Abstract
Introduction
It is unclear if direct oral anticoagulants (DOACs) are effective and safe alternatives to low-molecular-weight heparin (LMWHs) for the treatment of cancer-associated venous thromboembolism (VTE). We aim to synthesize existing literature that compared DOACs versus LMWHs in this high-risk population.
Materials and Methods
We conducted a systematic review using EMBASE, MEDLINE and CENTRAL for all observational studies and randomized controlled trials (RCTs) (PROSPERO: CRD42017080898). Two authors independently reviewed study eligibility, extracted data, and assessed bias. Primary outcomes included 6-month recurrent VTE and major bleeding. Secondary outcomes included clinically relevant non-major bleeding (CRNMB) and mortality.
Results
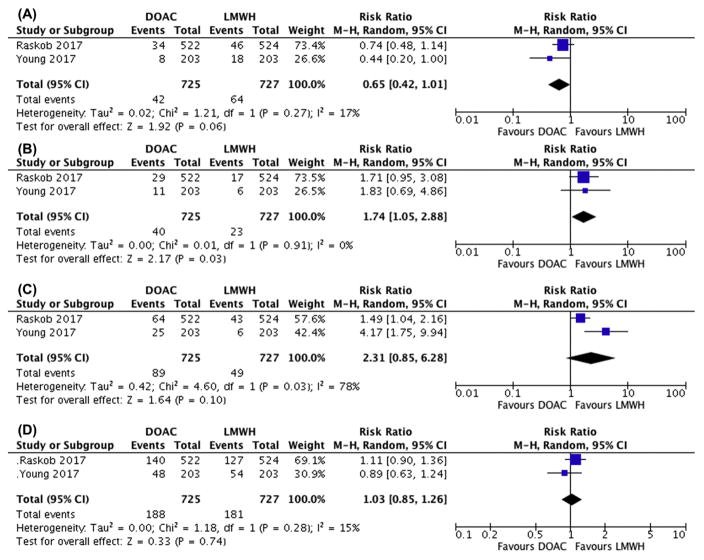
We screened 426 articles, reviewed 25 in full-text, and selected 13 and 2 for qualitative and quantitative synthesis, respectively. Based on a meta-analysis of the 2 RCTs, DOACs had lower 6-month recurrent VTE (42/725) when compared to LMWH (64/727) (RR: 0.65 (0.42–1.01)). However, DOACs had higher major bleeding (40/725) when compared to LMWH (23/727) (RR 1.74 (1.05–2.88)). Similarly, CRNMB was higher (RR 2.31 (0.85–6.28)) for patients receiving DOACs. There was no difference in mortality (RR 1.03 (0.85–1.26). Observational studies were heterogeneous with high risks of bias but showed recurrent VTE rates consistent with the meta-analysis.
Conclusions
DOACs were more effective than LMWHs to prevent recurrent VTE but were associated with a significantly increased risk of major bleeding as well as a trend toward more CRNMB. The absolute risk differences were small (2–3%) for both primary outcomes and may reflect better compliance with DOACs than LMWHs.
Keywords: neoplasms, venous thrombosis, heparin, low-molecular-weight, factor Xa inhibitors
Introduction
Cancer patients have a 4 to 7-fold increased risk of venous thromboembolism (VTE) which includes deep vein thrombosis (DVT) and pulmonary embolism (PE) [1]. The management of cancer-associated thrombosis (CAT) is challenging because cancer patients have higher risk of recurrent VTE and major bleeding episodes compared to patients without cancer [2,3]. For the past decade, subcutaneous low-molecular-weight heparin (LMWH) has been the recommended treatment for CAT [4,5]. However, only approximately 50% of patients adhere to long-term treatment with parenteral LMWH despite strong recommendations from clinical practice guidelines [6]. Direct oral anticoagulants (DOACs), including dabigatran, rivaroxaban, apixaban, and edoxaban, have been approved for the treatment of VTE in the general population. All have demonstrated comparable effectiveness and safety to vitamin K antagonists in the non-selected cancer subpopulation [7]. Network meta-analyses based on indirect comparisons also suggest that DOACs may also have similar effectiveness and safety to LMWHs for the management of CAT [8,9]. However, clinical guidelines continue to recommend LMWHs over DOACs as the preferred initial treatment of CAT due to the lack of high quality data from dedicated trials [10]. Recently, DOACs have been compared to LMWH in randomized controlled trials (RCTs) [11,12]. We hereby report the results of a systematic review of all observational studies and a meta-analysis of RCTs comparing the effectiveness and safety of DOACs versus LMWHs for the treatment of CAT.
Methods
Search Strategy
We conducted a systematic literature search using EMBASE, MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL) from all languages over a 10-year span (January 1st 2007 to December 14th 2017). The full search strategy is available in the Supplemental Appendix 1. We also performed a hand search of the American Society of Clinical Oncology and the American Society of Hematology annual meeting abstracts in 2017. References of included studies and narrative reviews were reviewed for additional studies. The systematic review protocol and search strategy were registered online (PROSPERO: CRD42017080898).
Study Selection
Two authors (AL and MC) independently identified studies eligible for inclusion based on an initial screen of reference titles and abstracts. Articles (including meeting abstracts) were included for further review if they directly compared a DOAC (dabigatran, rivaroxaban, apixaban or edoxaban) to a LMWH (dalteparin, enoxaparin, tinzaparin, nadroparin) for the treatment of CAT and reported the primary or secondary outcomes. Randomized controlled trials (RCTs), prospective and retrospective observational studies were included. Article records were independently reviewed for inclusion in duplicate, and discrepancies were resolved by consensus.
Data Extraction and Quality Assessment
Two authors (AL and MC) independently extracted the data. Primary outcomes of interest included 6-month incidence of recurrent VTE and major bleeding. Secondary outcomes of interest included incidence of clinically relevant non-major bleeding (CRNMB) and all-cause mortality. Outcomes were defined according to those used in the included studies. Major and CRNMB episodes were usually defined according to the criteria of the International Society on Thrombosis and Haemostasis [13,14]. The qualities of RCTs were assessed using the Cochrane Risk of Bias Tool and qualities of observational studies were assessed using the ROBINS-I tool from the Cochrane Method group [15,16]. Selective reporting bias for the included RCTs was assessed by identification of studies in trial registry and comparison of reported outcomes and those listed from the protocols. Publication bias was examined by funnel plots of study results plotted against sample size.
Statistical Analysis
Pooled proportions, relative risk (RR), risk difference (RD), and 95% confidence intervals of primary and secondary outcomes over a 6-month follow-up period were generated from included RCTs. Forest plots of comparative RRs (DOACs versus LMWH) were created using the Revman 5.3 software. Analyses were conducted using the Mantel-Haenszel random effects model (DerSimonian-Laird analysis) [17]. Heterogeneity between trials were assessed by visual inspection of forest plots and by the percentage of total variation across studies above chance alone (I2 statistic) [18].
Results
Study selection and characteristics
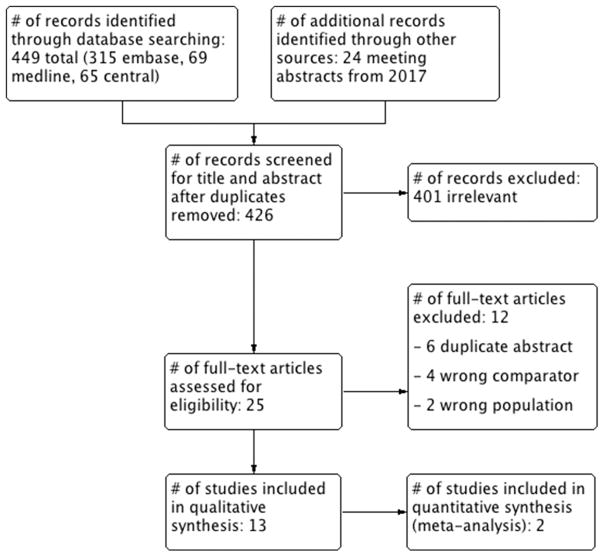
A total of 426 articles and abstracts met the initial search criteria. Out of the 426 screened articles, 25 were selected for full text review including 13 and 2 that were selected for qualitative and quantitative synthesis, respectively (Figure 1). The study characteristics are depicted in Table 1. There were 2 RCTs, 9 observational retrospective cohort studies, and 2 retrospective claims database studies.
Figure 1.
PRISMA flow diagram for study inclusion and exclusion.
Table 1.
Summary of studies from systematic review of DOAC vs. LMWH for the treatment of cancer-associated thrombosis
| Study | Design | Intervention | Outcome | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | DOAC | LMWH | Endpoint (Time) | DOAC | LMWH | ||
| Raskob Article 2017 [11] | RCT | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
522 (12 mo) 64, 53% male 11% heme, 53% met 32% incidental, 9% hx Edoxaban (6.9 mo) |
524 (12 mo) 64, 50% male 11% heme, 53% met 33% incidental, 12% hx Dalteparin (6.0 mo) |
VTE (6 mo) MB (6 mo) CRNMB (6 mo) Death (6 mo) VTE (12 mo) MB (12 mo) CRNMB (12 mo) Death (12 mo) |
6.5% (34/522) 5.6% (29/522) 12.3% (64/522) 26.8% (140/522) 7.9% (41/522) 6.9% (36/522) 14.6% (76/522) 39.5% (206/522) |
8.8% (46/524) 3.2% (17/524) 8.2% (43/524) 24.2% (127/524) 11.3% (59/524) 4.0% (21/524) 11.1% (58/524) 36.6% (192/524) |
| Young Abstract 2017 [12] | RCT | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
203 (6 mo) 67, 54% male 59% met 54% incidental Riva (55% at 6 mo) |
203 (6 mo) 67, 48% male 59% met 52% incidental Dalteparin (52% at 6 mo) |
VTE (6 mo) MB (6 mo) CRNMB (6 mo) Death (6 mo) |
3.9% (8/203) 5.4% (11/203) 12.3% (25/203) 24% (48/203) |
8.9% (18/203) 3.0% (6/203) 3.0% (6/203) 27% (54/203) |
| Ageno Article 2017 [19] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
146 (12 mo) 69, 52% male 8% heme, 14% GI CA 28% hx Riva (5.0 mo) |
223 (12 mo) 68, 47% male 10% heme, 29% GI CA 12% hx NR (5.4 mo) |
VTE (12 mo) MB (12 mo) CRNMB (12 mo) Death (12 mo) |
3.4% (5/146) 1.4% (2/146) NR 4.8% (7/146) |
4.5% (10/223) 3.6% (8/223) NR 24.7% (55/223) |
| Alzghari Article 2017 [20] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
48 (10.4 mo) 62, 50% male 33% met 100% DVT/PE Riva 92% (6.7 mo) |
23 (>6 mo) 62, 39% male 70% met 100% DVT/PE Enoxaparin (4.5 mo) |
VTE (6 mo) MB (6 mo) CRNMB (6 mo) Death (6 mo) |
2.1% (1/48) 6.3% (3/48) NR 10.4% (5/48) |
13.0% (3/23) 4.3% (1/23) NR 39.1% (9/23) |
| Chaudhury Article 2017 [21] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
107 (6 mo) 62, 52% male 20% heme, 68% met 100% DVT/PE, 10% hx Riva (56% at 6 mo) |
179 (6 mo) 59, 51% male 28% heme, 76% met 100% DVT/PE, 5% hx Dalteparin (54% at 6 mo) |
VTE (6 mo) MB (6 mo) CRNMB (6 mo) Death (6 mo) |
2.8% (3/107) 2.8% (3/107) 9.3% (10/107) NR |
6.1% (11/179) 1.1% (2/179) 4.5% (8/179) NR |
| Ross Article 2017 [22] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
30 (11.6 mo) 64, 43% male 24% heme, 31% met 17% CADVT Riva 90% (NR) |
123 (11.6 mo) 58, 44% male 27% heme, 54% met 30% CADVT Enoxaparin (NR) |
VTE (12 mo) MB (12 mo) CRNMB (12 mo) Death (12 mo) |
6.7% (2/30) 13.3% (4/30) 6.7% (2/30) NR |
8.1% (10/123) 10.6% (13/123) 7.3% (9/123) NR |
| Signorelli Article 2017 [23] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
18 (6 mo) 60, 100% female 100% GYN, 33% met 11% hx Riva, NR |
26 (6 mo) 60, 100% female 100% GYN, 35% met 27% hx Enoxaparin, NR |
VTE (6 mo) MB (6 mo) CRNMB (6 mo) Death (6 mo) |
0% (0/18) 16.7% (3/18) NR NR |
3.8% (1/26) 7.7% (2/26) NR NR |
| Phelps Abstract 2016 [25] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
190 (5.0 mo) 58 overall 32% heme 53% met overall Riva 88% (NR) |
290 (5.3 mo) 58 overall 19% heme 53% met overall Enoxaparin (NR) |
VTE (6 mo) MB (6 mo) CRNMB (6 mo) Death (6 mo) |
6.3% (12/190) 2.6% (5/190) 17.9% (34/190) 13.7% (26/190) |
7.2% (21/290) 7.6% (22/290) 26.2% (76/290) 22.8% (66/290) |
| Hummert Abstract 2017 [24] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
85 (NR) 65, 54% male 53% met overall NR Riva (7.1 mo) |
97 (NR) 57, 54% male 53% met overall NR Enoxaparin (3.1 mo) |
VTE (NR) MB (NR) CRNMB (NR) Death (NR) |
1.2% (1/85) 8.2% (7/85) 7.1% (6/85) NR |
2.1% (2/97) 7.2% (7/97) 3.1% (3/97) NR |
| Rahman Abstract 2017 [26] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
23 (NR) NR NR NR Riva (NR) |
149 (NR) NR NR NR Enoxaparin (NR) |
VTE (NR) MB (NR) CRNMB (NR) Death (NR) |
0% (0/23) NR NR NR |
7.4% (11/149) NR NR NR |
| Seo Abstract 2016 [27] | Cohort (record) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
78 (NR) NR 100% GI NR NR (NR) |
111 (NR) NR 100% GI NR NR (NR) |
VTE (NR) MB (NR) CRNMB (NR) Death (NR) |
5.1% (4/78) 16.7% (13/78) 12.8% (10/78) NR |
0.9% (1/111) 7.2% (8/111) 6.3% (7/111) NR |
| Khorana Abstract 2017 [28] | Cohort (claims) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
3370 (8.3 mo) NR NR NR Riva (5.3 mo) |
4313 (6.8 mo) NR NR NR NR (3.2 mo) |
VTE (6 mo) MB (6 mo) CRNMB (6 mo) Death (6 mo) |
8.7% 4.4% NR NR |
11.7% 4.9% NR NR |
| Streiff Abstract 2016 [29] | Cohort (claims) | Number (follow-up) Patient age, gender CA type, stage VTE type, history Drug name (duration) |
660 (5.6 mo) NR NR NR Riva (3 mo) |
707 (5.6 mo) NR NR NR NR (1 mo) |
VTE (6 mo) MB (6 mo) CRNMB (6 mo) Death (6 mo) |
NR 8.2% NR NR |
NR 8.3% NR NR |
NR: not reported, RCT: randomized controlled trial, cohort: retrospective cohort study (based on hospital records or claims databases), DVT: deep vein thrombosis, PE: pulmonary embolism, CADVT: catheter-associated DVT, VTE: venous thromboembolism, MB: major bleeding, CRNMB: clinically relevant non-major bleeding, heme: hematologic malignancy, GYN: gynecologic malignancy, GI: gastrointestinal malignancy, met: metastasis, hx: history, mo: month, riva: rivaroxaban
Observational studies
There were significant heterogeneities in the patients’ selection, outcome reporting, and duration of follow-up periods among different observational studies [19–29]. Therefore, pooled proportions of the primary and secondary outcome events were not generated. The outcomes of individual studies are summarized within Table 1. Most studies used rivaroxaban (DOAC) and enoxaparin (LMWH). The on-treatment duration of DOAC was usually longer than that of LMWH. All studies except one reported lower rates of recurrent VTE for patients using DOAC as compared to those on LMWH [27]. The major bleeding and CRNMB outcomes were heterogeneous across different studies. Two studies that only included gastrointestinal and gynecological cancers reported higher rates of major bleeding episodes for patients on a DOAC [23,27].
Synthesis of randomized controlled trials
Two RCTs (HOKUSAI-Cancer and SELECT-D) were included for the analysis [11,12]. Baseline characteristics for both trials are shown in Table 1. Approximately, 30 to 50% of the included CAT were incidentally detected and a majority of patients had metastatic disease. The HOKUSAI-Cancer and SELECT-D trials compared edoxaban and rivaroxaban to dalteparin, respectively. Overall, DOACs (42/725) had a lower incidence of 6-month recurrent VTE when compared to LMWHs (64/727) (RR: 0.65 (95% CI: 0.42–1.01; I2: 17%)) (RD: −0.03 (−0.06–0.00)) (Figure 2A). However, DOACs (40/725) had a higher incidence of 6-month major bleeding when compared to LMWHs (23/727) (RR: 1.74 (95% CI: 1.05–2.88; I2: 0%)) (RD: +0.02 (0.00–0.04)) (Figure 2B). Similarly, CRNMB was higher (RR: 2.31 (95% CI: 0.85–6.28; I2: 78%)) (RD: +0.06 (0.01–0.12)) for patients with CAT receiving a DOAC (Figure 2C). There was no difference in mortality (RR: 1.03 (95% CI: 0.85–1.26; I2: 15%) (RD: +0.01 (−0.04–0.06)) (Figure 2D). Finally, there did not appear to be a publication bias across studies based on visual inspection of the funnel plots (data not shown).
Figure 2.
Forest plots of relative risks (RRs) for pooled outcome comparisons between DOAC and LMWH from randomized controlled trials. (A) VTE recurrence by 6-month, (B) major bleeding by 6-month, (C) clinically relevant non-major bleeding (CRNMB) by 6-month, (D) overall mortality by 6-month. Gray boxes superimposing RR estimates are proportional to the weight of the included study. Heterogeneity between trials were assessed by the I2 statistic.
Qualitative assessment
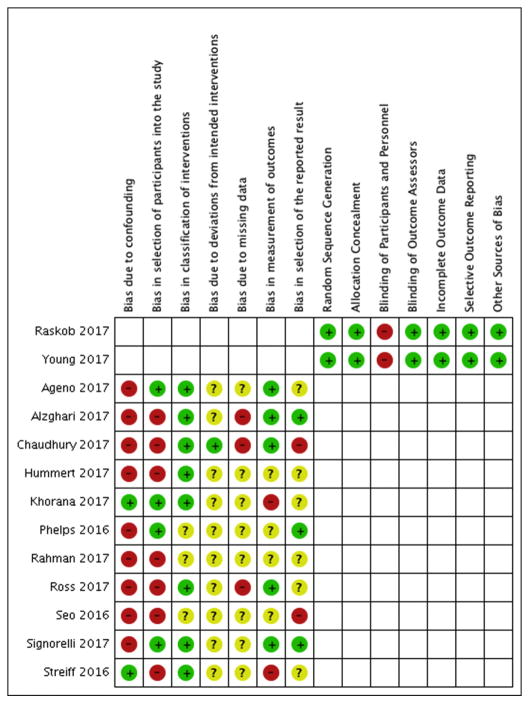
The risk of bias for each study is depicted in Figure 3. Both RCTs had a low risk for bias with the exception to blinding of participants and personnel due to the open study design; however, both had blinded and independent adjudication process for outcome assessment [11,12]. All 9 observational cohort studies had bias due to confounding and missing data [19–27]. Most studies also had selection bias due to treatment bias and inappropriate exclusion criteria (e.g. excluding patients who did not have certain duration of anticoagulation). Finally, the 2 claims database based studies had low risk for confounding given inverse weighting by the propensity score of treatment; however, both used recurrent VTE and major bleeding outcome measures that had not been appropriately validated in this specific patient population and could not be adjudicated [28,29].
Figure 3.
Risk of bias summary. Cochrane Risk of Bias Tool for randomized controlled trials is used to assess bias for the randomized trials (Raskob 2017 and Young 2017). ROBINS-I is used to assess bias for the observational studies. + low risk, − high risk, ? unclear/insufficient information.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to summarize the incidence of recurrent VTE and major bleeding episodes in over 5000 patients with CAT managed with DOACs when directly compared with LMWHs. While we included observational studies in the systematic review, our meta-analysis is only based on the RCTs. We believe that our literature review and data synthesis will provide clinicians with new insight to help decision making for patients with CAT.
The reported 6-month proportions of recurrent VTE and major bleeding episodes for patients receiving LMWH in our meta-analysis are similar to those previously reported in other CAT-related trials [4,5]. This provides reassurance about the generalizability of our findings to current clinical practice. Overall, the rates of recurrent VTE in patients treated with DOACs seemed to be lower to those receiving LMWHs. However, the risk difference of major bleeding and CRNMB episodes demonstrated a similarly higher risk of bleeding in patients treated with DOACs compared to those on LMWHs. It is important to note that although statistically important, the absolute risk differences between treatments with DOACs versus LMWHs are small for both recurrent VTE (−3% (−6% to 0%)) and major bleeding (+2% (0 to +4%)). Compliance with DOACs was generally better than compliance with LMWHs likely leading to longer time on treatment with DOACs. In the Hokusai-Cancer study, 15% of DOAC patients compared to 4% of LMWH patients discontinued study treatment due to “patient decision for inconvenience of dosing” [11]. Differential compliance could explain the differences in effectiveness and safety but would also reflect the real-world difficulty to adhering to long-term treatment with parenteral LMWHs. Furthermore, the risk of major bleeding differs between our study and that of Posch et al [9]. This difference is likely reflective of the heterogeneity of cancer patients and the confounding from indirect comparisons in the prior network meta-analysis.
The increase in major bleeding episodes related to DOACs seems to be limited to the upper gastrointestinal tract in the Hokusai-Cancer study. Subgroup analysis showed a significant interaction between edoxaban treatment and increased major bleeding in patients with gastrointestinal cancers [11]. Similarly, the Select-D trial stopped enrolling patients with gastroesophageal cancers at the recommendation of the independent Data Safety Monitoring Committee (DSMB) due to more than expected gastrointestinal bleeding [12]. The single observational study that only included all gastrointestinal cancer patients also reported a high incidence of major bleeding complications [27]. While we did not have sufficient data to perform a dedicated subgroup meta-analysis for patients with and without gastrointestinal cancers, this will warrant future exploration and confirmation for appropriate patient selection.
Strengths of our study include the inclusion of both interventional and observational trials and abstracts to assess for publication bias and generalizability. Limitations of our study include the relatively small number of RCTs included in the meta-analysis. However, there is little heterogeneity between the 2 studies for our primary outcomes of recurrent VTE and major bleeding episodes. CRNMB has demonstrated more heterogeneity and will require inclusion of more trials to determine its significance. Although we decided not to pool data from observational studies due to confounding and selection bias, the overall rates of recurrent VTE reported in these “real world” studies are consistent with the finding of our meta-analysis of RCTs. Furthermore, it is important to note that with exception of the largest Hokusai-Cancer trial which used edoxaban as the DOAC of choice, a majority of the other trials assessed rivaroxaban. Therefore, it remains unclear if the findings can be extrapolated to other DOACs including apixaban or dabigatran. Lastly, results of any meta-analysis should be interpreted in the context of the included studies. While both RCTs included patients with advanced cancer, very few had hematologic malignancies or hematopoietic cell transplantation and patients expected to have higher risk of bleeding (i.e. thrombocytopenia) were excluded from these trials. An individualized approach assessing the risk and benefits of the different anticoagulation regimens is required to tailor the management of CAT in these special patient populations [30].
In conclusion, for the treatment of CAT, DOACs (especially edoxaban and rivaroxaban) were more effective than LMWHs to prevent recurrent VTE but were associated with a small but significantly increased risk of major bleeding as well as a trend toward more CRNMB. Subgroup analyses from RCTs and observational studies suggest that patients with gastrointestinal cancer receiving DOACs may be at the highest risk for bleeding and DOACs should be used carefully in these patients. Future works should focus on assessing additional DOACs (e.g. apixaban) as well as the appropriate selection of cancer patients for the appropriate and safe use of DOACs for the treatment of CAT.
Supplementary Material
Highlights.
LMWHs have been the treatment of choice for cancer associated VTE
DOACs are more effective than LMWHs to prevent recurrent VTE in cancer patients
DOACs are associated with a higher risk of major bleeding compared to LMWHs
Effectiveness and safety may reflect better compliance with DOACs compared to LMWHs
Acknowledgments
Funding Sources:
This work was supported by grant from the National Heart, Lung, and Blood Institute, National Institutes of Health under award number T32HL007093 (AL).
Footnotes
Declarations of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P, Lensing AWa, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Cancer. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108.Reprints. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Lensing AWA, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 4.Lee AYY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 5.Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA CATCH Investigators. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA. 2015;314:677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 6.Khorana AA, Yannicelli D, McCrae KR, Milentijevic D, Crivera C, Nelson WW, Schein JR. Evaluation of US prescription patterns: Are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res. 2016;145:51–53. doi: 10.1016/j.thromres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147:475–483. doi: 10.1378/chest.14-0402. [DOI] [PubMed] [Google Scholar]
- 8.Carrier M, Cameron C, Delluc A, Castellucci L, Khorana AA, Lee AYY. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: a systematic review and meta-analysis. Thromb Res. 2014;134:1214–9. doi: 10.1016/j.thromres.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Posch F, Königsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582–9. doi: 10.1016/j.thromres.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farge D, Bounameaux H, Brenner B, Cajfinger F, Debourdeau P, Khorana AA, Pabinger I, Solymoss S, Douketis J, Kakkar A. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016;17:e452–e466. doi: 10.1016/S1470-2045(16)30369-2. [DOI] [PubMed] [Google Scholar]
- 11.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang T-F, Yeo E, Zhang G, Zwicker JI, Weitz JI, Büller HR Hokusai VTE Cancer Investigators. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2017 doi: 10.1056/NEJMoa1711948. NEJMoa1711948. [DOI] [Google Scholar]
- 12.Young A, Marshall A, Thirlwall J, Hill C, Hale D, Dunn J, Lokare A, Kakkar AK, Levine MN, Chapman O. Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism: Results of the Select-D™ Pilot Trial. Blood. 2017;130:625. [Google Scholar]
- 13.Schulman S, Kearon C Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–26. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ageno W, Mantovani LG, Haas S, Kreutz R, Monje D, Schneider J, Van Eickels M, Gebel M, Turpie AGG Subgroup Analysis of Patients with Cancer in XALIA. A Noninterventional Study of Rivaroxaban versus Standard Anticoagulation for VTE. TH Open. 2017;1:e33–e42. doi: 10.1055/s-0037-1603924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzghari SK, Seago SE, Garza JE, Hashimie YF, Baty KA, Evans MF, Shaver C, Herrington JD. Retrospective comparison of low molecular weight heparin vs. warfarin vs. oral Xa inhibitors for the prevention of recurrent venous thromboembolism in oncology patients: The Re-CLOT study. J Oncol Pharm Pract. 2017 doi: 10.1177/1078155217718382. 1078155217718382. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhury A, Balakrishnan A, Thai C, Holmstrom B, Nanjappa S, Ma Z, Jaglal MV. The Efficacy and Safety of Rivaroxaban and Dalteparin in the Treatment of Cancer Associated Venous Thrombosis. Indian J Hematol Blood Transfus. 2017:1–5. doi: 10.1007/s12288-017-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross JA, Miller MM, Rojas Hernandez CM. Comparative effectiveness and safety of direct oral anticoagulants (DOACs) versus conventional anticoagulation for the treatment of cancer-related venous thromboembolism: A retrospective analysis. Thromb Res. 2017;150:86–89. doi: 10.1016/j.thromres.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Signorelli JR, Gandhi AS. Evaluation of rivaroxaban use in patients with gynecologic malignancies at an academic medical center: A pilot study. J Oncol Pharm Pract. 2017 doi: 10.1177/1078155217739683. 107815521773968. [DOI] [PubMed] [Google Scholar]
- 24.Hummert SE, Gilreath J, Rodgers GM, Wilson N, Stenehjem DD. Comparative evaluation of the safety and effectiveness of rivaroxaban ( riva ) and enoxaparin ( enox ) for treatment of venous thromboembolism ( VTE ) in cancer patients. J Clin Oncol. 2017;35:e18268. doi: 10.1200/JCO.2017.35.15_suppl.e18268. [DOI] [Google Scholar]
- 25.Phelps MK, Wiczer TE, Erdeljac HP, Van Deusen KR, Porter K, Phillips G, Wang TF. Comparison of direct oral anticoagulants versus low-molecular-weight-heparins for the treatment of cancer associated thrombosis. Blood. 2016;128:5013. [Google Scholar]
- 26.Rahman S, Angelini DE, Elson P, Wilks ML, Pinkava V, O’Brien M, Tripp B, Song J-M, McCrae K, Khorana AA. Treatment of Cancer Associated Venous Thrombosis; The Cleveland Clinic Experience. Blood. 2017;130:4633. [Google Scholar]
- 27.Seo S, Ryu M-H, Kang Y-K, Kim K-P, Chang H-M, Ryoo B-Y, Kim SB, Lee J-L, Park SR. Oral rivaroxaban versus subcutaneous low molecular weight heparin treatment for venous thromboembolism in patients with upper gastrointestinal, hepatobiliary and pancreatic cancer. Ann Oncol. 2016;27:vi207–vi242. doi: 10.1093/annonc/mdw371.87. [DOI] [Google Scholar]
- 28.Khorana AA, McCrae K, Milentijevic D, McCormick N, Laliberté F, Crivera C, Lefebvre P, Lejeune D, Rozjabek H, Schein J, Streiff MB. VTE Recurrence and Safety of Anticoagulants Among Patients with Cancer Treated for Venous Thromboembolism. Blood. 2017;130:4631. [Google Scholar]
- 29.Streiff M, Milentijevic D, McCrae K, Yannicelli D, Fortier J, Nelson W, Laliberté F, Crivera C, Lefebvre P, Schein J, Khorana AA. Safety of anticoagulant therapies for treatment of venous thromboembolism in patients with cancer. Blood. 2016;128:1178. doi: 10.1002/ajh.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li A, Lopes RD, Garcia DA. Use of Direct Oral Anticoagulants in Special Populations. Hematol Oncol Clin North Am. 2016;30:1053–71. doi: 10.1016/j.hoc.2016.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.