Abstract
Rationale
Forkhead box M1 (FoxM1) is a transcription factor that promotes cell proliferation by regulating a broad spectrum of genes that participate in cell cycle regulation, such as Cyclin B, CDC25B, and Aurora B Kinase. We have shown that hypoxia, a well-known stimulus for pulmonary hypertension (PH), induces FoxM1 in pulmonary artery smooth muscle cells (PASMC) in a HIF-dependent pathway, resulting in PASMC proliferation, while the suppression of FoxM1 prevents hypoxia-induced PASMC proliferation. However, the implications of FoxM1 in the development of PH remain less known.
Methods
We determined FoxM1 levels in the lung samples of idiopathic PAH (pulmonary arterial hypertension) (IPAH) patients and hypoxia-induced PH mice. We generated constitutive and inducible smooth muscle cell (SMC)-specific FoxM1 knockdown or knockout mice as well as FoxM1 transgenic mice which overexpress FoxM1, and exposed them to hypoxia (10 % O2, 90% N2) or normoxia (Room air, 21 % oxygen) for four weeks, and measured PH indices. We also isolated mouse PASMC (mPASMC) and mouse embryonic fibroblasts (MEF) from these mice to examine the cell proliferation and expression levels of SMC contractile proteins.
Results
We showed that in hypertensive human lungs or mouse lungs, FoxM1 levels were elevated. Constitutive knockout of FoxM1 in mouse SMC caused early lethality, whereas constitutive knockdown of FoxM1 in mouse SMC prevented hypoxia-induced PH and PASMC proliferation. Inducible knockout of FoxM1 in SMC reversed hypoxia-induced pulmonary artery wall remodeling in existing PH. Overexpression of FoxM1 enhanced hypoxia-induced pulmonary artery wall remodeling and right ventricular hypertrophy in mice. Alteration of FoxM1 status did not affect hypoxia-induced hypoxia-inducible factor (HIF) activity in mice. Knockout of FoxM1 decreased PASMC proliferation and induced expression of SMC contractile proteins and TGF-β/Smad3 signaling.
Conclusions
Our studies provide clear evidence that altered FoxM1 expression in PASMC contributes to PH and uncover a correlation between Smad3-dependent signaling in FoxM1-mediated proliferation and de-differentiation of PASMC.
Introduction
Pulmonary arterial hypertension (PAH) is a devastating disease that results in a progressive increase in pulmonary vascular resistance, right ventricular failure, and ultimately death [1, 2]. Despite recent advances in the management of PAH, currently there is no cure for PAH. PAH is characterized by pulmonary arterial remodeling with vascular cell proliferation [1, 2]. Hypoxia is a well-established stimulus for the induction of pulmonary hypertension (PH) in several animal models that exhibit pulmonary artery smooth muscle cell (PASMC) proliferation and de-differentiation [3]. However, the mechanisms by which hypoxia mediates pulmonary artery remodeling and PH remain incompletely understood.
Recent evidence suggests that hypoxia induces Forkhead box M1 (FoxM1) in some cancer cell lines and that FoxM1 contains a putative hypoxia-inducible factor (HIF) response element in its promoter [4]. FoxM1 is a transcription factor that stimulates cell proliferation by regulating a broad spectrum of genes that participate in cell cycle regulation, such as Cyclin B, CDC25B, and Aurora B Kinase [5-7]. Elevated FoxM1 levels are reported in rapidly proliferating cells, including many human carcinomas [5, 7]. In the lungs, FoxM1 has been implicated in the tumorigenesis of lung cancer [8, 9] and the loss of FoxM1 prevents maturation of lung vasculature and attenuates proliferative epithelial and endothelial repair after lung injury [10-12]. However, the effect of Fox M1 on PASMC proliferation and its role in the pathogenesis of PH are less known.
In a recent study, we have shown that exposure to hypoxia up-regulates FoxM1 gene expression in an HIF-2α-dependent pathway, but not in an HIF-1a-dependent pathway [13]. We have also demonstrated that the inhibition of FoxM1 prevents the hypoxia-induced expression of Aurora A kinase and Cyclin D1 and the proliferation of human PASMC [13]. However, it is not known whether FoxM1 plays a role in the pathogenesis of PH in vivo.
In our current study, we generated constitutive and inducible smooth muscle cell (SMC)-specific FoxM1 knockdown and knockout mice as well as FoxM1 transgenic mice to investigate whether FoxM1 plays a causal role in the genesis of PH. We found that although constitutive knockout of FoxM1 in SMC is lethal, knockdown or inducible knockout of FoxM1 in SMC prevented or reversed hypoxia-induced PH indices, respectively. Overexpression of FoxM1 enhanced hypoxia-induced pulmonary artery remodeling and right ventricular hypertrophy. We also revealed that FoxM1-mediated PASMC proliferation and de-differentiation is correlated with a Smad3-dependent pathway.
Methods:
Lung samples from normal donors and PAH patients.
Lung tissue sections of four normal donors and four IPAH patients were kindly provided by Drs. Suzy A. A. Comhair and Serpil C. Erzurum at Department of Pathobiology, Respiratory Institute, Cleveland Clinic. The use of these lung tissue sections was approved by the University of Illinois at Chicago Institutional Review Board.
FoxM1 knockout mice and transgenic FoxM1 mice
We generated a strain of smooth muscle cell (SMC)-specific FoxM1 knockout (sm-FoxM1−/−) and knockdown mice (sm-FoxM1+/−) by crossing FoxM1fl/fl mice [14] with sm22α-Cre mice as previously described [15]. We also created a strain of inducible SMC-specific FoxM1 knockout mice by crossbreeding FoxM1fl/fl mice with SMMHC-CreERT2 mice [16]. In Rosa26-FoxM1 transgenic mice, the FoxM1 transgene is driven by the Rosa26 promoter and thus expresses ubiquitously [9].
To examine whether the knockdown of FoxM prevents hypoxia-induced PH, we exposed sm-FoxM1+/− mice and their wild type littermates to room air (normoxia) or 10% oxygen (hypoxia) for 4 weeks in a BioSpherix A-chamber (BioSpherix, Lacona, NY) in which the oxygen concentration (10%) was monitored with a Proox Model P110 oxygen controller (BioSpherix).
To examine whether the knockout of FoxM1 reverses existing PH, we first exposed SMMHC-CreERT2-FoxM1fl/fl mice to hypoxia and normoxia for nine days and then gave them 4-hydroxytamoxifen (4-OHT) or vehicle (corn oil) (i.p.) for 5 consecutive days, during which mice remained in normoxic or hypoxic conditions. After the last administration of 4-OHT or vehicle, we maintained these mice in hypoxia or normoxia for an additional two weeks.
To study the role of overexpression of FoxM1 in hypoxia-induced PH, we exposed rosa26-FoxM1 mice and Balb/c mice (purchased from the Jackson Laboratory as control for rosa26-FoxM1 mice) to hypoxia (10% O2) and normoxia for three weeks.
After normoxic or hypoxic exposure of these mice, we measured their right ventricular pressure (RVP) with a 1.4F pressure transducer catheter (Millar Instruments) and AcqKnowledge software (Biopac Systems Inc.) and calculated the right ventricular systolic pressure (RVSP) as a surrogate for pulmonary artery pressure. Blood was drawn and collected in BD Microtainer tubes with K2E (K2EDTA) (Becton, Dickinson and company, Franklin Lakes, NJ) and analyzed in a Siemens ADVIA 120 hematology analyzer (Siemens Healthcare GmbH, Erlangen, Germany). Mouse hearts were excised and dissected to determine the RV/(LV+S) ratio (right ventricle/(left ventricle + septum)) as a parameter of right ventricular (RV) hypertrophy. Mouse lung tissues were harvested, fixed, embedded, and sectioned, and stained with hematoxylin and eosin for morphometric analysis to quantify pulmonary arterial wall thickness on the arteries with a diameter of 50-100μm, which demonstrate significant remodeling during hypoxia [15, 17]. All animals were handled according to National Institutes of Health guidelines and the Institutional Animal Care and Use Committee-approved experimental protocols. Both male and female mice were included, but SMMHC-CreERT2-FoxM1fl/fl mice were all male as the SMMHC-Cre transgene was inserted on the Y Chromosome [18]. The animal studies were randomized and blinded.
Isolation of Mouse Pulmonary Artery Smooth Muscle Cells (mPASMC)
mPASMC were isolated from mouse lungs as we have previously described [15]. For SMMHC-FoxMfl/fl mice, we first injected these mice with 4-OHT or vehicle (corn oil) for five consecutive days, allowed them recover for one week before the mPASMC isolation. mPASMC were cultured and maintained in SmGM-2 medium (Lonza). Generally, we grew mPASMC for at least 2 weeks to obtain enough cells for experiments. The purity of mPASMC was validated with the co-immunofluorescence staining of SMA, Surfactant protein C (type II airway epithelial cell marker) and DAPI as we described [15].
Isolation and culture of mouse embryonic fibroblasts (MEF)
MEF were isolated from mice following published procedures [19]. Briefly, on day 14-16 of pregnancy, mice were sacrificed, and embryos were removed and rinsed in PBS. After decapitation and evisceration, the embryos were finely minced with a scalpel blade, incubated with dispase (2 mg/ml; Sigma-Aldrich) for 45 minutes, and plated into cell culture flasks in DMEM complete media. After fibroblasts had grown out from the tissue slices, we removed the tissue slices and grew and expanded the cell population. MEF were identified based on the morphology and expression of vimentin and collagen. Cell viability was checked by Trypan blue exclusion assay. All cells were maintained in a humidified incubator with a constant supply of 5% CO2 at 37°C.
Migration assay
1×104 cells were plated on uncoated inserts (BD Biosciences, Franklin Lakes, NJ) and incubated for 24 hours. The cells that migrated to the other side of the inserts were fixed, stained, and counted under the microscopic fields at 200x magnification using a calibrated ocular grid.
BrdU incorporation, Cell proliferation, and cell death
Briefly, mPASMC and MEF were plated at 5000 cells/well into 96-well plates and incubated overnight before the assays. Cell proliferation was determined by two methods: 1) the incorporation of 5-Bromo-2’-Deoxyuridine (BrdU) using the BrdU Proliferation Assay (Calbiochem, San Diego, CA); 2) cell proliferation assay by CellTiter 96® Aqueous One Solution Cell Viability Assay (Promega, Madison, WI), according to the manufacturer’s instructions. Cell death was measured by the release of lactate dehydrogenase (LDH) using the Cytotoxicity Detection Kit (LDH) (Roche, Indianapolis, IN).
Quantitative Real-time Reverse Transcription PCR (qRT-PCR)
We used a miRNeasy Mini Kit (Qiagen, Valencia, CA) and an RNase-Free DNase Set (Qiagen) to extract the total RNA. We determined the quantity and quality of total RNA with Nanodrop 2000 spectrophotometer (ThermoScientific, Rockford, IL) and then performed reverse transcription using High Capacity Reverse Transcription kits (Applied Biosystems, Foster City, CA). qRT-PCR was conducted with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on a StepOnePlus or a ViiA 7 Real-Time PCR System (Applied Biosystems). The abundance of Ribosomal protein L19 (RPL19) was used as internal control.
Western Blotting
After three washes with ice-cold phosphate-buffered saline (PBS), cells were lysed in an mRIPA buffer (50 mM Tris pH 7.4, 1% NP-40, 0.25% deoxycholate, 150 mM NaCl, and protease inhibitors), homogenized, and centrifuged at 13,000 g for 10 min at 4°C. We then determined the protein concentrations of the supernatants using Bio-Rad protein assay solution (Bio-Rad, Hercules, CA). Proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to BA85 nitrocellulose membrane (PROTRAN, Whatman, Dassel, Germany), incubated with primary and secondary antibodies, and detected with SuperSignal West Pico Chemiluminescent Substrate (ThermoScientific). The following antibodies were used in this study: α-tubulin (Cat#T5168), actin (Cat#A2228), α-smooth muscle actin (α-SMA)(Cat#A5228), calponin (Cat#C2687) (Sigma-Aldrich, St. Louis, MO), smooth muscle protein 22-α (SM22α) (Cat#ab10135, Abeam, Cambridge, MA), myocardin (Cat#MAB4028, R&D Systems), and proliferating cell nuclear antigen (PCNA) (Cat#10205-2-AP, Proteintech Group, Chicago, IL), Smad2/3, phospho-Smad2 (ser465/467) (Sigma-Aldrich and Protein Technologies, Inc.), phospho-Smad3 (ser423/425) (Sigma-Aldrich and Protein Technologies, Inc.), anti-mouse (Cat#172-1011), anti-rabbit (Cat#172-1034), and anti-goat (Cat#172-1019) IgG-HRP conjugates (Bio-Rad). The gray density of protein bands was quantified by the ImageJ software.
Immunohistochemistry (IHC)
IHC staining were performed as we described previously with multiple antibody dilution and two commercially available FoxM1 antibodies [13, 17]. To quantify IHC staining in pulmonary arteries, we converted the brown staining of these images into a single red color channel (RGB:255,0,0) in Photoshop. We then changed the red channels into greyscale images for quantification with the ImageJ software by measuring the intensity of the grey color.
Immunofluorescence (IF) staining
The lung tissue sections were de-paraffinized and rehydrated according to standard protocols. After antigen retrieval in 0.01 M citrate buffer (pH 6.0), the tissue sections were incubated with 1% Triton X-100 for 15 min and incubated with 5% BSA (in TBS, pH=7.4) for 30 min, followed by overnight incubation with the primary antibodies at 4 °C. After a wash with TBS, the sections were incubated with the secondary antibodies at room temperature for 1 h. After being washed three times with TBS, the sections were mounted using the 496-diamidino-2-phenylindole (DAPI) mounting medium. The fluorescence was recorded with a ZEISS Z1 AxioObserver fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Statistical Analysis
All experiments were repeated at least three to five times independently. Two-way ANOVA, One-way ANOVA, and t-test were performed using GraphPad Prism 4 (GraphPad, San Diego, CA) when applicable. Bonferroni posttests were carried out after ANOVA. For animal studies, we estimated the sample size with an online power analysis tool (http://www.datavis.ca/online/power/index.html), adopting a moderate to large difference (effect size 0.75). In 2×2 studies, n=3, 4, 5, 10 are sufficient to achieve a power of 0.416, 0.535, 0.636, and 0.911, respectively. Data are presented as mean ± SEM. The significant difference values were set at 0.05 and 0.01.
Results:
Hypertensive lungs express elevated levels of FoxM1.
To investigate whether hypertensive lungs express elevated levels of FoxM1, we determined FoxM1 expression in lung samples of normal and idiopathic PAH (IPAH) patients through immunohistochemistry staining. As shown in Fig 1A-B, lungs of IPAH patient express increased levels of FoxM1. We observed that in IPAH lungs, FoxM1 was located in both the cytosol and nucleus (Fig 1C). We further investigated the expression levels of FoxM1 in the lungs of mice with hypoxia-induced PH. We exposed C57BL/6 mice to hypoxia (10% O2) or room air for three weeks. We measured hematocrit (HCT), right ventricular systolic pressure (RVSP), and the RV/(LV+S) ratio (i.e., the mass ratio of the right ventricle (RV) to left ventricle(LV) and septum(S)). As expected, hypoxia increased the hematocrit in the mice (Fig 1D) and induced RVSP and the RV/(LV+S) ratio (Fig 1E-F), suggesting HIF activity and hypoxia-induced PH. These are consistent with previous reports [20, 21]. We determined the total amount of FoxM1 mRNA in the lungs of mice exposed to normoxia or hypoxia for one to three weeks. As shown in Fig 1G, hypoxia significantly induced FoxM1 mRNA expression, with peak expression one week after exposure to hypoxia. The FoxM1 protein expression was elevated one week after hypoxic exposure and stayed upregulated until the end of the two-week exposure (Fig 1H-I). These results show that FoxM1 is induced in hypertensive lungs.
Fig 1. Hypertensive lungs express elevated levels of FoxM1.
(A) Immunohistochemistry staining of FoxM1 on lung sections from normal and IPAH patients. The intensity of FoxM1 staining was shown in (B). C) Immunofluorescence staining of FoxM1 on lung sections from normal and IPAH patients. (D, E, F) C57BL/6 mice were exposed to normoxia or hypoxia (10%) for three weeks. The hematocrits, RVSP, and RV/(LV+S) of these mice were measured.n=3. (G-I) C57BL/6 mice were exposed to normoxia or hypoxia (10%) for 7, 14, and 21 days. The RNA from the whole lungs of these mice was extracted and the expression level of FoxM1 mRNA in these mice were determined by qRT-PCR. The mRNA levels of FoxM1 were normalized as a percentage of the amount of FoxM1 mRNA of mice exposed to normoxia after normalization to RPL19 (G). The protein levels of FoxM1in these mouse lungs were normalized to Actin (H-I). n=3. Data were expressed as mean ± SEM. **, p<0.01.
SMC-specific FoxM1 is required for normal development of mice.
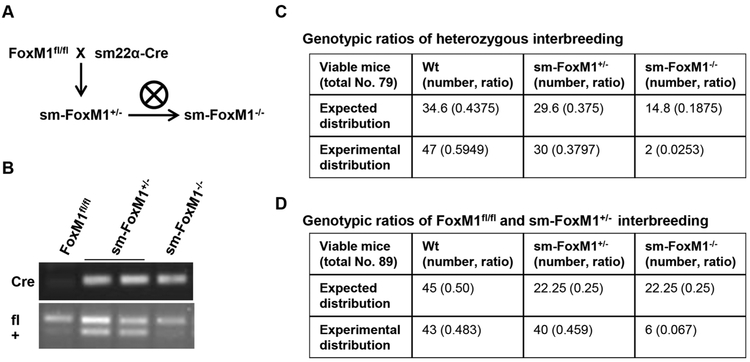
To address the role of FoxM1 in hypoxia-induced PH in vivo, we crossed the FoxM1-Floxed mice (FoxM1fl/fl) [14] with the SM22-Cre mice (the Cre recombinase is driven by sm22α promoter, thus the Cre recombinase is specifically expressed in smooth muscle cells (SMC) [15]) to specifically knock down or knock out FoxM1 in SMC (sm-FoxM1+/−and sm-FoxM1−/−) (Fig 2A). The genotype of these mice was confirmed by PCR of DNA samples isolated from mouse tails (Fig 2B). In both heterozygous interbreeding and crossing between FoxM1fl/fl and sm-FoxM1+/−, we found that the genotypes of the offspring did not exhibit the mendelian inheritance ratio, with extremely low occurrence of sm-FoxM1−/− mice (Fig 2C-2D). Moreover, out of 8 sm-FoxM1−/− mice, 4 of them died a month after birth, 2 were used to isolate mouse PASMC (mPASMC) two weeks after birth for studies in Fig 7, and 2 were used to measure RVSP after four weeks exposure to hypoxia (Fig 3A). This suggests that SMC-specific FoxM1 is required for the normal development of mice and the constitutive knockout of FoxM1 in SMC likely causes embryonic lethality of these mice.
Fig 2. Constitutive knockout of SMC-specific FoxM1 cause mice die early.
(A-B) cross-breeding of FoxM1fl/fl and sm22α-Cre mice (A) and genotype confirmation of these mice. (C-D) Genotypic ratio of interbreeding between mouse strains.
Fig 7. Loss of FoxM1 decreases PASMC proliferation.
(A-B) We isolated mPASMC from FoxM1fl/fl, sm-FoxM1+/−, and sm-FoxM1−/− and measured BrdU incorporation (A) and migration (B). (C-F) We isolated mouse embryonic fibroblasts (MEF) from these mice and measured the BrdU incorportaion (C), proliferation (D), LDH release (E). We measured the protein levels of PCNA and CCN1 in these cells with the amount of Tubulin as the loading control (F-G). Data are presented as mean ± SEM. * and #, p < 0.05; ** and ##, p < 0.01.
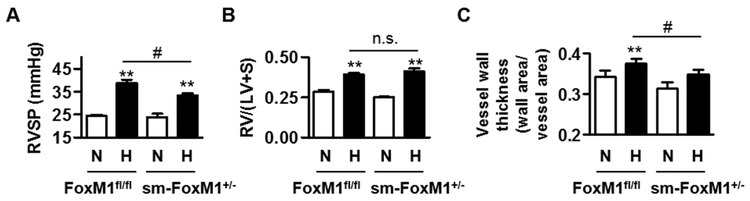
Fig 3. SMC-specific knockdown of FoxM1 is sufficient to attenuate hypoxia-induced PH in mice.
FoxM1fl/fl and SMC-specific knockdown FoxM1 mice (sm-Foxm1+/−) were exposed to normoxia (N) or hypoxia (H, 10% O2) for four weeks, and then measured for RVSP (A), RV/(LV+S) (B), and vessel wall thickness (C). There are three or four mice in each group. # and #, p<0.05; **, p<0.01; n.s., not significant.
Constitutive SMC-specific knockdown of FoxM1 inhibits hypoxia-induced PH.
Since we were unable to obtain a sufficient amount of mature sm-FoxM1−/− mice due to the requirement of FoxM1 in the development of mice, we instead used sm-FoxM1+/− mice to examine the participation of SMC-specific FoxM1 in PH, utilizing a well-established hypoxia-induced mouse model of PH. We exposed 8-10 week old adult sm-FoxM1+/− and FoxM1fl/fl littermates to hypoxia (10 % O2, 90% N2) or normoxia (Room air, 21 % oxygen) for 4 weeks. After normoxic or hypoxic exposure, we measured their RVSP, RV hypertrophy (RV/(LV+S) ratio), and pulmonary arterial wall thickness as described [20, 21]. As shown in Fig 3, constitutive knockdown of FoxM1 in SMC attenuated hypoxia-induced increase in RVSP (Fig 3A) and arterial wall thickness (Fig 3C), but not RV/(LV+S) ratio (Fig 3B). These results suggest that suppression of SMC-specific FoxM1 inhibits hypoxia-induced PH.
Inducible knockout of SMC-specific FoxM1 reverses existing vessel remodeling in hypoxic mice.
To address whether FoxM1 can be a therapeutic target for the treatment of PAH, we investigated whether the loss of FoxM1 reverses existing PH. We created an inducible SMC-specific FoxM1 knockout mouse model by crossing FoxM1fl/fl mice with SMMHC-CreERT2 mice in which Cre expression is inducible with the administration of tamoxifen (Fig 4A) [18]. We generated SMMHC-CreERT2-FoxM1fl/fl mice bearing SMMHC-CreERT2 and confirmed their genotypes by PCR with DNA samples isolated from mouse tails (Fig 4B). We also isolated mouse PASMC from these mice and confirmed the reduction of FoxM1 mRNA (Fig 4C). We exposed SMMHC-CreERT2-FoxM1fl/fl mice to hypoxia and normoxia to induce PH. On day 9 of hypoxia exposure, mice were given 4-hydroxytamoxifen (4-OHT) or vehicle (corn oil) (i.p.) for 5 consecutive days, during which these mice remained in nomoxic or hypoxic conditions. After the administration of 4-OHT or vehicle, mice remained in hypoxia or normoxia for an additional two weeks. The total duration of the experiment was four weeks of exposure to normoxia or hypoxia. We measured RVSP, RV/(LV+S), RV/Heart, Heart/Body, and pulmonary artery wall thickness. As shown in Fig 4D-4H, SMC-specific inducible knockout of FoxM1 has little effect on hypoxia-induced RVSP, RV/(LV+S), and RV/Heart, but decreased hypoxia-induced pulmonary artery wall thickening and Heart/Body ratio, suggesting that the inhibition of FoxM1 may partially reverse existing PH. In addition, we found that inducible knockout of FoxM1 had little effect on basal and hypoxia-induced heart rate (HR) (Fig 4I) right ventricular contractility as measured by the rate of rise of right ventricular pressure (dP/dt) (Fig 4J).
Fig 4. Inducible knockout of SMC-specific FoxM1 reverses existing vessel remodeling in hypoxic mice.
(A-C) Crossbreeding schema and genotyping of SMMHC-FoxM1 mice were shown in (A) and (B), respectively. SMMHC-FoxM1fl/fl mice were given 4-hydroxytamoxifen (4-OHT) or corn oil by intraperitoneal (i.p) injection for 5 consecutive days followed by two days of recovery. Mice were used for isolation of mouse PASMC (mPASMC) after one week of maintenance. The mRNA levels of FoxM1 in these freshly isolated mPASMC were measured by qRT-PCR with RPL19 as an internal control. n=4 each group (C). (D-J) 8-10-week-old SMMHC-FoxM1fl/fl mice were exposed to room air (normoxia) or 10% oxygen (hypoxia) for nine days to induce PH. Then, 4-OHT was administrated for 5 consecutive days to induce the knockout of FoxM1 while remaining in normoxia or hypoxia. Mice were allowed two days of recovery. These mice were then exposed to room air or 10% oxygen for another 2 weeks. In control groups, mice were injected with corn oil. After the experiment, mice were subjected to the measurement of RVSP (D), right ventricular hypertrophy (E), RV/heart ratio (F), heart/body weight ratio (G), pulmonary arterial wall thickness (H), heart rate (HR)(I), and the rate of rise of right ventricular pressure (dP/dt). n = 3-5 mice for each group. Data are presented as mean ± SEM. * and #, p < 0.05; **, p < 0.01; n.s., not significant.
Overexpression of FoxM1 enhances hypoxia-induced RV hypertrophy and vessel remodeling in vivo.
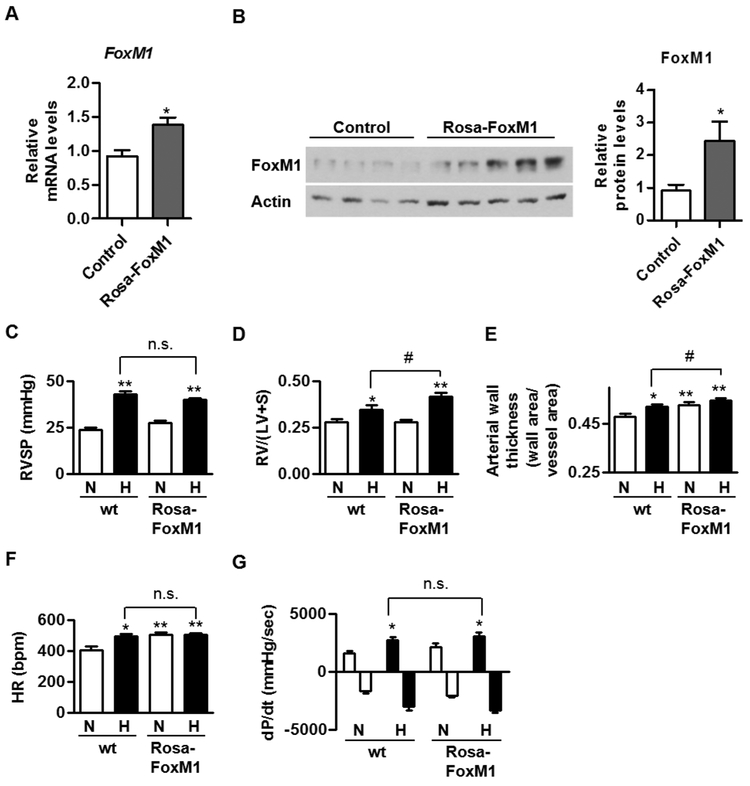
Since loss-of-function of SMC-specific FoxM1 limits hypoxia-induced PH (Fig 3-4), we then investigated whether the overexpression of FoxM1 induces PH or enhances hypoxia-induced PH. To address this, we utilized rosa26-FoxM1 mice in which FoxM1 expression is driven by rosa26 and expressed ubiquitously in the tissues [10, 22]. Rosa26-FoxM1 mice are viable, fertile, and do not exhibit abnormalties in normal conditions [10, 22]. We confirmed that FoxM1 was overexpressed in mouse lungs (Fig 5A-B). Since rosa26-FoxM1 is on Balb/c background, we purchased Balb/c mice (wt) from the Jackson Laboratory as a control for rosa26-FoxM1 mice. 8-10 week-old rosa26-FoxM1 and Balb/c were exposed to hypoxia (10% O2) and normoxia for three weeks to induce PH. After normoxic or hypoxic exposure, we examined their RVSP, RV hypertrophy (RV/(LV+S) ratio), and pulmonary arterial wall thickness. We found that overexpression of FoxM1 had little effect on RVSP or RV/(LV+S) ratio in normal condition, but increased basal pulmonary artery wall thickness (Fig 5C-E). More importantly, overexpression of FoxM1 enhanced hypoxia-induced RV hypertrophy and pulmonary artery wall thickening (Fig 5C-E). In addition, we found that overexpression of FoxM1 increased basal heart rate, but had little effect on hypoxia-induced heart rate and basal and hypoxia-induced right ventricular contractility (dP/dt) (Fig 5F-G).
Fig 5. Overexpression of FoxM1 enhances hypoxia-induced RV hypertrophy and vessel remodeling in vivo.
(A-B) The RNA from the whole lungs of these mice was extracted and the expression levels of FoxM1 mRNA in these mice were determined by qRT-PCR and normalized to RPL19 (A). The protein levels of FoxM1 in these mouse lungs were normalized to Actin (B). (C-G) We exposed 8-10 week-old rosa26-FoxM1 and Balb/c to hypoxia (10% O2) and normoxia for three weeks to induce PH. After normoxic or hypoxic exposure, we measured their RVSP (C), RV hypertrophy (RV/(LV+S) ratio (D), pulmonary arterial wall thickness (E), heart rate (F), and the rate of rise of right ventricular pressure (dP/dt). n = 5-7 mice for each group. Data are presented as mean ± SEM. * and #, p < 0.05; **, p < 0.01; n.s., not significant.
Knockdown of FoxM1 does not affect hypoxia-induced HIF activation.
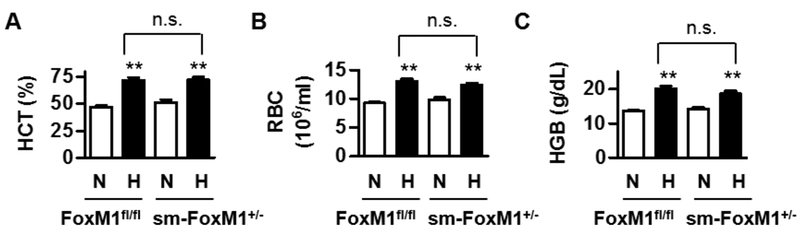
In hypoxia-induced PH, HIF is a key transcription factor that upregulates hematocrits (HCT), hemoglobin contents (HGB), and red blood cell counts (RBC) and thus participates in the development of PH [15, 16]. Previously, we have shown that during hypoxia, the stabilization of HIF can induce FoxM1 in PASMC [13], which indicates that HIF is an upstream regulator of FoxM1. To further address the relationship between HIF and FoxM1 in mice in vivo, we collected the blood samples from sm-FoxM1+/− and FoxM1fl/fl that were exposed to normoxia and hypoxia for four weeks as descibed in Fig 3 and measured HCT, RBC, and HGB. We found that the suppression of FoxM1 has little effect on hypoxia-induced HCT, RBC, and HGB (Fig 6), confirming that FoxM1 is downstream of HIF activation.
Fig 6. Knockdown of FoxM1 does not affect hypoxia-induced HIF activation.
We collected blood samples from sm-FoxM1+/− and FoxM1fl/fl mice that were exposed to normoxia and hypoxia for four weeks as descibed in Fig 3 and measured hematocrits (HCT) (A), red blood cell counts (RBC) (B), and hemoglobin contents (HGB) (C). There are three or four mice in each group. Data are presented as mean ± SEM. **, p < 0.01; n.s., not significant.
Loss of FoxM1 decreases PASMC proliferation.
A common feature in human IPAH samples and three animal experiments utilizing genetically altered FoxM1 mice is the correlation of the presence of FoxM1 and elevated pulmonary artery wall thickening (Fig 1, 3-5). This suggests that FoxM1 may be responsible for the heightened pulmonary artery wall thickening in PH. Our previous report also showed that FoxM1 is necessary for hypoxia-induced human PASMC proliferation [13]. To further investigate the role of FoxM1 in PASMC cell behavior, we isolated mouse PASMC (mPASMC) from sm-FoxM1−/−, sm-FoxM1+/−, and FoxM1fl/fl mice and measured BrdU incorporation and migration. We found that the loss of FoxM1 decreased mPASMC cell proliferation in a dose-dependent manner, whereas the migration of mPASMC was unchanged after knockout of knockdown of FoxM1 (Fig 7A-7B).
Due to the embryonic lethality of sm-FoxM1−/− mice, we have a limited source of mPASMC from these mice. We chose to isolate mouse embryonic fibroblasts (MEF) from sm-FoxM1−/−, sm-FoxM1+/−, and FoxM1fl/fl mice. First, we measured the BrdU incorportaion in MEFs from these mice and found that the loss of FoxM1 decreased MEF proliferation in a dose-dependent manner (Fig 7C), comparable to what was observed in mPASMC (Fig 7A). We then examined the proliferation and the LDH release (as an indicator of cell death) of MEF and found that the loss of FoxM1 decreased cell proliferation but not the LDH release (Fig 7D-7E). In addition, we determined protein levels of PCNA (Proliferating Cell Nuclear Antigen, a cell proliferation marker) and CCN1 (a FoxM1 downstream target) and found that the loss of FoxM1 decreased protein levels of PCNA and CCN1 (Fig 7F-G). These results confirm that indeed we have knocked out FoxM1 and suggest that the knockout of FoxM1 decreases PASMC proliferation but not migration or survival.
Knockout of FoxM1 increases Smad3 signaling and expression of SMC markers.
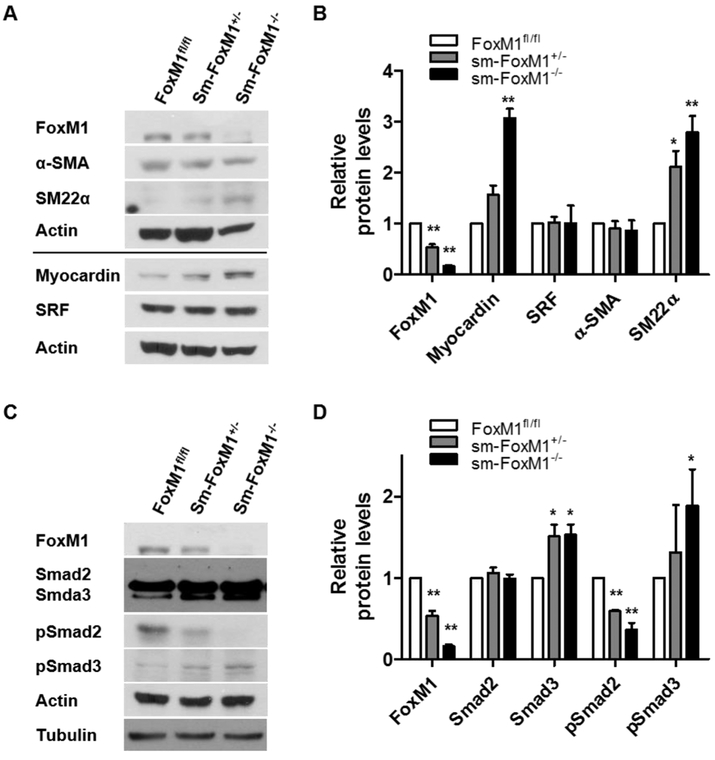
Vascular remodeling in PAH is associated with the phenotype change of PASMC -- from a contractile or differentiated phenotype to a proliferative or de-differentiated phenotype [23, 24] [15]. Since we have shown that FoxM1 is associated with PASMC proliferation (Fig 7) [13], we investigated whether FoxM1 status affects expression of SMC markers. We examined the expression levels of SMC marker proteins in MEF isolated from sm-FoxM1−/−, sm-FoxM1+/−, and FoxM1fl/fl mice. We found that loss of FoxM1 induced Myocardin and SM22α, but not α-SMA or SRF (Fig 8A-8B). We have shown that TGF-β/Smad signaling regulates SMC marker expression in PASMC [15, 25], thus we examined the Smad2/3 and phosphorylated Smad2/3 (pSmad2/3) levels and their relationship with SMC marker proteins in MEF isolated from these mice. We showed that the loss of FoxM1 induced Smad3 and pSmad3, but decreased pSmad2, while total Smad2 remained unchanged. These results suggest that the loss of FoxM1 is correlated with an increase in Smad3 signaling and contractile protein expression, resulting in differentiation of PASMC.
Fig 8. Loss of FoxM1 increases Smad3 signaling and the expression of SMC contractile proteins.
(A-B) mouse embryonic fibroblasts (MEF) from FoxM1fl/fl, sm-FoxM1+/−, and sm-FoxM1−/− mice were subjected to Western blotting for a panel of SMC contractile proteins. The quantification of these proteins was shown in (B). (C-D) mouse embryonic fibroblasts (MEF) from FoxM1fl/fl, sm-FoxM1+/−, and sm-FoxM1−/− mice were subjected to Western blotting for Smad and pSmad. The quantification of these proteins was shown in (D). n = 5. Data are presented as mean ± SEM. *, p < 0.05; **, p < 0.01.
Discussion
Previously we have shown that FoxM1 is essential for hypoxia-induced PASMC proliferation [13], however, the participation of FoxM1 in PAH remains elusive. In this study, we provide novel evidence to support the involvement of FoxM1 in PAH by demonstrating the following: 1) FoxM1 is overexpressed in PH tissues; 2) suppression of FoxM1 in SMC is sufficient to inhibit hypoxia-induced PH in mice, whereas overexpression of FoxM1 enhances hypoxia-induced vessel remodeling; 3) FoxM1 promotes PASMC proliferation and dedifferentiation. These results clearly indicate that the induction of FoxM1 is responsible for the PASMC proliferation and PH. Our study is consistent with recent studies that FoxM1 is a novel target for the therapeutic treatment of PH [26, 27] and that PASMC-specific but not endothelial cells-specific FoxM1 contributes to PAH [27].
Hypoxia is a well-established stimulus for PH and pulmonary artery remodeling. We have shown that hypoxia induces FoxM1 in PASMC and promotes PASMC proliferation [13]. Consistently, we show that in both human hypertensive lungs and lungs of experimental PH mouse, FoxM1 is upregulated (Fig 1). Our studies are in line with a recent report that FoxM1 is upregulated in human PASMC (HPASMC) isolated from both hereditary (HPAH) and idiopathic PAH (IPAH) subjects in an Affymetrix Human Gene ST 1.0 chip analysis [28]. Accordingly, polo-like kinase, a downstream target of FoxM1, is also upregulated in both HPAH and IPAH HPASMC [28]. Interestingly, we show that FoxM1 upregulation in the hypoxic mouse PH is transient, whereas FoxM1 is upregulated in human samples (Fig 1A-B and 1G-I). This may reflect the difference between the reversible PH in hypoxic mice and the progressive nature of PAH. Due to the lack of detailed clinical information regarding the severity of PAH, age, ethnicity, sex, etc. in these human samples, the relationship between these factors and FoxM1 upregulation remains unclear and warrants future investigation. We and others have reported that HIF is responsible for the induction of FoxM1 in various cell types [4, 13, 29]. Interestingly, in PASMC, HIF1 controls basal levels of FoxM1 whereas HIF2 is responsible for hypoxia-induced FoxM1 [13]. In both cell culture model and the animal model of hypoxia-induced PH, FoxM1 status does not affect HIF stabilization or activity (Fig 6)[13], confirming that HIF is upstream of FoxM1. Interestingly, a recent article shows that endothelial derived factors, such as PDGF-B, CXCL12, ET-1 and MIF, induce FoxM1 in PASMC [27], suggesting a complex network regulating FoxM1.
In order to address the role of SMC-specific FoxM1 in vivo, we generated a SMC-specific knockout of FoxM1 mice. Unortunately, homozygous deletion of FoxM1 in SMC results in compromised vascular development in the early stage of embryologic development, causing embryonic lethality of these mice (Fig 2 and reference [30]). To overcome this issue, we used both heterozygous deletion of FoxM1 mice (Fig 3) and inducible homozygous deletion of FoxM1 mice (Fig 4). We show that suppression of FoxM1 not only inhibits the hypoxia-induced RVSP and pulmonary artery wall thickening (Fig 3), it also alleviates pulmonary artery wall thickening in existing PH (Fig 4). Accordingly, overexpression of FoxM1 is sufficient to induce pulmonary artery wall thickening in normal conditions and enhances hypoxia-induced pulmonary artery wall thickening (Fig 5). It is worth to point out that the sample size is relatively small in some animal experiments due to difficulty to breed and high cost. We overcome this issue by conducting multiple animal experiments with both loss-of-function and gain-of-function approaches and the results are consistent and are in line with similar studies by Dai Z, et al. and Bourgeois A, et al. [26, 27]. Together, these results suggest that FoxM1 may be a novel therapeutic target for PAH.
Interestingly, in heterozygous deletion of FoxM1 mice, the decrease of hypoxia-induced RVSP is coupled with the reduction of vessel remodeling, whereas in inducible homozygous deletion of FoxM1 mice and transgenic FoxM1, hypoxia-induced vessel remodeling is uncoupled with RVSP (Fig 3-5), suggesting that FoxM1 may have additional functions related to the heart. Previous reports show that FoxM1 is required for heart development and that ablation of FoxM1 in cardiomyocytes induces cardiac hypertrophy and fibrosis in aged mice [31, 32]. RV remodeling and subsequent heart failure is a major determinant of the eventual outcome of PAH patients [33-35]. Nitrite, a known vasodilator and PAH therapeutic avenue, prevents pulmonary artery banding-induced RV remodeling and failure [36]. This raises the question of whether FoxM1 contributes to RV remodeling and heart failure in PAH. So far, we do not observe FoxM1 changes, hypoxia-induced heart rate, or right ventricular contractility (Fig 4-5). Interestingly, Dai and colleague show that FoxM1 inhibition by Thiostrepton alleviates RV fibrosis in experimental PH rats [27], suggesting that FoxM1 may contribute to PAH via its function relating to RV remodeling or fibrosis. Consistently, transaortic constriction induces FoxM1, which in turn promotes the Ace2-to-Ace enzyme switch, leading to cardiac hypertrophy and fibrosis [37], whereas Elabela/Toddler/Apela (ELA) peptide antagonizes RV fibrosis by downregulating FoxM1 [38]. Thus, it will be interesting to examine the participation of FoxM1 in RV remodeling and fibrosis during PH and elucidate the underlying molecular mechanisms in the future.
Recent studies suggest that thiazole antibiotics Siomycin A and thiostrepton can downregulate FoxM1 expression and inhibit FoxM1 activity as well as inhibit cell proliferation and induce apoptosis in some cancer cell lines [39]. Furthermore, a cell-penetrating ARF peptide can inhibit FoxM1 and its biological or pathological function [40, 41]. Thus, we reason that Siomycin A, thiostrepton, and ARF peptide might also inhibit PH and represent a novel therapeutic option for the treatment of PAH in the future studies. Indeed, in recent two separate studies, Drs. Youyang Zhao and Olivier Boucherat and colleagues have elegantly demonstrated that thiostrepton prevents and reverses pulmonary hypertension in multiple animal models [26, 27]. Further studies are warranted to identify novel drug candidates targeting FoxM1 and evaluate their clinical utility.
Numerous studies in cancer have shown the critical role of FoxM1 in regulation of cell cycle and cell proliferation. We show that in animal experiments the loss of FoxM1 decreases PASMC cell proliferation and vessel wall thickening, whereas overexpression of FoxM1 increases them (Fig 3-5), confirming our previous report that FoxM1 is necessary for PASMC proliferation in cultured cells [13]. Interestingly, in MEF, we also show that FoxM1 is required for cell proliferation (Fig 7C-7F), supporting a universal role of FoxM1 in regulating cell proliferation, which will also explain the embryonic lethality of FoxM1 knockout mice (Fig 2)[30].
PASMC is typically well-differentiated and express contractile proteins, which are shown to be down-regulated in PAH, representing a de-differentiated phenotype [15]. Myocardin and TGF-β/Smad signaling are known to regulate contractile protein expression in PASMC [15, 25]. Although we observed that the loss of FoxM1 induces Myocardin, it has little effects on levels of SRF, a co-factor of Myocardin for inducing contractile proteins (Fig 8). Thus, FoxM1-mediated PASMC de-differentiation is unlikely due to Myocardin/SRF. Interestingly we found that the loss of FoxM1 increases TGF-β/Smad signaling, particularly Smad3 (Fig 8). These results are consistent with our previous reports that Smad3 is critical in PASMC contractile protein expression [15, 25]. Although we don’t know how FoxM1 downregulates Smad3 signaling in PASMC, FoxM1 positively correlates with TGF-β/Smad signaling in cancers [42, 43]. It has been postulated that in breast cancer, FoxM1 interacts with Smad3, blocks the ubiquitination of Smad4, and stabilizes the Smad3/Smad4 complex, leading to breast cancer invasion and metastasis [44]. This suggests that there is a context-specific relationship between FoxM1 and TGF-β/Smad and further studies into the molecular mechanisms underlying FoxM1-mediated downregulation of Smad3 in PASMC are warranted.
Taken together, our study provides direct evidence that FoxM1 contributes to PAH, complementing and expanding existing literature on the participation of FoxM1 in the development of PAH. We also show that FoxM1-mediated PASMC differentiation correlates with a Smad3 signaling pathway, which will warrant further investigation.
Highlights:
FoxM1 levels are elevated in hypertensive human lungs or mouse lungs.
Constitutive and inducible suppression of SMC-specific FoxM1 inhibit experimental PH.
FoxM1-mediated proliferation and de-differentiation of PASMC inversely correlates with a Smad3-dependent signaling.
Acknowledgement
Normal and IPAH human PASMC were kindly provided by Drs. Suzy A. A. Comhair and Serpil C. Erzurum at the Department of Pathobiology, Respiratory Institute, Cleveland Clinic. This work was supported in part by NIH R01HL123804 (G. Zhou), a National Natural Science Foundation of China (NSFC) grant 81770050 (G. Zhou), and an American Lung Association Biomedical Research Grant RG-416135 (T. Chen). We would also like to thank Miranda Sun and Rongzhen Zhou for their proofreading of our manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Humbert M, Sitbon O, Simonneau G, Treatment of pulmonary arterial hypertension.[see comment], New England Journal of Medicine 351(14) (2004) 1425–36. [DOI] [PubMed] [Google Scholar]
- [2].McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Moliterno DJ, Mukherjee D, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ, Accf/Aha, ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association, Circulation 119(16) (2009) 2250–94. [DOI] [PubMed] [Google Scholar]
- [3].Stenmark KR, Fagan KA, Frid MG, Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms, Circ Res 99(7) (2006) 675–91. [DOI] [PubMed] [Google Scholar]
- [4].Xia LM, Huang WJ, Wang B, Liu M, Zhang Q, Yan W, Zhu Q, Luo M, Zhou ZZ, Tian DA, Transcriptional up-regulation of FoxM1 in response to hypoxia is mediated by HIF-1, J Cell Biochem 106(2) (2009) 247–56. [DOI] [PubMed] [Google Scholar]
- [5].Costa RH, FoxM1 dances with mitosis.[comment], Nat Cell Biol 7(2) (2005) 108–10. [DOI] [PubMed] [Google Scholar]
- [6].Park HJ, Costa RH, Lau LF, Tyner AL, Raychaudhuri P, Anaphase-promoting complex/cyclosome-CDH1-mediated proteolysis of the forkhead box M1 transcription factor is critical for regulated entry into S phase, Molecular & Cellular Biology 28(17) (2008) 5162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wierstra I, Alves J, FOXM1, a typical proliferation-associated transcription factor, Biol Chem 388(12) (2007) 1257–74. [DOI] [PubMed] [Google Scholar]
- [8].Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, Major ML, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV, The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer, Cancer Research 66(4) (2006) 2153–61. [DOI] [PubMed] [Google Scholar]
- [9].Wang IC, Meliton L, Tretiakova M, Costa RH, Kalinichenko VV, Kalin TV, Transgenic expression of the forkhead box M1 transcription factor induces formation of lung tumors, Oncogene 27(30) (2008) 4137–49. [DOI] [PubMed] [Google Scholar]
- [10].Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH, Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury.[erratum appears in J Biol Chem. 2003 Nov 28;278(48):48506 Note: Costal, RH [corrected to Costa, RH]], Journal of Biological Chemistry 278(39) (2003) 37888–94. [DOI] [PubMed] [Google Scholar]
- [11].Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV, The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature, Journal of Biological Chemistry 280(23) (2005) 22278–86. [DOI] [PubMed] [Google Scholar]
- [12].Kalin TV, Wang IC, Meliton L, Zhang Y, Wert SE, Ren X, Snyder J, Bell SM, Graf L Jr., Whitsett JA, Kalinichenko VV, Forkhead Box m1 transcription factor is required for perinatal lung function, Proceedings of the National Academy of Sciences of the United States of America 105(49) (2008) 19330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raghavan A, Zhou G, Zhou Q, Ibe JCF, Ramchandran R, Yang Q, Racherla H, Raychaudhuri P, Raj JU, Hypoxia-induced pulmonary arterial smooth muscle cell proliferation is controlled by forkhead box M1, Am J Respir Cell Mol Biol 46(4)(2012)431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ustiyan V, Wert SE, Ikegami M, Wang IC, Kalin TV, Whitsett JA, Kalinichenko VV, Foxm1 transcription factor is critical for proliferation and differentiation of Clara cells during development of conducting airways, Dev Biol 370(2) (2012) 198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen T, Zhou G, Zhou Q, Tang H, Ibe JCF, Cheng H, Gou D, Chen J, Yuan JXJ, Raj JU, Loss of MicroRNA-1792 in Smooth Muscle Cells Attenuates Experimental Pulmonary Hypertension via Induction of PDZ and LIM Domain 5, American journal of respiratory and critical care medicine 191(6) (2015) 678–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen T, Zhou Q, Tang H, Bozkanat M, Yuan JXJ, Raj JU, Zhou G, miR-17/20 Controls Prolyl Hydroxylase 2 (PHD2)/Hypoxia-Inducible Factor 1 (HIF1) to Regulate Pulmonary Artery Smooth Muscle Cell Proliferation, J Am Heart Assoc 5(12) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ibe JCF, Zhou Q, Chen T, Tang H, Yuan JXJ, Raj JU, Zhou G, Adenosine monophosphate-activated protein kinase is required for pulmonary artery smooth muscle cell survival and the development of hypoxic pulmonary hypertension, Am J Respir Cell Mol Biol 49(4) (2013) 609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schütz G, Gutkind S, Offermanns S, G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension, Nat Med 14(1) (2008) 64–8. [DOI] [PubMed] [Google Scholar]
- [19].Zhou Q, Pardo A, Konigshoff M, Eickelberg O, Budinger GRS, Thavarajah K, Gottardi CJ, Jones J, Varga J, Selman M, Sznajder JI, Raj JU, Zhou G, Role of von Hippel-Lindau protein in fibroblast proliferation and fibrosis, Faseb J 25(9) (2011) 3032–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P, Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia, J Clin Invest 111(10) (2003) 1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL, Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha, J Clin Invest 103(5) (1999) 691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yoshida Y, Wang IC, Yoder HM, Davidson NO, Costa RH, The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer, Gastroenterology 132(4) (2007) 1420–31. [DOI] [PubMed] [Google Scholar]
- [23].Owens GK, Kumar MS, Wamhoff BR, Molecular regulation of vascular smooth muscle cell differentiation in development and disease, Physiol Rev 84(3) (2004) 767–801. [DOI] [PubMed] [Google Scholar]
- [24].Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F, Mechanisms of disease: pulmonary arterial hypertension, Nat Rev Cardiol 8(8) (2011) 443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheng H, Chen T, Tor M, Park D, Zhou Q, Huang JB, Khatib N, Rong L, Zhou G, A High-Throughput Screening Platform Targeting PDLIM5 for Pulmonary Hypertension, J Biomol Screen 21(4) (2016) 333–41. [DOI] [PubMed] [Google Scholar]
- [26].Bourgeois A, Lambert C, Habbout K, Ranchoux B, Paquet-Marceau S, Trinh I, Breuils-Bonnet S, Paradis R, Nadeau V, Paulin R, Provencher S, Bonnet S, Boucherat O, FOXM1 promotes pulmonary artery smooth muscle cell expansion in pulmonary arterial hypertension, J Mol Med (Berl) 96(2) (2018) 223–235. [DOI] [PubMed] [Google Scholar]
- [27].Dai Z, Zhu MM, Peng Y, Jin H, Machireddy N, Qian Z, Zhang X, Zhao Y-Y, Endothelial and Smooth Muscle Cell Interaction via FoxM1 Signaling Mediates Vascular Remodeling and Pulmonary Hypertension, Am J Respir Crit Care Med (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.[] Yu J, Wilson J, Taylor L, Polgar P, DNA microarray and signal transduction analysis in pulmonary artery smooth muscle cells from heritable and idiopathic pulmonary arterial hypertension subjects, J Cell Biochem 116(3) (2015) 386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xia L, Mo P, Huang W, Zhang L, Wang Y, Zhu H, Tian D, Liu J, Chen Z, Zhang Y, Chen Z, Hu H, Fan D, Nie Y, Wu K, The TNF-alpha/ROS/HIF-1-induced upregulation of FoxMI expression promotes HCC proliferation and resistance to apoptosis, Carcinogenesis 33(11) (2012) 2250–9. [DOI] [PubMed] [Google Scholar]
- [30].Ustiyan V, Wang IC, Ren X, Zhang Y, Snyder J, Xu Y, Wert SE, Lessard JL, Kalin TV, Kalinichenko VV, Forkhead box M1 transcriptional factor is required for smooth muscle cells during embryonic development of blood vessels and esophagus, Dev Biol 336(2) (2009) 266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bolte C, Zhang Y, Wang IC, Kalin TV, Molkentin JD, Kalinichenko VV, Expression of Foxm1 transcription factor in cardiomyocytes is required for myocardial development, PLoS One 6(7) (2011) e22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bolte C, Zhang Y, York A, Kalin TV, Schultz JEJ, Molkentin JD, Kalinichenko VV, Postnatal ablation of Foxm1 from cardiomyocytes causes late onset cardiac hypertrophy and fibrosis without exacerbating pressure overload-induced cardiac remodeling, PLoS One 7(11) (2012) e48713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, Chaanine A, Torquato S, Naim C, Ibanez B, Pereda D, Garcia-Alvarez A, Fuster V, Sengupta PP, Leopold JA, Hajjar RJ, Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model, Am J Physiol Heart Circ Physiol 307(8) (2014) H1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ryan JJ, Archer SL, The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure, Circ Res 115(1) (2014) 176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sydykov A, Mamazhakypov A, Petrovic A, Kosanovic D, Sarybaev AS, Weissmann N, Ghofrani HA, Schermuly RT, Inflammatory Mediators Drive Adverse Right Ventricular Remodeling and Dysfunction and Serve as Potential Biomarkers, Front Physiol 9 (2018) 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hu J, Sharifi-Sanjani M, Tofovic SP, Nitrite Prevents Right Ventricular Failure and Remodeling Induced by Pulmonary Artery Banding, J Cardiovasc Pharmacol 69(2) (2017) 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang J, Feng X, Zhou Q, Cheng W, Shang C, Han P, Lin C-H, Chen H-SV, Quertermous T, Chang C-P, Pathological Ace2-to-Ace enzyme switch in the stressed heart is transcriptionally controlled by the endothelial Brg1-FoxM1 complex, Proceedings of the National Academy of Sciences of the United States of America 113(38) (2016) E5628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sato T, Sato C, Kadowaki A, Watanabe H, Ho L, Ishida J, Yamaguchi T, Kimura A, Fukamizu A, Penninger JM, Reversade B, Ito H, Imai Y, Kuba K, ELABELA-APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage, Cardiovasc Res 113(7) (2017) 760–769. [DOI] [PubMed] [Google Scholar]
- [39].Bhat UG, Zipfel PA, Tyler DS, Gartel AL, Novel anticancer compounds induce apoptosis in melanoma cells, Cell Cycle 7(12) (2008) 1851–5. [DOI] [PubMed] [Google Scholar]
- [40].Costa RH, Kalinichenko VV, Major ML, Raychaudhuri P, New and unexpected: forkhead meets ARF, Curr Opin Genet Dev 15(1) (2005) 42–8. [DOI] [PubMed] [Google Scholar]
- [41].Gusarova GA, Wang IC, Major ML, Kalinichenko VV, Ackerson T, Petrovic V, Costa RH, A cell-penetrating ARF peptide inhibitor of FoxM1 in mouse hepatocellular carcinoma treatment, The Journal of clinical investigation 117(1) (2007) 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lin B, Madan A, Yoon J-G, Fang X, Yan X, Kim T-K, Hwang D, Hood L, Foltz G, Massively parallel signature sequencing and bioinformatics analysis identifies up-regulation of TGFBI and SOX4 in human glioblastoma, PLoS One 5(4) (2010) e10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].De Luca A, Fiorillo M, Peiris-Pages M, Ozsvari B, Smith DL, Sanchez-Alvarez R, Martinez-Outschoorn UE, Cappello AR, Pezzi V, Lisanti MP, Sotgia F, Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells, Oncotarget 6(17) (2015) 14777–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xue J, Lin X, Chiu W-T, Chen Y-H, Yu G, Liu M, Feng X-H, Sawaya R, Medema RH, Hung M-C, Huang S, Sustained activation of SMAD3/SMAD4 by FOXM1 promotes TGF-beta-dependent cancer metastasis, The Journal of clinical investigation 124(2) (2014) 564–79. [DOI] [PMC free article] [PubMed] [Google Scholar]