Abstract
Evolving literature suggests that the epidemic of prescription opioid use affects the transplant population. We examined a novel database wherein national U.S. transplant registry records were linked to a large pharmaceutical claims warehouse (2007 to 2015) to characterize prescription opioid use before and after kidney transplant, and associations (adjusted hazard ratio, 95% LCLaHR95% UCL) with death and graft loss. Among 75,430 eligible patients, 43.1% filled opioids in the year before transplant. Use was more common among recipients who were women, white, unemployed, publicly insured, and with longer pre-transplant dialysis. Of those with the highest level of pre-transplant opioid use, 60% continued high-level use post-transplant. Pre-transplant opioid use had graded associations with 1-year post-transplant outcomes; the highest-level use predicted 46% increased risk of death (aHR 1.281.461.66) and 28% increased risk of all-cause graft failure (aHR 1.171.281.41). Effects of high-level opioid use in the first year after transplant were stronger, predicting twice the risk of death (aHR 1.932.242.60) and 68% higher all-cause graft loss risk (aHR 1.501.681.89) over the subsequent year; increased risk persisted over 5 years. While associations may, in part, reflect underlying conditions or behaviors, opioid use history is relevant in assessing and providing care to transplant candidates and recipients.
INTRODUCTION
Candidates for transplant undergo evaluation of the severity of end-stage organ failure, comorbid conditions, overall fitness for surgery, and psychosocial status including risk factors for poor adherence after transplant.(1, 2) Similarly, living donor candidates are carefully evaluated for medical and psychosocial suitability for donation.(3) Medication therapies are reviewed to assess treatment appropriateness and identify possible drug interactions that might occur after surgery. However, while substantial attention is invested in the evaluation clinic to identify traditional risk factors, we often overlook medications that might be markers for serious conditions, lack of adherence, or other behaviors that may affect patient and allograft survival. Until recently, use of pharmaceutical claims to quantitatively inform expectations for clinical outcomes before and after transplant and living organ donation has not been described.
Opioid analgesics serve an important role in management of acute and chronic pain, but recognition of an “epidemic” of complications related to the over-prescribing, misuse, and inherent potential toxicity of prescription opioids is growing.(4, 5) The daily news resounds with descriptions of this epidemic and the national surge in opioid-related deaths.(6–8) Pre-surgical use of opioid analgesics is increasingly recognized as a predictor of post-operative complications and resource utilization in diverse populations, including those undergoing general, orthopedic, and liver transplant surgeries, as well as living donor nephrectomy procedures (9–12). Concerns about opioid-related toxicity are even greater in patients with end-stage organ failure due to altered drug protein binding, metabolism, and excretion, leading to accumulation of parent agents and potentially toxic metabolites.(13, 14) Patients who use high levels of opioids may also have increased risk of nonadherence to prescribed care.
Recently, we developed a novel integrated database incorporating dispensed prescription records from a large pharmaceutical claims warehouse with the national transplant registry data. Using these linked data, we identified adverse clinical outcomes in transplant recipients who filled prescription opioids before surgery, including increased risk of death and graft failure after liver transplant,(11) and increased risk of readmission after living donor nephrectomy.(12) Among samples of kidney transplant recipients, 2005–2010, we found that compared with those who did not fill opioids, patients with the highest level of pre-transplant opioid use had a 63% increased risk of death and a 41% increased risk of all-cause graft failure,(15) and increased risks of posttransplant complications, such as cardiovascular, respiratory, or neurological events.(16) These findings resonate with a new report of graded increases in death rates with higher prescription opioid doses in dialysis patients.(17)
To address the effect of America’s opioid epidemic on kidney transplant recipients, we extended our previous work examining associations between pre-transplant opioid use and transplant outcomes by studying a larger, more contemporary cohort of recipients who underwent kidney transplant through 2015. In addition, we examined relationships of pre-transplant opioid use with opioid fills in the year after transplant, and associations of post-transplant use with subsequent outcomes. We hypothesized that pre- and post-transplant prescription opioid use are associated with patient and graft outcomes after kidney transplantation, and risk may increase with higher levels of use.
METHODS
Data Sources
We conducted a retrospective cohort study using linked healthcare databases in the U.S. to ascertain patient characteristics, pharmacy fill records, and outcome events for kidney transplant recipients. This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR system includes data on all donors, waitlist candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors.
Pharmacy fill data were assembled by linking SRTR records for kidney transplant recipients with billing claims from Symphony Health Solutions (SHS), a large U.S. pharmaceutical claims data warehouse that collects prescription drug fill records including self-paid fills and those reimbursed by private and public payers. SHS comprises National Council for Prescription Drug Program format prescription claims aggregated from multiple sources including claims warehouses, retail pharmacies, and prescription benefit managers for approximately 60% of U.S. retail pharmacy transactions. Individual claim records include the date of a given pharmacy fill with the national drug code identifying agent and dosage. After Institutional Review Board and HRSA approvals, SHS records were linked with SRTR records for kidney transplant recipients. We applied a deterministic de-identification strategy wherein patient identifiers (last name, first name, date of birth, sex, and ZIP code of residence) were transformed before delivery to the Saint Louis University researchers with Health Information Portability and Accountability Act and HITECH-certified encryption technology from SHS. The patient de-identification software employs multiple encryption algorithms in succession to guarantee that the resulting “token” containing encrypted patient identifiers can never be decrypted. However, the algorithm yields the same results for a given set of data elements, such that linkages by unique anonymous tokens are possible.
All direct identifiers were removed before the final dataset was available for analysis. Because of the large sample size, the anonymity of the patients studied, and the non-intrusive nature of the research, a waiver of informed consent was granted per the Department of Health and Human Services Code of Federal Regulations (Title 45, Part 46, Paragraph 46.116).
Population and Covariates
We included kidney transplant recipients who underwent transplant between 2007 and 2015 and who had 1 year of pretransplant pharmacy fill records in SHS. We examined a subgroup of the cohort with available data in the records to ascertain prescription opioid use in the 1 year before and after transplant. Transplant recipient clinical and demographic characteristics, and characteristics of the donated organ and other transplant factors, were defined by the OPTN Transplant Candidate Registration and Transplant Recipient Registration forms (Table 1).
Table 1.
Distributions of clinical traits of kidney transplant recipients by level of pre-transplant opioid use
| Baseline Characteristics at the Time of Transplant | No Use (N=42,947) | Level 1 (N=16,060) | Level 2 (N=5,184) | Level 3 (N=2,649) | Level 4 (N=8,590) |
|---|---|---|---|---|---|
| Age, years | ‡ | ‡ | ‡ | ‡ | |
| <18 | 6.8 | 3.3 | 2.0 | 1.9 | 2.0 |
| 18 to 30 | 7.0 | 9.1 | 9.5 | 9.1 | 8.0 |
| 31 to 44 | 17.8 | 21.4 | 22.3 | 22.5 | 22.6 |
| 45 to 59 | 35.3 | 36.9 | 37.9 | 37.9 | 42.5 |
| ≥60 | 33.1 | 29.2 | 28.3 | 28.7 | 24.8 |
| Female | 38.2 | 40.2‡ | 41.0‡ | 42.6‡ | 40.9‡ |
| Race | ‡ | ‡ | ‡ | ‡ | |
| White | 52.6 | 52.8 | 53.6 | 54.4 | 56.1 |
| African-American | 24.8 | 28.2 | 30.3 | 29.9 | 30.2 |
| Hispanic | 15.1 | 13.1 | 11.7 | 11.3 | 10.6 |
| Other | 7.6 | 6.0 | 4.5 | 4.4 | 3.1 |
| Highest level of education | ‡ | * | * | ‡ | |
| College or higher | 47.8 | 49.5 | 47.8 | 45.9 | 42.1 |
| Grade/High school | 42.7 | 42.7 | 43.8 | 45.9 | 49.1 |
| Missing | 9.6 | 7.9 | 8.4 | 8.2 | 8.8 |
| Employment status | ‡ | ‡ | ‡ | ‡ | |
| Working | 31.5 | 33.1 | 29.2 | 26.8 | 19.6 |
| Not working | 54.9 | 55.9 | 61.5 | 63.4 | 70.4 |
| Missing | 13.6 | 10.9 | 9.3 | 9.8 | 10.1 |
| Health insurance type | * | * | ‡ | ‡ | |
| Private | 37.7 | 39.1 | 35.5 | 32.2 | 25.2 |
| Public | 61.9 | 60.5 | 64.1 | 67.4 | 74.5 |
| Missing | 0.4 | 0.4 | 0.3 | 0.3 | 0.4 |
| Body mass index, kg/m2 | ‡ | ‡ | ‡ | ‡ | |
| <18.5 | 5.0 | 2.7 | 2.7 | 3.3 | 3.5 |
| 18.5 to 24.9 | 29.2 | 28.5 | 27.6 | 27.2 | 27.8 |
| 25 to 30 | 31.7 | 32.0 | 32.1 | 30.4 | 28.3 |
| >30 | 31.6 | 34.4 | 35.2 | 36.7 | 37.4 |
| Missing | 2.5 | 2.4 | 2.4 | 2.4 | 3.0 |
| Physical capacity status | ǂ | ǂ | ǂ | ǂ | |
| Not limited | 64.8 | 66.2 | 65.4 | 63.6 | 62.5 |
| Limited | 5.9 | 6.5 | 8.2 | 8.4 | 9.6 |
| Missing | 29.3 | 27.3 | 26.5 | 28.0 | 28.0 |
| Comorbid conditions | |||||
| Hypertension | 77.9 | 79.2* | 78.1 | 79.6* | 78.8 |
| Diabetes mellitus | 31.2 | 32.3* | 33.9‡ | 35.3‡ | 34.1‡ |
| Coronary artery disease | 5.4 | 5.7 | 6.3* | 6.8* | 6.9‡ |
| Cerebral vascular disease | 2.2 | 2.2 | 2.3 | 2.9* | 3.0‡ |
| PVD | 3.2 | 3.2 | 3.6 | 4.3 | 4.5‡ |
| COPD | 1.0 | 1.3* | 1.3 | 1.1 | 1.8‡ |
| Cause of ESRD | ‡ | ‡ | † | ‡ | |
| Hypertension | 25.3 | 25.3 | 25.1 | 25.2 | 25.2 |
| Diabetes mellitus | 22.8 | 23.6 | 24.6 | 25.5 | 24.7 |
| Glomerulonephritis | 23.0 | 24.9 | 23.8 | 23.3 | 23.0 |
| Polycystic kidney disease | 9.9 | 10.0 | 10.3 | 9.6 | 9.9 |
| Other | 19.1 | 16.3 | 16.2 | 16.4 | 17.3 |
| Duration of dialysis, months | ‡ | ‡ | ‡ | ‡ | |
| None (pre-emptive) | 21.3 | 16.4 | 11.8 | 10.9 | 8.9 |
| 0.1 to 24 | 26.9 | 35.9 | 37.9 | 36.0 | 28.1 |
| 25 to 60 | 31.3 | 28.1 | 29.0 | 29.8 | 35.0 |
| >60 | 19.6 | 18.8 | 20.3 | 22.6 | 27.3 |
| Missing | 0.8 | 0.9 | 1.0 | 0.6 | 0.8 |
| Peak PRA level | ‡ | ‡ | ‡ | ‡ | |
| <10 | 70.5 | 68.4 | 66.5 | 66.9 | 65.4 |
| 10 to 79 | 17.6 | 19.0 | 19.7 | 20.4 | 19.7 |
| ≥80 | 6.1 | 7.0 | 7.4 | 7.7 | 9.3 |
| Missing | 5.7 | 5.7 | 6.5 | 5.0 | 5.5 |
| HLA mismatches | ‡ | ||||
| Zero A, B, DR | 7.6 | 7.7 | 8.1 | 8.7 | 9.1 |
| Zero DR | 11.1 | 11.3 | 11.9 | 10.5 | 11.6 |
| Other | 81.3 | 81.0 | 80.0 | 80.8 | 79.3 |
| Previous organ transplant | 12.6 | 13.9 | 15.3 | 15.4 | 16.7 |
| Era of current transplant | ‡ | ‡ | * | ‡ | |
| 2007 to 2009 | 20.3 | 19.1 | 17.9 | 19.2 | 18.8 |
| 2010 to 2012 | 44.9 | 47.1 | 48.7 | 47.8 | 47.8 |
| 2013 to 2015 | 34.8 | 33.8 | 33.4 | 33.1 | 33.5 |
| Donor type | ‡ | * | ‡ | ||
| Living | 36.2 | 39.8 | 38.3 | 35.6 | 31.9 |
| Deceased (SCD) | 44.0 | 42.4 | 42.9 | 45.5 | 48.9 |
| Deceased (ECD) | 9.8 | 8.5 | 8.5 | 9.1 | 8.7 |
| Deceased (DCD) | 10.1 | 9.3 | 10.2 | 9.9 | 10.5 |
| Cold ischemia time, hours | ǂ | * | |||
| <12 | 49.8 | 52.1 | 50.6 | 49.3 | 47.7 |
| 13 to 24 | 31.3 | 29.9 | 31.6 | 33.6 | 34.7 |
| 25 to 36 | 9.3 | 8.7 | 9.2 | 8.7 | 9.8 |
| >36 | 2.6 | 2.3 | 2.1 | 2.3 | 2.0 |
| Missing | 7.0 | 6.9 | 6.5 | 6.1 | 5.8 |
Data presented as percentages (%)
p<0.05–0.002;
p=0.001–0.0002;
p<0.0001.
Level 1: 300 mg, Level 2: 301–600 mg, Level 3: 601–1000 mg, and Level 4: >1000 mg per year.
Abbreviations: COPD, chronic obstructive pulmonary disease; DCD, donation after cardiac death; ECD, expanded criteria donor; ESRD, end-stage renal disease; HLA, human leukocyte antigens; PRA, panel reactive antibody; PVD, peripheral vascular disease; SCD, standard criteria donor.
Pharmacy fills for opioid analgesics in the 1 year before and after transplant were ascertained from the SHS records. Opioid use was normalized to morphine equivalents (ME), according to conversion ratios, as previously described (Table S1).(7,8) Pre- and post-transplant ME were aggregated, separately within each period, for each recipient and expressed as dose (mg) of ME exposure over the year. We ranked annual ME exposure among recipients who filled opioid prescriptions by levels as: level 1, ≤300 mg; level 2, 301–600 mg; level 3, 601–1000 mg; and level 4, >1000 mg per year, similar to previous methods.(11, 12, 15, 16) We performed sensitivity analyses considering opioid filling patterns within 6 months and in months 7 to 12 prior to transplant.
Outcome Measurements
The primary outcomes were all-cause mortality and graft failure. Graft failure was defined as return to maintenance dialysis or re-transplant. All-cause graft failure included graft loss due to patient death. In the analysis of pre-transplant opioid use, outcomes were assessed at the 1-year posttransplant date. In the analysis of post-transplant opioid use, outcomes were assessed between the 1- and 2-year posttransplant dates. Observation time was censored at the end of follow-up (December 31, 2016).
Statistical Analyses
Datasets were merged and analyzed with SAS (Statistical Analysis Software) version 9.4 (SAS Institute Inc., Cary, NC). Distributions of clinical and demographic traits among recipients with each level of pre-transplant opioid exposure, compared with no opioid use, were compared by Chi-square test. Propensity models for the likelihood of any opioid use in the pre-transplant period and the first year posttransplant were constructed by multivariate logistic regression. Adjusted associations of pre- and posttransplant opioid use with posttransplant death and graft failure (adjusted hazard ratio with 95% upper and lower confidence limits, LCLaHRUCL) were quantified by multivariate Cox regression including adjustment for recipient, donor, and transplant clinical factors. In all outcome analyses, we interpreted two-tailed p-values <0.05 as statistically significant.
RESULTS
Baseline Characteristics of Kidney Transplant Recipients
A STROBE checklist for the study is provided in Table S1. Between 2007 and 2015, 117,931 adult kidney transplant recipients in the U.S. were recorded in the SRTR database. Of these, 75,430 (64.0%) also had available pre-transplant medication data in SHS (Table 1) and 76,187 (64.6%) had available posttransplant medication data in SHS (Table S3). In the study sample with available pretransplant medication data, mean age at the time of transplant was 49.8 years (standard deviation, 15.8 years); 39.3% were female, 53.1% were white, and 26.7% were African American. In the study sample, 32,483 recipients (43.1%) filled ≥1 opioid prescription in the year before transplant. Compared with recipients who did not use opioids before transplant, those with the highest level of use (level 4) were more often aged 45–59 years, women, of white race, unemployed, publicly insured, and less likely to be college educated (Table 1). Recipients with level 4 pre-transplant opioid use were also more likely to be obese (body mass index >30 kg/m2), and to have comorbid conditions (diabetes mellitus, coronary artery disease, cerebral vascular disease, peripheral vascular disease, chronic obstructive pulmonary disease), longer pre-transplant dialysis duration, and higher rates of sensitization.
Death and Graft Failure According to Pre-transplant Opioid Use Level
Overall, 2.5% of recipients died within the first year post-transplant. Incidence of 1-year death-censored graft failure and all-cause graft failure were 3.0% and 3.2%, respectively, occurring at median times from transplant of 54 and 62 days, respectively. Compared with recipients with no pre-transplant opioid use, those with level 4 use had an increased risk of death (3.9% vs. 2.5%, p<0.0001), death-censored graft failure (3.9% vs. 3.1%, p=0.0002), and all-cause graft loss (7.2% vs. 5.1%, p<0.0001) (Figure 1). After adjustment for demographic, clinical, and procedure factors, level 4 pre-transplant opioid use was associated with a 46% increased risk of death within the first year posttransplant (aHR 1.281.461.66, p<0.0001), and with an increased risk of death-censored graft failure (aHR 1.011.151.30, p=0.031) and all-cause graft failure (aHR 1.171.281.41, p<0.0001) compared with no use (Table 2, Figure 2). Considered in terms of fills within >0–6 months and 7–12 months before transplant, we also observed graded increased in the risks of death and graft loss with higher-level opioid use in either period, although risk is greatest with the combination of high-level use in both periods (Figure S1). For example, level 4 use in both pre-transplant periods was associated with 78% higher risk of death (aHR 1.611.781.96) and 51% higher risk of all-cause graft failure (aHR 1.341.511.69) in the year after transplant.
Figure 1.
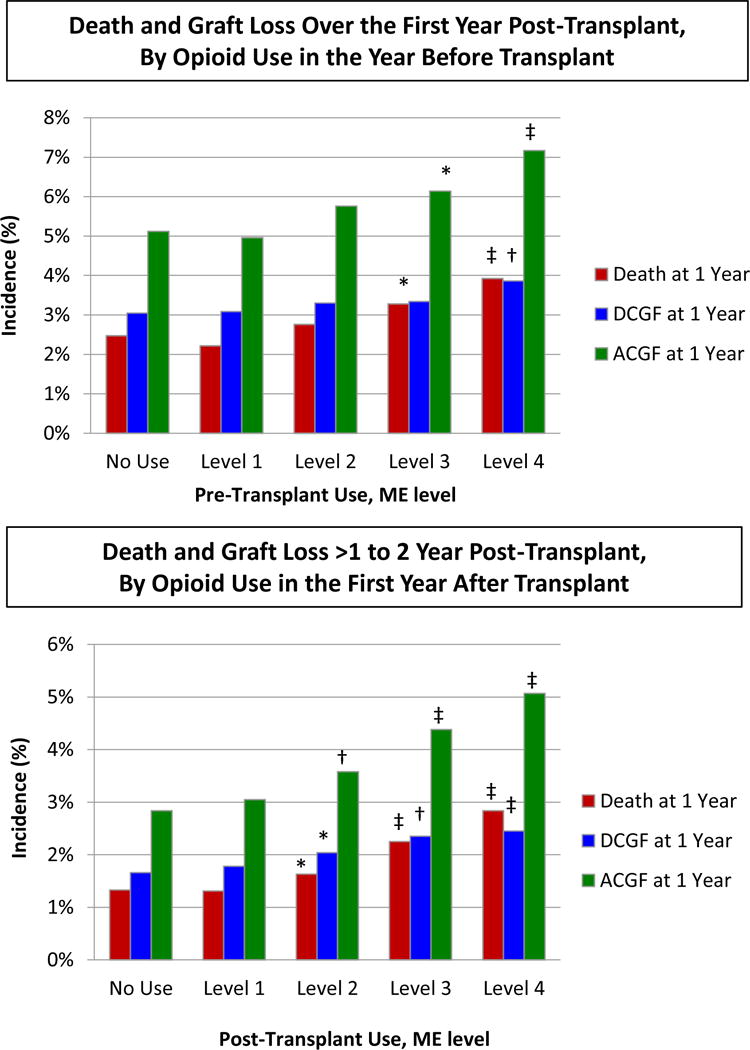
The incidence of death and graft failure according to prescription opioid use before and after kidney transplant. *p<0.05–0.002; †p=0.001–0.0002; ‡p<0.0001. Level 1, ≤300 mg; level 2, 301–600 mg; level 3, 601–1000 mg; level 4, >1000 mg per year. Abbreviations: ACGF, all-cause graft failure; DCGF, death-censored graft failure; ME, morphine equivalents.
Table 2.
Adjusted associations of clinical factors with death and graft failure within the first year post-transplant
| Clinical Factor | Death | Death-Censored Graft Failure | All-Cause Graft Failure |
|---|---|---|---|
| Pre-transplant opioid use | |||
| No use | Reference | Reference | Reference |
| Level 1 | 0.95 (0.84–1.07) | 1.03 (0.92–1.14) | 1.00 (0.92–1.09) |
| Level 2 | 1.12 (0.93–1.34) | 1.06 (0.90–1.24) | 1.11 (0.98–1.26) |
| Level 3 | 1.24 (0.99–1.55) | 1.02 (0.82–1.28) | 1.11 (0.95–1.31) |
| Level 4 | 1.46 (1.28–1.66)‡ | 1.15 (1.01–1.30)* | 1.28 (1.17–1.41)‡ |
| Age, years | |||
| <18 | 0.87 (0.44–1.72) | 1.23 (0.79–1.92) | 1.23 (0.86–1.77) |
| 18 to 30 | Reference | Reference | Reference |
| 31 to 44 | 1.52 (1.08–2.15)* | 0.83 (0.69–0.99)* | 0.94 (0.80–1.10) |
| 45 to 60 | 2.27 (1.61–3.19)‡ | 0.72 (0.60–0.87)† | 0.98 (0.83–1.15) |
| ≥60 | 4.22 (2.78–6.42)‡ | 0.67 (0.51–0.89)* | 1.25 (0.99–1.57) |
| Female | 0.88 (0.79–0.98)* | 1.10 (1.00–1.20) | 1.00 (0.93–1.08) |
| Race | |||
| White | Reference | Reference | Reference |
| African American | 0.85 (0.76–0.96)* | 1.07 (0.96–1.19) | 0.99 (0.92–1.08) |
| Hispanic | 0.69 (0.55–0.86)* | 0.76 (0.62–0.91)* | 0.73 (0.63–0.85)‡ |
| Other | 0.71 (0.52–0.96)* | 0.76 (0.58–0.99)* | 0.75 (0.61–0.92)* |
| Highest level of education | |||
| College or higher | Reference | Reference | Reference |
| Grade/high school | 0.87 (0.78–0.98)* | 0.96 (0.87–1.06) | 0.92 (0.85–0.99)* |
| Missing | 1.10 (0.93–1.29) | 0.87 (0.75–1.02) | 0.98 (0.87–1.10) |
| Employment status | |||
| Working | 0.77 (0.64–0.91)* | 0.82 (0.71–0.95)* | 0.80 (0.72–0.90)† |
| Not working | Reference | Reference | Reference |
| Missing | 0.91 (0.75–1.09) | 0.89 (0.75–1.04) | 0.89 (0.79–1.01) |
| Health insurance type | |||
| Private | Reference | Reference | Reference |
| Public | 0.83 (0.73–0.95)* | 0.99 (0.88–1.11) | 0.92 (0.84–1.00) |
| Missing | 0.00 (0–6.82×1063) | 0.18 (0.03–1.28) | 0.11 (0.02–0.81)* |
| Body mass index, kg/m2 | |||
| <18.5 | 1.22 (0.87–1.70) | 0.93 (0.72–1.20) | 0.98 (0.80–1.20) |
| 18.5 to 24.9 | Reference | Reference | Reference |
| 25 to 30 | 0.94 (0.83–1.06) | 1.22 (1.09–1.37)† | 1.08 (0.99–1.18) |
| >30 | 0.95 (0.83–1.08) | 1.41 (1.26–1.58)‡ | 1.18 (1.08–1.29)† |
| Missing | 1.50 (1.17–1.94)* | 1.19 (0.91–1.55) | 1.31 (1.08–1.59)* |
| Physical capacity status | |||
| Not limited | Reference | Reference | Reference |
| Limited | 1.55 (1.31–1.83)‡ | 1.12 (0.94–1.33) | 1.31 (1.16–1.48)‡ |
| Missing | 1.22 (1.09–1.36)† | 1.11 (1.00–1.22)* | 1.14 (1.05–1.23)* |
| Comorbid conditions | |||
| Hypertension | 0.96 (0.85–1.10) | 1.02 (0.91–1.14) | 1.00 (0.92–1.09) |
| Diabetes mellitus | 1.38 (1.18–1.62)‡ | 0.90 (0.77–1.06) | 1.10 (0.98–1.23) |
| Coronary artery disease | 1.34 (1.14–1.57)† | 1.11 (0.93–1.32) | 1.23 (1.09–1.39)† |
| Cerebral vascular disease | 1.12 (0.89–1.41) | 1.07 (0.83–1.38) | 1.10 (0.93–1.32) |
| PVD | 1.41 (1.18–1.67)† | 1.12 (0.91–1.39) | 1.30 (1.13–1.50)† |
| COPD | 1.75 (1.30–2.34)† | 1.58 (1.15–2.19)* | 1.67 (1.34–2.01)‡ |
| Cause of ESRD | |||
| Hypertension | 1.30 (1.10–1.53)* | 0.95 (0.83–1.08) | 1.04 (0.94–1.16) |
| Diabetes mellitus | 1.34 (1.10–1.63)* | 0.99 (0.82–1.20) | 1.10 (0.96–1.27) |
| Glomerulonephritis | Reference | Reference | Reference |
| Polycystic kidney disease | 0.92 (0.73–1.16) | 0.72 (0.60–0.87)† | 0.78 (0.67–0.90)† |
| Other | 1.26 (1.05–1.52)* | 1.02 (0.90–1.17) | 1.07 (0.96–1.20) |
| Duration of dialysis, months | |||
| None (pre-emptive) | 0.70 (0.58–0.85)† | 0.70 (0.60–0.83)‡ | 0.71 (0.63–0.81)‡ |
| 0.1 to 24 | Reference | Reference | Reference |
| 25 to 60 | 1.23 (1.08–1.40)* | 1.11 (0.99–1.25) | 1.16 (1.06–1.27)* |
| >60 | 1.74 (1.51–2.01)‡ | 1.46 (1.28–1.66)‡ | 1.55 (1.40–1.71)‡ |
| Missing | 1.22 (0.73–2.04) | 5.16 (4.06–6.57)‡ | 3.54 (2.83–4.42)‡ |
| Peak PRA level | |||
| <10 | Reference | Reference | Reference |
| 10 to 79 | 1.08 (0.95–1.22) | 1.04 (0.93–1.17) | 1.05 (0.97–1.15) |
| ≥80 | 1.26 (1.04–1.52)* | 1.25 (1.06–1.47)* | 1.21 (1.06–1.37)* |
| Missing | 0.96 (0.76–1.20) | 1.01 (0.82–1.24) | 0.97 (0.83–1.14) |
| HLA mismatches | |||
| Zero A, B, DR | 0.89 (0.74–1.08) | 0.76 (0.64–0.91)* | 0.82 (0.71–0.93)* |
| Zero DR | 0.98 (0.84–1.14) | 0.91 (0.79–1.04) | 0.95 (0.85–1.05) |
| Other | Reference | Reference | Reference |
| Previous organ transplant | 1.22 (1.05–1.42)* | 1.20 (1.05–1.36)* | 1.22 (1.10–1.35)† |
| Era of current transplant | |||
| 2007 to 2009 | Reference | Reference | Reference |
| 2010 to 2012 | 0.92 (0.80–1.05) | 0.87 (0.78–0.98)* | 0.89 (0.81–0.97)* |
| 2013 to 2015 | 0.90 (0.78–1.03) | 0.73 (0.65–0.83)‡ | 0.80 (0.73–0.88)‡ |
| Donor type | |||
| Living | 0.83 (0.71–0.97)* | 0.78 (0.68–0.90)† | 0.81 (0.73–0.90)† |
| Deceased (SCD) | Reference | Reference | Reference |
| Deceased (ECD) | 1.36 (1.19–1.54)‡ | 2.16 (1.92–2.43)‡ | 1.67 (1.53–1.83)‡ |
| Deceased (DCD) | 1.11 (0.96–1.28) | 1.37 (1.20–1.55)‡ | 1.26 (1.14–1.39)‡ |
| Cold ischemia time, hours | |||
| <12 | Reference | Reference | Reference |
| 13 to 24 | 1.09 (0.97–1.23) | 1.27 (1.14–1.42)‡ | 1.20 (1.10–1.31)‡ |
| 25 to 36 | 1.27 (1.09–1.49)* | 1.63 (1.42–1.88)‡ | 1.48 (1.33–1.66)‡ |
| >36 | 1.45 (1.12–1.86)* | 1.75 (1.40–2.18)‡ | 1.61 (1.35–1.91)‡ |
| Missing | 1.01 (0.79–1.27) | 1.05 (0.85–1.30) | 1.02 (0.87–1.20) |
Data presented as aHR (95% CI).
p<0.05–0.002;
p=0.001–0.0002;
p<0.0001.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DCD, donation after cardiac death; ECD, expanded criteria donor; ESRD, end-stage renal disease; HLA, human leukocyte antigens; PRA, panel reactive antibody; PVD, peripheral vascular disease; SCD, standard criteria donor.
Figure 2.
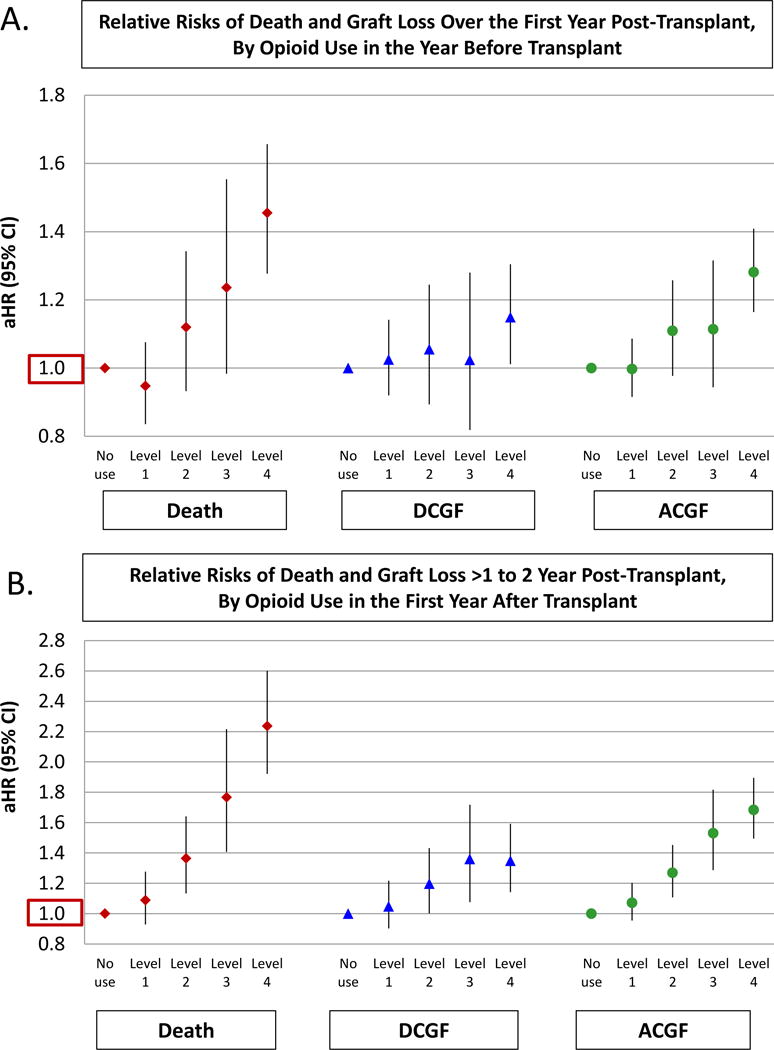
Adjusted associations of level 1–4 prescription opioid use before and after transplant with death and graft failure (referent=no use). Abbreviations: aHR, adjusted hazard ratio; ACGF, all-cause graft failure; CI, confidence interval; DCGF, death-censored graft failure.
Patterns of Opioid Use Before and After Transplant
We examined a subgroup of the cohort (n=58,154, 77.1%) with available data in the pharmacy records to determine prescription opioid use in the 1 year before and after transplant. Overall, 70.5% of recipients who filled an opioid prescription in the 1 year before transplant continued to fill opioid prescriptions in the 1 year after transplant. Recipients with higher levels of post-transplant opioid use were more likely to show higher levels of pre-transplant opioid use (Figure 3). Almost 60% of level 4 opioid users pre-transplant continued high level use posttransplant. Clinical correlates of posttransplant opioid use included factors associated with pre-transplant opioid use, such as female sex, African American race, less than college education, unemployment, obesity, and physical limitations (Table S4). Age 31–44 years was also associated with posttransplant opioid use.
Figure 3.
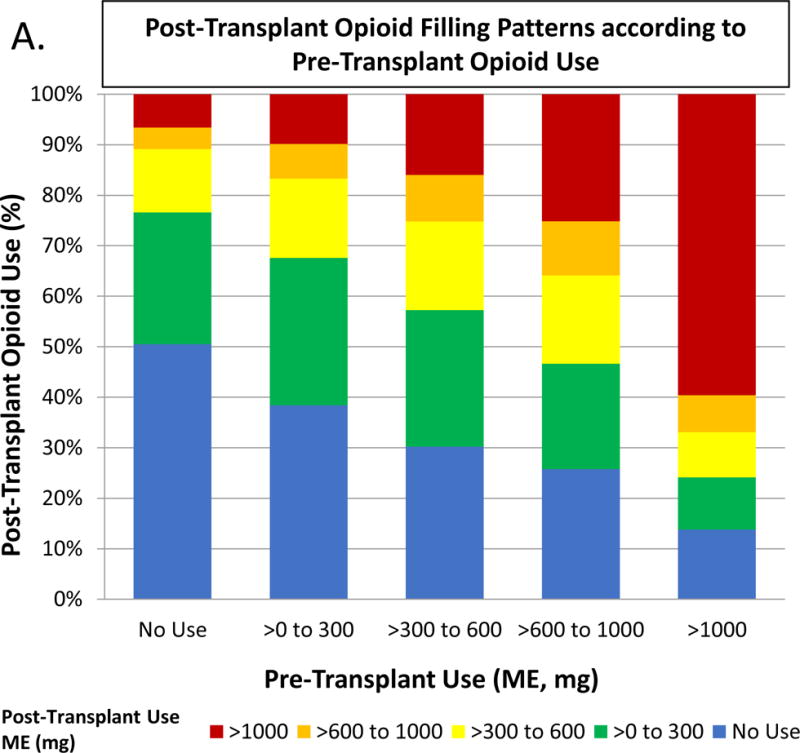
Posttransplant opioid use according to pretransplant opioid use. Abbreviations: ME, morphine equivalents.
Death and Graft Failure According to Post-transplant Opioid Use Level
Overall, 1.5% of recipients died in the first or second post-transplant year. During that time, incidence of death-censored graft failure was 1.7% and of all-cause graft failure 3.1%. Compared with non-users, recipients with level 2–4 opioid use in the first posttransplant year had an increased risk of death, death-censored graft failure, and all-cause graft failure (Figure 1). After adjustment for demographic, clinical, and procedure factors, level 4 post-transplant opioid use was associated with a 2-fold increased risk of death over the next post-transplant year (aHR 1.932.242.60, p<0.0001), 35% increased risk of death-censored graft failure (aHR 1.151.351.59, p=0.0003) and 68% increased risk of all-cause graft failure (aHR 1.501.68 1.89, p<0.0001) compared with no use (Table 3, Figure 2). Risk relationships persisted with extension of observation time over five years after the first anniversary (Figure S2). For example, level 4 post-transplant opioid use was associated with a 67% increased risk of death (aHR 1.551.671.80, p<0.0001), 13% increased risk of death-censored graft failure (aHR 1.031.131.24, p=0.008), and 39% increased risk of all-cause graft failure (aHR 1.311.39 1.48, p<0.0001) compared with no use.
Table 3.
Adjusted associations of clinical factors with death and graft failure within the first and second post-transplant years
| Clinical Factor | Death | Death-Censored Graft Failure | All-Cause Graft Failure |
|---|---|---|---|
| Posttransplant opioid use | |||
| No use | Reference | Reference | Reference |
| Level 1 | 1.09 (0.93–1.27) | 1.05 (0.91–1.21) | 1.07 (0.96–1.20) |
| Level 2 | 1.36 (1.14–1.64)† | 1.20 (1.01–1.43)* | 1.27 (1.11–1.45)† |
| Level 3 | 1.77 (1.41–2.21)‡ | 1.36 (1.08–1.71)* | 1.53 (1.29–1.81)‡ |
| Level 4 | 2.24 (1.93–2.60)‡ | 1.35 (1.15–1.59)† | 1.68 (1.50–1.89)‡ |
| Age, years | |||
| <18 | 0.41 (0.18–0.93)* | 0.91 (0.55–1.53) | 0.79 (0.53–1.20) |
| 18 to 30 | Reference | Reference | Reference |
| 31 to 44 | 1.11 (0.77–1.60) | 0.55 (0.45–0.66)‡ | 0.63 (0.53–0.75)‡ |
| 45 to 59 | 1.89 (1.34–2.65)† | 0.33 (0.27–0.40)‡ | 0.55 (0.47–0.65)‡ |
| ≥60 | 3.18 (2.01–5.04)‡ | 0.23 (0.17–0.33)‡ | 0.62 (0.48–0.82)† |
| Female | 0.86 (0.75–0.98)* | 1.00 (0.89–1.13) | 0.93 (0.85–1.03) |
| Race | |||
| White | Reference | Reference | Reference |
| African American | 0.76 (0.66–0.89)† | 1.54 (1.35–1.77)‡ | 1.16 (1.04–1.28)* |
| Hispanic | 0.51 (0.37–0.70)‡ | 0.63 (0.48–0.84)* | 0.57 (0.46–0.71)‡ |
| Other | 0.58 (0.41–0.83)* | 0.81 (0.57–1.13) | 0.66 (0.51–0.86)* |
| Highest level of education | |||
| College or higher | Reference | Reference | Reference |
| Grade/high school | 1.06 (0.93–1.21) | 1.12 (0.98–1.27) | 1.11 (1.01–1.23)* |
| Missing | 0.91 (0.74–1.13) | 1.10 (0.91–1.34) | 1.02 (0.88–1.19) |
| Employment status | |||
| Working | 0.66 (0.53–0.82)† | 0.72 (0.59–0.87)† | 0.70 (0.61–0.82)‡ |
| Not working | Reference | Reference | Reference |
| Missing | 0.86 (0.69–1.08) | 0.73 (0.58–0.92)* | 0.80 (0.67–0.94)* |
| Health insurance type | |||
| Private | Reference | Reference | Reference |
| Public | 0.93 (0.79–1.09) | 0.89 (0.76–1.04) | 0.90 (0.80–1.01) |
| Missing | 0.00 (0–4.76×1093) | 0.54 (0.08–3.93) | 0.34 (0.05–2.40) |
| Body mass index, kg/m2 | |||
| <18.5 | 1.34 (0.94–1.92) | 0.84 (0.63–1.14) | 0.97 (0.76–1.23) |
| 18.5 to 24.9 | Reference | Reference | Reference |
| 25 to 30 | 0.82 (0.71–0.95)* | 1.07 (0.92–1.25) | 0.95 (0.85–1.06) |
| >30 | 0.84 (0.71–1.00)* | 1.27 (1.08–1.49)* | 1.06 (0.94–1.20) |
| Missing | 0.69 (0.46–1.05) | 0.75 (0.50–1.13) | 0.73 (0.54–0.99)* |
| Physical capacity status | |||
| Not limited | Reference | Reference | Reference |
| Limited | 0.99 (0.81–1.22) | 1.35 (1.10–1.66)* | 1.15 (0.99–1.35) |
| Missing | 1.02 (0.90–1.17) | 0.96 (0.84–1.10) | 0.99 (0.90–1.10) |
| Comorbid conditions | |||
| Hypertension | 0.93 (0.80–1.10) | 0.94 (0.81–1.10) | 0.95 (0.84–1.07) |
| Diabetes mellitus | 1.45 (1.21–1.74)‡ | 1.03 (0.84–1.27) | 1.26 (1.10–1.45)* |
| Coronary artery disease | 1.23 (1.03–1.47)* | 1.15 (0.91–1.44) | 1.20 (1.04–1.40)* |
| Cerebral vascular disease | 1.16 (0.88–1.53) | 0.92 (0.63–1.34) | 1.04 (0.82–1.32) |
| PVD | 1.51 (1.23–1.86)‡ | 1.20 (0.88–1.62) | 1.32 (1.10–1.59)* |
| COPD | 1.57 (1.09–2.26)* | 1.08 (0.62–1.87) | 1.54 (1.13–2.11)* |
| Cause of ESRD | |||
| Hypertension | 1.31 (1.07–1.59)* | 1.04 (0.88–1.23) | 1.13 (0.99–1.29) |
| Diabetes mellitus | 1.21 (0.95–1.54) | 0.79 (0.61–1.02) | 0.93 (0.78–1.11) |
| Glomerulonephritis | Reference | Reference | Reference |
| Polycystic kidney disease | 0.87 (0.66–1.14) | 0.77 (0.59–1.00) | 0.82 (0.68–1.00)* |
| Other | 1.20 (0.97–1.48) | 0.82 (0.68–0.98)* | 0.91 (0.79–1.05) |
| Duration of dialysis, months | |||
| None (pre-emptive) | 0.68 (0.54–0.85)† | 0.66 (0.53–0.83)† | 0.68 (0.58–0.80)‡ |
| 0.1 to 24 | Reference | Reference | Reference |
| 25 to 60 | 1.19 (1.02–1.39)* | 1.10 (0.94–1.28) | 1.13 (1.01–1.27)* |
| >60 | 1.62 (1.36–1.93)‡ | 1.10 (0.92–1.31) | 1.28 (1.13–1.46)† |
| Missing | 1.42 (0.81–2.47) | 0.94 (0.48–1.81) | 1.22 (0.78–1.90) |
| Peak PRA level | |||
| <10 | Reference | Reference | Reference |
| 10 to 79 | 1.02 (0.88–1.19) | 1.10 (0.95–1.28) | 1.05 (0.94–1.18) |
| ≥80 | 0.98 (0.77–1.26) | 1.01 (0.79–1.29) | 0.98 (0.81–1.18) |
| Missing | 1.00 (0.72–1.38) | 1.17 (0.84–1.62) | 1.11 (0.88–1.41) |
| HLA mismatches | |||
| Zero A, B, DR | 1.10 (0.90–1.35) | 0.61 (0.47–0.79)† | 0.87 (0.74–1.03) |
| Zero DR | 0.77 (0.63–0.94)* | 0.82 (0.68–1.00)* | 0.83 (0.72–0.96)* |
| Other | Reference | Reference | Reference |
| Previous organ transplant | 1.51 (1.27–1.79)‡ | 1.13 (0.94–1.35) | 1.28 (1.12–1.45)† |
| Era of current transplant | |||
| 2007 to 2009 | Reference | Reference | Reference |
| 2010 to 2012 | 0.90 (0.80–1.02) | 0.84 (0.75–0.95)* | 0.88 (0.80–0.96)* |
| 2013 to 2015 | 1.02 (0.83–1.24) | 0.68 (0.55–0.85)† | 0.86 (0.73–1.00)* |
| Donor type | |||
| Living | 0.86 (0.71–1.04) | 0.71 (0.60–0.85)† | 0.75 (0.65–0.85)‡ |
| Deceased (SCD) | Reference | Reference | Reference |
| Deceased (ECD) | 1.48 (1.27–1.73)‡ | 1.92 (1.62–2.28)‡ | 1.56 (1.38–1.76)‡ |
| Deceased (DCD) | 1.24 (1.04–1.49)* | 0.84 (0.68–1.03) | 1.04 (0.90–1.20) |
| Cold ischemia time, hours | |||
| <12 | Reference | Reference | Reference |
| 13 to 24 | 1.11 (0.96–1.29) | 1.14 (0.99–1.33) | 1.10 (0.99–1.23) |
| 25 to 36 | 1.12 (0.92–1.37) | 1.21 (0.99–1.48) | 1.15 (0.99–1.34) |
| >36 | 1.29 (0.93–1.78) | 1.43 (1.05–1.95)* | 1.33 (1.05–1.69)* |
| Missing | 0.98 (0.75–1.28) | 1.21 (0.96–1.53) | 1.14 (0.95–1.37) |
Data presented as aHR (95% CI).
p<0.05–0.002;
p=0.001–0.0002;
p<0.0001.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DCD, donation after cardiac death; ECD, expanded criteria donor; ESRD, end-stage renal disease; HLA, human leukocyte antigens; PRA, panel reactive antibody; PVD, peripheral vascular disease; SCD, standard criteria donor.
DISCUSSION
The national epidemic of prescription opioid use is affecting patients across domains of care. Because of high-frequency use and potential for increased risks due to altered drug metabolism, prescription opioids are a particular concern among populations with end-stage organ failure, including transplant recipients. We examined prescription opioid fills among a contemporary sample of 75,000 kidney transplant recipients and observed several key findings: 1) Consistent with a prior report, pre-transplant prescription opioid use was associated with an approximately 45% increased risk of death and a 28% increased risk of all-cause graft failure over the first year posttransplant. 2) Most (70.5%) recipients who filled an opioid prescription in the year preceding transplant continue opioid use in the year after, and most high-level opioid users continued to fill at high levels. 3) Post-transplant prescription opioid use in the first year had ever stronger prognostic significance, predicting a 2-fold increased risk of death and a 68% increased risk of all-cause graft failure over the subsequent year. Increased risk persisted over five years of observation.
In this larger cohort of recipients including 5 years of more recent data, we confirmed our previous findings that high-level prescription opioid use in the year preceding kidney transplant was associated with increased risk of death and graft loss.(15) These findings are consistent with a retrospective study at a Midwest U.S. center by Barrantes et al.: kidney transplant recipients’ self-reported long-term opioid use before transplant was associated with a 2-fold higher risk of death but similar risk of graft loss; graft loss risk was not based on self-reported medication use but identified through chart review.(18) The kidney transplant evaluation period is an opportunity for the transplant team to review the candidate’s comorbid conditions and medication list to assess risks, and counsel and optimize care before surgery. Pretransplant opioid use may become more prevalent, since end-stage renal disease (ESRD) patients have multiple potential sources of acute and chronic pain, including underlying causes of ESRD (e.g., polycystic kidney disease), other comorbidity (e.g., diabetes mellitus), renal complications (e.g., calciphylaxis), and vascular access complications (e.g., steal syndrome with arteriovenous fistulas).(19–23) One Canadian survey of 205 hemodialysis patients reported that 50% were affected by pain, of which 83% reported moderate-severe pain.(22) A new study of prescription fills among Medicare-insured dialysis patients found that overall, more than 60% filled at least one opioid prescription every year.(17) Our results support the notion that pretransplant prescription opioid use is not only prevalent in this unique patient population, but it also has implications for posttransplant patient and graft survival.
Patients who used prescription opioids pre-transplant were likely to continue use posttransplant. In our current study, 60% of recipients with the highest level of pre-transplant prescription opioid use continued high levels of use posttransplant. Possibly, ongoing prescription opioid use posttransplant is due to persistent pain. In a cross-sectional study of 164 hemodialysis patients and 114 stable deceased donor kidney transplant recipients, prevalence of reported pain was similar (63% vs. 62%), suggesting that successful kidney transplant does not reduce the prevalence of chronic pain in patients with ESRD.(24) Similarly, at one U.S. center, pre-transplant opioid use was the strongest predictor of posttransplant use.(25) Due to the nature of our available data sources, we were unable to determine the reason for ongoing prescription opioid use. Thus, it is unknown whether kidney transplant recipients experience persistent pain posttransplant requiring opioid treatment, or whether pain management was not reassessed posttransplant and the pre-transplant regimen was continued. While the former may represent appropriate management, efforts should be made to prevent the latter and to review and attempt to wean opioids that are no longer needed.
In our study, prescription opioid use in the first year after transplant had a strong, graded associated with increased risk of death and graft loss in the subsequent year, and the highest level of use predicted 2-fold increased risk of death, 35% increased risk of death-censored graft failure, and 68% increased risk of all-cause graft failure compared with non-use. Poorer outcomes with opioid use in kidney transplant recipients may reflect comorbidity associated with chronic pain, such as diabetes mellitus, or psychiatric conditions such as depression.(26) In addition, opioid use may be related to riskier patient behaviors, such as non-compliance with appointments or immunosuppressive medications, which may increase the risk of adverse posttransplant outcomes.(27) At one center, Kulshrestha et al found that opioid use in the first year was associated with increased readmission rates but no difference in rejection rates.(25) Further research is needed to understand the factors contributing to the increased risk of death and graft failure in kidney transplant recipients who require opioids for ongoing pain management.
Regardless of the mechanisms of association, identification of novel markers of post-transplant outcomes is timely and can help transplant programs assess and manage risk at a programmatic level. Transplantation in the US is an increasingly regulated field with a high level of public reporting. Transplant centers are graded for recipient and graft survival using risk-adjusted equations developed by SRTR to predict expected 1-year post-transplant patient and graft survival.(28) Importantly, SRTR equations do not adjust for pain or medication use history as risk factors for post-transplant death or graft loss. Thus, centers performing transplants in patients who require prescription opioids before and after transplant should be aware of un-identified risk that will not be recognized by SRTR, and should consider extra monitoring and focused post-transplant care of these recipients.
Our study has a number of strengths, including use of a novel integrated national data registry that allows identification of prescription opioid fills. Electronic prescription fills have been shown to be highly accurate and may better represent the burden of opioid use compared with self-reported questionnaires.(29, 30) We used these data to understand the implications of pre- and post-transplant prescription opioid use for clinically relevant outcomes in a large cohort of kidney transplant recipients. We were also able to describe patterns of opioid use among recipients before and after transplant.
There are limitations to our study. We describe associations but are unable to prove causation between opioid use and adverse clinical outcomes. While use of pharmacy electronic records to identify prescription fills may be more accurate than self-report, opioid use and its impact may be underestimated as we were unable to account for illicit drug use, “pharmacy shopping” behaviors, or prescription fills at pharmacies not included in SHS. However, these patients would likely still be categorized as high-level users if they filled their opioid prescriptions at the same pharmacy as their immunosuppressive prescriptions. In addition, unmeasured confounders may have affected our findings, resulting in uncontrolled confounding by indication. We lacked information on laboratory values and transplant kidney biopsy pathology results. We were also unable to accurately identify patients who may have drug dependency or addiction issues, and we lacked data on patient compliance. Lastly, as noted, we were unable to determine whether opioid use pre- and post-transplant is continually monitored and managed for appropriateness.
In conclusion, our results reinforce growing concern about the outcome implications of prescription opioids, particularly in unique patient populations. We identified an increased risk of death and graft loss in kidney transplant recipients with evidence of high-level prescription opioid use before or after surgery. Transplant centers should assess opioid use in candidates being evaluated for transplant and reassess ongoing use after transplant. Given our findings, we believe that transplant candidates who receive opioids warrant careful evaluation of pain management strategies, perhaps by a multidisciplinary team including a pain management specialist, as well as focused monitoring of clinical status over time after surgery. Ongoing work is needed to develop strategies to mitigate the risk of opioid-related complications in this patient population.
Supplementary Material
Table S1. STROBE checklist.
Table S2. Conversions used to standardize doses of opioid medications as morphine equivalents.
Table S3. Distributions of clinical traits of kidney transplant recipients by level of post-transplant opioid use.
Table S4. Propensity model for associations of baseline factors with pre- and posttransplant opioid use.
Figure S1. Relative risks of death and graft loss over the first year post-transplant, according to opioid use by pre-transplant period.
Figure S2. Relative risks of death and graft loss >1 to 6 year post-transplant, according to opioid use in the first year after transplant.
Acknowledgments
This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a U.S. Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01-DK096008. NNL was supported by a KRESCENT New Investigator Award. The opinions, results, and conclusions reported in this article are those of the authors and are independent of the funding sources. The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for manuscript editing.
ABBREVIATIONS
- aHR
adjusted hazard ratio
- COPD
chronic obstructive pulmonary disease
- DCD
donation after cardiac death
- ECD
expanded criteria donor
- ESRD
end-stage renal disease
- HLA
human leukocyte antigen
- HRSA
Health Resources and Services Administration
- LCL
lower confidence limit
- ME
morphine equivalents
- OPTN
Organ Procurement and Transplantation Network
- PRA
panel reactive antibody
- SAS
Statistical Analysis Software
- SCD
standard criteria donor
- SHS
Symphony Health Solutions
- SRTR
Scientific Registry of Transplant Recipients
- UCL
upper confidence limit
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab of this article.
References
- 1.Dew MA, Switzer GE, DiMartini AF, Matukaitis J, Fitzgerald MG, Kormos RL. Psychosocial assessments and outcomes in organ transplantation. Prog Transplant. 2000;10(4):239–259. doi: 10.1177/152692480001000408. quiz 260–231. [DOI] [PubMed] [Google Scholar]
- 2.Pham PT, Pham PA, Pham PC, Parikh S, Danovitch G. Evaluation of adult kidney transplant candidates. Semin Dial. 2010;23(6):595–605. doi: 10.1111/j.1525-139X.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 3.Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberu J, Bakr MA, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101(8S Suppl 1):S1–S109. doi: 10.1097/TP.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manchikanti L, Helm S, 2nd, Fellows B, Janata JW, Pampati V, Grider JS, et al. Opioid epidemic in the United States. Pain physician. 2012;15(3 Suppl):ES9–38. [PubMed] [Google Scholar]
- 5.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 6.Drash W, Merica D. President Trump declares opioid crisis a national emergency. CNN Health News. 2017 Aug 11; Available at: http://www.cnn.com/2017/08/10/health/trump-opioid-emergency-declaration-bn/index.html. Accessed: September 30, 2017.
- 7.Pullen LC. The Opioid Epidemic Weaves Its Way Through the Transplant Community. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(9):2231–2232. doi: 10.1111/ajt.14424. [DOI] [PubMed] [Google Scholar]
- 8.NIH Director Francis Collins on America’s opioid crisis. Washington Post Live. 2017 Sep 20; Available at: https://www.washingtonpost.com/video/postlive/nih-directorfrancis-collins-on-opioid-crisis-in-the-us/2017/09/20/cfb04e68-9e41-11e7-b2a7-bc70b6f98089_video.html?utm_term=.9ef48436a9d8. Accessed: September 30, 2017.
- 9.Menendez ME, Ring D, Bateman BT. Preoperative Opioid Misuse is Associated With Increased Morbidity and Mortality After Elective Orthopaedic Surgery. Clinical orthopaedics and related research. 2015;473(7):2402–2412. doi: 10.1007/s11999-015-4173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cron DC, Englesbe MJ, Bolton CJ, Joseph MT, Carrier KL, Moser SE, et al. Preoperative Opioid Use is Independently Associated With Increased Costs and Worse Outcomes After Major Abdominal Surgery. Annals of surgery. 2016 doi: 10.1097/SLA.0000000000001901. [DOI] [PubMed] [Google Scholar]
- 11.Randall HB, Alhamad T, Schnitzler MA, Zhang Z, Ford-Glanton S, Axelrod DA, et al. Survival implications of opioid use before and after liver transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2017;23(3):305–314. doi: 10.1002/lt.24714. [DOI] [PubMed] [Google Scholar]
- 12.Lentine KL, Lam NN, Schnitzler MA, Hess GP, Kasiske BL, Xiao H, et al. Predonation Prescription Opioid Use: A Novel Risk Factor for Readmission After Living Kidney Donation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(3):744–753. doi: 10.1111/ajt.14033. [DOI] [PubMed] [Google Scholar]
- 13.Dean M. Opioids in renal failure and dialysis patients. Journal of pain and symptom management. 2004;28(5):497–504. doi: 10.1016/j.jpainsymman.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Niscola P, Scaramucci L, Vischini G, Giovannini M, Ferrannini M, Massa P, et al. The use of major analgesics in patients with renal dysfunction. Current drug targets. 2010;11(6):752–758. doi: 10.2174/138945010791170879. [DOI] [PubMed] [Google Scholar]
- 15.Lentine KL, Yuan H, Tuttle-Newhall JE, Xiao H, Chawa V, Axelrod D, et al. Quantifying prognostic impact of prescription opioid use before kidney transplantation through linked registry and pharmaceutical claims data. Transplantation. 2015;99(1):187–196. doi: 10.1097/TP.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 16.Lentine KL, Lam NN, Xiao H, Tuttle-Newhall JE, Axelrod D, Brennan DC, et al. Associations of pre-transplant prescription narcotic use with clinical complications after kidney transplantation. American journal of nephrology. 2015;41(2):165–176. doi: 10.1159/000377685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW. Opioid Prescription, Morbidity, and Mortality in United States Dialysis Patients. Journal of the American Society of Nephrology: JASN. 2017 doi: 10.1681/ASN.2017010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrantes F, Luan FL, Kommareddi M, Alazem K, Yaqub T, Roth RS, et al. A history of chronic opioid usage prior to kidney transplantation may be associated with increased mortality risk. Kidney international. 2013;84(2):390–396. doi: 10.1038/ki.2013.136. [DOI] [PubMed] [Google Scholar]
- 19.Salisbury EM, Game DS, Al-Shakarchi I, Chan M, Fishman L, Tookman L, et al. Changing practice to improve pain control for renal patients. Postgraduate medical journal. 2009;85(999):30–33. doi: 10.1136/pgmj.2008.071191. [DOI] [PubMed] [Google Scholar]
- 20.Weisbord SD, Fried LF, Arnold RM, Fine MJ, Levenson DJ, Peterson RA, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. Journal of the American Society of Nephrology: JASN. 2005;16(8):2487–2494. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]
- 21.Bailie GR, Mason NA, Bragg-Gresham JL, Gillespie BW, Young EW. Analgesic prescription patterns among hemodialysis patients in the DOPPS: potential for underprescription. Kidney international. 2004;65(6):2419–2425. doi: 10.1111/j.1523-1755.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 22.Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2003;42(6):1239–1247. doi: 10.1053/j.ajkd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: a review of available evidence. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2003;42(2):217–228. doi: 10.1016/s0272-6386(03)00645-0. [DOI] [PubMed] [Google Scholar]
- 24.Masajtis-Zagajewska A, Pietrasik P, Krawczyk J, Krakowska M, Jarzebski T, Pietrasiewicz B, et al. Similar prevalence but different characteristics of pain in kidney transplant recipients and chronic hemodialysis patients. Clinical transplantation. 2011;25(2):E144–151. doi: 10.1111/j.1399-0012.2010.01359.x. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Heroin overdose deaths increased in many states through 2012. http://www.cdc.gov/media/releases/2014/p1002-heroin-overdose.html (released October 4, 2014). Access date: May 11, 2016.
- 26.Wollschlaeger BA, Willson TM, Montejano LB, Ronquest NA, Nadipelli VR. Characteristics and treatment patterns of US commercially insured and Medicaid patients with opioid dependence or abuse. J Opioid Manag. 2017;13(4):207–220. doi: 10.5055/jom.2017.0389. [DOI] [PubMed] [Google Scholar]
- 27.Campbell CI, Kline-Simon AH, Von Korff M, Saunders KW, Weisner C. Alcohol and Drug Use and Aberrant Drug-Related Behavior Among Patients on Chronic Opioid Therapy. Subst Use Misuse. 2017;52(10):1283–1291. doi: 10.1080/10826084.2016.1276189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scientific Registry of Transplant Recipients (SRTR) Risk Adjustment Models. Available at: https://www.srtr.org/reports-tools/risk-adjustment-models-posttransplant-outcomes/ Accessed: January 20, 2018.
- 29.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10(2):67–71. [PubMed] [Google Scholar]
- 30.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–625. doi: 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. STROBE checklist.
Table S2. Conversions used to standardize doses of opioid medications as morphine equivalents.
Table S3. Distributions of clinical traits of kidney transplant recipients by level of post-transplant opioid use.
Table S4. Propensity model for associations of baseline factors with pre- and posttransplant opioid use.
Figure S1. Relative risks of death and graft loss over the first year post-transplant, according to opioid use by pre-transplant period.
Figure S2. Relative risks of death and graft loss >1 to 6 year post-transplant, according to opioid use in the first year after transplant.


