Summary
Objective
To characterize seizure semiology and the utility of antiepileptic drug (AED) therapy in leucine‐rich glioma inactivated‐1 ( LGI1‐Ab) autoimmune epilepsy (AE).
Methods
Patients with voltage‐gated potassium channel complex (VGKCc) titers higher than 0.02 nmol/L who were evaluated between May 2008 and June 2016 at the 3 Mayo Clinic sites (Arizona, Florida, or Minnesota) were identified. We then performed a retrospective review of those who were LGI1‐Ab positive and were treated for seizures.
Results
A total of 1,095 patients with VGKCc titers higher than 0.02 nmol/L were identified, in which 77 were LGI1 positive. Of these, 56 patients with seizures were included in the analysis. Mean age at symptom onset was 62.9 years; 66% (n = 37) were male. The most common seizure semiology was focal faciobrachial dystonic seizures with preserved awareness (FBDS) (n = 35, 63%), followed by focal with impaired awareness (FIA) (n = 29, 52%), generalized tonic–clonic (GTCs) (n = 28, 50%), and focal non‐motor seizures with preserved awareness (n = 28, 50%). The majority had more than one seizure type (n = 49, 88%; median = 2.5). Thirty‐eight patients (68%) became seizure free: 29 (76%) with immunotherapy, 3 (5%) with AEDs alone, 2 (3%) with AEDs before any immunotherapy, and 4 (7%) with AEDs after immunotherapy. Levetiracetam (n = 47, 84%) and valproic acid (n = 21, 38%) were the most commonly used AEDs, but neither were associated with seizure freedom. Sodium channel blocking (NCB) AEDs were associated with seizure freedom in 4 patients compared to none treated with non‐NCB AEDs. Regardless of class, AEDs prior to or apart from immunotherapy were associated with seizure freedom in only five patients (9%). In patients with FBDS, seizure freedom was more often associated with immunotherapy than AEDs (20/30 vs. 3/34, p = 0.001).
Significance
Although FBDS are the most characteristic seizure type seen in LGI1‐Ab AE, other seizure types including FIA and GTCs also occur. Immunotherapy was the treatment most frequently associated with seizure freedom in LGI1‐Ab AE. In general, AEDs seemed to confer a very low chance for seizure freedom, although AEDs with NCB‐blocking properties were associated with seizure freedom in a limited number. Levetiracetam in particular appears to be ineffective in this patient population.
Keywords: Autoimmune encephalitis, Drug‐resistant epilepsy, Faciobrachial dystonic seizures, Sodium channel blockers, Voltage‐gated potassium channel complex
Key Points.
In this study of 56 patients with LGI1‐Ab AE, immunotherapy was most frequently associated with seizure freedom (68%). Seizure freedom occurred in only 9 patients (16%) when immunotherapy was not used or used as an adjunct
Most patients with LGI1‐Ab AE (n = 49, 88%; median = 2.5) more than one seizure type
Regardless of the AED class used, AEDs prior to or apart from immunotherapy were associated with seizure freedom in only 5 patients (9%). Sodium channel blocking (NCB) AEDs were associated seizure freedom in 4 patients compared to none treated with non‐NCB AEDs
Focal faciobrachial dystonic seizures with preserved awareness (FBDS) seemed to respond more often to immunotherapy than to AEDs; non‐FBDS may respond to AEDs better than FBDS
A subset of patients with voltage‐gated potassium channel complex (VGKCc) autoimmunity harbors pathogenic antibodies directed against the extracellular domains of leucine‐rich glioma inactivated‐1 (LGI1) or contactin‐associated protein 2 (CASPR2).1, 2 The remaining patients who lack specific antibodies for LGI1 or CASPR2 are considered ‘double negative’; their antibodies target intracellular epitopes of proteins or channels in the complex and are not considered pathogenic.3, 4 LGI1 forms a trans‐synaptic complex with the presynaptic proteins ADAM11 (a disintegrin and metalloproteinase 11) and ADAM23, and the postsynaptic protein ADAM22, and is involved in synaptic transmission of neuronal excitability.1, 2, 3 Clinically, LGI1 antibodies are most commonly associated with limbic encephalitis, seizures and hyponatremia.1, 5 Focal faciobrachial dystonic seizures with preserved awareness (FBDS) are the most characteristic seizure type, occurring in 26–71% of cases.4, 6 However, LGI1 patients may manifest other seizure types as well.5, 7 Although tumor screening is indicated in these patients, tumors can be found in less than 10% of cases.4
There are no published randomized controlled trials evaluating the efficacy of immunotherapy in LGI1‐Ab associated autoimmune epilepsy (AE). However, immunotherapy is generally considered the cornerstone of treatment in these patients, particularly in those with FBDS and/or cognitive dysfunction.8, 9 Although the seizures in AE are often considered medication‐resistant, in select cases, AEDs may confer seizure control.10, 11 Previous studies suggest that AEDs with sodium channel blocking (NCB) properties may be more effective in AE than other AED types, raising the possibility of a class effect.11 In this study we aimed to evaluate the effect of seizure semiology in LGI1‐Ab AE on treatment response, and examine whether there is difference in AED efficacy based on AED mechanism.
Methods
Patients
Patients were identified through service neural autoantibody evaluation between May 2008 and June 2016 at the 3 principal Mayo Clinic sites (Arizona, Florida, or Minnesota). We identified 1,095 patients with VGKC titers higher than 0.02 nmol/L. Of these, 77 were confirmed LGI1 positive, 15 CASPR2 positive, and 3 positive for both. Of the LGI1‐positive patients, 56 were treated specifically for seizures. Patients with both LGI1 and CASPR2 antibodies were excluded from review in an attempt to avoid confounding variables. Figure 1 shows the study design. Clinical data captured included length of follow‐up, cerebrospinal fluid (CSF) and serum LGI‐1 antibody titers, seizure semiology, baseline seizure frequency, presence of cognitive dysfunction, magnetic resonance imaging (MRI) and electroencephalography (EEG) findings, AED type, immunotherapy, duration of each therapy, and seizure response.
Figure 1.
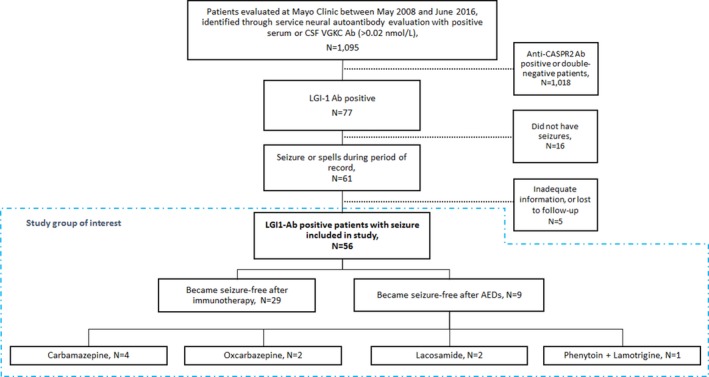
Study design.
Baseline seizure frequency was determined by reviewing the seizure frequency stated in the clinical record prior to initiation of treatment and was categorized as daily (>1 seizure per day), weekly (>1 seizure per week but not daily), or monthly (>1 seizure per month but not weekly). Seizure freedom response was attributed to therapy using information from clinical notes, which either (1) directly stated that a particular therapy resulted in seizure freedom or (2) that the seizure frequency was recorded as 0 in the treating neurologists’ clinical note within 4 weeks of initiation of a new antiseizure therapy if there were no other new therapies started in the same period. Seizure freedom was used as an endpoint, since information on partial response was not uniformly available.
Comparison to a non‐LGI1 cohort
A cohort of patients with non‐LGI1 antibody‐related AE was extracted from a recent study by our group.11 In this study consisting of 50 patients, 39 cases who tested negative for LGI1 antibody were identified and were used as controls. These cases presented with AE associated with VGKC‐complex antibody (n = 6) (CASPR2 [1], and double negative [5]); ganglionic acetylcholine receptor (n = 5); NMDA receptor (n = 3); P/Q‐type calcium channel (n = 1), GAD65 (n = 10), ANNA‐1 (n = 1), and ANNA‐2 (n = 1). Three patients had non‐neuronal antibodies (thyroid peroxidase [TPO], Ant‐Ro, and anti–dsDNA), and 9 patients were autoantibody negative.11 We compared demographics, seizure type and frequency, seizure freedom by AED class, as well as patterns of immunotherapy and AED use between the current and the non‐LGI1 cohort.
Assays for VGKC complex subtypes
All samples were screened for VGKC‐complex IgG by radioimmunoprecipitation assay as described previously.6 Those yielding positive results (VGKC‐complex IgG >0.02 nmol/L) were tested for LGI1and CASPR2 specificity by a clinically validated transfected cell‐based immunofluorescence assay (CBA; EUROIMMUN, Lubeck, Germany). CSF was tested undiluted and serum at 1:10 dilution. The results were scored as either negative or positive with no titration used. The CBA was run in duplicate and scored by at least 2 experienced readers (A.G., S.J.P., A.M., and C.J.K.); a third reader scored any assays that yielded discordant results (<1%).
Statistical analysis
Data were expressed as mean, median, and range for continuous variables, and counts (percentages) for categorical variables. Univariate analysis of nominal variables was performed using chi‐square test, and continuous variables were analyzed using independent sample t‐test or Mann‐Whitney U test. All analyses were performed using SAS software (version 9.3). All statistical tests were two sided, and p < .05 was considered statistically significant.
Seizure freedom by AED class was calculated as the number of patients who became seizure free with a particular class of AED (NCB vs. non‐NCB) divided by the total number of individual recorded uses of that AED class. Given the refractoriness of seizures in our cohort, most patients had multiple trials of various AEDs during the course of their illness. Comparison of seizure freedom by therapy (immunotherapy vs. AEDs) for the FBDS and non‐FBDS groups was calculated as the number of patients who became seizure free (numerator) divided by the number of those who received each type of therapy (denominator).
Standard protocol approvals, registrations, and patient consents
The study was approved by the Mayo Clinic Institutional Review Board and all patients consented to the use of their medical records for research purposes.
Results
Clinical characteristics
Sixty‐one of 77 positive LGI1 patients (79%) had seizures. Clinical data regarding medication, response to treatment, and long‐term follow‐up was available for 56. Mean age at onset of symptoms was 63 years (range 46–87), and 66% (n = 37) were male. Cognitive dysfunction of any kind was present at initial visit in the majority (n = 47, 84%). The most common seizure semiology was FBDS (n = 35; 63%) followed by focal with impaired awareness (FIA) (n = 29, 52%) and generalized tonic–clonic seizures (GTCs) (n = 28, 50%), with most patients having more than one seizure type (n = 49, 88%; median= 2.5). In 33/35 patients (94%), FBDS occurred with other seizure types, whereas only 2 were identified to have FBDS alone. Summary of seizure semiology for the cohort is provided in Figure 2. Half of the patients (n = 28, 50%) had ictal or interictal epileptiform abnormalities on EEG. Of the 50 patients that underwent brain MRI, 30 patients (60%) had abnormalities: unilateral or bilateral T2 hyperintensities in the medial temporal lobes in 23 (46%) and T2 signal changes in the basal ganglia in 7 (14%). Whole body positron emission tomography (PET) scan for tumor screening was performed in 30 patients (56%) and revealed evidence of tumors in none of our cohort. Summary of the clinical characteristics of the cohort is provided in Table 1.
Figure 2.
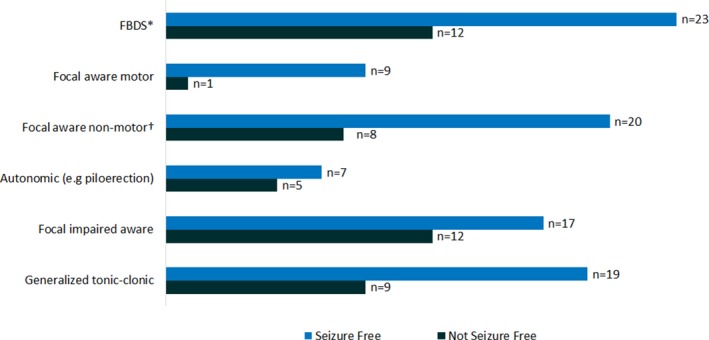
Seizure semiology and therapy response in 56 patients with anti‐LGI1 associated autoimmune epilepsy. *Faciobrachial dystonic seizures; †includes focal sensory, visual, and cognitive seizures.
Table 1.
Clinical Characteristics of the cohort
| N = 56, % | |
|---|---|
| Sex | |
| Male | 37 (66%) |
| Female | 19 (34%) |
| Mean age of onset, year | 62.9 |
| Cognitive dysfunction | 47 (84%) |
| Seizure type | |
| Faciobrachial dystonic seizures (FBDS) | 35 (63%) |
| Focal impaired awareness | 29 (52%) |
| Focal aware motor | 10 (18%) |
| Focal aware non‐motor | 28 (50%) |
| Autonomic (including piloerection) | 12 (21%) |
| Generalized tonic–clonic (GTC) | 28 (50%) |
| AEDs used or tried | 55 (98%) |
| 0 | 1 |
| 1 | 10 |
| 2 | 19 |
| ≥3 | 26 |
| Immunotherapy | 50 (89%) |
| IV Methylprednisolone | 47 (84%) |
| IV Immunoglobulin | 18 (32%) |
| Plasmapheresis | 5 (9%) |
| Rituximab | 2 (4%) |
| Mycophenolate | 27 (48%) |
| Azathioprine | 10 (18%) |
| Methotrexate | 5 (9%) |
| Prednisolone | 7 (13%) |
| Seizure freedom independent of therapy | |
| Yes | 38 (70%) |
| No | 18 (32%) |
| Seizure freedom by type of therapy | |
| With immunotherapy | 29 (52%) |
| With AEDs alone | 3 (5%) |
| With AEDs before immunotherapy | 2 (3%) |
| With AEDs after immunotherapy | 4 (7%) |
| EEG findings | 56 (100%) |
| With interictal epileptiform discharges | 10 (18%) |
| Seizures captured | 18 (32%) |
| MRI findings | 50 (89%) |
| Evidence of Inflammatory changes | 30 (60%) |
| No evidence of inflammation | 24 (40%) |
| VGKCc Antibody titer by RIA | 56 (100%) |
| ≤0.5 | 29 (52%) |
| >0.5 | 27 (48%) |
| ≤1.00 | 42 (75%) |
| >1.00 | 14 (25%) |
AED, antiepileptic drug; EEG, electroencephalography; MRI, magnetic resonance imaging: RIA, radioimmunoassay; VGKCc, voltage‐gated potassium channel complex.
Treatments
The majority (n = 50, 89%) received at least one form of immunotherapy in combination with AEDs, whereas the remainder (n = 6, 11%) received AEDs alone. Only one patient received immunotherapy alone without documented use of AEDs. Immunotherapy included intravenous methylprednisolone (n = 47), intravenous immunoglobulin (n = 18), and/or plasmapheresis (n = 5). Long‐term maintenance immunotherapy was initiated in several patients: mycophenolate mofetil (n = 27), azathioprine (n = 10), rituximab (n = 2), or methotrexate (n = 5). Levetiracetam was the most common AED used (n = 47, 83%), followed by valproic acid (n = 21), phenytoin (n = 15), lacosamide (n = 14), carbamazepine (n = 12), oxcarbazepine (n = 6), and lamotrigine (n = 6). Summary of AEDs used in the cohort is provided in Figure S1.
Seizure freedom outcome
The majority of patients (n = 38, 68%) became seizure‐free: 29 (76%) with immunotherapy, 3 (5%) with AEDs alone, 2 (3%) with AEDs before any immunotherapy, and 4 (7%) with AEDs after immunotherapy. The majority who responded to AEDs (6/9) had non‐FBDS. Seizure freedom was more often associated with immunotherapy than AEDs in patients with FBDS (20/30 vs. 3/34, p = 0.001). Although patients with FBDS seemed less likely to respond to AEDs than other seizure types, this did not reach statistical significance (p = 0.07). In contrast, there was no significant difference in the rate of response by therapy type for non‐FBDS (9/20 vs. 6/21, p = 0.34). Summary of seizure freedom by seizure semiology and type of therapy is provided in Table 2 and Figure 3.
Table 2.
Summary of seizure freedom by seizure semiology and type of therapy
| Total, N (%)a | Seizure free (% from total) | |
|---|---|---|
| Patients with FBDS | 35 | 23 |
| Received immunotherapyb | 30 (88) | 20 (67) |
| Received AEDsb | 34 (97) | 3 (9) |
| Patients with non‐FBDS | 21 | 15 |
| Received immunotherapyb | 20 (95) | 9 (42) |
| Received AED(s)b | 21 (100) | 6 (28) |
| Patients with use of NCB AEDb | 52 | 4c |
| Patients with use of non‐NCB AEDb | 83 | 0c |
AED, antiepileptic drug; FBDS, focal aware faciobrachial dystonic seizures; NCB AED, sodium channel blocking antiepileptic drugs.
Multiple therapies within the same patient were used in most cases.
Received therapy during the course of their illness.
Included only those patients who responded to either AEDs alone or with AEDs prior to initiation of immunotherapy.
Figure 3.
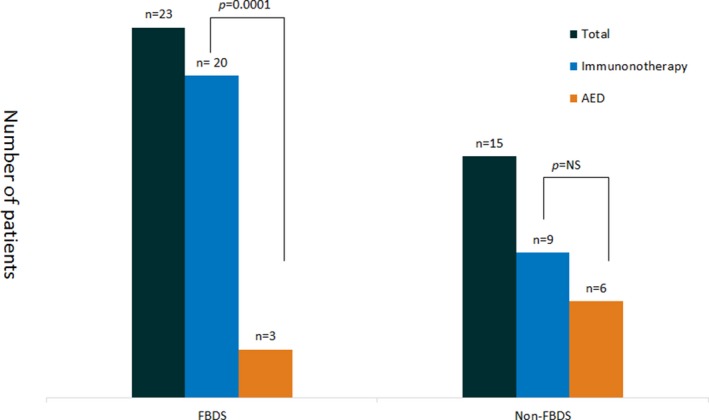
Seizure freedom by type of therapy and seizure.
Seizure freedom by AED class
Although levetiracetam (n = 47, 83.5%) and valproic acid (n = 21, 38%) were the most frequently used AEDs; neither were associated with seizure freedom. NCB AEDs were more often associated with seizure freedom than non‐NCB AEDs (4/52 vs. 0/83, p = 0.025). Seizure freedom was associated with 4/12 with carbamazepine, 2/14 with lacosamide, 2/6 with oxcarbazepine, and one with gabapentin and lamotrigine. All 3 patients with FBDS who became seizure‐free with AEDs responded to carbamazepine. However, 2 of these patients began receiving immunotherapy for cognitive dysfunction several weeks earlier. Time to seizure freedom following AED initiation was within 24 hours in 2 patients, within 2 weeks in 2, within 3 weeks in 3, and within 4 weeks in 2. Details of patients who responded to AEDs are summarized in Table 3. We have also provided the timeline of AED use in relation to immunotherapy for the 6 patients who responded to AEDs despite previous or concurrent immune therapies in Figure S2.
Table 3.
Clinical characteristic of nine patients who become seizure free with AEDs
| Age/Sex | Seizure type(s) | AED associated with seizure freedom | Immunotherapy used | Timeline of effective AED initiation in relation to immunotherapy | Duration of time between seizure onset and initiation of AED associated with seizure freedom (column 3) | Other AEDs tried | VGKCc Ab Titer (nmol/L) | Duration of seizure freedom (months) |
|---|---|---|---|---|---|---|---|---|
| 47/F | GTC; focal aware | CBZ | None | NA | 10 months | LEV | 0.88 | 66 |
| 51/M | GTC; focal aware motor; focal impaired aware; FBDS | CBZ | IVMP; Azathioprine; Prednisone; mycophenolate; IVIG | 16 months prior | 14 months | LEV, VPA, LCM | 0.41 | 10 |
| 73/M | FBDS; focal impaired aware; focal aware motor | CBZ | IVMP; mycophenolate; IVIG; Rituximab | 9 months prior | 8 months | LEV | 0.3 | 13 |
| 56/F | Focal impaired aware; FBDS | CBZ | None | NA | 3 months | LEV | 0.4 | 48 |
| 68/F | Focal aware‐autonomic | LCM | IVMP; IVIG; mycophenolate | 5 months prior | 8 months | LTG, LEV | 0.25 | 32 |
| 62/M | GTC; focal aware | LCM | None | NA | 5 months | PHTa, CBZ,a LEV | 0.04 | 36 |
| 71/M | GTC | GBT and LTG | IVIG | Added 2 months later | 3 months | LEV | 1.33 | 22 |
| 71/M | Focal aware‐autonomic; focal impaired aware; GTCs | OXC | IVMP; mycophenolate | Added 3 months later | 1 month | LEV | 0.62 | 66 |
| 46/M | Focal aware‐autonomic; focal impaired aware | OXC | IVMP; IVIG; mycophenolate | 11 months prior | 1 month | LEV, VPA | 0.37 | 10 |
AED, antiepileptic drug; CBZ, carbamazepine; F, female; IVIG, IV immunoglobulin; IVMP, IV methylprednisone; LEV, levetiracetam; M, male; OXC, oxcarbazepine; PHT, phenytoin; Sz, seizure; VPA, valproic acid.
Discontinued due to adverse effect(s).
Comparison to the non‐LGI1 cohort
In contrast to the current LGI1 cohort, the non‐LGI1 cohort were younger (35.4 vs. 62.9, p = 0001). The male‐to‐female ratio of the non‐LGI1 cohort was about 1:1 while in the LGI1 cohort it was 3:2. Seizure frequency of greater than 1 per week was higher in the LGI1 cohort (47/52 vs. 26/37, p = 0.02). Frequency of GTCs and rate of seizure freedom with NCB and non‐NCB AEDs were similar among the 2 cohorts. Levetiracetam was the most commonly prescribed AED in both groups and was not associated with seizure freedom in either. A detailed of comparison between the LGI1 and the non‐LGI1 cohorts (case–control) is provided in Table 4.
Table 4.
Comparison of clinical characteristics of LGI1 cohort to a non‐LGI1 cohort (control)
| Non‐LGI1 (control) cohort (n = 39)a | LGI1 cohort (n = 56) | p | |
|---|---|---|---|
| Age, years | 35.4 | 62.9 | 0.0001 |
| Sex, male | 19 | 37 | 0.20 |
| Seizure type | |||
| FBDS | 0 | 35 | 0.0001 |
| Non‐FBDS | 39 | 54 | 0.50 |
| GTCs | 15 | 28 | 0.30 |
| Seizure frequency | |||
| Unknown | 2 | 4 | 1.00 |
| >1/week | 26 | 47 | 0.02 |
| <1/week | 11 | 5 | 0.02 |
| Seizure freedom | |||
| With NCB‐AEDs | 3 | 4 | 1.00 |
| With non NCB‐AEDs | 0 | 0 | 1.00 |
| With immunotherapy | 15 | 29 | 0.22 |
| Patients who received immunotherapy | 32 | 50 | 0.37 |
| Patients who received levetiracetam | 31 | 47 | 0.59 |
FBDS, focal aware faciobrachial dystonic seizures; GTCs, generalized tonic–clonic seizures; LGI1, leucine‐rich glioma‐inactivated protein 1; NCB‐AED, sodium channel blocking antiepileptic drugs.
The p values are bold where they are significant.
Cohort extracted from a recent study by our group.11
Discussion
The results reinforce that immunotherapies are the most effective intervention for patients with LGI1‐Ab AE, and should be considered first‐line, as has been published previously. Our data also suggest that AED mechanism may impact their efficacy for seizure control in LGI1‐Ab AE.12
In our series, 8 patients (14%) became seizure free after initiation of NCB AEDs (5 without or prior to immunotherapy). It is not known whether there are unique aspects of the pathophysiology of seizure onset in these patients that render them more susceptible to sodium channel blockade. It is conceivable that the therapeutic response could be secondary to immunomodulatory properties of these medications.10, 12 For example, carbamazepine has been shown to reduce levels of pro‐inflammatory cytokines IL‐1β and TNF‐α in the hippocampus of rats.13 Carbamazepine has also been shown to inhibit the development of inflammation via dose‐dependent reduction of prostaglandin E2 and substance P.14, 15 NCB AEDs, including carbamazepine, have been noted to be efficacious in other VGKC channelopathies such as immune‐mediated neuromyotonia and hereditary episodic ataxia type 1.16, 17 Although these agents act primarily by either slow or fast inactivation of voltage‐gated sodium channels, at concentrations used to treat epilepsy, some of these have also been shown to modulate ATP‐sensitive potassium channels as well as calcium‐activated potassium channel activity.18, 19
Alternatively, the potential class effect with NCBs could be due to a delayed therapeutic response to immunotherapy instituted prior to or during the NCB‐AED use. The use of multiple types of therapy within short time spans increases the possibility of misattributing seizure freedom, especially since the effects of immunotherapy are often not immediate. A third possible mechanism that might explain class effect could be related to evolution of the seizures with delayed development of structural changes of the brain (eg, mesial temporal sclerosis or hippocampal atrophy) coinciding with the institution of NCB‐AEDs, which were often prescribed later in the course of illness. These medications are known to be effective in the setting of focal structural epilepsies, which may represent a late stage of LGI1 AE. Further studies are needed to elucidate whether these differences in efficacy are based on the anticonvulsant mechanism of action of these drugs or to their immunomodulatory properties.
Our findings reiterate that FBDS is the most common seizure type in LGI1‐Ab AE, and that other seizure types including FIA and GTCs may also occur. FBDS more frequently occurred along with other seizure types in LGI1‐Ab AE rather than on an isolated basis. Of course, our data are limited by clinical recognition of FBDS, which may be more likely to go unrecognized if not accompanied by other features of LGI1‐Ab AE. A high clinical suspicion for FBDS is necessary for diagnosis, particularly in patients who do not have specific MRI findings, or other seizure types present. Pre‐ictal contralateral frontal infra‐slow activity can be recognized on EEG as a signature of FBDS,20 but FBDS may be clinically misdiagnosed as hyperkinetic movement disorder, limb‐shaking TIA, or functional neurologic symptom disorder.
In our series, as in others, patients with FBDS were more likely to respond to immunotherapy than to AEDs. The efficacy of immunotherapy is emphasized by our series and others previously published and should be considered the cornerstone of therapy in patients with LGI1‐Ab AE. FBDS often resolve within days after initiation of intravenous methylprednisolone or immunoglobulin in LGI1‐Ab AE. In addition, early immunotherapy has been thought to help prevent progression to limbic encephalitis in these patients.5 Although immunotherapy is usually emphasized in these patients, others have also reported response of FBDS to AED therapy.21 In a study of 29 patients with FBDS, 4 patients showed a good (20–50%) or excellent (>50%) reduction in FBDS frequency within 1 month of treatment.22 Only 3 patients (3/35) with FBDS in our series became seizure free with the use of AED(s) alone. Of these, one did not receive subsequent immunotherapy. In patients with non‐FBDS, use of AEDs was more frequently associated with seizure freedom than in those with FBDS, although this did not reach statistical significance. Our observations indicate that AEDs may also play a role in the management of these patients if immunotherapy does not produce seizure freedom.
Levetiracetam (84%) and valproic acid (38%) were the most commonly used AEDs in our cohort; however, their use was never associated with seizure freedom. On the other hand, AEDs with NCB mechanisms, including carbamazepine and oxcarbazepine, were rarely used in this cohort. Similarly, levetiracetam was the most commonly used agent for our case‐control cohort (80%) (Table 4). One reason that might dissuade the use of carbamazepine and oxcarbazepine involves the potential for hyponatremia in LGI1 encephalitis.1, 21, 22 It is unclear why we observed a relative lack of efficacy with levetiracetam in the current and the non‐LGI1 cohort. Levetiracetam interacts with synaptic vesicle protein 2A (SV2A) at the presynaptic region.23 Presynaptic mechanisms alone may be insufficient for seizure suppression in this condition, which has targets in both the pre‐ and postsynaptic regions.
In comparison to the previous cohort of non‐LGI1 AE patients, the rate of seizure freedom by AED class and pattern of AED was similar to that of the non‐LGI1 cohort. The seizure frequency was greater than 1 per week in LGI1 versus non‐LGI1 patients, highlighting the very high frequency of FBDS and overall seizure burden of this patient population. Although the seizures were more frequent in LGI1 patients, they also more frequently responded to therapies (Table 4).
Our study has inherent limitations. First, data collection was based on extraction of patient information from the electronic medical records, which was not documented in a structured, prospective fashion. For instance, seizure frequency and precise timing of drug effect were not routinely recorded, which introduces possibility of confounding with use of multiple AEDs and immunotherapies simultaneously. We relied on the documented impressions of treating clinicians. Second, lack of specificity in documentation precluded precise examination of the AED response of non‐FBDS in those patients who had both FBDS and non‐FBDS resistant to AEDs. In addition, recall bias due to seizure‐associated amnesia and concurrent cognitive dysfunction, which was present in a significant proportion of our cohort (84%), could have affected the accuracy of patient reporting. We evaluated only seizure freedom and did not assess cognitive outcomes, which is an equally important aspect of care in these patients for which immunotherapies play a vital role that AEDs do not address. Third, the non‐LGI1 control also had autoimmune etiology for their epilepsy; therefore, the current study did not address therapy response by epilepsy etiology (autoimmune vs non‐autoimmune). Finally, the “class effect” observed with sodium channel blocking AEDs should be interpreted cautiously, given the low frequency of prescription of AEDs including topiramate and zonisamide among our cohort. Although the AED prescription practices in AE may reflect a high frequency of seizures and need for an AED with rapid uptitration, they may not necessarily be reflective of lower efficacy. In summary, a retrospective study of patients with AE is challenging due to variation in phenotype and seizure burden between patients of even the same disease, the need for multiple hospitalizations and urgent treatment, and non‐uniform documentation of clinical progress. Well‐designed future prospective studies are needed to clarify whether there is an optimal AED selection for this patient population.
Conclusions
At this time, there are no randomized controlled trials to guide therapy to reduce seizure frequency in patients with LGI1‐Ab AE. Our data, as others have also published, reinforce the current practice of immunotherapy as the first‐line therapy for patients with LGI1‐Ab AE. The response to AEDs in our cohort was limited (5/56) but might suggest that seizures in LGI1‐Ab AE resistant to immunotherapy may be more likely to respond to AEDs with sodium channel blocking properties (eg, carbamazepine) as compared to other AEDs. In most cases, the seizure frequency is often quite high; therefore, it usually does not take a long time to determine whether AEDs are conferring an effect in these patients. Unfortunately, some patients with LGI1‐Ab AE will be refractory to both AEDs and immunotherapy.6
Disclosure
Drs Feyissa, Lamb, Pittock, Gadoth, McKoen, Klein, and Britton: report no disclosures relevant to the manuscript. Dr. Feyissa is supported by the Mayo Clinic Neuroscience Focused Research Team Program. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Summary of AEDs used or tried in the cohort.
Figure S2. Timeline of AED use in relation to immunotherapy for the 6 patient who responded to AEDs despite previous or concurrent immune treatment.
Biography
Dr. Anteneh Mekonnen Feyissa is an Assistant Professor of Neurology at the Mayo Clinic, Jacksonville.

References
- 1. van Sonderen A, Petit‐Pedrol M, Dalmau J, et al. The value of LGI1, Caspr2 and voltage‐gated potassium channel antibodies in encephalitis. Nat Rev Neurol 2017;13:290–301. [DOI] [PubMed] [Google Scholar]
- 2. Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel‐complex proteins leucine‐rich, glioma inactivated 1 protein and contactin‐associated protein‐2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lang B, Makuch M, Moloney T, et al. Intracellular and non‐neuronal targets of voltage‐gated potassium channel complex antibodies. J Neurol Neurosurg Psychiatry 2017;88:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Sonderen A, Schreurs MW, de Bruijn MA, et al. The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology 2016;86:1692–1699. [DOI] [PubMed] [Google Scholar]
- 5. Bastiaansen AE, van Sonderen A, Titulaer MJ. Autoimmune encephalitis with anti‐leucine‐rich glioma‐inactivated 1 or anti‐contactin‐associated protein‐like 2 antibodies (formerly called voltage‐gated potassium channel‐complex antibodies). Curr Opin Neurol 2017;30:302–309. [DOI] [PubMed] [Google Scholar]
- 6. Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2‐IgG positive patients. Ann Neurol 2017;82:79–92. [DOI] [PubMed] [Google Scholar]
- 7. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
- 8. Finke C, Prüss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine‐rich, glioma‐inactivated 1 antibodies. JAMA Neurol 2017;74:50–59. [DOI] [PubMed] [Google Scholar]
- 9. Toledano M, Britton JW, McKeon A, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology 2014;82:1578–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beghi E, Shorvon S. Antiepileptic drugs and the immune system. Epilepsia 2011;52:40–44. [DOI] [PubMed] [Google Scholar]
- 11. Feyissa AM, Chiriboga ASL, Britton JW. Antiepileptic drug therapy in patients with autoimmune epilepsy. Neurol Neuroimmunol Neuroinflamm 2017;4:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li G, Bauer S, Nowak M, et al. Cytokines and epilepsy. Seizure 2011;20:249–256. [DOI] [PubMed] [Google Scholar]
- 13. Gómez CD, Buijs RM, Sitges M. The anti‐seizure drugs vinpocetine and carbamazepine, but not valproic acid, reduce inflammatory IL‐1β and TNF‐α expression in rat hippocampus. J Neurochem 2014;130:770–779. [DOI] [PubMed] [Google Scholar]
- 14. Himmerich H, Bartsch S, Hamer H, et al. Modulation of cytokine production by drugs with antiepileptic or mood stabilizer properties in anti‐CD3‐and anti‐Cd40‐stimulated blood in vitro. Oxid Med Cell Longev 2014;2014:806162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bianchi M, Rossoni G, Sacerdote P, et al. Carbamazepine exerts anti‐inflammatory effects in the rat. Eur J Pharmacol 1995;294:71–74. [DOI] [PubMed] [Google Scholar]
- 16. Ahmed A, Simmons Z. Isaacs syndrome: a review. Muscle Nerve 2015;52:5–12. [DOI] [PubMed] [Google Scholar]
- 17. Rajakulendran S, Schorge S, Kullmann DM, et al. Episodic ataxia type 1: a neuronal potassium channelopathy. Neurotherapeutics 2007;4:258–266. [DOI] [PubMed] [Google Scholar]
- 18. Thomas EA, Petrou S. Network‐specific mechanisms may explain the paradoxical effects of carbamazepine and phenytoin. Epilepsia 2013;54:1195–1202. [DOI] [PubMed] [Google Scholar]
- 19. Zhou Q, Chen PC, Devaraneni PK, et al. Carbamazepine inhibits ATP‐sensitive potassium channel activity by disrupting channel response to MgADP. Channels (Austin) 2014;8:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wennberg R, Steriade C, Chen R, et al. Frontal infraslow activity marks the motor spasms of anti‐LGI1 encephalitis. Clin Neurophysiol 2018;129:59–68. [DOI] [PubMed] [Google Scholar]
- 21. Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013;136:3151–3162. [DOI] [PubMed] [Google Scholar]
- 22. Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011;69:892–900. [DOI] [PubMed] [Google Scholar]
- 23. French JA, Gazzola DM. Antiepileptic drug treatment: new drugs and new strategies. Continuum (Minneap Minn) 2013;19:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary of AEDs used or tried in the cohort.
Figure S2. Timeline of AED use in relation to immunotherapy for the 6 patient who responded to AEDs despite previous or concurrent immune treatment.


