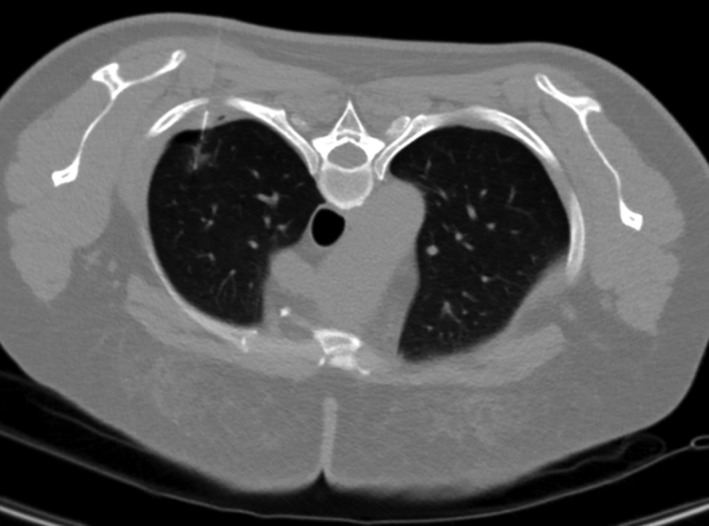
Asymptomatic minor pneumothorax was observed in a 39‐year old woman.
VATS procedure
Video‐assisted thoracoscopic surgery was performed within an hour of hook wire localization. All of the target nodules were accurately positioned and resected under VATS without requiring conversion to open thoracotomy. Among the 86 GGNs, 14 psGGNs could be identified by finger palpation (16%), while the left psGGNs and all pure pGGNs could not be definitely identified by finger palpation.
The median VATS duration was 49 ± 11 minutes (range: 28–89). Hospitalization ranged from 5 to 10 days (median 6.5). Twenty‐four wedge resections and 62 lobectomies were performed. Eight patients, including 6 with minimally invasive adenocarcinoma (MIA) and 2 with invasive adenocarcinoma (IA), underwent wedge resection because of poor cardiopulmonary function.
Hook wire dislodgement occurred in one female patient (1.6%) during VATS, but the pulmonary nodule was still successfully resected according to the focal subpleural hemorrhage surrounding the puncture point, without requiring conversion to thoracotomy.
Pathological results
The final pathological diagnoses of all 86 lesions are reported in Table 2. All operative margins were histologically free. Forty‐six psGGNs were IA, 9 psGGNs and 10 pGGNs were MIA, and 5 pGGNs were adenocarcinoma in situ. Two pGGNs were atypical adenomatous hyperplasia. Four psGGNs and 1 pGGN were metastatic tumors (2 from breast cancer, 2 from esophageal cancer, and 1 from hepatocellular carcinoma). Two psGGNs and 7 pGGNs were benign lesions. In cases where the hook wire penetrated the lesion, neither the nodule nor the pathological examination was affected.
Table 2.
Histopathologic results
| Variables | |
|---|---|
| Malignancy (%) | 77 (89.5%) |
| AAH | 2 |
| AIS | 5 |
| MIA | 19 |
| IA | 46 |
| Metastasis | 5 |
| Benign (%) | 9 (10.5%) |
| Focal inflammation | 7 |
| Fibrosis | 2 |
AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; IA, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma.
Discussion
The results of our study show a high technical success rate (100%) of preoperative CT‐guided hook wire localization of pulmonary GGNs, thus facilitating VATS for GGN resection. The complications experienced were minor and well tolerated.
Video‐assisted thoracoscopic surgery has been accepted as a standard procedure for the resection of peripheral small pulmonary nodules. The advantages of VATS include similar long‐term survival, less traumatic injury to the thoracic cavity, less postoperative pain, a lower complication rate, and shorter hospital stay than open thoracotomy.8 Nevertheless, some GGNs may be invisible or unpalpable during VATS. Therefore, it is crucial to accurately localize such faint lesions prior to VATS in case conversion to traditional thoracotomy is required.
In our study, 25 pGGNs and 61 psGGNs were preoperatively localized by hook wire for VATS. During VATS, all lesions were first palpated using the fingers. Only 16% GGNs (14/86) were identified by palpation. Our positive rate of palpation was lower than that reported by Kondo et al. (75% for psGGN, 12.1% for pGGN).9 The discrepancy may be associated with the clinicopathological characteristics of the target nodule, such as size, depth, pathological type, location, and time of puncture. When the nodule is deeper and smaller, it is more difficult to palpate. Some studies have focused on preoperative localization of GGO lesions. Huang et al. reported a success rate of 95.1% (39/41 pGGOs < 2 cm) using hook wire localization under CT guidance with three dislodgements.10 Bertolaccini et al. used a radio‐guided method to locate small GGOs (< 15 mm) with a success rate close to 100%.11 Hsu et al. detected 91 indeterminate pulmonary nodules (62 pGGNs, 27 psGGNs, and 2 solid nodules) with a success rate of 100%.12 Our results are consistent with these studies. Pathologically, a high ratio of malignancy was observed (89.5%) in our study, which may be because surgeons preoperatively screened the target nodules.
Hook wire localization is probably the most common technique for small lung nodule localization. The success rate of hook wire localization is reported as 93.6–97.6% in the literature. In our case, the success rate was close to 100%. The main concern of this method is hook wire dislodgement, which may cause difficulty locating the pulmonary lesion during VATS. In this study, hook wire dislodgement occurred in one case (1.6%) during lung deflation. In this case, the hook wire accidentally penetrated the horizontal fissure, which may have contributed to the dislodgement. The reported hook wire dislodgement rate is 0–6.9%. Klinkenberg et al. reported one case (0.7%) and Chen et al. two cases (4.9%) of dislodgement in their studies; thus, open thoracotomy was performed as an alternative.13, 14 Dislodgement could have occurred during transport to surgery, during positioning of the patient on the operating table, during deflation of the non‐ventilated lung, or as a result of the surgeon's manipulation during VATS.13, 14 Therefore, the following measures can be taken in clinical practice to prevent dislodgement: (i) patients should be instructed to avoid coughing and upper body movement to keep the hook wire relatively stationary and reduce the possibility of pneumothorax; (ii) the hook wire should be nearly perpendicular to the pleural surface and not close to the ribs, scapula, or fissure to reduce the resistance between the hook wire and chest wall; and (iii) during VATS, the surgeon should apply light traction on the hook wire to tent the lung during wedge resection. Furthermore, the time interval between puncture and VATS should be as short as possible.
Other complications, including minor pneumothorax (24%) and focal hemorrhage (21%), were observed. Pneumothorax is the most common complication, and is reported to occur in 7.5–40% of cases. In our study, pneumothorax occurred in 21/86 cases. Iguchi et al. evaluated the risk factors for pneumothorax in 267 hook wire placements and found that increased vital capacity, lower lobe lesions, solid lesions, prone positioning, a transfissural approach, and longer procedure duration were significant predictors of pneumothorax.15 Lung parenchyma hemorrhage is reported to occur in 13.9–36% of cases, and often can be limited. Serious complications, such as a large amount of hemothorax or massive air embolism, were not observed in our study.
Currently, many other techniques to localize small lung nodules are used in clinical practice, such as dye localization, microcoil and fiducial markers, contrast medium injection (e.g. barium sulfate and lipiodol), radiotracer, electromagnetic navigation bronchoscopy, and intraoperative ultrasonography.16 Each of these localization techniques has advantages and drawbacks. Methylene blue dye injection may rapidly diffuse into the surrounding lung parenchyma, and the failure rate is reported at approximately 13%. It may also induce hypotension and bronchospasm.17 Su et al. applied a preoperative CT‐guided microcoil localizing method whereby the microcoils were placed adjacent to the nodule lesion rather than through the nodule, and achieved an implantation success rate of 98%.18 The postprocedural complication rate was low, with 15.8% asymptomatic pneumothorax and 8.9% parenchymal hemorrhage. Liquid media such as barium, lipiodol, and polylactic acid, have also successfully been used in clinical trials.19, 20, 21 These liquid materials may carry a risk of focal inflammation and lesion structure disruption. An intraoperative ultrasonographic technique is sensitive to solid or semi‐solid nodules.9 When it comes to pGGNs, this method becomes more difficult. Moreover, the procedure is highly operator‐dependent and can only be successfully performed by experienced ultrasonography specialists. This method also has limited use in patients with emphysema, as their lungs cannot reach complete collapse. Electromagnetic navigation bronchoscopy enables real‐time navigation to locate small peripheral nodules using dye or a fiducial marker.22, 23 The procedure can be performed in the operating room under anesthesia without patient discomfort or complications. However, this technique is time‐consuming and is more sophisticated than commonly used percutaneous localization methods. Moreover, the contrast agent, metallic marker, and radiotracer methods require fluoroscopic guidance during VATS and increases unnecessary radiation exposure to the surgeon.
There are some limitations to the present study. Firstly, our sample was small; thus, a larger sample is required to evaluate the safety and long‐term effect of hook wire localization. Secondly, all GGNs in our study were screened by surgeons and were highly suspected of malignancy, which may account for the high ratio of malignancy observed (77/86, 89.5%). Thirdly, the mean distance from the nodule to the pleural surface was 7.3 ± 4.3 mm (range 2–19 mm), and deeper nodules were not included.
In conclusion, preoperative localization of small pulmonary GGNs using CT‐guided hook wire was safe and effective prior to VATS resection.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The Shandong Provincial Key Research and Development Program supported this study (grant numbers 2018GSF118087 and 2017GSF218010).
References
- 1. Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: Recommended interim guidelines for assessment and management. Radiology 2009; 253: 606–22. [DOI] [PubMed] [Google Scholar]
- 2. Yang ZG, Sone S, Takashima S et al. High‐resolution CT analysis of small peripheral lung adenocarcinomas revealed on screening helical CT. AJR Am J Roentgenol 2001; 176: 1399–407. [DOI] [PubMed] [Google Scholar]
- 3. Hu H, Wang Q, Tang H, Xiong L, Lin Q. Multi‐slice computed tomography characteristics of solitary pulmonary ground‐glass nodules: Differences between malignant and benign. Thoracic Cancer 2016; 7: 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC,AC o CP. Evidence for the treatment of patients with pulmonary nodules: When is it lung cancer?: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest 2007; 132 (3 Suppl): 94S–107S. [DOI] [PubMed] [Google Scholar]
- 5. Park CM, Goo JM, Lee HJ, Lee CH, Chun EJ, Im JG. Nodular ground‐glass opacity at thin‐section CT: Histologic correlation and evaluation of change at follow‐up. Radiographics 2007; 27: 391–408. [DOI] [PubMed] [Google Scholar]
- 6. Mack MJ, Hazelrigg SR, Landreneau RJ, Acuff TE. Thoracoscopy for the diagnosis of the indeterminate solitary pulmonary nodule. Ann Thorac Surg 1993; 56: 825–30. [DOI] [PubMed] [Google Scholar]
- 7. Travis WD, Brambilla E, Noguchi M et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011; 6: 244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakao M, Yoshida J, Goto K et al. Long‐term outcomes of 50 cases of limited‐resection trial for pulmonary ground‐glass opacity nodules. J Thorac Oncol 2012; 7: 1563–6. [DOI] [PubMed] [Google Scholar]
- 9. Kondo R, Yoshida K, Hamanaka K et al. Intraoperative ultrasonographic localization of pulmonary ground‐glass opacities. J Thorac Cardiovasc Surg 2009; 138: 837–42. [DOI] [PubMed] [Google Scholar]
- 10. Huang W, Ye H, Wu Y et al. Hook wire localization of pulmonary pure ground‐glass opacities for video‐assisted thoracoscopic surgery. Thorac Cardiovasc Surg 2014; 62: 174–8. [DOI] [PubMed] [Google Scholar]
- 11. Bertolaccini L, Salgarello M, Gorgoni G, Terzi A. Radioguided video‐assisted resection of non‐palpable solitary pulmonary nodule/ground glass opacity: How to do it. J Vis Surg 2015; 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu HH, Shen CH, Tsai WC et al. Localization of nonpalpable pulmonary nodules using CT‐guided needle puncture. World J Surg Oncol 2015; 13: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klinkenberg TJ, Dinjens L, Wolf RFE et al. CT‐guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol 2017; 115: 898–904. [DOI] [PubMed] [Google Scholar]
- 14. Chen S, Zhou J, Zhang J et al. Video‐assisted thoracoscopic solitary pulmonary nodule resection after CT‐guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011; 25: 1723–9. [DOI] [PubMed] [Google Scholar]
- 15. Iguchi T, Hiraki T, Gobara H et al. CT fluoroscopy‐guided preoperative short hook wire placement for small pulmonary lesions: Evaluation of safety and identification of risk factors for pneumothorax. Eur Radiol 2016; 26: 114–21. [DOI] [PubMed] [Google Scholar]
- 16. Lin MW, Chen JS. Image‐guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016; 8 (Suppl 9): S749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Partik BL, Leung AN, Müller MR et al Using a dedicated lung‐marker system for localization of pulmonary nodules before thoracoscopic surgery. AJR Am J Roentgenol 2003; 180: 805–9. [DOI] [PubMed] [Google Scholar]
- 18. Su TH, Fan YF, Jin L, He W, Hu LB. CT‐guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video‐assisted thoracoscopic surgical resection. Eur Radiol 2015; 25: 2627–33. [DOI] [PubMed] [Google Scholar]
- 19. Lee NK, Park CM, Kang CH et al. CT‐guided percutaneous transthoracic localization of pulmonary nodules prior to video‐assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012; 13: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miura H, Yamagami T, Tanaka O et al. CT findings after lipiodol marking performed before video‐assisted thoracoscopic surgery for small pulmonary nodules. Acta Radiol 2016; 57: 303–10. [DOI] [PubMed] [Google Scholar]
- 21. Hu M, Zhi X, Zhang J. Preoperative computed tomography‐guided percutaneous localization of ground glass pulmonary opacity with polylactic acid injection. Thoracic Cancer 2015; 6: 553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bolton WD, Howe H III, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014; 98: 471–5. [DOI] [PubMed] [Google Scholar]
- 23. Anantham D, Feller‐Kopman D, Shanmugham LN et al. Electromagnetic navigation bronchoscopy‐guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: A feasibility study. Chest 2007; 132: 930–5. [DOI] [PubMed] [Google Scholar]


