Abstract
Background
We compared the effects of thoracic epidural analgesia (TEA) to conventional patient‐controlled analgesia (PCA) on several postoperative parameters of recovery after elective video‐assisted thoracoscopic (VATS) lobectomy.
Methods
Ninety‐eight patients undergoing elective VATS lobectomy were enrolled. The primary endpoint was pain score. Recovery of bowel function, length of stay in the postanesthesia care unit (PACU), duration of postoperative hospital stay, and complications were assessed. Continuous variables were expressed and compared between groups using either a two‐tailed Student's t or Mann‐Whitney U test. Recovery of bowel function was compared using the log‐rank test.
Results
Baseline characteristics between the groups were similar. Dynamic pain scores on postoperative days (PODs) 0–2 were significantly lower in the TEA group, as were resting pain scores on PODs 1 and 2 (P < 0.05). The mean duration to first flatus (16 ± 0.7 vs. 26 ± 0.7 hours; P < 0.001) and the mean length of stay in the PACU (34 vs. 67 minutes; P = 0.027) were shorter in the TEA compared to the PCA group, respectively. The only difference in postoperative complications was regarding confusion (5 TEA vs. 18 PCA patients; P = 0.002). No difference in overall length of stay was noted.
Conclusions
Compared to PCA, TEA provided better postoperative pain control after VATS lobectomy and facilitated postoperative recovery of bowel function without increasing the length of hospital stay. This beneficial effect of TEA might be attributed to the attenuation of sympathetic hyperactivation, improved analgesia, and reduced opioid use.
Keywords: Bowel function, epidural analgesia, postoperative analgesia, video‐assisted thoracoscopic lobectomy
Introduction
Enhanced recovery after surgery (ERAS) protocols have become increasingly popular over the past decade. These pathways have proven to significantly reduce postoperative length of stay and costs after colorectal surgery.1, 2, 3 Fast‐track colorectal surgery has been comprehensively discussed,4, 5, 6 but few reports have examined the fast‐track program for VATS lobectomy.7, 8
Thoracic epidural analgesia (TEA) is generally considered the gold standard of analgesia after thoracotomy.9, 10 TEA can be a useful adjunct in fast‐track surgery, optimizing pain relief, freedom from pain attenuating the surgical stress response, the return of early of bowel function, and early mobilization. However, its acceptance among anesthetists remains low and its role remains controversial in laparoscopic surgery because few large studies have evaluated the role of TEA in minimally invasive surgeries.11
The aim of this study was therefore to test the hypothesis that TEA improves recovery after VATS lobectomy when compared to patient‐controlled analgesia (PCA).
Methods
Study design
This randomized, prospective, parallel‐group superiority study was performed to compare the clinical effects of TEA to sufentanil‐based PCA in patients undergoing VATS lobectomy from January to May 2017. The local medical ethical committee approved the study and all patients signed informed written consent before enrollment in the study. Our study was registered with Chinese Clinical Trial Registry (ChiCTR‐ IOR‐17010385).
Patients and setting
All patients undergoing elective VATS lobectomy at the Affiliated Hospital of Qingdao University, China were assessed for eligibility. Before admission to the operating room, patients were randomly assigned in a 1:1 ratio into parallel arms using a computer‐generated random number table with sealed envelopes to TEA or PCA groups. Blinding was not performed because it seemed neither feasible nor realistic for this study.
The following inclusion criteria were used: patients qualified for VATS lobectomy as a result of cancer; aged 18–70 years; of either gender; and American Society of Anesthesiologists (ASA) physiological status I–III. The exclusion criteria were: technical failure to insert an epidural catheter; conversion of VATS to thoracotomy; discontinuation of local anesthesia for technical reasons (e.g. catheter slipping out or damage); aged < 18 years; unable to provide informed consent; and medical contraindication for TEA according to institutional guidelines. Ninety‐eight patients scheduled for VATS lobectomy were enrolled.
Thoracic epidural
In the preoperative holding area just before surgery, a thoracic epidural catheter was inserted between the T4 and T7 interspaces at a level appropriate to the planned surgical incision. Using the loss‐of‐resistance‐to‐air technique, an epidural catheter was inserted 3–5 cm cephalad before the induction of anesthesia. Provided that neither cerebrospinal fluid nor blood was obtained on aspiration, a 3‐mL test dose of lidocaine 15 mg/mL was injected. Five minutes later, if there were no signs of intravascular or intrathecal administration, a 5–10 mL dose of ropivacaine 2.5 mg/mL (12.5–25 mg) was injected through the epidural catheter.
Interventions, anesthesia, and pain strategy
On the day of surgery, patients were randomly allocated to either a control group (PCA) that received general anesthesia and postoperative PCA; or a treatment group (TEA) that received light general anesthesia, intraoperative epidural anesthesia, and postoperative epidural analgesia. In the TEA group, a bolus of 5 mL of ropivacaine 0.25% was commenced as soon as the epidural catheter was in place, and continuous perfusion of ropivacaine 0.25% at 5 mL/hour was initiated until the completion of surgery.
Senior surgeons performed all surgical procedures. None of the patients required urinary catheters, and the use of drains was avoided if no errhysis occurred. In both groups, induction of anesthesia was performed with propofol (1–2 mg/kg), sufentanil (0.4–0.5 μg/kg), and cisatracurium (0.15–0.2 mg/kg) for muscle paralysis. After tracheal intubation, maintenance of anesthesia was performed with sevoflurane in a mixed oxygen/air fresh gas, and cisatracurium as needed. Analgesia was assured by the ropivacaine solution in the TEA group and by sufentanil as needed in the PCA group.
When the surgery was completed, a solution of ropivacaine (0.15%) and sufentanil (0.2 μg/mL) was initiated in the TEA group at a rate of 5–10 mL/hour (target: visual analogue scale [VAS] score < 4) with a bolus of 5 mL of the solution allowed every 40 minutes (patient‐controlled epidural analgesia). In the PCA group, sufentanil was inserted at 2 μg/hour. A bolus of 2 mL was allowed every 15 minutes up to a maximal dose of 10 μg/hour.
All patients received flurbiprofen axetil 50 mg/day as baseline analgesic treatment unless contraindicated. In case of an analgesic failure (VAS score persistently > 4), tramadol was used as a rescue medication. Pain assessment was conducted twice daily at rest (static) and on coughing (dynamic) by a dedicated institutional analgesia team.
Outcomes/study end points
The primary outcomes of this study were the resting and dynamic pain scores, recorded using a VAS. The secondary outcomes were the recovery of bowel function after surgery, evaluated as the time to first flatus; length of stay in the postanesthesia care unit (PACU); postoperative complications (nausea/vomiting requiring treatment with ondansetron, confusion, wound abscess, pneumonia); and postoperative hospital stay. Demographic information (age, gender, body mass index, and ASA grade) and pertinent surgical information (operation duration, estimated blood loss) were recorded.
The administration of postoperative analgesia was commenced after arrival in the PACU. Quality of pain relief at rest and on coughing was assessed by the patient using a VAS with a range of 0–10, with 0 representing “no pain” and 10 representing “the worst pain.”
Prospectively collected data included pain scores at rest and cough on postoperative day (POD) 0 (immediately after extubation) to POD 2, time to first passage of flatus, time to first stool, time to normal diet, length of stay in the PACU, complications, and length of stay. Patients in the TEA group received patient‐controlled epidural analgesia using a mixture of 0.15% ropivacaine and 0.2 μg/ml sufentanil at a constant rate of 5 mL/hour, with boluses of 5 mL and a 40 minute lockout time. The control group received PCA using a mixture of 1 μg/mL sufentanil and 0.08 mg/mL ondansetron with the pump set to deliver doses of 2 μg/hour intravenous sufentanil with a 15 minute lockout time. Epidural catheters were removed from all patients 48 hours after surgery. Nausea and vomiting were treated with intravenous 8 mg ondansetron. Oral fluids and feeding were commenced the day after surgery. All patients were subjected to enforced early mobilization. Perioperative management was similar in both groups, except for the route of analgesia.
Statistical analysis
Sample size computation was based on the mean recovery time of bowel function (3.8 ± 1.6 days) by use of TEA.12 To have a > 80% power with an overall two‐sided type I error rate of 5%, 49 patients were required in each group.
Continuous variables were expressed as the mean (± 1 standard deviation) or median (95% confidence interval [CI]) when data were not normally distributed and were compared between the two groups using either a two‐tailed Student's t or Mann‐Whitney U test.
Clinical parameters (recovery time of bowel function) were compared using the log‐rank test. Data were analyzed by using SPSS version 23.0 (IBM Corp., Armonk, NY, USA). P values < 0.05 were considered statistically significant.
Results
Between 6 January and 9 May 2017, 157 consecutive patients were assessed for eligibility. Fifty‐eight patients did not meet the inclusion criteria or refused to participate. The remaining 99 patients provided written consent to participate and were randomized to either TEA (n = 49) or PCA (n = 50) groups. One PCA patient dropped out after randomization. The final analysis therefore compared 49 TEA with 49 PCA patients (Fig 1). All subjects were included in the primary outcome analysis. There were no clinically significant differences in demographic data between the groups, except for the intraoperative consumption of sufentanil (Table 1).
Figure 1.

Protocol for patient enrolment in the study groups. Randomized controlled trial comparing thoracic epidural analgesia (TEA) versus patient‐controlled analgesia (PCA) for video‐assisted thoracoscopic surgery (VATS) lobectomy.
Table 1.
Demographic data
| Characteristics | TEA group (n = 49) | PCA group (n = 49) | P |
|---|---|---|---|
| Age (years) | 57.8 ± 8.1 | 54.9 ± 11.7 | 0.607 |
| Male, n (%) | 26 (67) | 30 (75) | 0.862 |
| BMI | 25.4 ±1.8 | 25.3 ±2.6 | 0.933 |
| ASA I/II/III | 23/13/3 | 24/12/4 | 0.651 |
| Duration of surgical procedure (min) | 112 ±33 | 120±47 | 0.718 |
| Estimated blood loss (mL) | 27 ± 11 | 23 ±9 | 0.420 |
| Intraoperative sufentanil, μg | 29 ±14 | 74 ± 10 | < 0.001 |
Values are shown as mean ± standard deviation or number (n) and %. ASA, American Society of Anesthesiologists; BMI, body mass index; PCA, patient‐controlled analgesia; TEA, thoracic epidural analgesia.
Pain scores
Visual analogue scale pain scores at rest and during coughing are shown in Figure 2. There was no difference in scores between the groups at rest on POD 0, whereas VAS pain scores with cough on POD 0 were lower in the TEA group (P < 0.001). Postoperative pain scores at rest and with cough on POD 1 and 2 were significantly lower in the TEA than in the PCA group. One (3%) TEA patient and 15 (38%) PCA patients received rescue medication after surgery (P < 0.001).
Figure 2.
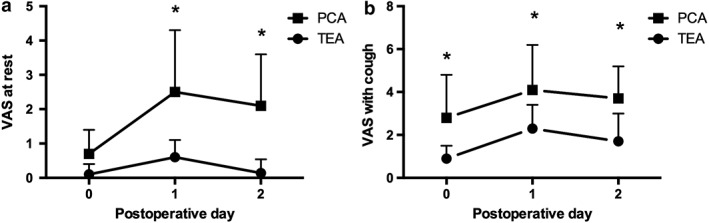
Postoperative pain scores (a) at rest and (b) during coughing assessed using a visual analogue scale (VAS) ranging from 0 to 10 on postoperative days (POD) 0–2 for TEA (dot) and PCA patients (rectangles), respectively. VAS scores at rest were significantly lower in the TEA group on PODs 1 and 2 than in the PCA group. VAS scores during coughing were significantly lower in the TEA group on PODs 0–2 than in the PCA group. *Statistical significance (P < 0.05). Data are expressed as mean ±standard deviation. PCA and TEA.
Bowel function
The duration from surgery to the first flatus was significantly shorter in the TEA group (P < 0.05) (Table 2). There was no significant difference in first passage of stool between the groups (62 in the TEA vs. 65 hours in the PCA; P = 0.145). Sixteen and 8 patients in the PCA group experienced nausea and vomiting compared to 5 and 0 patients in the TEA group, respectively (P < 0.01) (Table 2).
Table 2.
Postoperative recovery parameters and complications
| Parameters and complications | TEA group (n = 49) | PCA group (n = 49) | P |
|---|---|---|---|
| Time until flatus (hours) | 16 ± 0.7 | 26 ± 0.7 | < 0.001 |
| Time until stools (hours) | 62 ± 1.4 | 65 ± 1.2 | 0.145 |
| Time until return to full diet (hours) | 24 ± 0.4 | 24 ± 0.5 | 0.078 |
| Nausea, n (%) | 5 (13) | 16 (40) | 0.006 |
| Vomiting, n (%) | 0 | 8 (20) | 0.003 |
| Postoperative complications, n (%) | |||
| Wound abscess | 0 | 0 | NR |
| Atelectasis | 0 | 0 | NR |
| Subcutaneous emphysema, n (%) | 3 (8) | 4 (10) | 0.692 |
| Prolonged air leak, n (%) | 6 (15) | 5 (13) | 0.745 |
| Confusion, n (%) | 5 (13) | 18 (45) | 0.002 |
| Pneumonia | 0 | 0 | NR |
| Hospital stay | 5.0 (3.5–7.0) | 5.0 (4.0–8.5) | 0.94 |
Values are shown as mean ± standard error or median (interquartile range), as appropriate. NR, not related;
PCA, patient‐controlled analgesia; TEA, thoracic epidural analgesia.
Postanesthesia care unit and overall length of stay
The mean length of stay in the PACU was shorter in the TEA than in the PCA group (34 vs. 67 minutes, respectively; P = 0.027) (Fig 3). The median postoperative hospital stay was similar in both groups at 5 days (P = 0.94).
Figure 3.
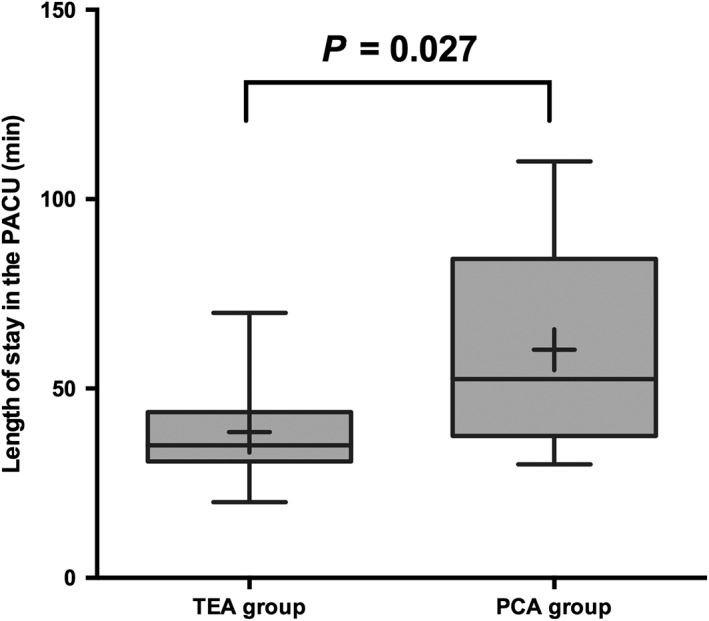
Length of stay in the postanesthesia care unit (PACU) in the thoracic epidural analgesia (TEA) and patient‐controlled analgesia (PCA) groups. Data are expressed as median (horizontal line with the box), interquartile range (upper and lower edges of the boxes), maximum and minimum (upper and lower bars), and means (black plus within the boxes).
Complications
Postoperative complications are shown in Table 2. Side effects were uncommon (0–45% frequency) and did not differ between groups, except for the incidence of confusion (5 TEA vs. 18 PCA patients; P = 0.002), which was treated by ceasing PCA infusion.
Discussion
Our results show that during elective VATS lobectomy within an enhanced rehabilitation program, TEA results in lower postoperative pain scores than PCA. In addition, earlier restoration of bowel function is achieved after TEA, as well as shortened length of stay in the PACU. Compared to the PCA group, the incidences of PCA‐related complications were lower in TEA group. The length of hospital stay was similar between the groups.
Unlike laparoscopic colorectal surgery, few prospective studies have focused on the effects of epidural analgesia on postoperative recovery after VATS. Optimal pain management, especially in the first three PODs, is the most important consideration after thoracic surgery.13, 14 Patient‐controlled epidural analgesia provided better analgesia and more rapid recovery from ileus after colon surgery than IV PCA morphine.15 Our results also show that the quality of postoperative analgesia with epidural was highly satisfactory, as demonstrated by the low VAS score at rest and during coughing in the first two days after patients underwent VATS lobectomy. This was significantly lower than the VAS with PCA IV opioids, consistent with the results of other studies.8 We also found that fewer patients in the TEA group required rescue medication after surgery than in the PCA group. This may have resulted from differences in the postoperative use of epidural analgesia, which has been shown to have opioid‐sparing effects.
Compared to PCA, TEA improved bowel function, which is regarded as playing a pivotal role for early rehabilitation after surgery. Postoperative ileus is among the common complications adversely affecting postoperative outcomes. The pathophysiology of postoperative ileus is complex and involves many factors, including surgical trauma, activation of inhibitory sympathetic reflexes, and the induction of local and systemic inflammatory mediators.16 Postoperative ileus has been identified as one of the most significant causes of patient discomfort, prolonging convalescence and length of hospital stay.17 Our results show that compared to PCA, TEA achieved a significantly shorter duration from VATS lobectomy to the first flatus and improved bowel functional recovery. Early return of bowel function in TEA has been attributed to the blockade of spinal reflexes that inhibit motility and the inhibition of sympathetic overactivity because of surgical trauma. These findings in VATS lobectomy are consistent with those of previous studies of TEA during fast‐track open colorectal surgery.18 This faster return of bowel function with TEA is likely a result of a combination of better analgesia, attenuated sympathetic tone to the gastrointestinal tract, and reduction in use of opioids for analgesia.
Multimodal analgesia is a means to improve analgesia, decrease side effects, and accelerate postoperative recovery. Both of our study groups received a potent non‐steroidal anti‐inflammatory drug (NSAID: flurbiprofen axetil) for multimodal analgesia. The addition of NSAIDs has been shown to enhance both epidural and IV PCA regimens by improving analgesia, decreasing analgesic consumption and side effects, and improving patient satisfaction.19, 20 We expected that the use of multimodal analgesia in both study groups would optimize analgesia and decrease potential differences between groups in analgesic efficacy. Nevertheless, the use of TEA still provided superior analgesia for the first two days in comparison to PCA, even with the use of multimodal analgesia.
As mentioned above, TEA plays an important role in ERAS. Although TEA can cause some adverse effects, such as nausea (13%) and confusion (13%), the incidence of postoperative complications was lower in the TEA group, compared to incidences of nausea (40%), vomiting (20%), and confusion (45%) in the PCA group. The suggested mechanism appears to be a block of the nociceptive afferent fibers and the thoracolumbar sympathetic efferent fibers with unopposed parasympathetic efferent fibers. In addition, TEA reduces the need for postoperative systemic opioids, which are known to cause adverse effects, such as confusion and nausea.
In contrast to previous studies,21, 22 we also found that TEA shortened the duration of PACU stay after VATS lobectomy. The provision of pain relief and sympathetic blockade of such a magnitude that allows patients to cough and breathe deeply can contribute to enhanced postoperative outcomes, reducing the duration of PACU stay. TEA also can be a useful adjunct in ERAS by optimizing pain relief, freedom from pain attenuating the surgical stress response, and allowing early mobilization. Although previous investigations have demonstrated a reduction in hospital stay when TEA is incorporated into the analgesic plan,23, 24 the length of stay was similar in both groups in our study. Hospital stay relies on various factors, which may modify the effect of perioperative care to a certain extent and different analgesic regimens in particular. This again supports the supposition that it is the multimodal components of enhanced recovery protocols combined that have an effect on recovery rather than one isolated factor.
Several limitations need to be addressed. First, for medical and logistic reasons, blinding was not performed because it seemed neither feasible nor realistic for this study. However, the main outcomes of the present study were pain scores and recovery outcomes. Second, the use of patient‐controlled intravenous sufentanil in addition to the PCEA in TEA group could have influenced the between‐group difference in functional recovery. Third, the surgical procedures performed were not homogeneous. The same team of surgeons performed the surgeries, yet the individual characteristics of patients and anatomical conditions necessitated some modifications of the techniques used that can be associated with slight differences in the extent of surgical injuries. Likewise, although only patients requiring lobectomies were enrolled, the distribution of the excised lobes differed; because of randomization, the differences were unavoidable.
The results of our study suggest that compared to PCA, TEA and postoperative analgesia provides significant pain relief, a faster return of bowel function, and shorter length of stay in the PACU, but does not affect overall length of stay, which is multifactorial, such as patient‐related factors, after VATS lobectomy within an enhanced rehabilitation program.
Disclosure
No authors report any conflict of interest.
Acknowledgment
The authors would like to thank Dr. Wenjie Jiao for helpful discussion. The study was supported by the Young Science Foundation of the Affiliated Hospital of Qingdao University.
References
- 1. Lassen K, Soop M, Nygren J et al Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144: 961–9. [DOI] [PubMed] [Google Scholar]
- 2. Greco M, Capretti G, Beretta L et al Enhanced recovery program in colorectal surgery: A meta‐analysis of randomized controlled trials. World J Surg 2014; 38: 1531–41. [DOI] [PubMed] [Google Scholar]
- 3. Pędziwiatr M, Wierdak M, Nowakowski M et al Cost minimization analysis of laparoscopic surgery for colorectal cancer within the enhanced recovery after surgery (ERAS) protocol: A single‐centre, case‐matched study. Wideochir Inne Tech Maloinwazyjne 2016; 11: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grass F, Slieker J, Frauche P et al Postoperative urinary retention in colorectal surgery within an enhanced recovery pathway. J Surg Res 2017; 207: 70–6. [DOI] [PubMed] [Google Scholar]
- 5. Pędziwiatr M, Pisarska M, Major P et al Laparoscopic colorectal cancer surgery combined with enhanced recovery after surgery protocol (ERAS) reduces the negative impact of sarcopenia on short‐term outcomes. Eur J Surg Oncol 2016; 42: 779–87. [DOI] [PubMed] [Google Scholar]
- 6. Taupyk Y, Cao X, Zhao Y et al Fast‐track laparoscopic surgery: A better option for treating colorectal cancer than conventional laparoscopic surgery. Oncol Lett 2015; 10: 443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Das‐Neves‐Pereira JC, Bagan P, Coimbra‐Israel AP et al Fast‐track rehabilitation for lung cancer lobectomy: A five‐year experience. Eur J Cardiothorac Surg 2009; 36: 383–91. [DOI] [PubMed] [Google Scholar]
- 8. El‐Tahan MR. Role of thoracic epidural analgesia for thoracic surgery and its perioperative effects. J Cardiothorac Vasc Anesth 2017; 31: 1417–26. [DOI] [PubMed] [Google Scholar]
- 9. Dango S, Harris S, Offner K et al Combined paravertebral and intrathecal vs thoracic epidural analgesia for post‐thoracotomy pain relief. Br J Anaesth 2013; 110: 443–9. [DOI] [PubMed] [Google Scholar]
- 10. Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: A systematic review and meta‐analysis. Interact Cardiovasc Thorac Surg 2014; 18: 626–35. [DOI] [PubMed] [Google Scholar]
- 11. Freise H, Fischer LG. Intestinal effects of thoracic epidural anesthesia. Curr Opin Anaesthesiol 2009; 22: 644–8. [DOI] [PubMed] [Google Scholar]
- 12. Kampe S, Weinreich G, Darr C et al The impact of epidural analgesia compared to systemic opioid‐based analgesia with regard to length of hospital stay and recovery of bowel function: Retrospective evaluation of 1555 patients undergoing thoracotomy. J Cardiothorac Surg 2014; 9: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osseis M, Weyrech J, Gayat E et al Epidural analgesia combined with a comprehensive physiotherapy program after cytoreductive surgery and HIPEC is associated with enhanced post‐operative recovery and reduces intensive care unit stay: A retrospective study of 124 patients. Eur J Surg Oncol 2016; 42: 1938–43. [DOI] [PubMed] [Google Scholar]
- 14. Raspé C, Flöther L, Schneider R et al Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur J Surg Oncol 2017; 43: 1013–27. [DOI] [PubMed] [Google Scholar]
- 15. Yeh CC, Jao SW, Huh BK et al Preincisional dextromethorphan combined with thoracic epidural anesthesia and analgesia improves postoperative pain and bowel function in patients undergoing colonic surgery. Anesth Analg 2005; 100: 1384–9. [DOI] [PubMed] [Google Scholar]
- 16. Holte K, Kehlet H. Postoperative ileus: A preventable event. Br J Surg 2000; 87: 1480–93. [DOI] [PubMed] [Google Scholar]
- 17. Spreng UJ, Dahl V, Hjall A et al High‐volume local infiltration analgesia combined with intravenous or local ketorolac+morphine compared with epidural analgesia after total knee arthroplasty. Br J Anaesth 2010; 105: 675–82. [DOI] [PubMed] [Google Scholar]
- 18. Kummer A, Slieker J, Grass F et al Enhanced recovery pathway for right and left colectomy: Comparison of functional recovery. World J Surg 2016; 40: 2519–27. [DOI] [PubMed] [Google Scholar]
- 19. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep 2016; 20: 62. [DOI] [PubMed] [Google Scholar]
- 20. Jouve P, Bazin JE, Petit A et al Epidural versus continuous preperitoneal analgesia during fast‐track open colorectal surgery: A randomized controlled trial. Anesthesiology 2013; 118: 622–30. [DOI] [PubMed] [Google Scholar]
- 21. Muller S, Zalunardo MP, Hubner M et al A fast‐track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology 2009; 136: 842–7. [DOI] [PubMed] [Google Scholar]
- 22. Hübner M, Blanc C, Roulin D et al Randomized clinical trial on epidural versus patient‐controlled analgesia for laparoscopic colorectal surgery within an enhanced recovery pathway. Ann Surg 2015; 261: 648–53. [DOI] [PubMed] [Google Scholar]
- 23. Nishimori M, Low JH, Zheng H et al Epidural pain relief versus systemic opioid‐based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev 2012; 7: CD005059. [DOI] [PubMed] [Google Scholar]
- 24. Pöpping DM, Elia N, Marret E et al Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: A meta‐analysis. Arch Surg 2008; 143: 990–9. [DOI] [PubMed] [Google Scholar]


