Summary
Intestinal microbial flora, known as the second gene pool of the human body, play an important role in immune function, nutrient uptake, and various activities of host cells, as well as in human disease. Intestinal microorganisms are involved in a variety of mechanisms that affect bone health. Gut microbes are closely related to genetic variation, and gene regulation plays an important part in the development of bone-related diseases such as osteoporosis. Intestinal microorganisms can disrupt the balance between bone formation and resorption by indirectly stimulating or inhibiting osteoblasts and osteoclasts. In addition, intestinal microorganisms affect bone metabolism by regulating growth factors or altering bone immune status and can also alter the metabolism of serotonin, cortisol, and sex hormones, thereby affecting bone mass in mice. Moreover, probiotics, antibiotics, and diet can change the composition of the intestinal microbial flora, thus affecting bone health and also potentially helping to treat bone disease. Studying the relationship between intestinal flora and osteoblasts, osteoclasts, and bone marrow mesenchymal stem cells may provide a basis for preventing and treating bone diseases. This paper reviews recent advances in the study of the relationship between intestinal microflora and bone disease.
Keywords: Intestinal microbial flora, hereditary bone disease, bone health, bone-related cells
1. Introduction
Microbial groups, which are symbiotic in the human body or on the surface of the human body and cause various diseases under certain conditions, are collectively known as the human microbiome. This concept was first proposed by Lederberg et al. (1). There are many types of bacteria that populate the intestines of humans and animals. For example, the number of microorganisms in the body and the proportion of cells can reach a ratio of 10:1, and there are more than 10 trillion bacteria (2) that encode 100-fold more genes than those in the human genome (2). Thus, the intestinal microbial flora are known as the "second gene pool" of the human body (2).
Like human organs, human intestinal microflora have significant effects on immune function, nutrient uptake, and various life activities of host cells (3) as well as on various diseases and conditions in the human body. The microbial flora of the gut affect an organism by changing the balance of bacteria and metabolites (4), which can lead to changes in metabolic processes and induce the development of various diseases such as ulcerative colitis in inflammatory bowel disease (5) and Crohn's disease (6). Microbial flora can also cause many other common diseases including obesity (7), diabetes (8), and other endocrine system diseases, as well as cardiovascular diseases (9). Intestinal flora also affect diseases such as immune system-induced rheumatoid arthritis (10) and systemic lupus erythematosus (10). A study on cancer found that Bifidobacterium can improve anti-tumor immunity in mice (11). Conversely, Fusobacterium nucleatum can promote colorectal cancer resistance chemotherapy in cancer treatment (12). In addition, changes in intestinal microbial flora can induce inflammatory reactions in the host and change neurotransmitter metabolism, resulting in neurological dysfunction (13), depression, mental decline, and other problems (14,15). An imbalance in intestinal flora affects many diseases, whereas intestinal flora in homeostasis can prevent diseases. For example, intestinal microbial flora attach to the intestinal mucosa to form a protective barrier to defend against invasion by exotic pathogenic microorganisms (10). Intestinal flora can also stimulate an organism to produce an immune response to microbial antigens and to produce more lymphocytes (16). The resulting immunoglobulin G (IgG) antibodies can induce the organism to eliminate pathogenic microorganisms by identifying Gram-negative bacteria and neutralizing toxins and viruses (17), thereby promoting maturation of the immune system. This protects against inflammation and infection and improves immune function in the host (6,17). The current review provides updated information on the intestinal microbial flora and their affect on bone health.
2. Intestinal microbial flora and bone disease
2.1. Intestinal microbial flora and genetic factors
The relationship between human intestinal microbes and host genetic variation (18-22) is one in which the latter affects the composition of human intestinal microbes (19,20). A single-nucleotide polymorphism (SNP) (C/T-13910) of the European lactase (LCT) gene and SNPs (G/C-14010, T/G-13915, and C/G-13907) in the African LCT gene are associated with the abundance of Bifidobacterium in the gastrointestinal tract. These SNPs were found to significantly enhance transcription of the LCT gene promoter in vitro and to facilitate the hydrolysis of lactose in the gastrointestinal tract by lactase, thus directly affecting the persistence of LCT (23). Bifidobacterium in the gastrointestinal tract can also metabolize host lactose (24). LCT gene mutations may indirectly regulate the abundance of Bifidobacterium in the gastrointestinal tract by altering its lactose levels. Ruminococcaceae and Lachnospiraceae are the two main families of human intestinal microorganisms and are more similar in identical twins than in fraternal twins (25). The host genotype can regulate the abundance of many microbial flora.
Microbial diversity is controlled by both environmental and host genetic factors and is related to multiple diseases. Toll-like receptor (TLR) 5 gene-deficient mice have signs of metabolic syndrome including hyperlipidemia, hypertension, and obesity. After intestinal microflora were transferred from TLR5 gene-deficient mice to wild-type aseptic mice, the aseptic mice also exhibited the characteristics of metabolic syndrome, which were related to changes in microbial flora in the gut (26). Next-generation sequencing of genes in the gastrointestinal tract and quantitative trait loci (QTL) mapping have revealed that some host genes can change gut immunological profiles and modulate the balance between gut microbial communities. For example, the interferon gene-rich QTL region located on chromatin 4 modulates Firmicutes and Bacteroidetes, which are dominant BXD strains among gut microbes. Interleukin-1 receptor-associated kinase (IRAK) 4 modulates Rikenellaceae while TGF-β3 modulates Prevotellaceae (27).
Genetic variations in the genome may occur during the process of adapting to the environment, causes specific changes in microbial groups. Some loci can control a single microbial species, the associated taxa of certain microorganisms, and some of the associated microbial populations that are presumed to be more efficient and widely distributed (27). In addition, the gut flora have certain effects on host gene mutations. Some strains of Escherichia coli have a polyketide synthase (PKS) gene island that induces DNA mismatch repair in host intestinal epithelial cells by encoding gene toxins that cause tumors (28). Moreover, the superoxide anion produced by Enterococcus also causes host DNA damage and genomic instability, resulting in intestinal epithelial cell mutations that trigger colorectal cancer (29).
2.2. Intestinal microbial flora and osteoporosis
Osteoporosis is a metabolic bone disease that results in decreased bone mass and bone mineral density and that induces changes in bone microstructure (30). This disease is mainly influenced by heredity and environmental factors, and many studies have confirmed that there is a certain relationship between intestinal microbial flora and osteoporosis (30). An estrogen deficiency leads to bone loss-induced osteoporosis, and the use of surgical ovarian resection (OVX) or sexual hormone inhibition causes a lack of estrogen in mice (31). When OVX mice were treated with Lactobacillus acidophilus, the level of bone resorption markers decreased and osteoclast formation was inhibited (31). In addition, the number of T lymphocytes in OVX mice decreased and osteoclast formation was inhibited by Lactobacillus reuteri (32). Type I diabetes can also induce osteoporosis. A study reported that L. reuteri can inhibit the expression of tumor necrosis factor (TNF) and Wnt10b, preventing bone loss and bone marrow adiposity in a mouse model of type I diabetes (33).
Recent studies have found that intestinal microflora can regulate growth factor insulin-like growth factor (IGF)-1 levels, and thus regulate bone formation and absorption in young and middle-aged mice. The levels of IGF-1 decreased when antibiotics were used to destroy intestinal microflora in young mice (34). In addition, intestinal microbes were able to mediate the regulation of bone metabolism by altering bone immune status (35). Sterile mice had a significant increase in bone mass and a significant decrease in the number of osteoclasts, the number of CD4+ T cells derived from the bone marrow, and the number of osteoclast precursor cells compared to normal mice. While the number of osteoclasts, CD4+ T cells, and osteoclast precursor cells returned to normal in 3-week-old sterile mice treated with intestinal microbial flora and the quality of bone trabecular and cortical bone decreased, the expression of inflammatory cytokines in bone decreased significantly (35).
Intestinal disorders can cause inflammatory bowel disease (36), which can increase the risk of osteoporosis and related brittle fractures (37). The reduction in bone associated with inflammatory bowel disease is mainly due to insufficient calcium absorption and a decrease in the cycling levels of vitamins D and K (38). In addition, the inflammatory reaction caused by the lack of sex steroids can promote bone resorption, resulting in the loss of trabecular bone. The probiotic Lactobacillus rhamnosus GG (LGG) can reduce inflammation in the intestine and bone, improve intestinal permeability, and prevent bone loss (39,40).
When an imbalance of intestinal flora homeostasis occurs during intestinal inflammation, the intestinal absorption of vitamin D can help to treat enteritis, thereby reducing intestinal permeability and avoiding the impact of that imbalance on bone health (41). Vitamin D has beneficial effects in bones (42). When calcium supply is sufficient, the absorption of vitamin D and its metabolites in the gut can maintain the homeostasis of intestinal flora, thus improving the calcium balance and promoting mineral deposition in the bone matrix. In the absence of calcium, vitamin D can enhance bone resorption while inhibiting bone mineralization, thus maintaining blood calcium homeostasis at the expense of bone mass. Therefore, adding a proper amount of calcium to the diet can help treat inflammation and maintain the homeostasis of intestinal microbes to prevent osteoporosis mediated by vitamin D (42,43).
3. The mechanism(s) by which intestinal microbes affect bone health
3.1. Immune-mediated mechanisms
Intestinal microflora affect bone remodeling, bone mass accumulation (44), and bone health in a variety of ways (Figure 1). They can impact bone health via the immune system (35), which regulates the development of bone marrow cells and inflammatory cytokines. In germ-free (GF) mice, the number of myeloid cell progenitors decreased and the response to Listeria monocytogenes was impaired. These defects were remedied by transplantation of complex microbiota. Therefore, gut bacteria mediate innate immune cell development by increasing hematopoiesis (45). GF mice have fewer granulocyte-monocyte progenitors (GMPs). GF mice also have fewer CD11b+ Ly6C+ mononuclear cells and CD11b+ Ly6G+ granulocytes in the bone marrow than do specific pathogen-free (SPF) mice, but the formation of bone marrow was improved by the administration of serum isolated from SPF mice (46).
Figure 1.
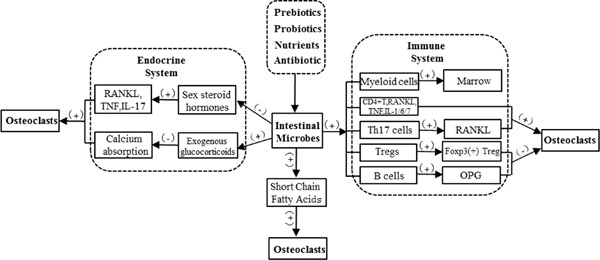
Mechanisms by which intestinal microbes affect bone health.
In addition, many studies have recently revealed the close relationship between intestinal microflora and regulatory T cells (Tregs) and helper T cells (Th cells) (31,47,49). Clostridium colonization in gnotobiotic mice resulted in preferential accumulation of Tregs in colonic lamina propria (50). The presence of intestinal bacteria might affect both the number and function of Tregs. Transforming growth factor beta (TGF-β), a key regulator of Treg development, is abundant in the colon in its active form. Similar findings were obtained with regard to other Treg-inducing molecules within the colon (50). Moreover, the induction of interleukin (IL)10- expressing Foxp3+ Tregs was specifically restricted in Clostridium but not in other bacteria. In addition, CD4+, CD25+, and Foxp3+Treg cells suppress macrophage colony-stimulating factor and osteoclast formation. This action is mainly through cytotoxic T-lymphocyte associated protein 4, an anti-osteoclastogenic molecule that binds osteoclast precursor cells and that inhibits its differentiation (51-53).
The bacterial segment in the gut is the driving force for Th17-assisted cell differentiation, which plays an important role in bone loss induced by rheumatoid arthritis (54,55). Intestinal flora disorders disrupt the balance of the pro-osteoclastogenic pathway and induce osteoclast-mediated bone loss in multiple ways, including the differentiation and inhibition of anti-osteoclastogenic Th1, Th2, and Treg subsets. This induces the differentiation of Th17 cells, which produce and increase RANKL expression on stromal cells, much like inflammatory cytokines (52,56,58). In addition, the number of osteoclast precursor cells also increases, thus promoting osteoclast differentiation (47). Microbial populations affect B cell development as well as bone resorption by osteoprotegerin, an inhibitor of osteoclasts produced by B cells (59).
3.2. Endocrine-mediated mechanisms
Intestinal microflora as a virtual "endocrine organ" (60) have certain effects on bone health. Sex hormones are vital in maintaining bone homeostasis, and a lack of these hormones can result in a decrease of microbial flora in the gut, thus increasing bone loss and affecting bone formation (40). In addition, a lack of sexual hormones increases the activity of osteoclasts and osteoblasts, although osteoclasts are more affected and bone loss is significant (61).
Sex steroid deficiency increases intestinal permeability and levels of the osteoclastogenic cytokines TNF, RANKL, and IL-17 in a murine model while GF mice were protected from bone loss (39). Supplementing the indigenous microbiota with the probiotic LGG prevents sex steroid-induced bone loss by inhibiting intestinal permeability. However, ingestion of nonprobiotic or mutant LGG eliminates this protective action. These findings indicate that gut microbiota serve as a dual role in sex steroid deficiency-induced bone loss (39). Moreover, excess glucocorticoid reduces the number of both osteoblasts and osteoclasts, it prolongs the lifespan of osteoclasts, and promotes apoptosis of osteoblasts and osteocytes (62,63).
3.3. Changes in intestinal flora affect bone health
Changes in intestinal microbial flora and their metabolites affect bone health directly or indirectly (47). The effects of microbial flora (64), probiotics (65), antibiotics (66), and dietary nutrition (67) also affect bone health. Probiotic dietary supplements containing Bacillus licheniformis and B. subtilis increased the diversity of intestinal flora, causing the tibial density in chickens to significantly increase compared to chickens fed a normal diet (68). Antibiotics can affect intestinal flora, and these effects are related to age, sex, and duration of treatment. The bone density of 3-week-old weaning mice dramatically increased when they were exposed to low doses of penicillin, chlortetracycline, or vancomycin compared to 7-week-old weaning mice (69). As a result of continuous treatment with low-dose penicillin (LDP), bone mass decreased in all male mice, regardless of whether or not they were exposed to LDP post-weaning or if the pregnant mother was exposed. These controversial results were replicated in several types of female mice administered LDP. LDP treatment in early life transiently disturbed the microbiota and altered the composition of the microbial community (66,69).
In addition, intestinal flora and different levels of nutrition affect bone health. Several studies transplanted the intestinal microflora of healthy and malnourished infants into sterile mice and found that malnourished infants with transplanted intestinal microorganisms had delayed development and abnormal skeletal muscle development (70). The effect of intestinal flora on bone health can by mediated by metabolites. The intestinal flora can digest soluble grain fiber in the diet into short-chain fatty acids (SCFAs), which lower the pH of the gut, contribute to calcium uptake, and inhibit the formation of osteoclasts (71). The G-protein-coupled receptors GPR40 and GPR120 superficially bind medium- and long-chain fatty acids, and a GPR40/120 agonist inhibits fatty acids in osteoclastogenesis. GPR120 acts as a mediator of the anti-osteoclastogenic action of C16 and C18 fatty acids (72).
4. Intestinal microbial groups and osteogenic/ osteoclasts and bone marrow mesenchymal cells
Bone marrow mesenchymal stem cells are pluripotent stem cells that can differentiate into various mesodermal lineages (73). Osteoblasts are the main cells that promote bone development and bone remodeling (74). Osteoblasts directly interact with bone cells, osteoclasts, and hematopoietic stem cells (74), thereby maintaining homeostasis between bone formation and bone resorption (74,75).
Studies have described the interaction between intestinal microflora and bone-related cells. Intestinal microflora influence the expression of peripheral and central serotonin (76). Peripheral serotonin is primarily produced in the gut, and intestinal flora such as indigenous spore-forming bacteria may play a catalytic role in the production of serotonin (77). Peripheral serotonin functions as a hormone to inhibit osteoblast proliferation through 5-hydroxytryptamine receptor 1B (5-HT1BR) and cAMP response element binding protein (CREB) (78), thereby reducing bone density and bone formation. The level of serotonin expression is lower in the intestinal tract of sterile mice and can be increased by transplanting E. coli Nissle 1917 into ileal tissue ex vivo, where it interacts with compounds secreted by host tissues (79). The use of probiotics can improve intestinal flora, reduce serotonin content, and alleviate bone disease (65,80). Central serotonin acts as a neurotransmitter to increase bone mass by calmodulin kinase (CaMK)- dependent signaling involving CaMKKβ and CaMKV, which are mediated by CREB (76).
Osteoclasts are functionally related to osteoblasts and are involved in bone resorption (35). Intestinal bone signaling pathways and microbial populations play an important role in regulating bone health (64). Decreases in estrogen in postmenopausal women result in an increase in inflammatory factors and osteoclast formation (81). In a model of osteoporosis in OVZ rats, expression of the inflammatory cytokines TNFα and IL-1 decreased, osteoprotegerin levels increased, and osteoclast formation was inhibited (35) when Lactobacillus strains and other probiotics were given to OVZ rats (35).
A reduction in the diversity of the intestinal microbiota has been noted and specific taxonomic preferences have been identified in Crohn's disease and ulcerative colitis (6). In a mouse model of ulcerative colitis induced with 2, 4, 6-trinitrobenzene sulfonic acid, bone marrow stromal cells (BMSCs) were implanted in intestinal mucosa, where they repaired damaged intestinal tissue. This may be because BMSCs differentiate into colonic stromal cells and express vascular endothelial growth factor and TGF-β1 in the injured region (82,83). BMSCs also have immune-regulatory effects on antigen-specific T cells in Crohn's disease through direct cell-cell contact (84), inhibiting allogeneic antigen-specific responses and mitogen-induced proliferation (85), preventing the production of cytotoxic T lymphocytes. In general, there is growing evidence that BMSCs could be used to treat inflammatory bowel disease caused by a disorder in intestinal flora, but the precise molecules and mechanisms responsible for immune regulation need to be ascertained (86).
5. Conclusion
Understanding the correlation between intestinal microbial flora and bone health paves the way for further studies on the treatment of bone diseases by intestinal microbes. Intestinal microbial flora influence bone health through the immune system, endocrine system, and various bone-related cells. An increasing number of studies on osteoporosis have suggested the beneficial effects of probiotics in terms of increasing bone mass. This provides a basis for treating hereditary bone diseases, which involve abnormal bone, cartilage, joint, and other related tissues due to pathogenic mutations in genes. The relationship between pathogenic mutations that cause genetic bone diseases and intestinal microbial flora remains unclear and needs to be studied further.
Acknowledgements
This project was supported by Grants-in-Aid from the Shandong Government (nos. 2016ZDJS07A10 and 2016GSF201222) and the State Major Infectious Disease Research Program (Chinese Central Government, no. 2017ZX10103004-007).
References
- 1. Lederberg J, McCray AT. 'Ome Sweet 'Omics - A genealogical treasury of words. Scientist. 2001; 15:8. [Google Scholar]
- 2. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010; 464:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001; 292:1115-1118. [DOI] [PubMed] [Google Scholar]
- 4. Serino M, Luche E, Gres S, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012; 61:543-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009; 361:2066-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright EK, Kamm MA, Teo SM, Inouye M, Wagner J, Kirkwood CD. Recent advances in characterizing the gastrointestinal microbiome in Crohn's disease: A systematic review. Inflamm Bowel Dis. 2015; 21:1219-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sze MA, Schloss PD. Looking for a signal in the noise: Revisiting obesity and the microbiome. MBio. 2016; 7:>pii: e01018-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ussar S, Fujisaka S, Kahn CR. Interactions between host genetics and gut microbiome in diabetes and metabolic syndrome. Mol Metab. 2016; 5:795-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pevsner-Fischer M, Blacher E, Tatirovsky E, Ben-Dov IZ, Elinav E. The gut microbiome and hypertension. Curr Opin Nephrol Hypertens. 2017; 26:1-8. [DOI] [PubMed] [Google Scholar]
- 10. Rosser EC, Mauri C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J Autoimmun. 2016; 74:85-93. [DOI] [PubMed] [Google Scholar]
- 11. Gill IS, Canes D, Aron M, Haber GP, Goldfarb DA, Flechner S, Desai MR, Kaouk JH, Desai MM. Single port transumbilical (E-NOTES) donor nephrectomy. J Urol. 2008; 180:637-641. [DOI] [PubMed] [Google Scholar]
- 12. Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017; 170:548-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: Focus on depression. Curr Opin Psychiatry. 2015; 28:1-6. [DOI] [PubMed] [Google Scholar]
- 14. Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, Guarner F. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013; 4:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015; 37:984-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. The maternal microbiota drives early postnatal innate immune development. Science. 2016; 351:1296-1302. [DOI] [PubMed] [Google Scholar]
- 17. Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Núñez G. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity. 2016; 44:647-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdul-Aziz MA, Cooper A, Weyrich LS. Exploring relationships between host genome and microbiome: New insights from genome-wide association studies. Front Microbiol. 2016; 7:1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, Spector TD, Keinan A, Ley RE, Gevers D, Clark AG. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015; 16:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dabrowska K, Witkiewicz W. Correlations of host genetics and gut microbiome composition. Front Microbiol. 2016; 7:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davenport ER. Elucidating the role of the host genome in shaping microbiome composition. Gut Microbes. 2016; 7:178-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khachatryan ZA, Ktsoyan ZA, Manukyan GP, Kelly D, Ghazaryan KA, Aminov RI. Predominant role of host genetics in controlling the composition of gut microbiota. PLoS One. 2008; 3:e3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tishkoff SA, Reed FA, Ranciaro A, et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2007; 39:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parche S, Beleut M, Rezzonico E, Jacobs D, Arigoni F, Titgemeyer F, Jankovic I. Lactose-over-glucose preference in Bifidobacterium longum NCC2705: glcP, encoding a glucose transporter, is subject to lactose repression. J Bacteriol. 2006; 188:1260-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell. 2014; 159:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010; 328:228-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKnite AM, Perez-Munoz ME, Lu L, Williams EG, Brewer S, Andreux PA, Bastiaansen JW, Wang X, Kachman SD, Auwerx J, Williams RW, Benson AK, Peterson DA, Ciobanu DC. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS One. 2012; 7:e39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012; 338:120-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beukers AG, Zaheer R, Goji N, Amoako KK, Chaves AV, Ward MP, McAllister TA. Comparative genomics of Enterococcus spp. isolated from bovine feces. BMC Microbiol. 2017; 17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marini F, Cianferotti L, Brandi ML. Epigenetic mechanisms in bone biology and osteoporosis: Can they drive therapeutic choices? Int J Mol Sci. 2016; 17:E1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjogren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014; 9:e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014; 229:1822-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015; 156:3169-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016; 113:e7554-e7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012; 27:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007; 104:13780-13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009; 122:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghishan FK, Kiela PR. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol. 2011; 300:G191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016; 126:2049-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carson JA, Manolagas SC. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone. 2015; 80:67-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larmonier CB, McFadden RM, Hill FM, Schreiner R, Ramalingam R, Besselsen DG, Ghishan FK, Kiela PR. High vitamin D3 diet administered during active colitis negatively affects bone metabolism in an adoptive T cell transfer model. Am J Physiol Gastrointest Liver Physiol. 2013; 305:G35-G46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eisman JA, Bouillon R. Vitamin D: Direct effects of vitamin D metabolites on bone: Lessons from genetically modified mice. Bonekey Rep. 2014; 3:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van de Peppel J, van Leeuwen JP. Vitamin D and gene networks in human osteoblasts. Front Physiol. 2014; 5:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hernandez CJ, Guss JD, Luna M, Goldring SR. Links between the microbiome and bone. J Bone Miner Res. 2016; 31:1638-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014; 15:374-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Balmer ML, Schurch CM, Saito Y, Geuking MB, Li H, Cuenca M, Kovtonyuk LV, McCoy KD, Hapfelmeier S, Ochsenbein AF, Manz MG, Slack E, Macpherson AJ. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol. 2014; 193:5273-5283. [DOI] [PubMed] [Google Scholar]
- 47. Charles JF, Ermann J, Aliprantis AO. The intestinal microbiome and skeletal fitness: Connecting bugs and bones. Clin Immunol. 2015; 159:163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asquith M, Elewaut D, Lin P, Rosenbaum JT. The role of the gut and microbes in the pathogenesis of spondyloarthritis. Best Pract Res Clin Rheumatol. 2014; 28:687-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sjögren K1 EC, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012 ; 27:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011; 331:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zaiss MM, Axmann R, Zwerina J, Polzer K, Guckel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheum. 2007; 56:4104-4112. [DOI] [PubMed] [Google Scholar]
- 52. Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999; 402:304-309. [DOI] [PubMed] [Google Scholar]
- 53. Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, McClanahan T, Bowman EP. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther. 2010; 12:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010; 32:815-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Farkas AM, Panea C, Goto Y, Nakato G, Galan-Diez M, Narushima S, Honda K, Ivanov II. Induction of Th17 cells by segmented filamentous bacteria in the murine intestine. J Immunol Methods. 2015; 421:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006; 203:2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lam J TS, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF- α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000; 106:1481-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wei S, Wang WM, Teitelbaum SK, Ross FP. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF- κB and mitogen-activated protein kinase signaling. J Biol Chem. 2002; 277:6622-6630. [DOI] [PubMed] [Google Scholar]
- 59. Wesemann DR. Microbes and B cell development. Adv Immunol. 2015; 125:155-178. [DOI] [PubMed] [Google Scholar]
- 60. Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: The neglected endocrine organ. Mol Endocrinol. 2014; 28:1221-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013; 9:699-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weinstein RS. Glucocorticoids, osteocytes, and skeletal fragility: The role of bone vascularity. Bone. 2010; 46:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhou H, Cooper MS, Seibel MJ. Endogenous glucocorticoids and bone. Bone Res. 2013; 2:107-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S, Ahmad Z, Ibrahim Z, Jamaluddin R. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int. 2015; 2015:897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Collins S, Reid G. Distant site effects of ingested prebiotics. Nutrients. 2016; 8:E523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012; 488:621-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bonjour JP, Kraenzlin M, Levasseur R, Warren M, Whiting S. Dairy in adulthood: From foods to nutrient interactions on bone and skeletal muscle health. J Am Coll Nutr. 2013; 32:251-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mutus R, Kocabagli N, Alp M, Acar N, Eren M, Gezen SS. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci. 2006; 85:1621-1625. [DOI] [PubMed] [Google Scholar]
- 69. Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014; 158:705-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blanton LV, Charbonneau MR, Salih T, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016; 351:6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Iwami K, Moriyama T. Effects of short chain fatty acid, sodium butyrate, on osteoblastic cells and osteoclastic cells. Int J Biochem. 1993; 25:1631-1635. [DOI] [PubMed] [Google Scholar]
- 72. Cornish J, MacGibbon A, Lin JM, Watson M, Callon KE, Tong PC, Dunford JE, van der Does Y, Williams GA, Grey AB, Naot D, Reid IR. Modulation of osteoclastogenesis by fatty acids. Endocrinology. 2008; 149:5688-5695. [DOI] [PubMed] [Google Scholar]
- 73. Pittenger MF MA, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:5143-5417. [DOI] [PubMed] [Google Scholar]
- 74. Ottewell PD. The role of osteoblasts in bone metastasis. J Bone Oncol. 2016; 5:124-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prideaux M, Findlay DM, Atkins GJ. Osteocytes: The master cells in bone remodelling. Curr Opin Pharmacol. 2016; 28:24-30. [DOI] [PubMed] [Google Scholar]
- 76. Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol. 2010; 191:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015; 161:264-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008; 135:825-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nzakizwanayo J, Dedi C, Standen G, Macfarlane WM, Patel BA, Jones BV. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci Rep. 2015; 5:17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yun HM, Park KR, Hong JT, Kim EC. Peripheral serotonin-mediated system suppresses bone development and regeneration via serotonin 6 G-protein-coupled receptor. Sci Rep. 2016; 6:30985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Whisner CM, Martin BR, Schoterman MH, Nakatsu CH, McCabe LD, McCabe GP, Wastney ME, van den Heuvel EG, Weaver CM. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: A double-blind cross-over trial. Br J Nutr. 2013; 110:1292-1303. [DOI] [PubMed] [Google Scholar]
- 82. Bonomo A, Monteiro AC, Goncalves-Silva T, Cordeiro- Spinetti E, Galvani RG, Balduino A. A T cell view of the bone marrow. Front Immunol. 2016; 7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011; 17:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ciccocioppo R, Cangemi GC, Kruzliak P, et al. Ex vivo immunosuppressive effects of mesenchymal stem cells on Crohn's disease mucosal T cells are largely dependent on indoleamine 2,3-dioxygenase activity and cell-cell contact. Stem Cell Res Ther. 2015; 6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99:3838-3843. [DOI] [PubMed] [Google Scholar]
- 86. Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 2011; 10:410-415. [DOI] [PubMed] [Google Scholar]


