Abstract
Thapsigargin (3) is a potent inhibitor of the SERCA-pump protein, with potential for application in a variety of medicinal areas. The efficient and scalable syntheses of thapsigargin (3) and nortrilobolide (2) have been disclosed previously. To demonstrate the modularity of the previous routes, three natural products (compounds 6, 13, 15) and four analogs (compounds 17–20) have been divergently prepared from a common building block featuring varied acyl chains at the C2, C3, and C8 positions. Biological tests revealed that all of the compounds prepared displayed promising activity profiles.
Keywords: Natural products, Total Synthesis, SERCA inhibition, Divergent synthesis
Graphical Abstract
The strategy of divergent synthesis was first codified by Boger in 1984 with his landmark syntheses of azafluoranthene alkaloids.1 As defined, divergence “requires that an identical intermediate (preferably an advanced intermediate) be converted, separately to at least two members of the class of compounds. Divergent total syntheses are distinct from partial total synthesis in which one member is interconverted to a second member of the class of compounds.” Since then numerous new terms have been developed to describe exactly the same idea. The essence of a divergent synthesis strategy requires the design of a key intermediate that can be further diversified to a set of molecules related to the target scaffold. This maneuver is widely used by all medicinal chemists to rapidly interrogate SAR. In the arena of natural product synthesis, such an approach can facilitate access to multiple members and analogs within a class.
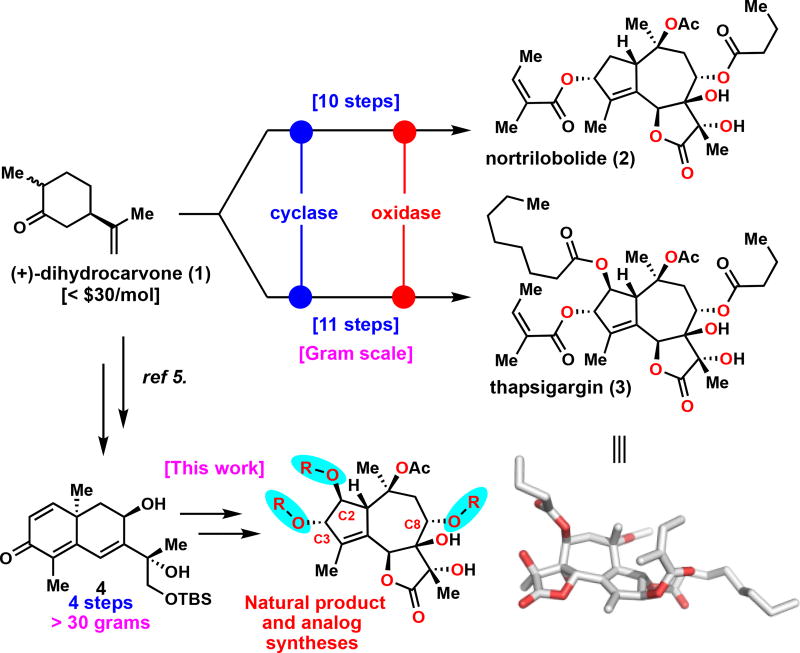
In 2009 our lab described a divergent approach to terpenes that mimics the two-phase biosynthetic pathway of this large class of natural products.2 The practical advantage of the two-phase terpene synthesis strategy resides in its capability to deliver analogs with diverse oxygenation patterns for biological evaluation, without the need to alter the parent route. The utility of this strategy was demonstrated multiple times in various total syntheses3 and also in the elucidation of the ingenol oxidase phase “barcode”.4 Similarly, the previously disclosed two-phase routes to access of nortrilobolide (2) and thapsigargin (3) from (+)-dihydrocarvone (1)5 (Figure 1) can be repurposed for the synthesis of medicinally relevant analogs.6 The therapeutic potential of thapsigargin analogs is demonstrated in mipsargargin,7a a prodrug of the parent natural product that is currently in PhII clinical trials in several cancer diseases.7b The employed route to access the parent guaianolide natural products featured modular installation of individual oxygen atoms along with their attached acyl groups, which in turn effectively generated a suitable template for facile analog synthesis. In the case of thapsigargin (3), for instance, the angelate and the butyrate group found at the C2 and C8 position, respectively, can easily be swapped via simple acylation transforms. Additionally, the C2 acyl group was installed via an alpha oxidation reaction.8 Changing the acid and anhydride components during this oxidation would enable access to a variety of C2 acyl analogs.
Figure 1.
Two-phase terpene synthesis strategy enables divergent, scalable, and modular access of complex guaianolide natural products and analogs.
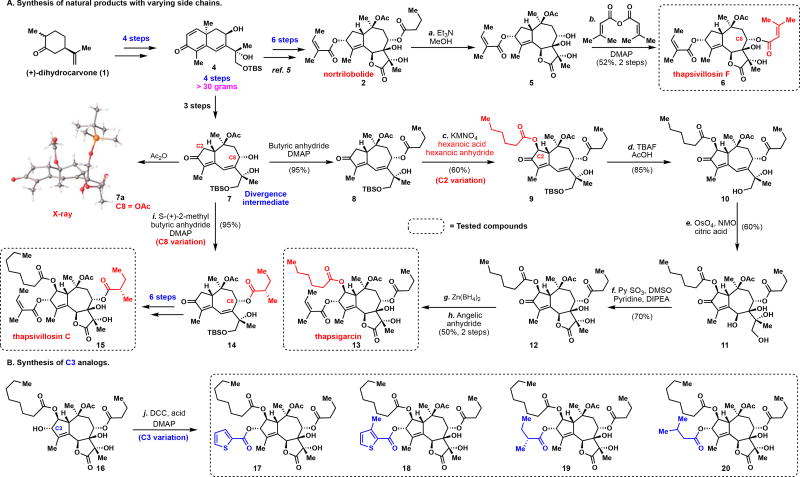
The syntheses of compounds 6, 13, 15, and 17–20 were accomplished by a two-phase strategy similar to that previously reported.5 Since intermediate 4 (prepared from (+)-dihydrocarvone (1) in 4 steps) has been scalably prepared in greater than 30-gram batches by an outside vendor, it was used as the starting point for analog synthesis. From this intermediate, nortrilobolide (2) was accessed in 6 steps.5 Heating 2 in methanol in the presence of triethylamine9 selectively cleaved the butyrate at the C8 position leading to an intermediate secondary alcohol. This compound was subsequently acylated with senecioic anhydride to afford thapsivillosin F (6) in 52% yield over 2 steps. Next, the C2 oxidized natural products were targeted with variations at the C2 and C8 positions. In 3 steps,10 compound 4 was converted to the [5.7] enone 7 by way of a Barton photochemical rearrangement11. This intermediate would serve as the divergence point to access two natural products of the thapsigargin family with varying acyl chains at the C2 and C8 positions. The identity of the photo-rearranged product was confirmed via x-ray crystallography of the acylated compound 7a. Acylation with butyric anhydride in the presence of 4-dimethylamino pyridine (DMAP) led to butyrate 8 in near quantitative yield. Subsequent permanganate mediated alpha oxidation8 in the presence of hexanoic acid and hexanoic anhydride furnished the corresponding hexanoate 9 in 60% yield. Acidic deprotection furnished diol 10, which underwent a smooth diastereoselective dihydroxylation12 to afford tetra-ol 11 in 51% over 2 steps. Parikh-Doering oxidation13 led to lactone 12. In two further steps, Thapsigarcin (13) could be accessed in 50% yield by way of a stereoselective reduction14 (Zn(BH4)2) and an acylation. Thapsivillosin C (15), the C8 variant in this family of natural products, was accessed in an analogous manner. Thus, starting from 7, acylation with S-(+)-2-methylbutyric anhydride provided the C8 acylated congener 14 that was processed in 6 steps to thapsivillosin C (15).
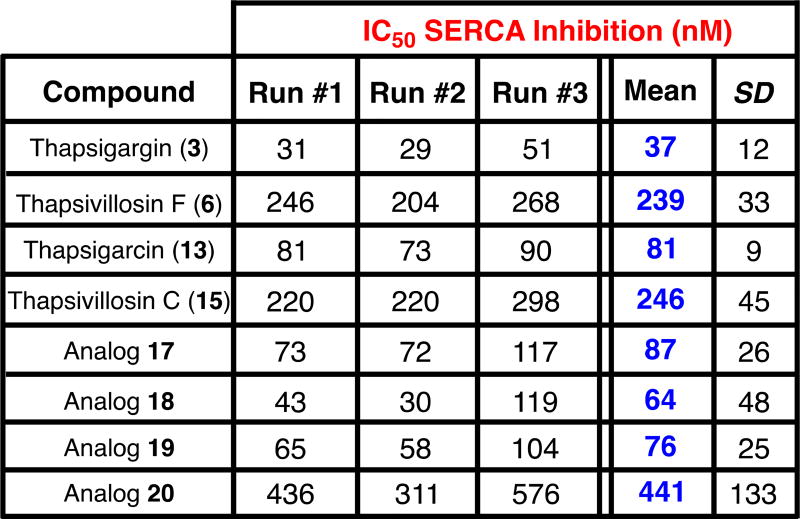
A significant amount of SAR information has been gathered on the role of the C8 acyl group on the bioactivity of thapsigargin (3).15 In contrast, the C3 allylic alcohol has received less attention. It is known in literature as well as from in-house experience that the C3 alcohol is notoriously resistant to various angeloylation conditions.16 In addition, esterification to generate angelate esters without isomerization to the thermodynamically more stable tiglate ester can be a challenge for production of active pharmaceutical ingredients.17 As a result, an equipotent analog without the C3-angelate ester moiety, but otherwise similar properties as the natural product, would serve as a stronger starting point for a discovery program. In this vein, 4 additional analogs (17–20) were prepared using simple acylation conditions from allylic alcohol 16. Indeed, cellular characterization of analogs 17–20 for their ability to increase cytosolic calcium concentration via SERCA inhibition18 revealed three of them (17–19) to have potency comparable to thapsigargin (3) and thapsigarcin (13). In contrast, a significant, approx. 10-fold loss of potency was observed for analogue 20.
In summary, the concise divergent approach to thapsigargin and related natural products has been leveraged to access a series of potent non-competitive SERCA inhibitors. The two-phase approach utilized herein strategically enabled various acyl chains to be directly embedded in the parent route without unnecessary redox or protecting group manipulations. Since the most relevant sites of SAR studies reside in the acyl chains of the thapsigargins, an efficient route to easily install these groups is of high value. Finally, compound 18 and 19 are examples of highly potent analogs of thapsigargin without the epimerization-prone C3-angelate moiety, thus rendering them attractive starting points for further manipulations.
Supplementary Material
Figure 2.
Divergent access of the thapsigargin family of natural products and analogs
Reagents and conditions: (a) Et3N, MeOH, 60 °C, 30 min; (b) Senecioic anhydride, DMAP, dichloromethane (52% yield, 2 steps); (c) KMnO4 (2.1 equiv), hexanoic acid (35 equiv), hexanoic anhydride (9 equiv), PhH (1.95 mL), 20 h, 85 °C (60%); (d) TBAF/AcOH (10 equiv), THF (0.1 M), 0 °C (85%); (e) Citric acid (2 equiv), NMO (2 equiv), OsO4 (0.1 equiv), tBuOH/H2O/Acetone (0.1 M), 50 °C, 4.5h (60%); (f) DIPEA (20 equiv), pyridine (30 equiv), PySO3 (20 equiv), DMSO, dichloromethane, 0 °C, 0.5 h (70%); (g) Zn(BH4)2, Et2O, 4 h, - 20 °C (84%); (h) angelic anhydride, NaHCO3, 80 °C, 16 h (60%); (i) (S)-(+)-2-methylbutyric anhydride (1.3 equiv), DMAP (0.8 equiv), dichloromethane (0.1 M), 25 °C, 12 h (95%); (j) DCC (4 equiv), DMAP (2 equiv), 25 °C (80–85%).
Figure 3.
IC50 SERCA inhibition values (nM) of analogs. SD = standard deviation.
Acknowledgments
This paper is dedicated to Professor Dale L. Boger for his friendship, collegiality and inspirational contributions to science and education. Financial support for this work was provided by LEO Pharma and NIH (GM-118176). The authors thank Prof. Arnold L. Rheingold and Dr. Curtis E. Moore for X-ray crystallographic analysis. We are grateful to Dr. Ravi Natarajan (KemXTree) for the scale-up of intermediate 4.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brotherton CE, Boger DL. J. Org. Chem. 1984;49:4050–4055. [Google Scholar]
- 2.a) Chen K, Baran PS. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]; b) Ishihara Y, Baran PS. Synlett. 2010;12:1733–1745. [Google Scholar]
- 3.a) Jørgensen L, McKerrall SJ, Kuttruff CA, Ungeheuer F, Felding J, Baran PS. Science. 2013;341:878–882. doi: 10.1126/science.1241606. [DOI] [PubMed] [Google Scholar]; b) McKerrall SJ, Jørgensen L, Kuttruff CA, Ungeheuer F, Baran PS. J. Am. Chem. Soc. 2014;136:5799–5810. doi: 10.1021/ja501881p. [DOI] [PubMed] [Google Scholar]; c) Mendoza A, Ishihara Y, Baran PS. Nat. Chem. 2011;4:21–25. doi: 10.1038/nchem.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wilde NC, Isomura M, Mendoza A, Baran PS. J. Am. Chem. Soc. 2014;136:4909–4912. doi: 10.1021/ja501782r. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Yuan C, Jin Y, Wilde NC, Baran PS. Angew. Chem., Int. Ed. 2016;55:8280–8284. doi: 10.1002/anie.201602235. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Chuang KV, Xu C, Reisman SE. Science. 2016;353:912–915. doi: 10.1126/science.aag1028. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Kawamura S, Chu H, Felding J, Baran PS. Nature. 2016;532:90–93. doi: 10.1038/nature17153. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Condakes ML, Hung K, Harwood SJ, Maimone TJ. J. Am. Chem. Soc. 2017;139:17783–17786. doi: 10.1021/jacs.7b11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y, Yeh C-H, Kuttruff CA, Jorgensen L, Dünstl G, Felding J, Natarajan SR, Baran PS. Angew. Chem., Int. Ed. 2015;54:14044–14048. doi: 10.1002/anie.201507977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu H, Smith JM, Felding J, Baran PS. ACS. Cent. Sci. 2017;3:47–51. doi: 10.1021/acscentsci.6b00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For a recent review of approaches to guaianolide-terpene natural products, see: Santana A, Molinillo JMG, Macias FA. Eur. J. Org. Chem. 2015:2093–2110.For recent syntheses in this family, see: Chen Dezhi, Andrew Evans P. J. Am. Chem. Soc. 2017;139:6046–6049. doi: 10.1021/jacs.7b01734.Hu X, Xu S, Maimone TJ. Angew. Chem. Int. Ed. 2017;56:1624. doi: 10.1002/anie.201611078.Johnson TC, Chin MR, Han T, Shen JP, Rana T, Siegel D. J. Am. Chem. Soc. 2016;138:6068–6073. doi: 10.1021/jacs.6b03055.
- 7.a) Andersen TB, Loṕez CQ, Manczak T, Martinez K, Simonsen HT. Molecules. 2015;20:6113–6127. doi: 10.3390/molecules20046113. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Denmeade SR, Mhaka AM, Rosen DM, Brennen WN, Dalrymple S, Dach I, Olesen C, Gurel B, DeMarzo AM, Wilding G, Carducci MA, Dionne CA, Møller JV, Nissen P, Christensen SB, Isaacs JT. Sci. Transl. Med. 2012;4:140ra86. doi: 10.2174/1871520610909030276. No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marín-Barrios R, García-Cabeza AL, Moreno-Dorado FJ, Guerra FM, Massanet GM. J. Org. Chem. 2014;79:6501–6509. doi: 10.1021/jo500915r. [DOI] [PubMed] [Google Scholar]
- 9.Andersen A, Cornett C, Lauridsen A, Olsen CE, Christensen SB. Acta Chemica Scandinavica. 1994;48:340–346. [Google Scholar]
- 10.See supporting information of ACS. Cent. Sci. 2017;3:47–51. doi: 10.1021/acscentsci.6b00313.
- 11.Barton DHR, de Mayo P, Shafiq M. J. Chem. Soc. 1957:929–935. [Google Scholar]
- 12.Dupau P, Epple R, Thomas AA, Fokin VV, Sharpless KB. Adv. Synth. Catal. 2002;344:421–433. [Google Scholar]
- 13.Chen L, Lee S, Renner M, Tian Q, Nayyar N. Org. Process Res. Dev. 2006;10:163–164. [Google Scholar]
- 14.Ball M, Andrews SP, Wierschem F, Cleator E, Smith MD, Ley SV. Org. Lett. 2007;9:663–666. doi: 10.1021/ol062947x. [DOI] [PubMed] [Google Scholar]
- 15.Doan NTQ, Christensen SB. Current Pharmaceutical Design. 2015;21:5501–5517. doi: 10.2174/1381612821666151002112824. [DOI] [PubMed] [Google Scholar]
- 16.a) Mixed anhydride condition: Andrews SP, Ball M, Wierschem F, Cleator E, Oliver S, Högenauer K, Simic O, Antonello A, Hünger U, Smith MD, Ley SV. Chem. - Eur. J. 2007;13:5688–5712. doi: 10.1002/chem.200700302.b) angelic anhydride condition: ref. 5.
- 17.Liang X, Grue-Sørensen G, Petersen AK, Högberg, Thomas Synlett. 2012;23:2647–2652. [Google Scholar]
- 18.Li S, Hao B, Lu Y, Yu P, Lee H-C, Yue J. PLoSOne. 2012;7:e310905. doi: 10.1371/journal.pone.0031905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.