Abstract
The under-reporting of pain and atypical manifestations of painful syndromes within the elderly population have been well-documented, however, the specific relationship between pain and aging remains ambiguous. Previous studies have reported degenerative changes in primary afferents with aging. In this study, we questioned whether there is any change in the density of primary afferent endings within the epidermis of aged animals. Rats were categorically assessed in four age groups, each representing a key developmental stage across their life span: juvenile (2 months); adult (7 months); aged (18 months); and senescent (24–26 months). The plantar hind paw skin was removed, post-fixed, cut, and immunostained for protein gene product 9.5 and type IV collagen. Rats in the adult aged groups had significantly increased epidermal nerve densities and total lengths of immunoreactive nerve fibers, compared to both juvenile and senescent rats. However, the paw withdrawal thresholds to punctate mechanical stimulation progressively increased with age, and did not exhibit a clear relationship with epidermal innervation. We conclude a non-linear, inverted-U shaped relationship between rat plantar epidermal nerve density with aging, which does not correlate with mechanically-induced paw withdrawal behaviors.
Keywords: Aging, PGP 9.5, rat, collagen, Fisher-344, epidermal nerve
INTRODUCTION
There is a growing concern in the international medical community about inadequacies in pain assessment and management of elderly people. A retrospective study of 231 patients found that 66% of elderly patients received analgesia compared to 80% of their younger counterparts 22. Under-reporting of pain and atypical manifestation of painful syndromes in the elderly population are well documented in the previous literature 7, however, the specific relationship between pain and aging remains to be inconclusive 4, 14.
Evidence suggests that acute nociception, a key mechanism utilized by our body to warn of impending tissue damage, is altered with age 2. Despite significant disagreement within literature, most studies reported either an increased pain threshold with old age or an absence of an aging effect 16, 35. There are considerable differences in methods, experimental design, stimulus parameters, the age of old subjects, and animal strains (F344 vs Sprague-Dawley), which make it difficult to compare results and form a conclusion 14. However, studies those investigated structural and morphologic changes in the nervous system, consistently observed significant degeneration in both central and peripheral nervous system 12, 57. Aged primary afferents (in humans or rodents) show wallerian degeneration 43, decreased neurotransmitter content 10, 26, and reduced expression of transduction proteins 58. The morphologic study of the myelinated nerve fibers showed a decrease in size, circularity, myelin thickness in the aged animals 57.
The epidermal nerve fibers, which are endings of Aδ and C-fibers, have also been examined in the previous literature, however, results are inconsistent in that they showed age-related decline 9, increase 6, and no changes 38. This seems to be due to site-specific changes by aging 6 since aging decreases nerve density in the facial skin, but has no influence on abdominal skin, and an age-associated increase of nerve density was observed in mammary skin 6. To this end, the age-specific changes in epidermal nerve fiber density and their relationships with nociception have yet to be completely understood.
A major function of the epidermal nerve terminals is to convert external stimuli into electrical signals. Therefore, they are important for acute nociception 11, 51. A decrease of epidermal nerve density is paralleled by decreased sensitivity to noxious heat and mechanical stimuli, as well as decreased tactile and cold sensations 27, 32, 33, 41, 45, 48, 52, 55. In this study, we utilized an assay developed by Kennedy and colleagues 24 for human skin. The methods involve labeling and quantification of epidermal nerve densities using double immunohistochemistry with the pan-neuronal marker, protein gene product (PGP) 9.5, and type IV collagen to visualize nerve fibers and dermo-epidermal boundary, respectively. We used confocal laser scanning microscopy to capture a series of 1-μm optical sections to construct a three-dimensional image (https://youtu.be/IzBwCz-Eu3Q), which was quantified using NIS elements software. We used four age groups of rats, each representing a developmental phase across the life span, which allowed us to examine changes in epidermal innervation and the critical periods when changes began to occur.
2. METHODS
2.1. Animals
Twelve male F344 rats (2, 7, 18 and 24–26 months old (mo) obtained from the National Institute of Aging were used. In this study, 2 months old (p60) was selected for representation of young adolescent age. Male rats develops sexual maturity at approximately at around P45-4834, which marks the beginning of their adolescence. It should be noted that the definition of young adolescent animals vary widely across the studies as they were labeled for ages P21-22 to P9239. The 7 months and 18 months age groups are selected for representation of early adult and middle age groups. Based on previous studies, these two age groups may be equivalent to human age of 20 years and 45 years, respectedly50. For senescent age, we chose 24–26 months old rats. These rats correlate with humans ranging from 60–70 years of age50, and have senescent changes of biomarkers47.
Two or three rats were housed together in each 43 × 21.5 × 25.5-cm cage and kept on a 12-hr light/dark cycle. Food and water were available ad libitum. All rats were first tested for pain behaviors (see below) and then used for immunohistochemical investigations. These studies adhered to the proposals of the Committee for Research and Ethical Issues of the IASP 59 and were approved by the Seton Hall University Animal Care and Use Committee.
2.2. Withdrawal responses to mechanical stimuli
Rats were placed on metal grid floor within Plexiglas (12 × 20 × 17-cm) compartments and allowed to acclimate for 30 minutes prior to testing. Withdrawal responses were obtained using an electronic von Frey esthesiometer (IITC Life Science, Woodland Hills, CA), which consists of a handheld force transducer with polypropylene tips. Force was applied using each of these tips in an ascending order of stiffness to the mid-plantar surface (avoiding the less sensitive footpads) of the right hind paw. Each stimulus was applied for approximately 2–3 seconds, with a 5-minute interval between each subsequent application. Three trials were performed to obtain an average withdrawal threshold force for paw withdrawal. The 2nd and 3rd trials each began with the application of a tip two grades below the tip that evoked a withdrawal response on the first trial. The results were expressed as the mean threshold (in grams) that evoked a withdrawal threshold (PWT).
2.3. Immunohistochemical analyses of plantar hind paw skin
Tissue processing
Following behavioral testing, blinded immunohistochemical analyses of plantar skin samples (same tissue evaluated in behavioral studies, Figure 1) from the hind paws to examine interactions between age and ENF length and density (number of ENFs per cubic mm of epidermis). Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused with 0.1 M phosphate-buffered saline, followed by Zamboni’s solution (2% paraformaldehyde, 0.2% picric acid in PBS, pH 7.4). Plantar skin samples were removed from both hind paws and post-fixed in the same fixative for 1-hr, cryoprotected in 30% sucrose in 0.1 M PBS (pH 7.4) overnight, and stored at 4°C for batch processing of samples. The stored tissue samples were alphanumerically labeled to blind the investigator who performed sectioning, immunohistochemistry, and analysis of the images.
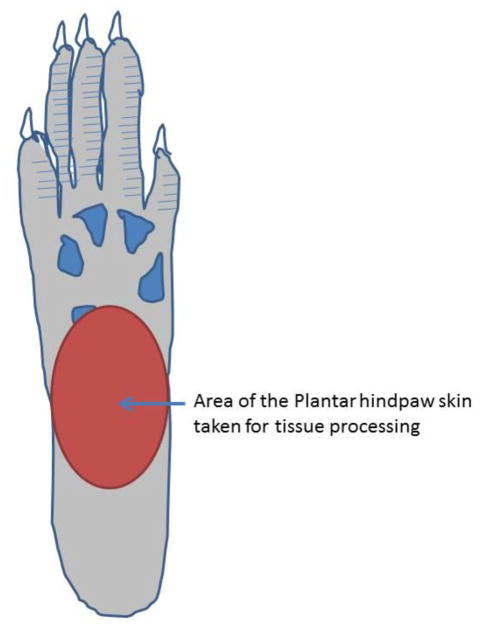
Figure 1.
Area of mid-plantar glabrous surface of rat hind paw used for von Frey hair stimulation and tissue collection for immunohistochemistry experiments.
For sectioning, tissues were immersed in Cryo-gel OCT compound (Electron Microscopy Sciences, Hatfield, PA, USA) and cut into 20-μm thick sections. The sections from all groups were processed simultaneously, using the same antisera solutions and buffers that were prepared for a single batch of experiments. Incubation and washing times were consistent between groups. Batch processing reduces the possibility of differences in solutions or incubation times creating differences between age groups.
Immunohistochemistry protocol
The cut sections were washed free-floating in a washing buffer containing TRIS-buffered saline 0.1 M, pH 7.4, 0.8% NaCl, and 0.8% Triton-X-100, and were incubated for one hour with a blocking buffer consisting of washing buffer, 10% normal donkey serum, and 0.001% sodium azide. The sections were then incubated in a mixture of two primary antisera at 4°C for 36 hours. The polyclonal rabbit antisera to protein gene product (PGP) 9.5 (Ultraclone, Isle of Wight, UK) at 1:1000 dilution and polyclonal goat antisera to type IV collagen (Millipore, Billerica, MA, USA) at 1:500 dilution were used. The sections were washed three times with washing buffer, and then incubated with Cy-2-conjugated affinity-pure Donkey anti-Rabbit antibody (Jackson ImmunoResearch, Grove, PA, USA; 1:200) and Cy-3-conjugated affinity-pure donkey anti-goat antibody (Millipore, Billerica, MA, USA; 1: 1000) for 2 hours at room temperature. After several rinses in the washing buffer, any dehydration or cleansing sections were mounted onto slides with DPX (Fluka, Buchs, Switzerland). Fluorescent sections were viewed with a Nikon Eclipse E800 microscope with appropriate filters. The selected sections were imaged using a Zeiss LSM 510 laser scanning confocal microscope equipped with a 25x water immersion objective (numerical aperture, 0.8 mm). Digitized images were collected in successive frames of 1-μm serial optical sections (Z-series) throughout the thickness of the sections and projected into a single image. Some sections were processed without the primary antibody to serve as controls.
2.4. Quantification of ENFs
The method of counting ENFs has been described previously25, 30. Epidermal nerves were counted as they crossed the basement membrane of the epidermis. The immunostaining of type IV collagen was used to localize the dermo-epidermal boundary. Nerves that branched after crossing the basement membrane were counted as single fibers, whereas nerves that split prior to crossing the basement membrane were counted as two fibers. Nerve fibers that approached, but did not cross the basement membrane were not counted. In addition, nerve fragments in the epidermis that had no visible connection to the basement membrane were not included in density measurements.
Previous studies have evinced epidermal nerve fiber length (ENFL) to be an important measure for determining the severity of diabetic neuropathy48. For ENFL measurements, all images were exported to NIS elements software (Nikon, Melville, NY). As the exported images were uncalibrated; each image was individually calibrated using a reference scale (20μm bar) for conversion of pixel data into micrometer values: 0.62μm equated to 100 pixels. ENFL was measured by drawing a polygonal line (poly line) along the length of each identified epidermal nerve fiber and fragment. All polyline measurements (in μm) were exported to Microsoft Excel for further analysis. Other PGP 9.5 positive structures, such as dermal cells, were excluded from counting on the basis of morphological differences. The images were examined by three independent observers blinded to their sources. The density and length of ENFs were standardized for section thickness and expressed as numbers per cubic millimeter of epidermis (0.6-mm section length X 0.02-mm thickness).
3. RESULTS
3.1. Characteristics of epidermal innervation in the rat plantar skin
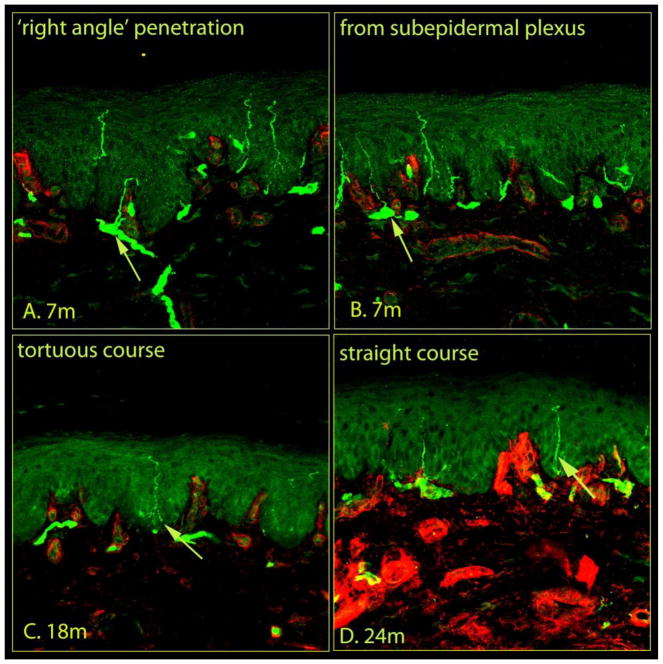
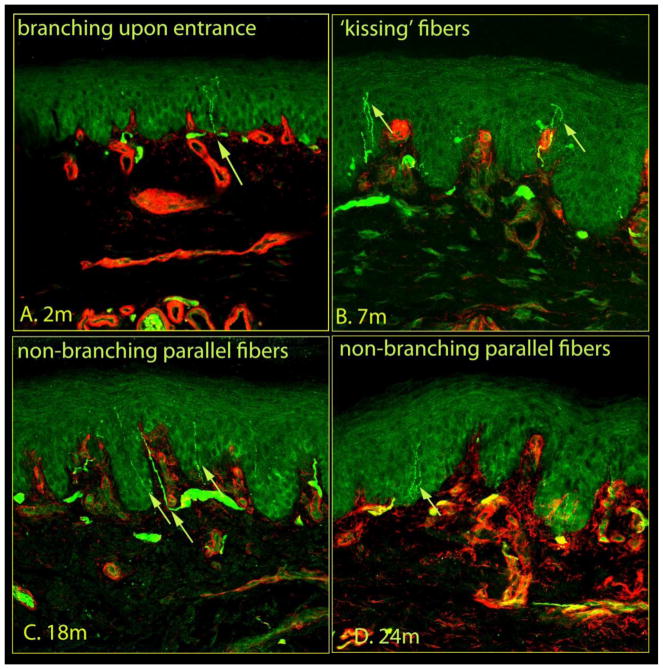
Confocal images of immunostained skin samples taken from glabrous hind paw skin (Fig. 1) are shown in Figures 2 and 3. PGP 9.5-labeled nerve-like structures are visible within both the dermis and epidermis, with type IV collagen labeling that distinguishes the dermo-epidermal junction. Dermal nerves are situated along the basement membrane and send fine branches through the membrane and into the epidermis (Fig. 2a, S1–43). Epidermal nerves also arise from the sub-epidermal neural plexus (Fig. 2b, S53), and penetrate the basement membrane. Upon entry into the epidermis, axons follow either a tortuous (Fig 2c, S6–83), or straight course (Fig. 2d, S33, S93). Occasionally, these intraepidermal fibers give rise to branches (Fig 3a, S43), or cross adjacent fibers to form a ‘kissing fiber’ configuration (Fig 3b, S10–113). Within the epidermis, fibers either extend up to the superficial layers without branching (Fig, 3c, S93), or run in parallel to the skin surface (Fig 3d).
Figure 2.
Morphological features of epidermal nerves within rat plantar skin. Confocal images of glabrous skin biopsies taken from control rats of different age groups with nerves (green) and the basement membrane and dermal structures (red) indicated. The plantar skin samples were removed, post-fixed in the same fixative, cryoprotected in 30% sucrose overnight, and stored at 4°C for batch processing. The stored tissue samples were coded to blind the investigator who performed sectioning, immunohistochemistry, and analysis of the images. Tissues were immersed in Cryo-gel OCT compound and cut into sections 20-μm thick. The sections from all groups were processed simultaneously using standard protocols (See Material and Methods). A series of 1-μm optical sections of the immunostained sections were captured in successive frames with a confocal laser scanning microscope to construct a 3D image (see video, https://youtu.be/IzBwCz-Eu3Q), and stacked into a single image. A, the dermal nerves run along the basement membrane into the dermo-epidermal junction and send fine branches into the epidermis at right angles. B, nerve fibers enter the epidermis from the subepidermal neural plexus located at the dermo-epidermal junction. The nerve endings within the epidermis followed either a tortuous (C) or straight (D) course. Each image captured 634 μm of skin samples.
Figure 3.
The morphological features of epidermal nerves within rat plantar skin, in addition to Figure 2. Within the epidermis, occasionally, a nerve fiber branched into two fibers (A) or crossed with another fibers (B) giving a ‘kissing fiber’ configuration. The epidermal nerve fibers were also seen to run in parallel with other fibers following a non-branching (C) and straight (D) course. Each image captured 634 μm of skin samples.
3.2. Age differences in epidermal innervation
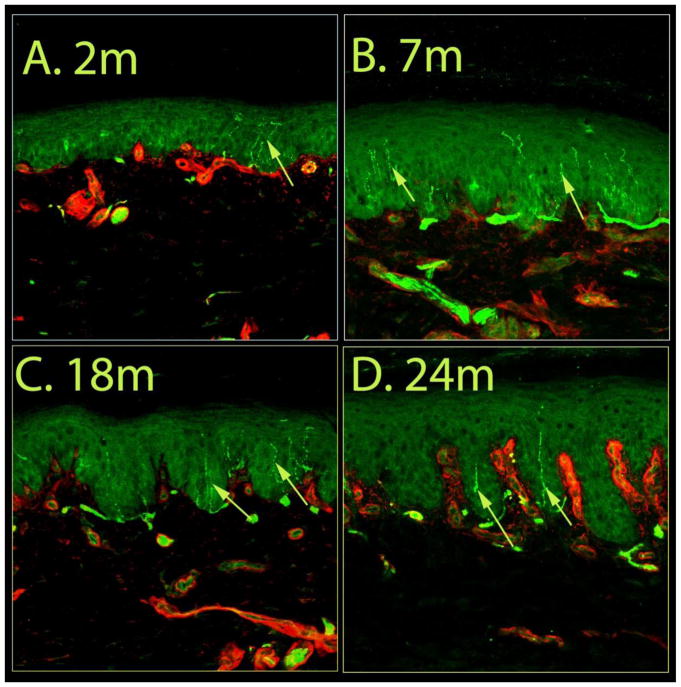
Representative confocal images of immunostained skin tissue from each of the four age groups are presented in Figure 4. The most pronounced reduction in epidermal innervation was observed in samples from the >24 mo rats, as they lacked intact fibers within the epidermis, although dermal fibers were present (Fig. 4d, S12–133). The median density of epidermal nerves in the samples from 2-mo animals was 236/mm2 of epidermis (Fig. 5a), and was increased in samples from 7-mo animals (315/mm2 of epidermis; P<0.01, Krushkal-Wallis test). The median ENF density from the 18-mo group was similar to that of the 2-mo group, and not significantly different from those in the 7-mo group. However, the median nerve ENF density of ‘>24 mo’ animals (157/mm2 of epidermis) was significantly lower compared to all other age groups (Fig. 4d, 5a; P<0.05, Krushkal-Walis followed by Dunn’s test).
Figure 4.
Effects of age on epidermal nerve fiber density. Confocal images of glabrous skin biopsies taken from 2, 7, 18 and 24–26 months old control rats. The number of epidermal nerves in the image from the 7 months old animal is relatively higher than that from the other groups. The skin samples from the 24–26 month old rats displayed the most pronounced reduction of in epidermal innervation. Each image captured 634 μm of skin samples.
Figure 5.
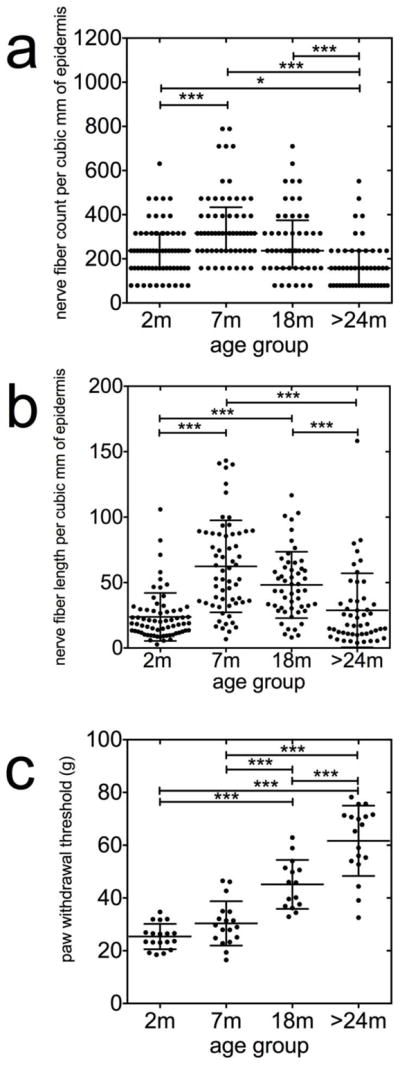
Nonlinear inverted-U shaped relationship between epidermal nerve fiber density and aging. Epidermal nerves were counted as they crossed the basement membrane of the epidermis. For details, see Methods. For measurements of epidermal nerve fiber lengths, images were exported to NIS elements software calibrated to convert pixels to micrometer values, and a polyline was drawn along the length of each identified epidermal nerve fiber and fragment. The density and length of nerve fibers within the epidermis were standardized for section thickness and expressed as numbers per cubic millimeter of epidermis. The median density (a) and total length (b) of the epidermal nerves are significantly increased in samples from the 7-months old (mo) animals and decreased in samples from the >24-mo animals (P<0.05, Krushkal-Walis followed by Dunn’s test). (c) The threshold force required to evoke paw withdrawal threshold (PWT) behaviors were significantly higher in the senescent animals (24–26 mo) compared with the 2-mo (n=18), 7-mo (n=18) and 18-mo (n=15) animals (P<0.0001, ANOVA followed by Newman-Keuls test).
We next assessed and compared ENF length between the different age groups. The total length of immunoreactive ENFs in the 2-mo samples was 24±18 mm/mm2 epidermis, and was increased in the 7-mo (62±35 mm/mm2) and 18-mo (48±25 mm/mm2) age groups. Nerve fiber length was significantly decreased in the >24-mo animals compared with samples from the 7-mo and 18-mo rats (Fig. 4, 5b; P<0.001, Krushkal-Walis test followed by Dunn’s test).
3.3. Age-related differences in PWT
The threshold force required to evoke PWT behaviors was significantly higher in the senescent animals (24–26 mo) compared with the 2-mo (n=18), 7-mo (n=18) and 18-mo (n=15) animals (Fig. 5c; P<0.0001, ANOVA followed by Newman-Keuls test).
DISCUSSION
In this study, we examined changes in density and length of ENFs at various ages across the lifespan: youth, middle aged, aged, and senescence. Using double immunolabeling and confocal laser scanning microscopy, we found that middle-aged rats (7-mo) have significantly increased epidermal nerve densities compared to youth (2-mo) and senescent rats (24–26 mo). The inverted U-shaped pattern of epidermal innervation over the life span of rats does not, however, correlate with mechanically-induced PWT, which progressively increased with age.
4.1. Nonlinear Inverted-U shaped relationship between epidermal nerve fiber density and aging
The most striking observation of this study was the inverted-U shaped relationship of epidermal innervation with age. There are few studies that investigated effects of aging on epidermal innervation in rodents 32. It is difficult to compare our observations in rats with the many previous studies in humans 6, 9, 17, 29, 56 because of potential species differences, and differences in correlating age between the different species. Moreover, the present study examined plantar (glabrous) skin (Fig. 1), which has not been extensively examined in most of the previous studies. Nevertheless, most human studies 6, 9, 17, 29, 56, with a few exceptions 31, 38, are in agreement with the results of the present study that advanced age is associated with a decreased density of ENFs.
Studies have showed epidermal innervation to gradually decrease from the rostral to the caudal area, perhaps due to the distance of the anatomical sites from the spinal nerve origin in the trunk 31. Supporting this hypothesis, it has been shown that the longest myelinated fibers are more prone to degeneration with age, and that this effect is most apparent at the most distal nerve endings, presumably as a consequence of failing axonal transport mechanisms 6, 15. It is therefore possible that the effects of aging are more robust in the plantar skin than in other areas, and therefore displayed exaggerated age-related effects on epidermal innervation, as is the case for length-dependent neuropathy.
Interestingly, our result that Inverted-U shaped relationship exists between epidermal nerve fiber density and aging, is consistent with the conclusions of Finkel and colleagues 13. In their studies, authors stimulated mouse plantar skin with electrical stimuli to determine vocalization thresholds. Electrical stimulation at 2,000, 250, and 5 Hz, which respectively stimulate Aβ, Aδ, and C sensory nerve fibers, revealed that the vocalization threshold changes across a mouse’s life span follow a U-shaped pattern 13. Specifically, middle-aged mice had lower current vocalization thresholds compared with young and senescent mice 13. Moreover, anatomical evidence from both humans and rodents show that myelin thickness gradually increases from birth to middle age, and decreases from old age to senescence following a typical U-pattern8. In sural nerve biopsies from human subjects, axon diameter and myelin thickness are lowest at birth, and increase with age until the second decade, at which point they remain almost completely static until the fifth decade21. Degenerative changes due to aging begin at approximately 60 years of age, with reductions in the densities of myelinated fibers, thinning myelin sheaths, myelin sheath irregularities and clusters of regenerated fibers 21. Similarly, myelin thickness of peripheral nerves in mice increase from birth until 12 months of age, and decrease between 12 and 22 months of age 8.
It is possible that the defects in the pain mechanisms including decreased epidermal innervation results decreased occurrence of pain in the aged individuals. The persistent pain problems including surgical procedures have been observed to present with unusually painless manifestations in elderly population. Approximately 40% of patients over 65 years report little or no pain associated with peritonitis, intestinal obstruction, pneumothorax36, and peptic ulcer disease19. Several studies have suggested that elderly adults report less postoperative pain than younger patients experiencing the same surgical procedures 5, 46.
4.2. Relationship between epidermal innervation and acute mechanical PWT
Previous studies have shown the ENF density is related to pain detection thresholds under physio-pathological conditions. Increased epidermal innervation has been described within the hypersensitive skin areas following exposure to ultraviolet light, or during psoralen UVA therapy 49. Moreover, it has been shown that patients with painful vulvodynia have an increase in vulval epithelial innervation 54. Conversely, decreased epidermal innervation is observed in peripheral neuropathy patients with hyposensitive skin areas 24, 25, 27, 30, 48. A decrease in ENF density following topical applications or intradermal injection of capsaicin41, 51, or application of shock-waves 44 were paralleled by decreased pain sensitivity on the exposed skin.
In this study, there was no clear relationship between the density of ENFs and withdrawal response thresholds for mechanical stimuli. There was a marked reduction in ENFs in the senescent animals, which correlated with an increase in PWT. However, while ENF density was highest in the adult (7 mo) animals, the average PWT of this group was higher than in young (2 mo) animals. This lack of correlation raises a question whether innocuous punctate stimuli can excite ENFs, which are unmyelinated and generally responsive to noxious stimulation 20. Studies showed that following capsaicin infiltration in the rat skin, which has been shown to deplete most ENFs in human skin 41, there is minimal or no effect (unpublished observation) on PWT by von Frey hair stimulation 18. Likely, von Frey hairs may excite thick myelinated Aβ-fibers and thinly myelinated Aδ-fibers that located in the dermis to evoke PWT.
4.3. Alteration of PWT in senescent rats
In this study, there was a gradual increase PWT with aging. This observation is comparable with earlier studies 1 who also used F344 rats to show decreased sensitivity to pain with age using the paw pressure test. These results, however, are in disagreement with studies that used Lou/C/Jall rats (4–29 months old) to show decreased von Frey thresholds in aged subjects 23. In those studies, the sensitivity to non-noxious stimulation with von Frey filaments was much higher in Lou rats (threshold of 254±0.9 g) than in Sprague- Dawley (SD) rats (25.0±2.5 g). Similarly, Taguchi and colleagues did not find any differences between in PWT using von Frey monofilaments in SD rats of various ages that received calorie-restricted diets 53. On the other hand, graded intraesophageal balloon distension show an age-related decrease in human visceral pain threshold28. Studies in mice also display disparate results. Vocalization thresholds evoked by electrical stimulation were higher in older (103 weeks old) mice compared with middle-aged (44, 64, and 84 weeks old) mice. However, in another study, PWTs using von Frey monofilaments of young and older mice were shown to be similar 58.
4.4. Conclusion
The study showed an objective method of detecting ENF density in rat skin. The method could be used in future studies for detection of experimentally induced small-fiber neuropathy in animal models. The decreased epidermal innervation in hind paw plantar skin of senescent rats may contribute to age related decreased nociception reported in previous studies 2, 15. However, there are other mechanisms involved including facilitation of central inhibitory pathways 53, loss of temporal inhibition40, and cognitive inhibition 37. Interestingly, decreased ENF has also been shown to be related to painful neuropathic pain 42. While the nonlinear inverted-U shaped relationship between aging and the ENF density is in agreement with the behavioral findings of a previous study 13, the possible physiological significance is unclear 48. Overall, the paper raises interesting questions about the relationship of innocuous punctate stimuli to the density and excitation of unmyelinated ENFs and of myelinated dermal nerves. It should be noted that we report measurements of ENFs, but not of dermal nerves to aging. The authors infer that careful future studies are warranted to correlate innocuous punctate stimuli to both the density and morphology of the ENFs, but also to the myelinated nerves in the dermis.
PERSPECTIVE.
This article presents age-related decreased epidermal innervation in rat hind paw skin, which partly explains mechanisms underlying decreased pain sensitivity in aged subjects. The article may help clinicians to understand that any compromise of pain-sensing pathway can lead to under-reporting of pain, inadequate analgesia, and slower recovery from a painful condition.
Highlights.
Laser-scanning microscopy is used for quantification of rat epidermal nerves
Advanced age is associated with decreased density of epidermal nerves
Middle-aged rats have increased epidermal nerves.
An inverted-U shaped relationship between aging and epidermal nerve density
Epidermal nerve density is not correlated with paw withdrawal thresholds
Acknowledgments
This work was performed in and supported by the NJ Neuroscience Institute and Seton Hall University School of Graduate Medical Education, JFK Medical Center, Edison, NJ, USA. The authors declare no conflict of interest for this study. The authors thank Dr. Jensen and four anonymous reviewers of Journal of Pain for their comments, which immensely improved our manuscript. This work is also supported in part by the American Federation for Aging Research and the National Institutes of Health grants AG030352 and AG030352-02S2 to RKB.
Footnotes
Name: Sankaranarayanan Kaliappan MD.
Contribution: This author conducted part of the experiments.
Attestation: Sankaranarayanan Kaliappan approved the final manuscript.
Name: Donald A. Simone, PhD
Contribution: This author helped to prepare the manuscript.
Attestation: Donald A. Simone approved the final manuscript.
Name: Ratan K. Banik, MD, PhD.
Contribution: This author helped to design, conduct part of the experiments, and prepare the manuscript.
Attestation: Ratan K. Banik approved the final manuscript.
Disclosures
This work was performed in and supported by the NJ Neuroscience Institute and Seton Hall University School of Graduate Medical Education, JFK Medical Center, Edison, NJ, USA. The authors declare no conflict of interest for this study. The authors thank Dr. Jensen and four anonymous reviewers of Journal of Pain for their comments, which immensely improved our manuscript. This work is also supported in part by the American Federation for Aging Research and the National Institutes of Health grants AG030352 and AG030352-02S2 to RKB.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akunne HC, Soliman KF. Serotonin modulation of pain responsiveness in the aged rat. Pharmacology, biochemistry, and behavior. 1994;48:411–416. doi: 10.1016/0091-3057(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 2.Banik RK. Aging: blessing or danger for individuals with painful conditions. Pain. 2007;132:337–338. doi: 10.1016/j.pain.2007.08.026. author reply 338. [DOI] [PubMed] [Google Scholar]
- 3.Banik RK. [Accessed 2/27/18];2018 http://supplementaryfigure.blogspot.com/2018/02/supplementary-figure-1-4.html.
- 4.Beckman M. The burden of pain on the shoulders of aging. Sci Aging Knowledge Environ. 2002;2002:oa1. doi: 10.1126/sageke.2002.50.oa1. [DOI] [PubMed] [Google Scholar]
- 5.Bellville JW, Forrest WH, Jr, Miller E, Brown BW., Jr Influence of age on pain relief from analgesics. A study of postoperative patients. JAMA. 1971;217:1835–1841. [PubMed] [Google Scholar]
- 6.Besne I, Descombes C, Breton L. Effect of age and anatomical site on density of sensory innervation in human epidermis. Arch Dermatol. 2002;138:1445–1450. doi: 10.1001/archderm.138.11.1445. [DOI] [PubMed] [Google Scholar]
- 7.Cavalieri TA. Management of pain in older adults. The Journal of the American Osteopathic Association. 2005;105:S12–17. [PubMed] [Google Scholar]
- 8.Ceballos D, Cuadras J, Verdu E, Navarro X. Morphometric and ultrastructural changes with ageing in mouse peripheral nerve. J Anat. 1999;195(Pt 4):563–576. doi: 10.1046/j.1469-7580.1999.19540563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang YC, Lin WM, Hsieh ST. Effects of aging on human skin innervation. Neuroreport. 2004;15:149–153. doi: 10.1097/00001756-200401190-00029. [DOI] [PubMed] [Google Scholar]
- 10.Doutova EA, Moss NG. Age-related changes in calcitonin gene-related peptide and substance P in renal afferent nerve soma in the rat. Association with afferent renal nerve activity. Brain research. 1996;97:260–268. doi: 10.1016/s0165-3806(96)00157-5. [DOI] [PubMed] [Google Scholar]
- 11.Dux M, Sann H, Schemann M, Jancso G. Changes in fibre populations of the rat hairy skin following selective chemodenervation by capsaicin. Cell and tissue research. 1999;296:471–477. doi: 10.1007/s004410051307. [DOI] [PubMed] [Google Scholar]
- 12.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 13.Finkel JC, Besch VG, Hergen A, Kakareka J, Pohida T, Melzer JM, Koziol D, Wesley R, Quezado ZM. Effects of aging on current vocalization threshold in mice measured by a novel nociception assay. Anesthesiology. 2006;105:360–369. doi: 10.1097/00000542-200608000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagliese L. Pain and aging: the emergence of a new subfield of pain research. J Pain. 2009;10:343–353. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227–239. doi: 10.1097/00002508-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med. 2001;17:433–456. v–vi. doi: 10.1016/s0749-0690(05)70079-3. [DOI] [PubMed] [Google Scholar]
- 17.Goransson LG, Mellgren SI, Lindal S, Omdal R. The effect of age and gender on epidermal nerve fiber density. Neurology. 2004;62:774–777. doi: 10.1212/01.wnl.0000113732.41127.8f. [DOI] [PubMed] [Google Scholar]
- 18.Hamalainen MM, Subieta A, Arpey C, Brennan TJ. Differential effect of capsaicin treatment on pain-related behaviors after plantar incision. J Pain. 2009;10:637–645. doi: 10.1016/j.jpain.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilton D, Iman N, Burke GJ, Moore A, O’Mara G, Signorini D, Lyons D, Banerjee AK, Clinch D. Absence of abdominal pain in older persons with endoscopic ulcers: a prospective study. Am J Gastroenterol. 2001;96:380–384. doi: 10.1111/j.1572-0241.2001.03455.x. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh ST, Choi S, Lin WM, Chang YC, McArthur JC, Griffin JW. Epidermal denervation and its effects on keratinocytes and Langerhans cells. Journal of neurocytology. 1996;25:513–524. doi: 10.1007/BF02284819. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108(Pt 4):897–924. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- 22.Jones JS, Johnson K, McNinch M. Age as a risk factor for inadequate emergency department analgesia. The American journal of emergency medicine. 1996;14:157–160. doi: 10.1016/S0735-6757(96)90123-0. [DOI] [PubMed] [Google Scholar]
- 23.Jourdan D, Boghossian S, Alloui A, Veyrat-Durebex C, Coudore MA, Eschalier A, Alliot J. Age-related changes in nociception and effect of morphine in the Lou rat. Eur J Pain. 2000;4:291–300. doi: 10.1053/eujp.2000.0188. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy WR, Wendelschafer-Crabb G. Utility of skin biopsy in diabetic neuropathy. Seminars in neurology. 1996;16:163–171. doi: 10.1055/s-2008-1040972. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- 26.Khalil Z, Ralevic V, Bassirat M, Dusting GJ, Helme RD. Effects of ageing on sensory nerve function in rat skin. Brain Res. 1994;641:265–272. doi: 10.1016/0006-8993(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 27.Koskinen M, Hietaharju A, Kylaniemi M, Peltola J, Rantala I, Udd B, Haapasalo H. A quantitative method for the assessment of intraepidermal nerve fibers in small-fiber neuropathy. Journal of neurology. 2005;252:789–794. doi: 10.1007/s00415-005-0743-x. [DOI] [PubMed] [Google Scholar]
- 28.Lasch H, Castell DO, Castell JA. Evidence for diminished visceral pain with aging: studies using graded intraesophageal balloon distension. Am J Physiol. 1997;272:G1–3. doi: 10.1152/ajpgi.1997.272.1.G1. [DOI] [PubMed] [Google Scholar]
- 29.Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh ST, Mellgren SI, Umapathi T, Ziegler D, Faber CG, Merkies IS. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 15:202–207. doi: 10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- 30.Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–758. doi: 10.1111/j.1468-1331.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 31.Lauria G, Holland N, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Epidermal innervation: changes with aging, topographic location, and in sensory neuropathy. J Neurol Sci. 1999;164:172–178. doi: 10.1016/s0022-510x(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 32.Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. J Peripher Nerv Syst. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- 33.Lauria G, McArthur JC, Hauer PE, Griffin JW, Cornblath DR. Neuropathological alterations in diabetic truncal neuropathy: evaluation by skin biopsy. Journal of neurology, neurosurgery, and psychiatry. 1998;65:762–766. doi: 10.1136/jnnp.65.5.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis EM, Barnett JF, Jr, Freshwater L, Hoberman AM, Christian MS. Sexual maturation data for Crl Sprague-Dawley rats: criteria and confounding factors. Drug Chem Toxicol. 2002;25:437–458. doi: 10.1081/dct-120014794. [DOI] [PubMed] [Google Scholar]
- 35.Lin YH, Hsieh SC, Chao CC, Chang YC, Hsieh ST. Influence of aging on thermal and vibratory thresholds of quantitative sensory testing. J Peripher Nerv Syst. 2005;10:269–281. doi: 10.1111/j.1085-9489.2005.10305.x. [DOI] [PubMed] [Google Scholar]
- 36.Liston R, McLoughlin R, Clinch D. Acute pneumothorax: a comparison of elderly with younger patients. Age Ageing. 1994;23:393–395. doi: 10.1093/ageing/23.5.393. [DOI] [PubMed] [Google Scholar]
- 37.Marouf R, Caron S, Lussier M, Bherer L, Piche M, Rainville P. Reduced pain inhibition is associated with reduced cognitive inhibition in healthy aging. Pain. 2014;155:494–502. doi: 10.1016/j.pain.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 38.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- 39.McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naugle KM, Cruz-Almeida Y, Fillingim RB, Riley JL., 3rd Loss of Temporal Inhibition of Nociceptive Information is Associated with Aging and Bodily Pain. J Pain. 2017 doi: 10.1016/j.jpain.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–145. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 42.Oaklander AL. Immunotherapy Prospects for Painful Small-fiber Sensory Neuropathies and Ganglionopathies. Neurotherapeutics. 2016;13:108–117. doi: 10.1007/s13311-015-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochoa J, Mair WG. The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol (Berl) 1969;13:217–239. doi: 10.1007/BF00690643. [DOI] [PubMed] [Google Scholar]
- 44.Ohtori S, Inoue G, Mannoji C, Saisu T, Takahashi K, Mitsuhashi S, Wada Y, Takahashi K, Yamagata M, Moriya H. Shock wave application to rat skin induces degeneration and reinnervation of sensory nerve fibres. Neuroscience letters. 2001;315:57–60. doi: 10.1016/s0304-3940(01)02320-5. [DOI] [PubMed] [Google Scholar]
- 45.Periquet MI, Novak V, Collins MP, Nagaraja HN, Erdem S, Nash SM, Freimer ML, Sahenk Z, Kissel JT, Mendell JR. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology. 1999;53:1641–1647. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- 46.Persons AGSPoPPiO. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:S205–224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 47.Piechota M, Sunderland P, Wysocka A, Nalberczak M, Sliwinska MA, Radwanska K, Sikora E. Is senescence-associated beta-galactosidase a marker of neuronal senescence? Oncotarget. 2016;7:81099–81109. doi: 10.18632/oncotarget.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes care. 2004;27:1974–1979. doi: 10.2337/diacare.27.8.1974. [DOI] [PubMed] [Google Scholar]
- 49.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiological reviews. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 50.Sengupta P. The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 51.Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18:8947–8959. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes care. 2006;29:883–887. doi: 10.2337/diacare.29.04.06.dc05-2180. [DOI] [PubMed] [Google Scholar]
- 53.Taguchi T, Ota H, Matsuda T, Murase S, Mizumura K. Cutaneous C-fiber nociceptor responses and nociceptive behaviors in aged Sprague-Dawley rats. Pain. 2010;151:771–782. doi: 10.1016/j.pain.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. The British journal of dermatology. 2003;148:1021–1027. doi: 10.1046/j.1365-2133.2003.05308.x. [DOI] [PubMed] [Google Scholar]
- 55.Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle & nerve. 2007;35:591–598. doi: 10.1002/mus.20732. [DOI] [PubMed] [Google Scholar]
- 56.Umapathi T, Tan WL, Tan NC, Chan YH. Determinants of epidermal nerve fiber density in normal individuals. Muscle & nerve. 2006;33:742–746. doi: 10.1002/mus.20528. [DOI] [PubMed] [Google Scholar]
- 57.Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006;27:895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]