Abstract
Tuberculosis (TB) is the leading killer due to a single infectious disease worldwide. With the aging of the global population, the case rate and deaths due to TB are highest in the elderly population. While general immunosenescence associated with old age is thought to contribute to the susceptibility of the elderly to develop active TB disease, very few studies of immune function in elderly individuals with Mycobacterium tuberculosis (M.tb) infection or disease have been performed. In particular, impaired adaptive T cell immunity to M.tb is one proposed mechanism for the elderly’s increased susceptibility primarily on the basis of the decreased delayed type hypersensitivity response to tuberculin-purified protein derivative in the skin of elderly individuals. To investigate immunological reasons why the elderly are susceptible to develop active TB disease, we performed a cross-sectional observational study over a five year period (2012–2016) enrolling participants from 2 age groups (adults: 25–44 years; elderly: 65 and older) and 3 M.tb infection statuses (active TB, latent TB infection, and healthy controls without history of M.tb infection). We hypothesized that impaired peripheral T cell immunity plays a role in the biological susceptibility of the elderly to TB. Contrary to our hypothesis, we observed no evidence of impaired M.tb specific T cell frequency or altered production of cytokines implicated in M.tb control (IFN-γ, IL-10) in peripheral blood in the elderly. Instead, we observed alterations in monocyte proportion and phenotype with age and M.tb infection that suggest their potential role in the susceptibility of the elderly to develop active TB. Our results suggest a potential link between the known widespread low-grade systemic inflammation of old age, termed “inflammaging,” with the elderly’s specific susceptibility to developing active TB. Moreover, our results highlight the need for further research into the biological reasons why the elderly are more susceptible to disease and death from TB, so that public health systems can be better equipped to face the present and future problem of TB in an aging global population.
Keywords: Tuberculosis, Geriatrics, T cell, Monocyte, Inflammaging, Immunosenescence
1. Introduction
The global population is aging. It is estimated that by the year 2050, 17% of the global population will be 65 years of age or older, compared to 8.5% in 2015 (US Census Bureau, 2016). Increasing age is the major risk factor for a host of diseases including diabetes, cardiovascular disease, cancer and neurodegenerative disease (López-Otín et al., 2013; Niccoli and Partridge, 2012). Increasing age is also a major risk factor for infectious diseases (Gavazzi and Krause, 2002; Yoshikawa, 1997). Tuberculosis (TB), caused by the airborne pathogen Mycobacterium tuberculosis (M.tb), is currently the leading killer due to an infectious disease, and increasing age is a significant risk factor for disease and death due to TB. For example, in the United States, the case rate of symptomatic, active TB is highest in those ≥ 65 years of age, and this age group is the most likely to die from TB (Centers for Disease Control and Prevention [CDC], 2017). Worldwide, the case rate is similarly highest among those ≥ 55 years of age (World Health Organization [WHO], 2017). Moreover, according to the 2016 Global Burden of Disease estimate, 62% of deaths due to TB globally occurred among people older than 50, with more than half of these deaths occurring in those ≥ 65 years of age (Institute for Health Metrics and Evaluation, 2016). We can expect that as the global population continues to age, the global burden of TB in the elderly will also increase (Negin et al., 2015).
Infection with M.tb is thought to confer a ~10% lifetime risk of developing symptomatic, transmissible active TB, with the remainder of individuals controlling the bacteria in a state known as latent TB infection (LTBI) (Vynnycky and Fine, 2000). While the prevalence of active TB has decreased in the last 50 years, the increased risk of the elderly to convert from LTBI to active TB poses an additional challenge to TB elimination efforts in the foreseeable future (Glaziou et al., 2015; Hochberg and Horsburgh, 2013; Horsburgh et al., 2010; Vynnycky and Fine, 2000). Research into the clinical and biological reasons for why the elderly are especially susceptible to develop active TB can help inform strategies to combat this present and future public health problem.
General immunosenescence associated with old age has been cited as one biological reason for the increased susceptibility of the elderly to develop active TB (Byng-Maddick and Noursadeghi, 2016; Guzzetta and Kirschner, 2013; Menon et al., 2016; Rajagopalan, 2016; Thrupp et al., 2004). However, very few studies of immune function in elderly individuals with M.tb infection (LTBI or active TB) have been performed. A historical hypothesis in the field is that impaired adaptive T cell immunity to M.tb plays a role in this biological susceptibility. This hypothesis stems from observations that reactivity to tuberculin-purified protein derivative (PPD) in the tuberculin skin test (TST), a measurement of a delayed type hypersensitivity (DTH) response for LTBI diagnosis, is reduced with advancing age past 65 years despite epidemiological evidence of higher exposure earlier in life (Dorken et al., 1987; Van den Brande and Demedts, 1992). A few articles have shown that elderly patients with active TB had increased T-suppressor cell activity, or suppression of lymphocyte proliferation in vitro (Adambekov and Morozov, 1989; Harada, 1989). More recently, T cell responses to specific latency-associated antigens of M.tb have been investigated in the elderly, though without specific comparison to younger cohorts (Commandeur et al., 2011). These studies, taken together, do not provide definitive proof that age-related changes in T cell function contribute to risk of active TB in the elderly.
To provide additional investigation of immunological reasons why the elderly are susceptible to develop active TB, we performed a cross-sectional observational study over a five year period (2012–2016) enrolling participants from 2 age groups (adults: 25–44 years; elderly: 65 and older) and 3 M.tb infection statuses (active TB, LTBI, and healthy controls without history of M.tb infection). We hypothesized that impaired peripheral T cell immunity plays a role in the biological susceptibility of the elderly to TB. Contrary to our hypothesis, we observed no evidence of impaired M.tb specific T cell frequency or altered production of cytokines important for M.tb control in peripheral blood in the elderly. Instead, we observed alterations in monocyte proportion and phenotype with age and M.tb infection that suggest their potential role in the susceptibility of the elderly to develop active TB. Our results suggest a potential link between the known widespread low-grade systemic inflammation of old age, termed “inflammaging,” with the elderly’s specific susceptibility to developing active TB (Franceschi and Campisi, 2014; Piergallini and Turner, 2017).
2. Materials and Methods
2.1 Subject Recruitment
Study participants were from two age groups, 25–44 years and 65 years and older. Subjects were recruited from The Ohio State University Wexner Medical Center and Columbus Public Health. Eligible subjects were enrolled in the inpatient and outpatient setting and consented according to an IRB approved protocol. Subjects’ medical histories were obtained by a questionnaire at study enrollment, and this information was verified and additional information obtained by review of their medical records. Clinical data collected for active TB/LTBI diagnosis included signs and symptoms of active TB, physical examination, QuantiFERON®TB-Gold Test (QFT) (Qiagen, USA), acid fast bacilli smears, M.tb PCR, cultures and imaging studies if available. Most subjects with active TB/LTBI were recruited at or before the day they started receiving treatment for active TB/LTBI. Upon study enrollment, peripheral blood was collected in lithium heparin coated tubes (Becton, Dickinson, NJ). Time from blood collection to peripheral blood mononuclear cell (PBMC) isolation was ≤ 4 hours.
2.2 PBMC Isolation
PBMCs were isolated by Ficoll-Paque (GE Healthcare Life Sciences) density centrifugation and washed twice before use in subsequent assays.
2.3 Measurement of ESAT-6 and CFP-10 specific IFN-γ+ T cells
The T-SPOT®.TB (Oxford Immunotec, UK) ELISPOT kit was used to measure the frequency of ESAT-6 and CFP-10 specific IFN-γ+ T cells in PBMCs, per the manufacturer’s protocol. Spots were counted manually by two lab members independently. The results gave identical trends between counters (results of one count are shown). In accordance with the manufacturer’s protocol, the data shown are from counts with the count of the negative control well subtracted.
2.4 PBMC Culture and Stimulation
2.5 × 105 PBMCs were cultured in triplicate in AIM-V media (with Albumax, 50 μg/mL Streptomycin, 10 μg/mL Gentamycin) (GIBCO) with 10 μg/mL H37Rv culture filtrate protein (CFP) (BEI Resources) or either media only or 10 μg/mL concanavalin A (Sigma-Aldrich) as negative/positive controls, respectively. Cell-free supernatants were removed after 48 hours culture at 37°C, 5% CO2 and stored at −20 °C until further analysis.
2.5 Measurement of Cytokine Levels
Human IFN-γ, IL-10 and IL-4 were measured in PBMC culture supernatants using ELISA kits (BD Biosciences), according to manufacturer’s instructions. Samples outside the linear range were serially diluted and re-assayed. Some samples were thawed twice. IFN-γ data from the first 72 recruited subjects were excluded from analysis due to the assay falling outside the linear range of the standards and samples being insufficient for further testing.
2.6 Flow Cytometry
5 × 105 PBMCs were washed in flow buffer (RPMI-1640, 10.4 mM HEPES, 0.1% sodium azide), stained for 20 minutes at 4°C with antibody and washed twice more. Antibodies used were: CD4-APC-Cy7 (Clone: RPA-T4), CD8-PE (Clone: HIT8a), CCR7-PerCP-Cy5.5 (Clone: 150503), PD-1-APC (Clone: MIH4), CD16-FITC (Clone: 3G8) and CD14-PerCP-Cy5.5 (Clone: MΦ9). All antibodies were purchased from BD Pharmingen. Data were acquired on a BD FACS Canto II with FACS Diva software (BD Biosciences). 50,000 events per sample were acquired. The data were analyzed using FlowJo software (Version 10.3.0). Lymphocytes were identified by FSC/SSC before gating on CD4 and CD8. The CD4/CD8 ratio was calculated from these gates. CCR7 and PD-1 positivity were determined using isotype controls. Monocytes were identified by gating on PBMCs using FSC/SSC before gating on CD14+ PBMCs. Monocyte subsets were subsequently identified by CD14 and CD16: classical (CD14++CD16−), intermediate (CD14++CD16+) and nonclassical (CD14dimCD16+).
2.7 Statistical Analysis
Data were analyzed using R v. 3.4.1 (R Foundation for Statistical Computing, Vienna, AUT). The chi-squared test or ANOVA were used to assess univariate differences in clinical covariates between our main study groups, for categorical and continuous variables, respectively. The non-parametric Mann-Whitney U-test was used in all pair-wise comparisons for T cell function assay data. For our T cell and monocyte flow cytometry data, the non-parametric Kruskal-Wallis test was used, after which Dunn’s post hoc test was used for pair-wise comparisons if the Kruskal-Wallis test gave p < 0.05. To assess the contribution of confounding clinical covariates to these comparisons, multivariate linear models were fit using the “lm” function in R and analysis of variance performed using the “ANOVA” function. IFN-γ ELISA data were log-transformed before performing multivariate analysis.
3. Results
3.1 Cohort Description
We recruited a total of 205 subjects to our study, and a total of 169 subjects were included in the final analysis. Subjects excluded from analysis mostly included those whose samples were used for assay standardization or who had a previous history of treatment for active TB or LTBI (see Figure S1). 6 study groups were defined for our primary analysis, with 2 age groups (adults: 25–44 years; elderly: 65 and older) and 3 M.tb infection statuses (active TB, LTBI, healthy controls without history of M.tb infection). All but 3 subjects with active TB were culture confirmed to have M.tb complex, with the other three diagnoses being clinical TB uveitis disease which improved on TB treatment and two subjects with pleural TB diagnosed based on abnormal chest x-ray and chest-computed tomography (CT) and improvement following TB treatment. Subjects with LTBI were either clinically diagnosed by a positive QFT (n=39), or had a positive TST (n=1). All were deemed absent of active TB based on symptoms, chest x-ray, CT and/or negative sputum samples. All control subjects had a negative QFT result (n=18) or no known QFT result with no prior history of a positive TST (n=98). No control subjects had a clinical history of active TB. Moreover, most control subjects originated from a location of very low TB incidence.
Table 1 shows clinical covariates for our study subjects, and Table 2 shows culture confirmation and tissue site affected in our subjects with active TB. Due to our complex study design and available patient populations, not all covariates are balanced across all 6 study groups. For several of our immunological assays showing significant differences between age groups, we used multivariate analysis to assess the effects of our known clinical confounding variables.
Table 1. Study Population Characteristics.
Due to our complex study design and available patient populations, not all covariates are balanced across all 6 study groups. p-values are from chi-squared test for categorical data and ANOVA for continuous variables.
| Adult Control | Elderly Control | Adult Latent TB | Elderly Latent TB | Adult Active TB | Elderly Active TB | All groups p-value | TB/LTBI groups p-value | |
|---|---|---|---|---|---|---|---|---|
| n | 63 | 53 | 31 | 9 | 6 | 7 | ||
| Sex (%) | 24 (38.1) | 24 (45.3) | 16 (51.6) | 4 (44.4) | 3 (50.0) | 4 (57.1) | 0.818 | |
| Age (mean (range)) | 34.21 (25–44) | 73.23 (65–92) | 31.48 (25–42) | 75.56 (66–87) | 33.33 (26–38) | 76.29 (65–89) | ||
| Ethnicity (%) | <0.001 | 0.383 | ||||||
| Asian | 6 (9.5) | 2 (3.8) | 17 (54.8) | 4 (44.4) | 1 (16.7) | 5 (71.4) | ||
| Black | 10 (15.9) | 7 (13.2) | 9 (29.0) | 4 (44.4) | 3 (50.0) | 0 (0.0) | ||
| Other | 0 (0.0) | 2 (3.8) | 3 (9.7) | 1 (11.1) | 2 (33.3) | 1 (14.3) | ||
| White | 47 (74.6) | 42 (79.2) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 1 (14.3) | ||
| Previous BCG Vaccination (%) | 0.078 | 0.424 | ||||||
| No | 30 (47.6) | 15 (28.3) | 12 (38.7) | 5 (55.6) | 1 (16.7) | 1 (14.3) | ||
| Unknown | 13 (20.6) | 14 (26.4) | 13 (41.9) | 3 (33.3) | 2 (33.3) | 4 (57.1) | ||
| Yes | 20 (31.7) | 24 (45.3) | 6 (19.4) | 1 (11.1) | 3 (50.0) | 2 (28.6) | ||
| Tobacco Use (%) | 0.002 | 0.178 | ||||||
| No | 36 (57.1) | 23 (43.4) | 15 (48.4) | 4 (44.4) | 4 (66.7) | 2 (28.6) | ||
| Previous | 3 (4.8) | 13 (24.5) | 1 (3.2) | 2 (22.2) | 2 (33.3) | 2 (28.6) | ||
| Unknown | 15 (23.8) | 6 (11.3) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 1 (14.3) | ||
| Yes | 9 (14.3) | 11 (20.8) | 14 (45.2) | 3 (33.3) | 0 (0.0) | 2 (28.6) | ||
| Alcohol Use (%) | <0.001 | 0.054 | ||||||
| No | 18 (28.6) | 28 (52.8) | 21 (67.7) | 5 (55.6) | 6 (100.0) | 3 (42.9) | ||
| Previous | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 0 (0.0) | ||
| Unknown | 17 (27.0) | 6 (11.3) | 4 (12.9) | 0 (0.0) | 0 (0.0) | 2 (28.6) | ||
| Yes | 28 (44.4) | 19 (35.8) | 6 (19.4) | 2 (22.2) | 0 (0.0) | 2 (28.6) | ||
| On TB/LTBI treatment | 29 (93.5) | 9 (100.0) | 6 (100.0) | 7 (100.0) | 0.688 | |||
| Enrolled within 30 days of TB/LTBI treatment start (%) | 29 (93.5) | 4 (44.4) | 6 (100.0) | 4 (57.1) | 0.002 | |||
| Diabetes Mellitus (%) | 2 (3.2) | 14 (26.4) | 0 (0.0) | 3 (33.3) | 1 (16.7) | 1 (14.3) | <0.001 | 0.020 |
| WBC (mean (sd)) | 7.25 (2.58) | 7.71 (2.90) | 7.35 (2.68) | 7.60 (2.92) | 6.88 (0.69) | 7.47 (2.34) | 0.964 | 0.960 |
| HCT (mean (sd)) | 40.17 (5.18) | 39.61 (9.69) | 43.46 (4.15) | 37.11 (4.00) | 38.07 (7.60) | 38.11 (7.42) | 0.125 | 0.005 |
| Albumin (mean (sd)) | 4.10 (0.75) | 4.03 (0.35) | 4.47 (0.29) | 3.76 (0.71) | 4.10 (0.91) | 3.65 (0.61) | 0.002 | <0.001 |
| On Immunosuppressive Therapy (%) | 2 (3.2) | 16 (30.2) | 1 (3.2) | 0 (0.0) | 1 (16.7) | 1 (14.3) | <0.001 | 0.359 |
| Positive HIV Status (%) | 1 (1.6) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0.131 | |
| Hepatitis B Infection (%) | 0 (0.0) | 0 (0.0) | 3 (9.7) | 3 (33.3) | 0 (0.0) | 0 (0.0) | <0.001 | 0.108 |
| History of Cancer (%) | 1 (1.6) | 12 (22.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | <0.001 | |
| Has Other Comorbidity † (%) | 9 (14.3) | 15 (28.3) | 3 (9.7) | 1 (11.1) | 2 (33.3) | 3 (42.9) | 0.102 | 0.119 |
Other recorded comorbidities included asthma, rheumatoid arthritis, chronic kidney disease, COPD, bronchiectasis, frequent pneumonias, emphysema, psoriasis (1 subject), depressive disorder (1), narcotic painkiller use (1), thoracic aneurysm (1), microcytic anemia-thalassemia (1), Bechet’s syndrome (1), CNS Toxoplasmosis (1 Adult Active TB), SIADH (1), gastrointestinal bypass surgery (1) and liver transplant (1).
Table 2. Active TB Subject Characteristics.
p-value is from chi-squared test.
| Adult Active TB | Elderly Active TB | p-value | |
|---|---|---|---|
| n | 6 | 7 | |
| Culture Confirmed M.tb (%) | 4 (66.7) | 6 (85.7) | 0.879 |
| Primary Body Sites Affected (%) | |||
| Pulmonary | 1 (16.7) | 5 (71.4) | |
| Pleural | 1 (16.7) | 2 (28.6) | |
| Pulmonary and Musculoskeletal | 1 (16.7) | 0 (0.0) | |
| Musculoskeletal | 1 (16.7) | 0 (0.0) | |
| CNS TB and Pulmonary NTM | 1 (16.7) | 0 (0.0) | |
| Uveitis | 1 (16.7) | 0 (0.0) |
3.2 T cell cytokine responses to M.tb antigens are not altered by age in those with LTBI or active TB
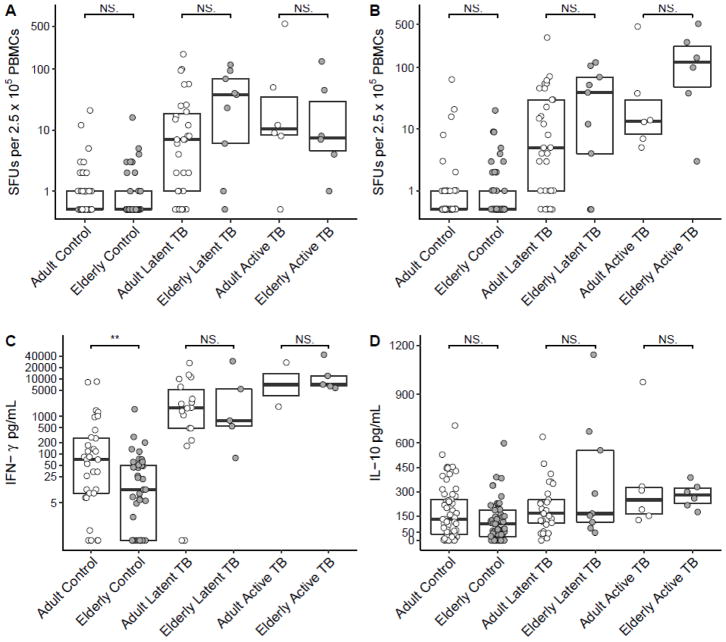
We first sought to determine whether among M.tb infected individuals, the elderly had decreased peripheral M.tb specific T cell numbers and function compared to adults. We measured the frequency of ESAT-6 and CFP-10 specific T cells using the T-SPOT.TB ELISPOT kit. Of note, peptide lengths (15-mer) were designed to contain MHC II epitopes (Li et al., 2012; Richeldi, 2006). We observed no significant differences in numbers of both ESAT-6 and CFP-10 specific IFN-γ+ T cells in the elderly relative to adults, in both latent and active TB groups (p>0.05, see Figure 1A–B). In fact, we observed a trend for increased ESAT-6 and CFP-10 specific IFN-γ+ T cells in elderly subjects with LTBI compared to adults. It is possible that while the elderly have increased CFP-10 and ESAT-6 specific T cells in the blood, the amount of IFN-γ that these and other cells produce is decreased. Indeed, the ability of T cells to produce IFN-γ in response to the large collection of M.tb antigens contained in M.tb culture filtrate protein (CFP) is decreased in adult active TB cases with higher clinical disease severity (Jamil et al., 2007). To test whether M.tb-specific IFN-γ production was reduced in the elderly, we stimulated PBMCs with M.tb CFP and measured supernatant IFN-γ by ELISA. Contrary to our hypothesis, there was no significant difference in CFP-specific IFN-γ production with age, either among subjects with LTBI or active TB (p>0.05, see Figure 1C). We also measured IL-10 and IL-4 production by CFP-stimulated PBMCs. We observed a trend for an increase in IL-10 production by CFP-stimulated PBMCs from subjects with active TB relative to those with latent TB, consistent with what is known in the TB literature (Beamer et al., 2008a; Bonecini-Almeida et al., 2004; Jamil et al., 2007) (Figure 1D). However, we saw no significant differences in IL-10 production between age groups among those with LTBI or active TB (p>0.05). Moreover, we observed no change in IL-4 production between age groups, neither basally nor with CFP-stimulation (data not shown). Taken together, our data on M.tb-specific T cell frequency and cytokine production provide no evidence of M.tb-specific T cell dysfunction in the periphery in elderly people ≥ 65 years of age.
Figure 1. T cell cytokine responses to M.tb antigens are not altered by age in those with LTBI or active TB.
Number of IFN-γ+ T cells, measured in spot forming units (SFUs), responding to ESAT-6 (A) and CFP-10 (B) from M.tb. IFN-γ (C) and IL-10 (D) from culture supernatants of PBMCs stimulated with M.tb culture filtrate protein. Boxplots are medians with interquartile ranges. Total subjects for each panel are 157 (A), 157 (B), 100 (C) and 164 (D). ** p<0.01 by Mann-Whitney U test.
Interestingly, we did observe a statistically significant decrease in IFN-γ production in response to CFP in the elderly who had no history of M.tb infection, relative to adults (Figure 1C). This suggests potential hypo-responsiveness of the innate immune system to mycobacterial antigens in the elderly. This observation was not confounded by diabetes, cancer, immunosuppressive treatment or the presence of another comorbidity, using graphical analysis (data not shown). Accordingly, the effect of age alone remained significant using a multivariate linear model including these variables (p=0.007) or all categorical clinical covariates from Table 1 (p=0.005). In addition, BCG vaccination status had no effect (p>0.05).
3.3 Global T cell phenotypes are altered by M.tb infection and disease independent of age
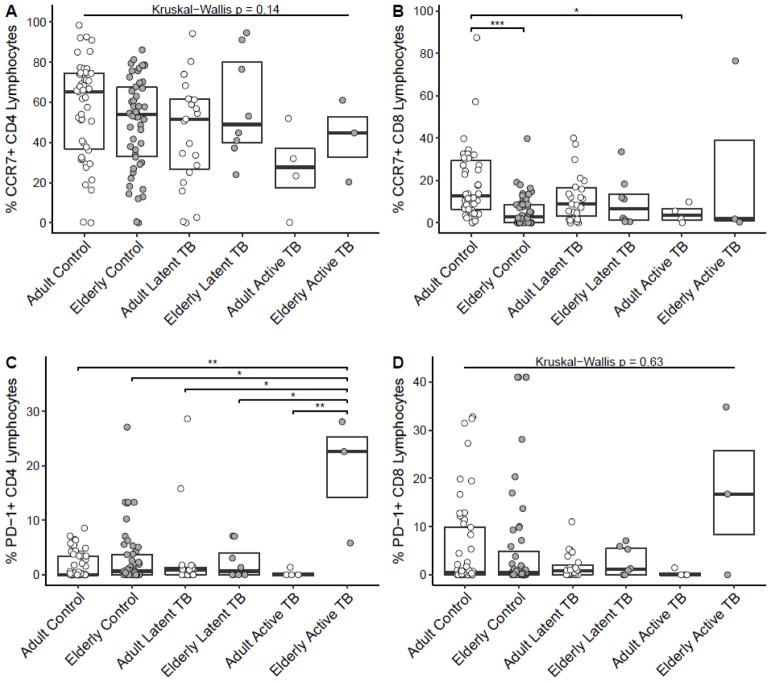
We also studied global T cell phenotypes by flow cytometry in our cohort. Consistent with what is known in the clinical literature, the CD4/CD8 ratio was decreased in subjects with LTBI and was further decreased in subjects with active TB relative to controls (data not shown). There was no significant difference in the CD4/CD8 ratio with age in any group (data not shown). We further sought to determine whether global T cell memory phenotype or exhaustion status was altered by M.tb infection and age. We observed a trend for a decrease in both CD4+CCR7+ and CD8+CCR7+ lymphocytes in adults with LTBI and a trend for a decrease in adults with active TB, with significance reached for CD8+CCR7+ lymphocytes (p<0.05), relative to adult controls (Figure 2A–B). However, we observed no significant difference in these parameters between the elderly and adults among those with LTBI or active TB (Figure 2A–B). Among our controls without history of M.tb infection, we did observe a significant decrease in CD8+CCR7+ lymphocytes and a trend for decreased CD4+CCR7+ lymphocytes with age, consistent with the aging literature (Koch et al., 2008; Yan et al., 2010) (Figures 2A–B). As for markers of T cell exhaustion, in a majority of subjects we measured no PD-1+ CD4+ or CD8+ lymphocytes (Figures 2C–D). There was a trend for increased PD1+ cells in both CD4+ and CD8+ lymphocytes in elderly with active TB relative to all other groups, though our low sample size in this group makes this conclusion tentative. Taken together with our cytokine production data, our data do not suggest a clear mechanism for impaired peripheral T cell immunity as a dominant cause of potential biological susceptibility of the elderly to active TB.
Figure 2. Global T cell CCR7 phenotype is not altered by age in those with LTBI or active TB but PD-1 phenotype may be increased in elderly with active TB.
Percent of CD4+ (A) and CD8+ (B) lymphocytes expressing CCR7. Percent of CD4+ (C) and CD8+ (D) lymphocytes expressing PD-1. For all comparisons between age groups not displayed, there were no significant differences (p>0.05). Boxplots are medians with interquartile ranges. Total subjects for each panel are 135 (A), 134 (B), 143 (C) and 142 (D). Other Kruskal-Wallis p values were p=0.0001 (B) and p=0.03 (C). * p<0.05, ** p<0.01, ***p<0.001 by Dunn’s test.
3.4 Old age and M.tb infection alter the monocyte/lymphocyte ratio and skew monocytes towards a nonclassical phenotype
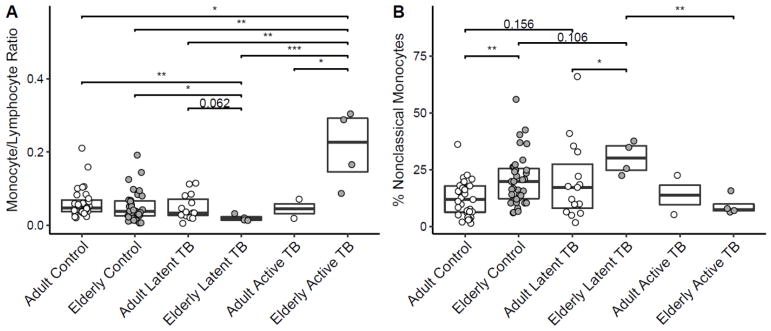
It is increasingly appreciated that monocyte phenotypes correlate with susceptibility to a variety of diseases (Berry et al., 2010; Puchta et al., 2016; Rogacev et al., 2012; Shaw et al., 2010; Wong et al., 2012). In addition to our examination of T cell phenotype and function, we sought to determine whether monocyte proportions and phenotypes were altered by old age and M.tb infection. In children and adults, the monocyte/lymphocyte ratio is associated with risk of active TB and disease severity, with a higher ratio being associated with highest risk and a very low ratio also associated with increased risk (Naranbhai et al., 2014a, 2014b). Interestingly, we observed an increased monocyte/lymphocyte ratio in the elderly with active TB, relative to all groups (p<0.05) (Figure 3A). We also observed a decreased monocyte/lymphocyte ratio in the elderly with LTBI, relative to both control groups and adults with LTBI (p<0.05 for controls and p=0.062 for adults with LTBI). In our examination of monocyte subset proportions defined by CD14 and CD16 expression, we observed a significant increase in the proportion of nonclassical (CD14dimCD16+) monocytes in elderly controls relative to adults, which has been observed by others (Hearps et al., 2012; Nyugen et al., 2010; Pence and Yarbro, 2018) (Figure 3B). This increase remained significant when taking into account diabetes, cancer, immunosuppressive treatment and the presence of another comorbidity (p<0.001) or all categorical clinical covariates from Table 1 (p<0.001) (see Table 3). Even more interesting, we observed a marginally significant increase in nonclassical monocytes in the elderly with LTBI, compared to elderly controls and adults with LTBI (p=0.106 and p<0.05, respectively). This suggests the hypothesis of an age-dependent increase in nonclassical monocytes in LTBI. Mirroring the results in nonclassical monocytes, we observed a trend for a decreased classical monocyte (CD14++CD16−) proportion in elderly controls relative to adults and a trend for a decreased proportion in elderly with LTBI relative to elderly controls and adults with LTBI (data not shown). We observed no significant differences in intermediate (CD14++CD16+) monocyte proportions with age or M.tb infection status (data not shown). Contrary to our results examining M.tb-specific T cell function in the elderly, our monocyte data suggest the hypothesis that changes in monocyte proportion and phenotype may contribute to susceptibility to develop active TB in the elderly.
Figure 3. Monocyte/lymphocyte ratio is increased in elderly with active TB and decreased in elderly with LTBI, and old age and M.tb infection skew monocytes towards a nonclassical phenotype.
Monocyte/lymphocyte ratio (A) and percent monocytes with nonclassical (CD14dimCD16+) phenotype (B). Boxplots are medians with interquartile ranges. Total subjects for each panel are 91 (A) and 91 (B). Kruskal-Wallis p values were p=0.002 (A) and p=0.003 (B). * p<0.05, ** p<0.01, ***p<0.001 by Dunn’s test, or p value otherwise displayed.
Table 3.
Multivariate linear models of nonclassical monocyte proportion in adult (n=31) and elderly (n=35) controls based on clinical covariates.
| Independent Variable | Sum of Squares | F Value | P Value |
|---|---|---|---|
| Model 1. Dependent variable: Nonclassical monocyte proportion | |||
| Age Group | 1127.2 | 12.79 | 0.0007 |
| Diabetes | 855.5 | 9.71 | 0.0028 |
| History of Cancer | 69.2 | 0.79 | 0.38 |
| On Immunosuppressive Therapy | 66.0 | 0.75 | 0.39 |
| Has Other Comorbidity | 19.9 | 0.23 | 0.64 |
| Residuals | 5287.6 | ||
| Model 2. Dependent variable: Nonclassical monocyte proportion | |||
| Age Group | 1127.2 | 13.58 | 0.0006 |
| Diabetes | 855.5 | 10.31 | 0.0024 |
| Ethnicity | 926.6 | 2.79 | 0.037 |
| Alcohol Use | 315.7 | 1.90 | 0.16 |
| History of Cancer | 69.2 | 0.83 | 0.37 |
| On Immunosuppressive Therapy | 66.0 | 0.80 | 0.38 |
| Has Other Comorbidity | 19.9 | 0.24 | 0.63 |
| HIV Status | 17.6 | 0.21 | 0.65 |
| Sex | 13.1 | 0.16 | 0.69 |
| Previous BCG Vaccination | 38.1 | 0.23 | 0.80 |
| Tobacco Use | 76.6 | 0.31 | 0.82 |
| Residuals | 3900.0 |
4. Discussion
The global burden of TB in the elderly will increase as the global population continues to age (Negin et al., 2015). From available epidemiologic data (CDC, 2017; Negin et al., 2015; WHO, 2017), it is clear that changes incident to age contribute to the likelihood of the elderly to die from an episode of active TB. In areas of low TB incidence, the increased likelihood of the elderly to develop active TB could be due to two effects. Epidemiologically, the elderly had higher exposure to TB in earlier years of life, when TB was more prevalent, compared to younger birth cohorts. Thus, a higher case rate in the elderly would simply reflect a larger reservoir of LTBI that could reactivate to active TB (Powell and Farer, 1980; Vynnycky and Fine, 2000; Wiker et al., 2010). Conversely, the increased case rate of TB in the elderly could be due to an age-related biological susceptibility to transition from a latent TB infection to active TB (Hochberg and Horsburgh, 2013; Horsburgh et al., 2010; Tocque et al., 1998). In South-East Asia, where new and relapse TB case notification rates are in general on par with case rates in Africa, those older than 55 years of age still have the highest incidence rate of TB, suggesting that the elderly are biologically susceptible to developing active TB (Tocque et al., 1998; WHO, 2017; Yew Wing W. et al., 2018). Our cross-sectional observational study assumes that the elderly are biologically more susceptible to developing active TB.
We hypothesized that impaired T cell immunity to M.tb plays a role in the biological susceptibility of the elderly to develop active TB. This has been a historical hypothesis in the field, stemming mainly from observations that reactivity to the PPD skin test, a measurement of a DTH response for LTBI diagnosis, is reduced with advancing age past 65 years despite epidemiological evidence of higher exposure earlier in life (Dorken et al., 1987; Van den Brande and Demedts, 1992). Contrary to our hypothesis, we observed no difference between adults and elderly people with LTBI or active TB when we measured the frequency of IFN-γ producing T cells specific to ESAT-6 or CFP-10 or the amount of IFN-γ or IL-10 produced in response to M.tb culture filtrate protein in PBMCs. Similarly, we saw no statistically significant effect of age on CCR7+ subsets of CD4 or CD8 T cells among those with LTBI or active TB. Therefore, among these measurements of M.tb specific and global T cell phenotype and function, we saw no evidence of impaired peripheral T cell immunity as a dominant cause of biological susceptibility of the elderly to develop active TB. The discordance of our functional T cell data with these historical PPD results in the elderly is consistent with the work of Mori and Kobashi which demonstrated that though PPD positive results are dramatically reduced in patients over the age of 80 who have culture-positive TB, their reactivity to a CFP-10 and ESAT-6 IFN-γ release assay using peripheral blood is not diminished relative to younger adults (Kobashi et al., 2008; Mori et al., 2004). It is important to note that ESAT-6 and CFP-10 are immunodominant M.tb antigens and are not present in BCG or most nontuberculous mycobacteria (NTM), including M. avium. We chose M.tb culture filtrate protein, which does contain many epitopes in NTM and BCG, to measure total cytokine production in subject’s PBMCs to assess the wider antigenic response to M.tb. Importantly, the median LTBI and active TB subject IFN-γ response to culture filtrate protein was more than 10-fold higher than that of controls (Figure 1C). This suggests that most of the response to culture filtrate protein was specific to M.tb complex. Interestingly, we observed decreased IFN-γ production in response to CFP in the elderly who had no history of M.tb infection, relative to adults (Figure 1C). Because BCG status had no effect on this observation and we do not expect significantly different prior NTM exposure between the adult and elderly controls, we believe this suggests hypo-responsiveness of the innate immune system of the elderly to the mycobacterial antigens in CFP rather than a decreased adaptive response.
We cannot rule out the possibility that M.tb-specific T cell function in the elderly may be impaired within the microenvironment of M.tb infected tissues, especially the lung. However, published data for adaptive immune responses in the lungs of aged human subjects are extremely limited. Our lab has previously shown that ex vivo responses to M.tb antigens are comparable between blood and lung among different strains of mice with different TB disease susceptibility, suggesting that our observations of no difference in M.tb-specific T cell function in the blood of different age groups may hold true in the lung (Beamer et al., 2008b). While previous work in an intravenous route M.tb infection model has shown that aged mice are more likely than young mice to die from a primary M.tb infection and delayed recruitment of CD4 T cells to the spleen contributes to this susceptibility, no study has demonstrated a clear defect in CD4 or CD8 T cell immunity to M.tb in aged mice using a pulmonary infection model (Orme, 1987; Orme et al., 1993; Wang et al., 2012).
In the case of general in vivo T cell immune function in elderly humans, several studies have previously shown that the impaired DTH response in the skin to recall antigens in the elderly is a general phenomenon to numerous antigens, not just PPD from M.tb (Castle et al., 1990; Agius et al., 2009). Agius et al have shown that in the case of Candida albicans antigens, the reduced skin DTH response in the elderly is due to defective activation of dermal blood vessels resulting from decreased TNF-α secretion by cutaneous macrophages (Agius et al., 2009). In this study, the intrinsic ability of peripheral blood T cells to migrate or of isolated cutaneous macrophages to secrete TNF-α upon in vitro stimulation were not impaired in the elderly. More recently, Vukmanovic-Stejic et al. showed that the impaired DTH to varicella zoster virus (VZV) in the elderly is associated with an antigen-nonspecific over exuberant proinflammatory response to injected saline, which was particularly associated with transient increased HLA-DR+, CD14+ and/or CD16+ mononuclear phagocytes likely recruited from the blood (Vukmanovic-Stejic et al., 2017). Contrary to the DTR to Candida albicans, early dermal blood vessel activation in response to VZV antigens was not impaired in the elderly (Agius et al., 2009; Vukmanovic-Stejic et al., 2017). Treatment with a p38 MAP kinase inhibitor significantly reduced plasma C reactive protein levels, decreased peripheral blood monocyte secretion of IL-6 and TNF-α and enhanced the VZV-specific DTR response in the skin of old subjects (Vukmanovic-Stejic et al., 2017).
Interestingly, we observed an age-dependent increase in nonclassical monocyte proportion in elderly subjects with LTBI. We hypothesize that this monocyte subset may play a role in the susceptibility of the elderly to develop active TB, possibly by impairing T cell responses at the site of infection, as in the cited studies in the skin (Vukmanovic-Stejic et al., 2017). It has been shown that in adults with pulmonary TB, the proportion of CD16+ monocytes is increased and this correlates with disease severity and TNF-α plasma levels (Balboa et al., 2011; Lastrucci et al., 2015). Moreover, this monocyte subpopulation is more permissive for M.tb growth, more migratory and is inhibited in the ability to stimulate T cell proliferation and cytokine production (Lastrucci et al., 2015). Therefore, we hypothesize that nonclassical monocytes in the elderly may play an excessively inflammatory role at the site of infection, be more permissive to M.tb growth and impair T cell responses at the site of infection. Current studies in our laboratory are ongoing to assess this hypothesis in the elderly. Our work does not preclude the possibility that the anti-mycobacterial functions of classical and intermediate monocytes are also impaired in the elderly, which has been suggested by one study (Guerra-Laso et al., 2013). Our work has the potential to link the known widespread low-grade systemic inflammation of old age, termed “inflammaging,” with the elderly’s specific susceptibility to developing active TB (Franceschi and Campisi, 2014; Piergallini and Turner, 2017).
Our study is limited by sample size and available outcomes data, and thus our ability to comment on immunological correlates of susceptibility of the elderly to die from active TB is limited. We did observe a significantly increased monocyte/lymphocyte ratio in the elderly with active TB, relative to all other groups. In adults and children, an elevated monocyte/lymphocyte ratio has prospectively been associated with risk of developing active TB and dying from disease (Naranbhai et al., 2014a, 2014b). In adults, a very low monocyte/lymphocyte ratio has also been associated with risk of developing active TB (Naranbhai et al., 2014a). Thus, our additional observation of a decreased monocyte/lymphocyte ratio in elderly with LTBI relative to controls and adults with LTBI suggests its potential role in the risk of the elderly to develop active TB. Further research is needed to identify biomarkers that associate with poor outcomes in the elderly with TB, together with partially known risk factors for their more difficult diagnosis and treatment (Oshi et al., 2014; Velayutham et al., 2014).
Our study is one of the first in decades to study immune function in the elderly with LTBI and active TB. Importantly, our study also included a M.tb-uninfected cohort to account for biological effects due solely to age. With regard to M.tb specific T cell frequency and production of cytokines important for M.tb control, our data show no evidence for M.tb-specific T cell dysfunction in the elderly with M.tb infection relative to adults, and our data is suggestive of an age-dependent alteration in monocyte proportion and phenotype that may correlate with susceptibility to active TB. While we do not provide a quantification due to our low sample size, our data are also consistent with the conclusion that the QFT and T-SPOT.TB tests provide concordant results in the elderly with LTBI or active TB. Although the sensitivities of these tests have been previously compared for diagnosing active TB in the elderly (Bae et al., 2016; Du et al., 2018), no published study has directly compared these tests in the elderly population with LTBI.
Our study has several limitations. The principle limitation is our low sample size in our elderly LTBI and both active TB groups. Therefore, our results are suggestive but not definitive for the parameters we measured. Together with this limitation, it was also difficult to control for the higher prevalence of diabetes, cancer and immunosuppressive treatment in the elderly in study recruitment. However, our multivariate analyses showed that most differences we observed between age groups among controls were due to age, independent of these three confounders and others for which we have data. Importantly, diabetes, cancer and immunosuppressive treatment did not confound our conclusions for monocyte phenotypes in the elderly with LTBI, as only 1 subject from this group for whom we collected monocyte data had diabetes, and this subject had the lowest nonclassical monocyte proportion of the group (data not shown).
Additionally, in our small cohort, we could not distinguish between active TB developed soon after exposure to M.tb, termed primary TB, or disease which occurred years after initial exposure, termed reactivation TB. Finally, our elderly LTBI and active TB subjects were on average treated longer with TB medications before study recruitment than the adults, and the tissue distribution of TB disease was different between our adult and elderly subjects. Our low sample size precludes our ability to estimate the effect of these differences in M.tb infected subjects for our monocyte data.
Overall, our study highlights the need for further research into the biological reasons why the elderly are more susceptible to TB, so that public health systems can be better equipped to face the present and future problem of TB in an aging global population.
Highlights.
Human immune function stratified by age and infection or disease from tuberculosis
Tuberculosis-specific T cell number and cytokine production not impaired in elderly
Alterations in monocyte proportion and phenotype with age and tuberculosis status
Acknowledgments
We thank study participants as well as clinic staff at The Ohio State University Wexner Medical Center and Columbus Public Health for assistance in subject recruitment. We additionally thank Oxford Immunotec for providing discounted T-SPOT®.TB kits.
Funding
This work was supported by the National Institutes of Health [grant numbers R03-AG041129, P01-AG051428]. R.A. was supported by the Ohio State University Dean’s Distinguished University Fellowship.
Abbreviations
- CFP
culture filtrate protein
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- DTH
delayed type hypersensitivity
- HCT
hematocrit
- NTM
nontuberculous mycobacteria
- PBMC
peripheral blood mononuclear cell
- PPD
tuberculin-purified protein derivative
- QFT
QuantiFERON®TB-Gold Test
- SFUs
spot forming units
- SIADH
syndrome of inappropriate antidiuretic hormone secretion
- TST
tuberculin skin test
- VZV
varicella zoster virus
- WBC
white blood cell count
Footnotes
Declarations of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adambekov DA, Morozov VL. T-suppressors and their functional activity in tuberculosis in middle-aged and elderly subjects. Probl Tuberk. 1989:32–34. [PubMed] [Google Scholar]
- Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, Reed JR, Curnow SJ, Fuentes-Duculan J, Buckley CD, Salmon M, Taams LS, Krueger J, Greenwood J, Klein N, Rustin MHA, Akbar AN. Decreased TNF-α synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae W, Park KU, Song EY, Kim SJ, Lee YJ, Park JS, Cho YJ, Yoon HI, Yim JJ, Lee CT, Lee JH. Comparison of the Sensitivity of QuantiFERON-TB Gold In-Tube and T-SPOT.TB According to Patient Age. PLOS ONE. 2016;11:e0156917. doi: 10.1371/journal.pone.0156917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa L, Romero MM, Basile JI, García CASy, Schierloh P, Yokobori N, Geffner L, Musella RM, Castagnino J, Abbate E, de la Barrera S, Sasiain MC, Alemán M. Paradoxical role of CD16+CCR2+CCR5+ monocytes in tuberculosis: efficient APC in pleural effusion but also mark disease severity in blood. J Leukoc Biol. 2011;90:69–75. doi: 10.1189/jlb.1010577. [DOI] [PubMed] [Google Scholar]
- Beamer GL, Flaherty DK, Assogba BD, Stromberg P, Gonzalez-Juarrero M, de Malefyt RW, Vesosky B, Turner J. Interleukin-10 Promotes Mycobacterium tuberculosis Disease Progression in CBA/J Mice. J Immunol. 2008a;181:5545–5550. doi: 10.4049/jimmunol.181.8.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer GL, Flaherty DK, Vesosky B, Turner J. Peripheral Blood Gamma Interferon Release Assays Predict Lung Responses and Mycobacterium tuberculosis Disease Outcome in Mice. Clin Vaccine Immunol. 2008b;15:474–483. doi: 10.1128/CVI.00408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MPR, Graham CM, McNab FW, Xu Z, Bloch SAA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O’Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonecini-Almeida MG, Ho JL, Boéchat N, Huard RC, Chitale S, Doo H, Geng J, Rego L, Lazzarini LCO, Kritski AL, Johnson WD, McCaffrey TA, Silva JRLe. Down-Modulation of Lung Immune Responses by Interleukin-10 and Transforming Growth Factor β (TGF-β) and Analysis of TGF-β Receptors I and II in Active Tuberculosis. Infect Immun. 2004;72:2628–2634. doi: 10.1128/IAI.72.5.2628-2634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byng-Maddick R, Noursadeghi M. Does tuberculosis threaten our ageing populations? BMC Infect Dis. 2016;16:119. doi: 10.1186/s12879-016-1451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle SC, Norman DC, Perls TT, Chang MP, Yoshikawa TT, Makinodan T. Analysis of cutaneous delayed-type hypersensitivity reaction and T cell proliferative response in elderly nursing home patients: an approach to identifying immunodeficient patients. Gerontology. 1990;36:217–229. doi: 10.1159/000213203. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] [accessed 3.6.18];Reported Tuberculosis in the United States, 2016 [WWW Document] 2017 URL https://www.cdc.gov/tb/statistics/reports/2016/pdfs/2016_Surveillance_FullReport.pdf.
- Commandeur S, van Meijgaarden KE, Lin MY, Franken KLMC, Friggen AH, Drijfhout JW, Oftung F, Korsvold GE, Geluk A, Ottenhoff THM. Identification of Human T-Cell Responses to Mycobacterium tuberculosis Resuscitation-Promoting Factors in Long-Term Latently Infected Individuals. Clin Vaccine Immunol. 2011;18:676–683. doi: 10.1128/CVI.00492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorken E, Grzybowski S, Allen EA. Significance of the Tuberculin Test in the Elderly. Chest. 1987;92:237–240. doi: 10.1378/chest.92.2.237. [DOI] [PubMed] [Google Scholar]
- Du F, Xie L, Zhang Y, Gao F, Zhang H, Chen W, Sun B, Sha W, Fang Y, Jia H, Xing A, Du B, Zheng L, Gao M, Zhang Z. Prospective Comparison of QFT-GIT and T-SPOT. TB Assays for Diagnosis of Active Tuberculosis. Sci Rep. 2018;8:5882. doi: 10.1038/s41598-018-24285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J Gerontol Ser A. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–666. doi: 10.1016/S1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- Glaziou P, Sismanidis C, Floyd K, Raviglione M. Global Epidemiology of Tuberculosis. Cold Spring Harb Perspect Med. 2015;5:a017798. doi: 10.1101/cshperspect.a017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Laso JM, González-García S, González-Cortés C, Diez-Tascón C, López-Medrano R, Rivero-Lezcano OM. Macrophages from elders are more permissive to intracellular multiplication of Mycobacterium tuberculosis. AGE. 2013;35:1235–1250. doi: 10.1007/s11357-012-9451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetta G, Kirschner D. The Roles of Immune Memory and Aging in Protective Immunity and Endogenous Reactivation of Tuberculosis. PLOS ONE. 2013;8:e60425. doi: 10.1371/journal.pone.0060425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S. Lymphocyte function of pulmonary tuberculosis in the elderly. Kekkaku. 1989;64:669–670. [PubMed] [Google Scholar]
- Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–875. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- Hochberg NS, Horsburgh CR. Prevention of Tuberculosis in Older Adults in the United States: Obstacles and Opportunities. Clin Infect Dis. 2013;56:1240–1247. doi: 10.1093/cid/cit027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh CR, O’Donnell M, Chamblee S, Moreland JL, Johnson J, Marsh BJ, Narita M, Johnson LS, von Reyn CF. Revisiting Rates of Reactivation Tuberculosis. Am J Respir Crit Care Med. 2010;182:420–425. doi: 10.1164/rccm.200909-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation. [accessed 3.6.18];Global Burden of Disease [WWW Document] 2016 URL http://www.healthdata.org/gbd.
- Jamil B, Shahid F, Hasan Z, Nasir N, Razzaki T, Dawood G, Hussain R. Interferonγ/IL10 ratio defines the disease severity in pulmonary and extra pulmonary tuberculosis. Tuberculosis. 2007;87:279–287. doi: 10.1016/j.tube.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kobashi Y, Mouri K, Yagi S, Obase Y, Miyashita N, Okimoto N, Matsushima T, Kageoka T, Oka M. Clinical Utility of the QuantiFERON TB-2G Test for Elderly Patients With Active Tuberculosis. Chest. 2008;133:1196–1202. doi: 10.1378/chest.07-1995. [DOI] [PubMed] [Google Scholar]
- Koch S, Larbi A, Derhovanessian E, Özcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastrucci C, Bénard A, Balboa L, Pingris K, Souriant S, Poincloux R, Al Saati T, Rasolofo V, González-Montaner P, Inwentarz S, Moraña EJ, Kondova I, Verreck FA, del Sasiain MC, Neyrolles O, Maridonneau-Parini I, Lugo-Villarino G, Cougoule C. Tuberculosis is associated with expansion of a motile, permissive and immunomodulatory CD16+ monocyte population via the IL-10/STAT3 axis. Cell Res. 2015;25:1333–1351. doi: 10.1038/cr.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang L, Liu X, Wang L, Wang X, Wang J, Qian J. The role of in vitro interferonγ-release assay in differentiating intestinal tuberculosis from Crohn’s disease in China. J Crohns Colitis. 2012;6:317–323. doi: 10.1016/j.crohns.2011.09.002. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Rossi R, Nshimyumukiza L, Wusiman A, Zdraveska N, Eldin MS. Convergence of a diabetes mellitus, protein energy malnutrition, and TB epidemic: the neglected elderly population. BMC Infect Dis. 2016;16:361. doi: 10.1186/s12879-016-1718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M, Suzuki K, Inoue Y, Tsuyuguchi K, Sasaki Y, Mazurek GH, Tsuyuguchi I. Specific Detection of Tuberculosis Infection. Am J Respir Crit Care Med. 2004;170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- Naranbhai V, Hill AVS, Karim A, SS, Naidoo K, Abdool Karim Q, Warimwe GM, McShane H, Fletcher H. Ratio of Monocytes to Lymphocytes in Peripheral Blood Identifies Adults at Risk of Incident Tuberculosis Among HIV-Infected Adults Initiating Antiretroviral Therapy. J Infect Dis. 2014a;209:500–509. doi: 10.1093/infdis/jit494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranbhai V, Kim S, Fletcher H, Cotton MF, Violari A, Mitchell C, Nachman S, McSherry G, McShane H, Hill AV, Madhi SA. The association between the ratio of monocytes:lymphocytes at age 3 months and risk of tuberculosis (TB) in the first two years of life. BMC Med. 2014b;12:120. doi: 10.1186/s12916-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults – time to take notice. Int J Infect Dis, Special Issue: Commemorating World Tuberculosis Day 2015. 2015;32:135–137. doi: 10.1016/j.ijid.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a Risk Factor for Disease. Curr Biol. 2012;22:R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired Functions of Peripheral Blood Monocyte Subpopulations in Aged Humans. J Clin Immunol. 2010;30:806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme IM. Aging and immunity to tuberculosis: increased susceptibility of old mice reflects a decreased capacity to generate mediator T lymphocytes. J Immunol. 1987;138:4414–4418. [PubMed] [Google Scholar]
- Orme IM, Griffin JP, Roberts AD, Ernst DN. Evidence for a Defective Accumulation of Protective T Cells in Old Mice Infected with Mycobacterium tuberculosis. Cell Immunol. 1993;147:222–229. doi: 10.1006/cimm.1993.1062. [DOI] [PubMed] [Google Scholar]
- Oshi DC, Oshi SN, Alobu I, Ukwaja KN. Profile and treatment outcomes of tuberculosis in the elderly in southeastern Nigeria, 2011–2012. PloS One. 2014;9:e111910. doi: 10.1371/journal.pone.0111910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BD, Yarbro JR. Aging impairs mitochondrial respiratory capacity in classical monocytes. Exp Gerontol. 2018;108:112–117. doi: 10.1016/j.exger.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Piergallini TJ, Turner J. Tuberculosis in the elderly: Why inflammation matters. Exp Gerontol. 2017 doi: 10.1016/j.exger.2017.12.021. [DOI] [PMC free article] [PubMed]
- Powell KE, Farer LS. The Rising Age of the Tuberculosis Patient: A Sign of Success and Failure. J Infect Dis. 1980;142:946–948. doi: 10.1093/infdis/142.6.946. [DOI] [PubMed] [Google Scholar]
- Puchta A, Naidoo A, Verschoor CP, Loukov D, Thevaranjan N, Mandur TS, Nguyen P, Jordana M, Loeb M, Xing Z, Kobzik L, Larché MJ, Bowdish DME. TNF Drives Monocyte Dysfunction with Age and Results in Impaired Anti-pneumococcal Immunity. PLOS Pathog. 2016;12:e1005368. doi: 10.1371/journal.ppat.1005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S. Tuberculosis in Older Adults. Clin Geriatr Med, Infectious Diseases in Geriatric Medicine. 2016;32:479–491. doi: 10.1016/j.cger.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Richeldi L. Tuberculosis Diagnostic Tests: Sensitivity, Specificity, and Comparing Apples with Apples. Am J Respir Crit Care Med. 2006;174:953a–954. doi: 10.1164/ajrccm.174.8.953a. [DOI] [PubMed] [Google Scholar]
- Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Große-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Böhm M, Fliser D, Heine GH. CD14++CD16+ Monocytes Independently Predict Cardiovascular Events: A Cohort Study of 951 Patients Referred for Elective Coronary Angiography. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol, Host pathogens • Immune senescence. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrupp L, Bradley S, Smith P, Simor A, Gantz N, Crossley K, Loeb M, Strausbaugh L, Nicolle L, Committee SL. Tuberculosis Prevention and Control in Long-Term–Care Facilities for Older Adults. Infect Control Hosp Epidemiol. 2004;25:1097–1108. doi: 10.1086/502350. [DOI] [PubMed] [Google Scholar]
- Tocque K, Bellis MA, Tam CM, Chan SL, Syed Q, Remmington T, Davies PDO. Long-term Trends in Tuberculosis. Am J Respir Crit Care Med. 1998;158:484–488. doi: 10.1164/ajrccm.158.2.9709125. [DOI] [PubMed] [Google Scholar]
- US Census Bureau. [accessed 3.15.18];An Aging World: 2015 [WWW Document] 2016 URL https://www.census.gov/library/publications/2016/demo/P95-16-1.html.
- Van den Brande P, Demedts M. Four-Stage Tuberculin Testing in Elderly Subjects Induces Age-Dependent Progressive Boosting. Chest. 1992;101:447–450. doi: 10.1378/chest.101.2.447. [DOI] [PubMed] [Google Scholar]
- Velayutham BRV, Nair D, Chandrasekaran V, Raman B, Sekar G, Watson B, Charles N, Malaisamy M, Thomas A, Swaminathan S. Profile and response to anti-tuberculosis treatment among elderly tuberculosis patients treated under the TB Control programme in South India. PloS One. 2014;9:e88045. doi: 10.1371/journal.pone.0088045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Chambers ES, Suárez-Fariñas M, Sandhu D, Fuentes-Duculan J, Patel N, Agius E, Lacy KE, Turner CT, Larbi A, Birault V, Noursadeghi M, Mabbott NA, Rustin MHA, Krueger JG, Akbar AN. Enhancement of cutaneous immunity during aging by blocking p38 mitogen-activated protein (MAP) kinase–induced inflammation. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.10.032. [DOI] [PMC free article] [PubMed]
- Vynnycky E, Fine PEM. Lifetime Risks, Incubation Period, and Serial Interval of Tuberculosis. Am J Epidemiol. 2000;152:247–263. doi: 10.1093/aje/152.3.247. [DOI] [PubMed] [Google Scholar]
- Wang S-H, Carruthers B, Turner J. The Influence of Increasing Age on Susceptibility of the Elderly to Tuberculosis. Open Longev Sci. 2012:6. [Google Scholar]
- Wiker HG, Mustafa T, Bjune GA, Harboe M. Evidence for waning of latency in a cohort study of tuberculosis. BMC Infect Dis. 2010;10:37. doi: 10.1186/1471-2334-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KL, Yeap WH, Tai JJY, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization [WHO] [accessed 3.6.18];Global tuberculosis report 2017 [WWW Document] 2017 URL http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1.
- Yan J, Greer JM, Hull R, O’Sullivan JD, Henderson RD, Read SJ, McCombe PA. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew Wing W, Takashi Yoshiyama, Leung Chi C, Chan Denise P. Epidemiological, clinical and mechanistic perspectives of tuberculosis in older people. Respirology. 2018 doi: 10.1111/resp.13303. [DOI] [PubMed]
- Yoshikawa TT. Perspective: Aging and Infectious Diseases: Past, Present, and Future. J Infect Dis. 1997;176:1053–1057. doi: 10.1086/516547. [DOI] [PubMed] [Google Scholar]