Abstract
Squamous cancer (SqCC) of the lung has a poor prognosis. With the advent of immunotherapy, prognosis has tended to improve; however, pseudoprogression poses a challenge to the management of immunotherapy. Herein, we discuss the case of a 47‐year‐old heavy smoker with advanced SqCC. The patient had recurrent disease after initial successful control of the tumor by concurrent radiochemotherapy, together with ample pleural effusion. Pleural effusion was well controlled with systematic nivolumab and intra‐thoracic recombinant endostatin; however with simultaneous deterioration of performance and tumor progression. Nivolumab was maintained with the addition of nab‐paclitaxel. The combination soon led to a partial response and rapid improvement of the patient's performance. During treatment of this case, we advocated the early control of pleural effusion as an indicator for pseudoprogression. Our experience might be helpful to identify pseudoprogression for the clinical management of immunotherapy.
Keywords: Chemotherapy, lung cancer, nivolumab, pleural effusion, pseudoprogression
Introduction
Squamous cancer (SqCC), which accounts for a quarter of new cases of non‐small cell lung cancer (NSCLC), has a notoriously poor prognosis with a five‐year survival rate of ≤ 15%, even after surgery, radiotherapy, or chemotherapy.1 Targeted therapy is far less applicable for SqCC than adenocarcinoma. With the advent of immunotherapy, prognosis has tended to improve. The United States Food and Drug Administration (FDA) approved nivolumab, a fully humanized IgG4 PD‐1 antibody, for the second‐line treatment of SqCC based on the superior survival rate over docetaxel in the CheckMate 017 trial.2 Nivolumab blocks interaction between PD‐1 and its ligands, PD‐L1 and PD‐L2, and disrupts the negative signal that regulates T‐cell activation and proliferation.3 However, pseudoprogression in immunotherapy where initial tumor growth was followed by regression poses a challenge to the management of immunotherapy.4, 5 Herein, we report a case of a patient with advanced SqCC successfully treated with nivolumab.
Case presentation
A 47‐year‐old man with a 30 pack‐year history of smoking presented with hemoptysis in September 2012. An enhanced computed tomography (CT) scan showed a 3.7 cm mass located in the lower right hilum, wrapped by the right intermediate bronchus, and accompanied by mediastinum and hilar lymph node enlargement. A bronchoscopy was performed, and a pathological diagnosis of clinical stage cT4N3M0 (stage III) SqCC was confirmed. No EGFR mutations were identified. He was prescribed with concurrent chemoradiotherapy consisting of four cycles of paclitaxel plus cisplatin chemotherapy and 66Gy/33f radiotherapy and achieved a partial response (PR).
The patient experienced recurrent hemoptysis in March 2017. He was in poor condition, with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 2. A chest CT showed a 7.3 cm mass in the lower right hilum, an enlarged 6.8 cm lymph node in the left axillary, and pleural effusion on the right side. Serum tumor marker levels, including carcinoembryonic antigen, cytokeratin 19 fragments, and neuron‐specific enolase, were high. We recommended immunotherapy with nivolumab at a dose of 3 mg/kg, once every two weeks from 3 May 2017 and thoracic perfusion treatment with recombinant endostatin. Pleural effusion was well controlled after four cycles of nivolumab and endostar, but the tumor continued to progress. The patient's condition deteriorated further, to an ECOG PS score of 3. At this time, DNA profiling was introduced, but no mutations in known driver genes (EGFR, ALK, ERBB2, BRAF, MET, RET, ROS1, and KRAS) were identified. This profiling also showed that the patient had a high tumor mutation burden. Nivolumab plus nab‐paclitaxel were administered. Two cycles of therapy led to a PR in his tumor, sharply decreased tumor markers (Fig 1), and an improved ECOG PS score of 1. Another two cycles were implemented and the lesion shrank further (Fig 2). Nivolumab therapy was maintained and the patient was regularly followed‐up. The response was stable up to January 2018.
Figure 1.
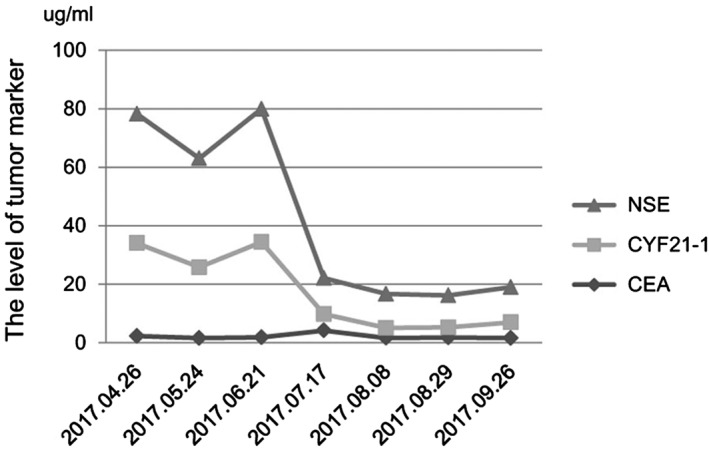
The serum tumor marker levels were stable after three cycles of nivolumab. After chemotherapy is administered, the serum tumor marker level continues to reduce.
Figure 2.
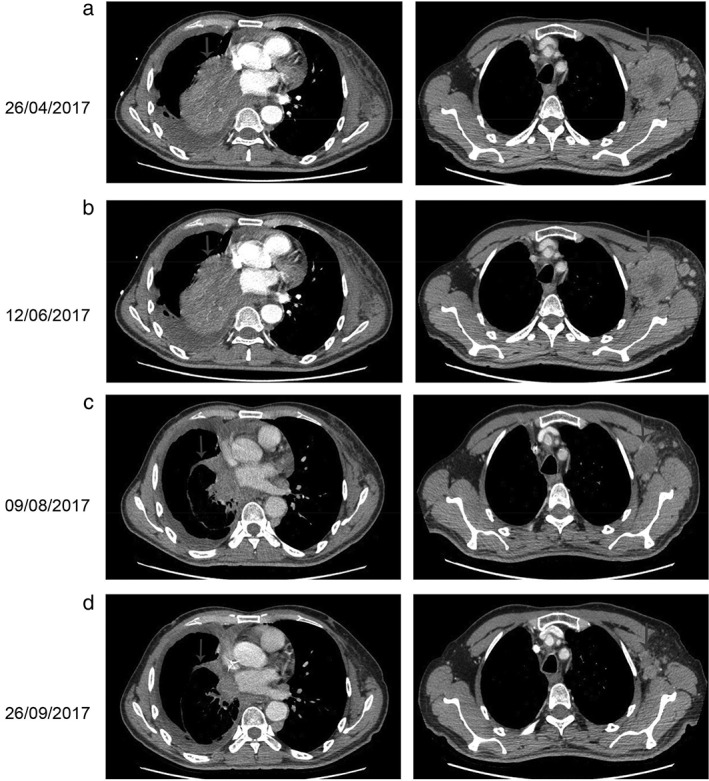
Computed tomography (CT) findings. (a) The CT scan taken on 26 April 2017 shows a lesion in the right lung and left axillary (arrows). (b) The following CT scan shows no effect after three cycles of nivolumab (arrows). (c,d) A restaging CT scan shows the lesion in the right lung and left axillary has reduced after two and four courses of nivolumab plus chemotherapy, respectively (arrows).
Discussion
Pleural effusion can be caused by a variety of malignancies and is accompanied by poor survival of approximately three months. The common treatment strategy is chemotherapy to reduce the tumor and adsorb effusion, which is rarely successful in NSCLC.6, 7 Anti‐angiogenesis was proposed in this case because of the angiogenic nature of the pleural effusion.8 Endostar inhibits angiogenesis mainly by counteracting the effects of vascular endothelial growth factor. It was approved by the Chinese Food and Drug Administration for the treatment of NSCLC and was expected to play a role in effusion control; however, its effectiveness for pleural control has not been confirmed by subsequent clinical analyses.9, 10, 11 As a consequence, endostar is rarely used as monotherapy. The long‐term control of pleural effusion in this patient was reasonably attributed to nivolumab.
Previously, docetaxel, pemetrexed, or erlotinib monotherapy was established as the standard of care in second‐line therapy, with an objective response rate (ORR) ranging from 8.2% to 9.1%.12, 13, 14 Nab‐paclitaxel, another chemotherapy agent, achieved a better but still unsatisfactory ORR of 14.5%.15 However a disappointing ORR of 0% was reported for patients with recurrent or platinum‐refractory SqCC.16 A good response was observed in our patient after combination treatment of nab‐paclitaxel and nivolumab. In this regard, the effect was achieved by nivolumab, or a possible synergy between chemotherapy and nivolumab. This conclusion is supported by the results of the phase III CheckMate057 study and others.17, 18
Immunotherapy response patterns differ from those of cytotoxic agents. Pseudoprogression in immunotherapy, where initial tumor growth is followed by regression, has been reported in 6.7–12% of melanoma patients.19 Another study reported that 13% of NSCLC patients experienced pseudoprogression during immunotherapy.20 The underlying mechanism was either continued tumor growth until a sufficient immune response occurred, or a transient immune‐cell infiltrate. Regardless, pseudoprogression in immunotherapy poses a great challenge to response evaluation using the current Response Evaluation Criteria in Solid Tumors (RECIST) or World Health Organization criteria, thus novel criteria such as immune‐related response criteria (irRC) or immune‐related RECIST (iRECIST) have been proposed.4 Pseudoprogression is established in a post hoc fashion, that is, recognition of pseudoprogression is extremely difficult at the first sign of tumor progression.
It was interesting to explore scenarios to explain the dissociation of primary tumor progression from effusion control in our case. Firstly, pleural effusion was attributed to pleural tumor dissemination, which is typically manifested as small loci (2–3 cm)21 compared to the large mass of the primary tumor, providing suitable options for immunotherapy. Secondly, pseudoprogression cannot be ruled out after primary tumor progression.4, 22 In a recent report, the larger tumor mass detected after the administration of nivolumab only contained immune cells and no tumor cells.23 Thirdly, the later reduction of the primary tumor was possibly a result of the addition of chemotherapy, which modulated the inflammation millieu.24, 25
In conclusion, we report a case of the successful treatment of advanced SqCC with nivolumab and chemotherapy. The early control of pleural effusion preceded that of the primary tumor, and is proposed as an indicator for pseudoprogression. Our experience might be helpful to identify pseudoprogression for the clinical management of immunotherapy.
Disclosure
No authors report any conflict of interest.
References
- 1. Cetin K, Ettinger DS, Hei YJ, O'Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results program. Clin Epidemiol 2011; 3: 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kazandjian D, Suzman DL, Blumenthal G et al FDA approval summary: Nivolumab for the treatment of metastatic non‐small cell lung cancer with progression on or after platinum‐based chemotherapy. Oncologist 2016; 21: 634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ozaki Y, Shindoh J, Miura Y et al Serial pseudoprogression of metastatic malignant melanoma in a patient treated with nivolumab: A case report. BMC Cancer 2017; 17 (778): 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolchok JD, Hoos A, O'Day S et al Guidelines for the evaluation of immune therapy activity in solid tumors: Immune‐related response criteria. Clin Cancer Res 2009; 15: 7412–20. [DOI] [PubMed] [Google Scholar]
- 5. Sarfaty M, Moore A, Dudnik E, Peled N. Not only for melanoma. Subcutaneous pseudoprogression in lung squamous‐cell carcinoma treated with nivolumab: A case report. Medicine (Baltimore) 2017; 96: e5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: Tumor‐host interactions unleashed. Am J Respir Crit Care Med 2012; 186: 487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marquez‐Medina D, Popat S. Closing faucets: The role of anti‐angiogenic therapies in malignant pleural diseases. Clin Transl Oncol 2016; 18: 760–8. [DOI] [PubMed] [Google Scholar]
- 8. Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep 2013; 15: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biaoxue R, Xiguang C, Hua L, Wenlong G, Shuanying Y. Thoracic perfusion of recombinant human endostatin (Endostar) combined with chemotherapeutic agents versus chemotherapeutic agents alone for treating malignant pleural effusions: A systematic evaluation and meta‐analysis. BMC Cancer 2016; 16: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma EQ, Zou LL, Yuan D. Clinical observation on the treatment of malignant pleural effusion with endostar and endostar combined with chemotherapy. Chin Med Pharm 2012; 2: 52–3. [Google Scholar]
- 11. Wen J, Ge W, Li G et al Clinical observation of pleural hyperthermic perfusion chemotherapy with endostar and lobaplatin for treatment of malignant pleural effusion. BME & Clin Med 2014; 18: 540–3. [Google Scholar]
- 12. Shepherd FA, Dancey J, Ramlau R et al Prospective randomized trial of docetaxel versus best supportive care in patients with non‐small‐cell lung cancer previously treated with platinum‐based chemotherapy. J Clin Oncol 2000; 18: 2095–103. [DOI] [PubMed] [Google Scholar]
- 13. Hanna N, Shepherd FA, Fossella FV et al Randomized phase III trial of pemetrexed versus docetaxel in patients with non‐small‐cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22: 1589–97. [DOI] [PubMed] [Google Scholar]
- 14. Shepherd FA, Rodrigues Pereira J, Ciuleanu T et al Elotinib in previously treated non‐small‐cell lung cancer. N Engl J Med 2005; 353: 123–32. [DOI] [PubMed] [Google Scholar]
- 15. Liu Z, Wei Z, Hu Y et al A phase II open‐label clinical study of comparing nab‐paclitaxel with pemetrexed as second‐line chemotherapy for patients with stage IIIB/IV non‐small‐cell lung cancer. Med Oncol 2015; 32: 316. [DOI] [PubMed] [Google Scholar]
- 16. Saxena A, Schneider BJ, Christos PJ, Audibert LF, Cagney JM, Scheff RJ. Treatment of recurrent and platinum‐refractory stage IV non‐small cell lung cancer with nanoparticle albumin‐bound paclitaxel (nab‐paclitaxel) as a single agent. Med Oncol 2016; 33: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rizvi NA, Hellmann MD, Brahmer JR et al Nivolumab in combination with platinum‐based doublet chemotherapy for first‐line treatment of advanced non‐small‐cell lung cancer. J Clin Oncol 2016; 34: 2969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiou VL, Burotto M. Pseudoprogression and immune‐related response in solid tumors. J Clin Oncol 2015; 33: 3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tazdait M, Mezquita L, Lahmar J et al Patterns of responses in metastatic NSCLC during PD‐1 or PDL‐1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018; 88: 38–47. [DOI] [PubMed] [Google Scholar]
- 21. Shen Y, Zhong M, Jiang W, Fan H, Wang H, Wang Q. Video‐assisted radiofrequency ablation for pleural disseminated non‐small cell lung cancer. BMC Surg 2013; 13: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodi FS, Hwu WJ, Kefford R et al Evaluation of immune‐related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016; 34: 1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forde PM, Chaft JE, Smith KN et al Neoadjuvant PD‐1 blockade in resectable lung cancer. N Engl J Med 2018; 378: 1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: Immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11: 215–33. [DOI] [PubMed] [Google Scholar]
- 25. Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature 2017; 541: 321–30. [DOI] [PubMed] [Google Scholar]


