Summary
Traumatic neuroma of the breast after cancer surgery is a very rare clinical entity with only a few cases having been reported to date. We herein present a very rare case of traumatic breast neuroma in a postmenopausal patient with a history of breast-conserving surgery, who presented with a four-month history of intractable neuropathic breast pain. Diagnostic evaluation and management are discussed along with a review of the literature. Traumatic breast neuromas are very rare benign lesions that have been reported mainly after mastectomy. Our literature review yielded only 35 cases of traumatic breast neuromas in 28 patients, reported so far. Although imaging features may be indicative of a benign lesion, surgical excision is necessary to obtain a definitive diagnosis and to rule out a recurrent breast cancer. Conservative treatment is feasible in properly selected cases with asymptomatic neuromas after an accurate tissue sampling. The case presented herein underlines the necessity to consider traumatic neuroma in the differential diagnosis in patients with a history of breast surgery presenting with refractory neuropathic breast pain. A high index of suspicion is required because the lesion may be too small and can be missed on imaging investigations.
Keywords: Neuroma, breast, traumatic, neuropathic, pain
1. Introduction
Traumatic neuroma (TN) is a nonneoplastic reactive proliferation of the proximal end of a partially transected or severed nerve as a result of trauma or surgery (1,2). It is a disorganized tangled mass of axons, Schwann cells and perineural fibroblasts that is formed as a result of a failed attempt to reestablish axonal continuity following disruption of neuronal axons (3). Although TN is a well-established finding in patients who have undergone amputation (4), it, however, may occur in almost any anatomical site of the body and may resemble a peripheral nerve sheath tumor on imaging (5). Histologically, TN is characterized by a nonencapsulated, disordered proliferation of small nerve fascicles, composed of axons, Schwann cells, endoneurial and perineurial cells, embedded in dense fibrous stroma (6,7). TN may produce severe refractory neuropathic pain, functional impairment, and phycological distress thus decreasing the quality of life (8,9). TN of the breast following cancer surgery is a very rare clinical entity with only a few cases reported in the literature so far. We herein present a very rare case of traumatic breast neuroma in a postmenopausal patient with a history of breast-conserving cancer surgery followed by radiotherapy, who presented with a four-month history of severe neuropathic refractory breast pain. Diagnostic evaluation and management are discussed along with a review of the literature.
2. Case Report
A 65-year old woman presented with a four-month history of left breast pain. She stated that the pain was severe and constant, with paroxysms of burning and electrical shock-like sensations, was exacerbated with pressure and was refractory to analgesics.
Her medical history was significant for a breast-conserving surgery, due to a ductal carcinoma in situ (DCIS) two years ago, followed by adjuvant radiation therapy and hormonal treatment with tamoxifen.
Clinical examination revealed severe left breast tenderness that was exacerbating with pressure over the area of the surgical scar. The mammogram revealed clustered microcalcifications in the area of the previously resected DCIS, along with a small area of architectural distortion. Ultrasonography was unremarkable.
In order to exclude the possibility of a recurrent DCIS a complete surgical excision of the mammographic lesions after wire localization was performed. Specimen radiography confirmed that the mammographic lesions were entirely removed (Figure 1). Immediately after surgery, the patient experienced complete pain relief.
Figure 1.
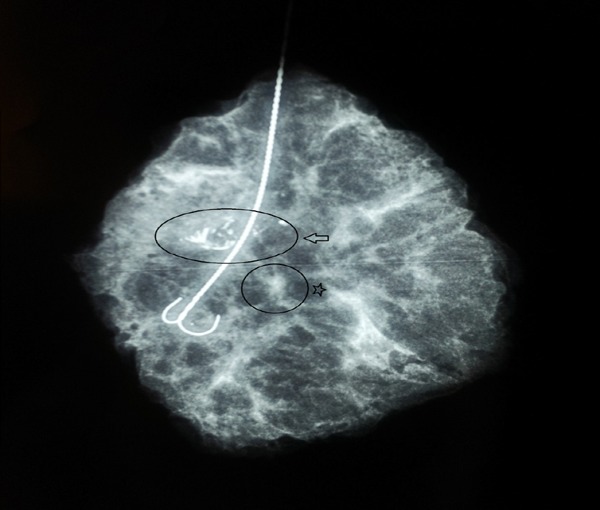
Specimen radiography showing clustered microcalcifications (arrow), along with a small area of architectural distortion (star).
The histopathological findings were consistent with fat necrosis and confluent microcalcifications. In addition, a small eosinophilic tumor measuring 0.2 cm was detected in the area of the architectural distortion. The tumor consisted of haphazardly arranged, eosinophilic cells with oval to spindle nuclei, without atypia, necrosis or mitosis. The periphery of the nodule was composed of perineural cells, while the inside consisted of proliferated Schwann cells (Figure 2). No evidence of malignancy was noted. The histological findings, in correlation with the clinical history and the complete relief of the breast pain immediately after surgery, indicated the diagnosis of traumatic breast neuroma.
Figure 2.
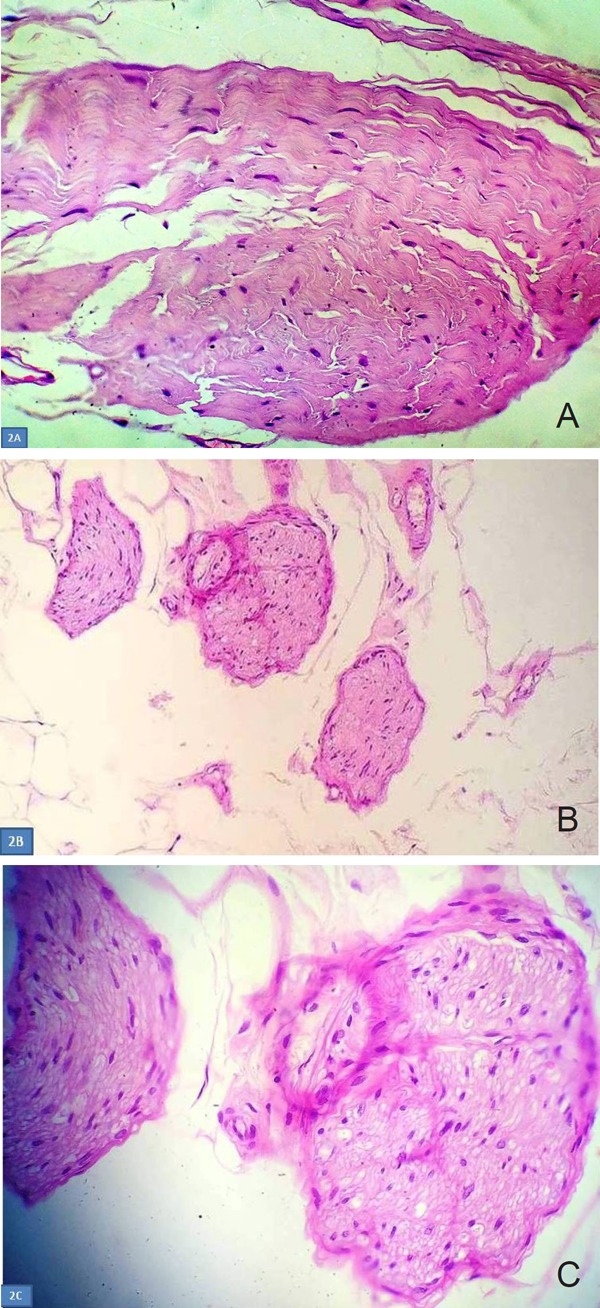
Histopathological findings of traumatic breast neuroma. (A) The tumor consists of haphazardly arranged, eosinophilic cells with oval to spindle nuclei, without atypia, necrosis or mitosis. (Hematoxylin and Eosin ×400); (B) Tangles of small and medium, well circumscribed nerve fiber bundles, that do not invade the surrounding fibroadiposal tissue.(Hematoxylin and Eosin ×100); (C) High power photomicrograph, of eosinophilic cells, with bubbly cytoplasm, bland, oval to spindle nuclei, without significant atypia. The periphery of the nodule is composed of perineural cells, while the inside consists of proliferated Swann cells. (Hematoxylin and Eosin ×400).
The patient is completely asymptomatic, without any suspicious mammographic findings four years after surgery.
3. Discussion
TN is not a true tumor but a reactive proliferation of nerve tissue of the proximal end of a severed nerve in an unsuccessful attempt to reestablish axonal continuity (7). TNs are divided into two major categories: spindle neuromas that are focal swellings caused by chronic irritation to an injured but non severed nerve and terminal neuromas that have a bulbous-end morphology as a result of a partial disruption or total transection of the nerve as a result of surgery (5,7,10).
The exact pathogenesis of TN has not been clearly defined. Foltan et al. (11), suggested that the development of a TN may be divided into five phases and caused by simultaneous regeneration of nerve fibers and excessive fibrous tissue proliferation which results in contraction of nerve fibers within the scar tissue and the establishment of a chronic defensive proliferation process involving nerve fibers and scar fibrous tissue.
TN may develop 1-12 months after transection or injury secondary to a variety of surgical procedures such as radical neck dissections, abdominal surgery, limb amputations, orthognathic surgery, parotidectomy and tooth extractions (11). TN most commonly occurs in the lower extremities followed by head and neck, radial nerve and brachial plexus (7). In an early study by Tapas et al. (12), involving 67 cancer patients with TN the most frequently reported sites were radically dissected necks followed by upper and lower extremities.
TN typically presents as a firm, slowly growing painful or tender nodule not larger than 2 cm (3,11). Pain may be evoked by palpation or tapping over the lesion (Tinel sign) and may be associated with burning, stabbing or gnawing sensations (7,9).
Postoperative breast pain is a frequent issue reported in up to 60% of patients undergoing breast surgery (13-15) and may interfere with sexual activity, exercise, social activity and employment (13). Severe postoperative pain persists for 1 month and for 6-12 months in 25% and 10% of the breast surgery patients respectively (14).
Chronic post mastectomy pain is a chronic pain affecting the anterior chest wall, axilla and the upper half of the arm that begins after mastectomy or quadrantectomy and persists for more than three months after surgery (16). Although its exact pathogenesis is unclear, it is however believed, that an injury of the intercostobrachial nerve is the most common cause. The most common distribution of pain is the axilla and the arm (20-60%) followed by pain in the surgical scar (23-49%) (16). Pain can be experienced as a burning sensation or tenderness with paroxysms of lancinating shock-like pain, is exacerbated by pressure or movement and may be associated with discomfort or paresthesia (16). Neuromas trapped in the scar tissue may cause chronic neuropathic pain (15).
Jung et al. (8), classified chronic neuropathic pain following breast surgery into four categories: I phantom breast pain, II intercostobrachial neuralgia as a result of injury to the intercostobrachial nerve, associated with sensory changes in the distribution of the intercostobrachial nerve, III neuroma pain in the region of scar of the breast that is provoked or exacerbated by percussion, and IV other nerve injury pain secondary to injury to medial or lateral pectoral, long thoracic or thoracodorsal nerves. Neuroma pain has been more frequently encountered following lumpectomy plus radiotherapy than mastectomy (8).
In a retrospective review of 57 patients with postsurgical chronic breast pain Ducic et al. (13), described five zones of nerve injury. The lateral zone was the most commonly injured area (79%) followed by inferior (10.5%), medial (5%), central (3.5%) and superior (2%) zones.
Traumatic neuroma of the breast (TBN) following cancer surgery is a very rare clinical entity (18,21). The first case was reported in 2000 (17). Our literature review yielded only 35 TBNs in 28 patients reported so far (1,2,4,6,10,14,24) (Table 1).
Table 1. Description of factors and variables used to assess ARSACS patients for functional capacity and physical fitness evaluations.
| Reference | Patients | Age | Neuromas (n) | Neuroma size (cm) | Time after surgery (months) | Palpable | Pain/Tenderness | Ultrasound features | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Rosso et al. (17), 2000 | 2 | 55, 67 | 2 | 0.4-0.6 | 22, 50 | Yes | Yes | N/A | Surgical excision |
| Haj et al. (19), 2004 | 1 | 47 | 1 | N/A | 120 | No | No | N/A | Stereotactic vaccum -assisted core needle biopsy |
| Baltalarli et al. (2), 2004 | 1 | 54 | 1 | 1 | 15 | Yes | No | Well-circumscribed, homogeneous, hypoechoic | Surgical excision |
| Wang et al. (23), 2007 | 6 | 33-61 | 8 | 1 | 33-96 | Yes | No | Well-circumscribed, homogeneous, Hypoechoic (4) Poorly defined hypoechoic (2) | Surgical excision |
| Kim et al. (20), 2011 | 1 | 47 | 1 | 1 | 168 | No | No | Oval circumscribed hypoechoic | Surgical excision |
| Li et al. (21). 2012 | 1 | 43 | 1 | 0.5 | 24 | Yes | N/A | Well-circumscribed, echo-heterogeneous | Surgical excision |
| Ashkar et al. (1) 2013 | 1 | 42 | 1 | 0.7 | 36 | No | Yes | Well-defined, homogeneous, Hypoechoic | Ultrasound-guided core biopsy |
| Shin et al. (22), 2013 | 1 | 7 | 1 | 0.5 | 144 | N/A | N/A | Oval circumscribed hypoechoic | Surgical excision |
| Zhu et al. (24), 2015 | 1 | 65 | 2 | N/A | 24 | No | Yes | N/A | Surgical excision |
| Al Sharif et al. (6), 2016 | 6 | 48-71 mean:56 | 8 | 0.2-0.9 mean:0.46 | 24-264 mean:123 | 3/8 (38%) | 1/8 (13%) | Hypoechoic with parallel orientation (7), Oval shape with circumscribed margins (1) | Ultrasound-guided core biopsy |
| Messinger et al. (4). 2017 | 1 | 74 | 2 | 0.6, 1.6 | N/A | No | No | Parallel, oval, hypoechoic masses | Ultrasound-guided core biopsy |
| Fitzpatrick et al. (18), 2017 | 1 | 73 | 1 | 0.7 | 192 | No | No | Hypoechoic, oval with parallel orientation and circumscribed margins | Ultrasound-guided core biopsy |
| Sung et al. (10), 2017 | 5 | 33-63 mean:44 | 6 | 0.39-0.55 Mean:0.48 | 23-133 mean:67 | No | No | Oval shape with a circumscribed margin (4), Irregular shape and an indistinct margin (2) | Surgical excision |
| Salemis (present case), 2018 | 1 | 65 | 1 | 0.2 | 24 | No | Yes | N/A | Surgical excision |
In a retrospective review Al Sharif et al. (6), reported a 0.09% incidence of TBN among 9,293 ultrasound-guided breast biopsies performed over a 10 year period. TBN can occur either after mastectomy or lumpectomy (8) and may develop 2-22 years after surgery (6).
Clinically TBN may present as a palpable mass or may be identified incidentally (1,6,10). It is most commonly encountered in the upper outer quadrant of the breast (6,10). Although in some cases the mass is painless (18,21), however, persistent chronic pain has been reported (19,24). In all six cases presented by Sung et al. (10), the TBN was located within the pectoralis major muscle near the mastectomy scar. TBN may be occult on mammogram (6,18).
On ultrasonography, TBN may appear as a homogenous well circumscribed hypoechoic mass with no internal vascular flow on color Doppler imaging (6,18,20,21). In some cases, however, TBN may appear as an irregularly shaped mass with indistinct margins and nonparallel orientation (10,23). A tail sign on ultrasonography has been reported in 50% of the cases reported by Al Sharif et al. (6). In the same study, 40% of the cases were occult on MRI evaluation. The most common MRI finding in the remaining cases was an isointense foci on T1 weighted images with a benign type I enhancement curve (2). On positron emission tomography (PET) computed tomography (CT) scan no fluorodeoxyglucose (FDG) focal uptake of TBN has been reported (10,20).
TBN should always be distinguished from recurrent breast cancer (21,23). Clinically, the location of TBN in the pectoral muscle layer may be helpful in the differential diagnosis, since a recurrent breast cancer is most commonly detected in the subcutaneous fat layer of the chest wall (10,20). The definitive diagnosis of TBN is, however, obtained by histopathological evaluation (1,4,18,21,23).
Treatment approaches for TBN include conservative management or surgery. The conservative approach, which has been reported to be successful in 50% of the cases, includes injection of a long-acting local anesthetic, corticosteroids, anti-inflammatory and antidepressant medications, opioids, acupuncture, physical therapy and electrical stimulation (2,6,7,9,16,18,24).
An image-guided percutaneous biopsy is the standard of care for TBN (2,4,6,19). The procedure may be associated with severe pain despite generous local anesthesia (6). If a correct diagnosis is established, there is no need for further treatment, unless the TBN is painful (18). Sonographic surveillance is, however, indicated (2,6). Fine needle aspiration (FNA) cytology of TBN may be inconclusive (2,4,17).
Surgical treatment should be considered in cases not responding to conservative management presenting with chronic neuropathic pain (9,18,24). Surgical treatment includes resection of the neuroma, neurorrhaphy, and implantation of the nerve stump into adjacent muscles (6,9) and can result in complete pain relief (24), as in our case.
Long-standing traumatic neuromas may undergo osseous metaplasia (19), or granular cell changes thus constituting a granular cell traumatic neuroma (17). The latter should be differentiated from malignant tumors such as apocrine carcinoma and alveolar soft part sarcoma. Kos et al. (3), reported a malignant peripheral nerve sheath tumor that arose within a long-standing traumatic neuroma.
In our case, similarly to the cases reported in the literature the neuroma was detected in the area of the surgical scar. The lesion was diagnosed 20 months after the primary surgery, while the reported cases have been reported to occur 24 months to 22 years after surgery. The size of the neuroma in our case was too small and was not detected on ultrasonography but was only seen as a small architectural distortion on mammography. Another different aspect in our case was the fact that the lesion was non palpable and provoked neuropathic breast pain refractory to analgesics, while in the literature most neuromas refer to palpable and painless nodules (18,21). In addition, a characteristic feature in our case was that our patient experienced complete relief of the breast pain immediately after surgery.
In conclusion, traumatic neuroma after breast cancer surgery is a very rare clinical entity with only a few cases reported in the literature so far. It should always be considered as a potential diagnosis in patients with a history of breast cancer surgery presenting with refractory neuropathic breast pain. Although the imaging features may be suggestive of a benign lesion, surgical excision is necessary to obtain a definitive diagnosis and to exclude a recurrent breast cancer. Conservative treatment is feasible in properly selected cases with asymptomatic neuromas after an accurate tissue sampling.
Acknowledgements
The author would like to thank Dr. Katikaridis I, and Dr. Sambaziotis D, from the Department of Pathology for providing the histology slides.
References
- 1. Ashkar L, Omeroglu A, Halwani F, Alsharif S, Loutfi A, Mesurolle B. Post-traumatic neuroma following breast surgery. Breast J. 2013; 19:671-672. [DOI] [PubMed] [Google Scholar]
- 2. Baltalarli B, Demirkan N, Yağci B. Traumatic neuroma: Unusual benign lesion occurring in the mastectomy scar. Clin Oncol (R Coll Radiol). 2004; 16:503-504. [DOI] [PubMed] [Google Scholar]
- 3. Kos Z, Robertson SJ, Purgina BM, Verma S, Gravel DH. Malignant peripheral nerve sheath tumor arising in a traumatic neuroma: A case report. Hum Pathol. 2013; 44:2360-2364. [DOI] [PubMed] [Google Scholar]
- 4. Messinger JD, Crawford SM. Traumatic neuroma in axillary dissection scar bed following mastectomy. Appl Radiol. 2017; 46:38-39. [Google Scholar]
- 5. Ahlawat S, Belzberg AJ, Montgomery EA, Fayad LM. MRI features of peripheral traumatic neuromas. Eur Radiol. 2016; 26:1204-1212. [DOI] [PubMed] [Google Scholar]
- 6. AlSharif S, Ferré R, Omeroglu A, El Khoury M, Mesurolle B. Imaging Features Associated With Posttraumatic Breast Neuromas. AJR Am J Roentgenol. 2016; 206:660-665. [DOI] [PubMed] [Google Scholar]
- 7. Kransdorf MJ, Murphey MD. Neurogenic tumors. In: Imaging of soft tissue tumors. Kransdorf MJ, Murphey MD (eds). Philadelphia PA, Lippincott, Williams &Wilkins, 2006; pp:328-380. [Google Scholar]
- 8. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: Proposed classification and research update. Pain. 2003; 104:1-13. [DOI] [PubMed] [Google Scholar]
- 9. Yao C, Zhou X, Zhao B, Sun C, Poonit K, Yan H. Treatments of traumatic neuropathic pain: A systematic review. Oncotarget. 2017; 8:57670-57679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sung HS, Kim YS. Ultrasonographic features of traumatic neuromas in breast cancer patients after mastectomy. Ultrasonography. 2017; 36:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foltán R, Klíma K, Spacková J, Sedý J. Mechanism of traumatic neuroma development. Med Hypotheses. 2008; 71:572-576. [DOI] [PubMed] [Google Scholar]
- 12. Das Gupta TK, Brasfield RD. Amputation neuromas in cancer patients. NY State J Med. 1969; 1969; 2129-2132. [PubMed] [Google Scholar]
- 13. Ducic I, Seiboth LA, Iorio ML. Chronic postoperative breast pain: Danger zones for nerve injuries. Plast Reconstr Surg. 2011; 127:41-46. [DOI] [PubMed] [Google Scholar]
- 14. Fecho K, Miller NR, Merritt SA, Klauber-Demore N, Hultman CS, Blau WS. Acute and persistent postoperative pain after breast surgery. Pain Med. 2009; 10:708-715. [DOI] [PubMed] [Google Scholar]
- 15. Ramesh, Shukla NK, Bhatnagar S. Phantom breast syndrome. Indian J Palliat Care. 2009; 15:103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaur N, Jain A. Postmastectomy chronic pain in breast cancer survivors: An update on definition, pathogenesis, risk Factors, treatment and prevention. Clin Oncol. 2017; 2:1300. [Google Scholar]
- 17. Rosso R, Scelsi M, Carnevali L. Granular cell traumatic neuroma: A lesion occurring in mastectomy scars. Arch Pathol Lab Med. 2000; 124:709-711. [DOI] [PubMed] [Google Scholar]
- 18. Fitzpatrick KA, Borders MH, MacKerricher WS, Bracamonte ER. Traumatic neuroma of the breast after mastectomy. Appl Radiol. 2017; 46:26-28. [Google Scholar]
- 19. Haj M, Bickel A, Cohen I. Osseous metaplasia of breast neuroma: Diagnosis with stereotactic core biopsy. Breast J. 2004; 10:366-367. [DOI] [PubMed] [Google Scholar]
- 20. Kim EY, Kang DK, Kim TH, Kim KS, Yim H. Traumatic neuroma in a breast cancer patient after modified radical mastectomy: A case report. J Korean Soc Radiol. 2011; 64:515-518. [Google Scholar]
- 21. Li Q, Gao EL, Yang YL, Hu HY, Hu XQ. Traumatic neuroma in a patient with breast cancer after mastectomy: A case report and review of the literature. World J Surg Oncol. 2012; 10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shin S. A traumatic neuroma in breast cancer patient after mastectomy: A case report. J Korean Soc Radiol. 2013; 69:405-407. [Google Scholar]
- 23. Wang X, Cao X, Ning L. Traumatic neuromas after mastectomy. ANZ J Surg. 2007; 77:704-705. [DOI] [PubMed] [Google Scholar]
- 24. Zhu L, Batdorf NJ, Meares AL, Sukov WR, Lemaine V. Bilateral thoracodorsal neuromas: A cause of persistent breast pain after bilateral latissimus dorsi breast reconstruction. Arch Plast Surg. 2015; 42:499-502. [DOI] [PMC free article] [PubMed] [Google Scholar]


