Summary
Clobazam (CLB) is a commonly used oral antiepileptic drug (AED) that has been shown to be effective in various forms of epilepsy. Given its distinct 1,5‐benzodiazepine structure, rapid absorption, minimal drug interactions, and favorable safety profile, CLB displays unique properties when compared to other commonly used benzodiazepines. Recent evidence has shown that CLB may demonstrate therapeutic efficacy in status epilepticus (SE). The objective of this systematic review was to summarize the available evidence pertaining to the efficacy of CLB use in SE. An electronic literature search of Medline (1946 to November 6, 2017), Embase (1974 to November 6, 2017), and the Cochrane Library (1999 to November 6, 2017) databases was performed to identify reports of CLB use in SE. After screening and full text review, a total of 15 articles were included: 8 retrospective studies, 2 case series, and 5 case reports. Efficacy rates for CLB have varied among reports. Overall, based on the retrospective studies, a total of 76 patients with SE have been reported. CLB was introduced within 2–4 days from SE onset and has been reported to contribute to remission in 36 patients (47%). CLB maintenance dose ranged from 10 to 60 mg/day. However, the results need to be interpreted carefully because SE patients are a heterogeneous group with different etiologies and disease severities, and the response to CLB might vary in different patient population or seizure types. In conclusion, there is not sufficient evidence to determine the safety and efficacy of clobazam in the setting of SE. However, the current limited evidence combined with the unique characteristics of CLB suggest that the drug might be considered as an add‐on option in SE patients, with a suggested dosage range of 10–60 mg/day. Prospective studies are needed to fully establish the role of CLB in the management of SE.
Keywords: Clobazam, Status epilepticus, Refractory status epilepticus, Seizure
Key Points.
A systematic review of the use of clobazam in status epilepticus
A total of 15 articles were included: 8 retrospective studies, 2 case series, and 5 case reports
Based on the retrospective studies, a total of 76 patients with SE have been reported
Clobazam was introduced within 2–4 days from SE onset and has been reported to contribute to remission in 36 patients (47%)
Clobazam might be considered as an add‐on option in SE patients, with a suggested dosage range of 10–60 mg/day
Clobazam (CLB) is a commonly used oral antiepileptic drug (AED) that has been shown to be effective in treating various forms of refractory epilepsy.1 It is indicated as an adjunctive therapy for seizures in Lennox‐Gastaut syndrome (LGS) and other forms of epilepsy. Given its distinct 1,5‐benzodiazepine structure, CLB displays unique properties when compared to other commonly used benzodiazepines (BDZs).2 Recent evidence has shown that clobazam may demonstrate therapeutic efficacy in status epilepticus (SE).3, 4 CLB's rapid and complete absorption, minimal drug interactions, low propensity for sedation, and possibility of being administered by enteral feeding tube without affecting its absorption make CLB a plausible option in the setting of SE.5 The International League Against Epilepsy (ILAE) has defined SE as “a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms which lead to abnormally prolonged seizures (after time point t 1). It is a condition that can have long‐term consequences (after time point t 2), including neuronal death, neuronal injury, and alteration of neuronal networks, depending on the type and duration of seizures.”6 Generally, the presence of ongoing clinical and/or electroencephalographic seizure activity for >5 minutes or the presence of multiple seizures with no return to baseline in‐between the attacks is a trigger for SE management.7 Various agents have been utilized in patients with SE such as BDZs, phenytoin, valproic acid, levetiracetam, phenobarbital, and intravenous anesthetics, and the choice often varies by institution or clinician preference. If one drug fails to induce seizure remission, other alternate therapies are used in an attempt to control seizure activity. Due to the significant amount of morbidity and mortality associated with SE, determination of other effective therapies, particularly for refractory forms of SE, is essential. Guidelines have been published to guide clinicians for the proper management of patients with SE.7, 8 Despite that, compelling evidence for the use of other AEDs including CLB is still lacking. As a result, evaluating the potential use of clobazam in patients with SE is necessary. The objective of this review was to summarize the current evidence pertaining to the efficacy and safety of clobazam use in patients with SE. To our knowledge, this is the first systematic review summarizing the use of CLB in SE.
Methods
This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist.9
Search strategy
A literature search was performed in MEDLINE (1946 to May 3, 2017), EMBASE (1974 to May 3, 2017), and the Cochrane Library (1999 to May 3, 2017). The search was repeated on November 6, 2017, to include any articles published since the original search. The following keywords were used: “clobazam,” “clobazamum,” “Apo‐clobazam,” “clobazam‐10,” “Dom‐clobazam,” “N‐desmethylclobazam,” “NCLB,” “Norclobazam,” “Novo‐clobazam,” “PMS‐clobazam,” “4′‐hydroxyclobazam,” “Onfi,” “Frisium,” “Urbanol,” “Tapclob,” “Aedon,” “Castilium,” “Clobam,” “Clobamax,” “Grifoclobam,” “Mystan,” “Noiafren,” “Sederlona,” “Urbanil,” “Urbanyl,” “Venium,” “1‐phenyl‐5‐methyl‐8‐chloro‐1,2,4,5‐tetrahydro‐2,4‐dioxo‐3H‐1,5‐benzodiazepine,” “7‐chloro‐1‐methyl‐5‐phenyl‐1,5‐dihydro‐benzo[b][1,4]diazepine‐2,4‐dione,” “1,5‐benzodiazepine,” and “status epilepticus,” “SE,” “Long adj3 Seizure,” “Continuous adj3 Seizure,” “Unremitting adj3 Seizure,” “Nonconvulsive adj3 Seizure,” “Epilepsy Partialis Continua,” “Epilepsia Partialis Continua,” “Generalized Convulsive SE,” “Petit mal status,” “absence status,” “Subtle SE,” “Nonconvulsive SE,” “Absence SE,” “Complex Partial SE,” and “Simple Partial SE.” Keywords were selected in conjunction with a library information specialist to ensure that the search comprehensively covered variations in drug name and condition subtypes. The reference lists of the included articles were also searched manually to find additional eligible articles.
Study selection
Human studies were selected based on the reported use of CLB in SE. Titles and abstracts of articles were screened to exclude any nonhuman studies, non–English‐language studies that could not be interpreted using Google Translate, and other nonrelevant studies. Commentaries, opinion articles, editorials, and review articles were excluded. The full texts of the remaining articles were then assessed for inclusion in the systematic review. The level of evidence was graded using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group criteria.10 In case of any discrepancies between the authors, further discussion was done to reach a consensus.
Data collection
Data collected included year of publication, study type, number of participants treated with CLB, and participants’ age and sex, SE type, and previous history of seizures and epilepsy. When available, CLB dosing information, order of initiation, and time from SE onset to CLB initiation were also collected. Outcome data collected included CLB success in causing remission of SE and adverse reactions attributed to CLB use. Data extraction from studies was conducted and confirmed by both authors.
Results and Discussion
As depicted in Figure 1, the search resulted in a total of 644 records. Of these records, 555 came from Embase, 81 from Medline, 4 from the Cochrane Library, and 4 additional records came from other sources. After screening, full‐text review, and applying the exclusion criteria, 15 articles were included (Tables 1 and 2): 8 retrospective studies, 2 case series, and 5 case reports. Three of the retrospective studies specifically looked at CLB in SE; however, one of them was a conference abstract. Based on that, the available evidence is considered low or very low using the GRADE working group criteria.10
Figure 1.
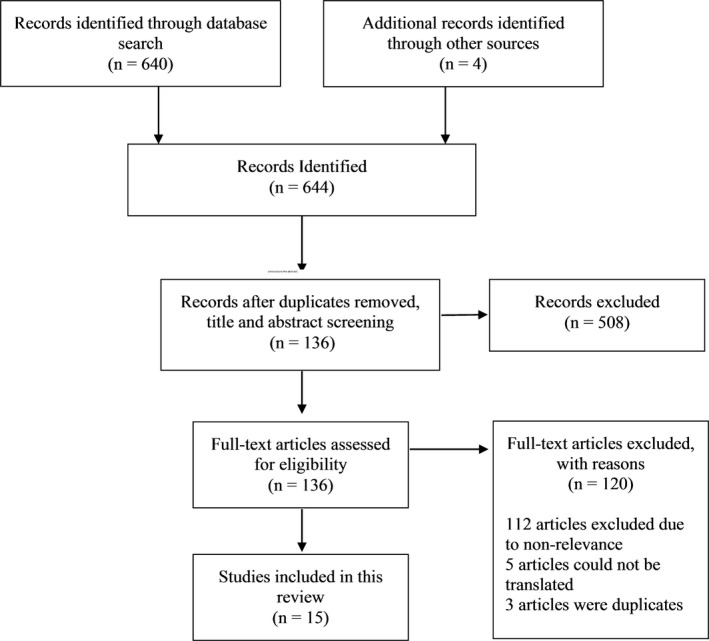
Flow diagram for the search strategy
Table 1.
Summary of the retrospective studies regarding clobazam efficacy in status epilepticus
| Author (year) | Study type | n | Male, n (%) | Age (years) | History of seizures/epilepsy, n (%) | SE type, n (%) | Clobazam dose | Order of initiation (excluding initial BDZ) | Time from SE onset to clobazam initiation (day) | SE control in CLB responders | Response, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Madzar et al.3 (2016) | Retrospective study of CLB use in SE | 24 | 8 (33) | 64 (52–72) | 10 (41.7) |
RSE NCSE 4 (16.7) GCSE 8 (33.3) CPSE 12 (50) |
MD 20 (14–23) mg/day | 4th (3rd–6th) | 2 (3–6) |
Duration of SE 6 (4–36) days CLB was the last agent added |
6 (25) |
| Sivakumar et al.4 (2015) | Retrospective study of CLB use in SE | 17 | 10 (59) | 63 (14–75) | 11 (65) |
RSE FSE 15 (88) GCSE 2 (12) |
Initial 10 (10–40) mg/day MD 20 (10–60) mg/day |
4th (3rd–7th) | 4 (1–61) | Remission within 24 h of CLB initiation | 13 (76.5) |
| Lu et al.13 (2016) | Retrospective study of pregnancy related SE | 1 | 0 (0) | 23 | 0 (0) | GCSE progressed to NCSE 1 (100) | NR | NR | NR | N/A | 0 (0) |
| Kravljanac et al.16 (2015) | Retrospective study of children with SE (n = 392) | 5 | NR | NR | NR | NR | 0.5–1.5 mg/kg | NR | NR | Seizure remission while on CLB | 4 (80) |
| Holzer et al.14 (2012) | Retrospective study of patients with antibody mediated SE (n = 13) | 3 | 0 (0) | 30 (17–37) | 0 (0) |
NCSE 2 (66.7) GCSE 1 (33.3) |
NR | NR | NR | N/A | 0 (0) |
| Mameniskiene et al.15 (2011) | Retrospective study of patients with FSE (n = 65) | 8 | NR | NR | NR | FSE 8 (100) | NR | NR | NR | N/A | 0 (0) |
| Manning and Rosenbloom17 (1987) | Retrospective study of children with SE | 1 | NR | NR | NR | Myoclonic SE | NR | NR | NR | CLB was reported successful | 1 (100) |
|
Swisher et al.12 (2017) (Abstract) |
Retrospective study of CLB use in refractory NCS, NCSE | 17 | NR | NR | 7 (41) |
NCS 7 (41) NCSE 10 (59) |
MD 20 (10–60) mg/day | 3rd–6th | 2 (1–55) | CLB was the last agent added | 12 (71) |
BDZ, benzodiazepine; CLB, clobazam; CPSE, complex partial status epilepticus; FSE, focal status epilepticus; GCSE, generalized convulsive status epilepticus; MD, maintenance dose; N/A, not applicable; NCS, nonconvulsive seizures; NCSE, nonconvulsive status epilepticus; NR, not reported; RSE, refractory status epilepticus; SE, status epilepticus.
Table 2.
Summary of the case series and case reports regarding clobazam efficacy in status epilepticus
| Author (year) | Study type | n | Sex | Age (years) | History of seizures/epilepsy | SE type | Clobazam dose | Order of initiation (excluding initial BDZ) | Time from SE onset to clobazam initiation (day) | SE control in CLB responders | Response |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Corman et al.19 (1998) | Case series | 4 |
M (n = 1) F (n = 3) |
49 (30–71) |
Yes 2 No 2 |
RSE FSE 4 (100%) |
Single dose of 60–70 mg to induce remission MD 20 mg/day in one case |
2nd–4th | 1–2 | Seizure activity controlled within 2 h in 3 cases (75%) and 2 days in the 4th case | Yes n = 4 (100%) |
|
Tinuper et al.18 (1986). Note: Gastaut et al.25 (1984) reported the same findings in French |
Case series | 16 |
M (n = 6) F (n = 10) |
22 (3–62) | Yes 100% |
Absence SE 6 (38%) Myoclonic Absence SE 1 (6%) Myoclonic SE 1 (6%) Tonic SE 1 (6%) FSE 7 (44%) |
Single dose: 1.08 (0.51.70) mg/kg | NR | NR | Seizure activity controlled within 30 min in 15 cases (94%) and 7 h in one patient | YES n = 16 (100%) |
| Mutis et al.24 (2017) | Case report | 1 | F | 62 | No |
NCSE FSE |
20 mg/day in 2 divided doses | 2nd | NR | Added with lacosamide | Yes |
|
Sawicka et al.26 (2016) (Abstract) |
Case report | 1 | F | 18 | No | RSE | NR | NR | NR | CLB was part of multidrug regimen | Yes – Not clear if it had added benefit |
| Tran et al.27 (2013) | Case report | 1 | M | 49 | No | FSE | NR | NR | NR | CLB was part of multidrug regimen | No |
| Reuber et al.28 (2002) | Case report | 1 | F | 68 | No | CPSE | 30 mg daily | 2nd | NR | N/A | No |
| Murchison et al.29 (1995) | Case report | 1 | F | 31 | Yes | NCSE | 10 mg daily | 2nd | NR | Remission in 3 days | Yes |
BDZ, benzodiazepine; CLB, clobazam; CPSE, complex partial status epilepticus; FSE, focal status epilepticus; MD, maintenance dose; N/A, not applicable; NCSE, nonconvulsive status epilepticus; NR, not reported; RSE, refractory status epilepticus; SE, status epilepticus.
Efficacy of clobazam in status epilepticus
CLB use has been reported in refractory status epilepticus (RSE).3, 4 RSE is commonly defined as SE that continues to occur despite treatment with a benzodiazepine and an additional AED, or SE that requires general anesthesia.11 If patients reach this point, where electroencephalographic activity continues despite treatment with first‐line agents, they will rarely return to their prior baseline level of functioning. Clinicians will often see rapid deterioration, and in some cases, even death of the patient, despite rapidly administered treatment with a variety of different AEDs.
Three retrospective cohort studies specifically looked at CLB in RSE3, 4, 12 (Table 1). In the retrospective study by Sivakumar et al.,4 a cohort of patients with RSE who were treated with CLB has been reported. Patients who were on CLB before admission were excluded. CLB response was defined as termination of seizure activity within 24 hours of CLB initiation without alteration of the other AEDs regimens, and successful weaning of anesthetic infusions. Sivakumar et al. have reported a successful treatment response to clobazam in 13/17 (76.5%) patients, and CLB was the last agent added for 94% of the patients. CLB was initiated after 2 or more AEDs, with a median start of 4 days following RSE diagnosis. The starting dose was 10 mg/day, with a median maintenance dose of 20 mg/day. The most common adverse event reported with CLB administration was sedation (38%) resulting in its discontinuation following RSE control. Because many of these patients were also receiving several other AEDs prior to CLB initiation, it is uncertain whether this side effect was caused explicitly by CLB. Similarly, a conference abstract by Swisher et al.,12 has reported successful remission with CLB in 71% of the patients with refractory nonconvulsive seizures and nonconvulsive status epilepticus (NCSE).
On the other hand, in the retrospective study by Madzar et al.3 where a cohort of RSE patients treated with CLB has been reported, the treatment success rate was not as high. Patients were considered responders to CLB if it was the last AED given throughout the course of therapy before RSE termination. They have identified 71 episodes of RSE in 65 patients, 24 (34%) of whom were treated with CLB. Clobazam was initiated within 2 days from RSE diagnosis, with a median duration of 8 days and a maintenance dose of 20 mg/day. CLB was the fourth‐line AED given to the patients and was followed by 2 AEDs if RSE was not controlled. CLB was the last agent in 25% of the patients who achieved remission, and hence those patients were considered responders according to the authors’ definition. Of interest, at 12‐week follow‐up, 78% of CLB‐treated patients had a poor prognostic outcome, which could have been confounded by disease severity.
The rationale for the discrepancy in the reported magnitude of treatment effects with CLB could be attributed to several factors. First, despite the definition of SE being similar between studies, defined as continuous epileptic activity for >5 minutes or multiple seizures with no return to baseline status among seizures, the definition of RSE has varied. Because these studies are of retrospective design, variations in study definitions could affect the inclusion and exclusion criteria. Although Madzar et al. have defined RSE as SE refractory to administration of at least 2 properly dosed AEDs, the study by Sivakumar et al. defined RSE as SE refractory to a BDZ and one AED with or without general anesthesia. Second, although Madzar et al. excluded patients with focal RSE without impairment of awareness, absence RSE, and RSE secondary to anoxic brain damage, Sivakumar et al. did not have such exclusion criteria and 88% of the patients had focal status epilepticus making it difficult to compare both cohorts. Third, as discussed above, the definition of response to CLB was different between studies. Finally, the dosing of CLB, concomitantly administered drugs, and etiology of SE differed among patients. Therefore, endeavoring to directly compare each treatment effect may not be feasible.
Furthermore, other retrospective studies not specifically designed to look at CLB in SE have reported conflicting results. Although 3 studies have reported treatment failure with CLB,13, 14, 15 2 studies have reported treatment success.16, 17 Due to the retrospective nature of those studies it is unknown whether remission was due to the effect of CLB, or rather its effect in combination with other AEDs.
In addition to the above evidence, CLB use in SE has been reported in earlier case series (Table 2). Tinuper et al.18 reported successful SE control in a series of 16 patients, mainly with absence or focal SE. A single loading dose of CLB (average 1.08 mg/kg) was successful in controlling SE in all patients. Remission was achieved within 30 minutes of CLB administration in 94% of the patients with no significant sedation or hemodynamic instability noted. However, there was not enough follow‐up reported, making it unclear if remission was sustained. Similarly, Corman et al.19 reported successful seizure control in 4 patients with focal SE refractory to initial BDZ and phenytoin using a single 60–70 mg CLB loading dose.
Overall, based on the retrospective studies described earlier, a total of 76 patients with SE have been reported. CLB was introduced within 2–4 days from SE onset and has been reported to contribute to remission in 36 patients (47%). When combining the results of the retrospective studies specifically looked at the efficacy of CLB in SE, 58 patients have been reported. CLB was the last AED added before SE remission in 24/58 patients (59% response). With regard to CLB efficacy in particular seizure types, 3 studies have reported CLB to be effective in 14/35 (40%) patients with focal SE.3, 4, 15 In addition, CLB was successful in terminating SE in 4/12 (33.3%) patients with generalized convulsive SE.3, 4, 14 However, the results need to be carefully interpreted because SE patients are a heterogeneous group of patients with different etiologies and disease severities, and the response to CLB might vary in different patient populations or seizure types.
The possibility of tolerance development and lack of efficacy need to be considered when interpreting the efficacy of CLB. The clinical response to CLB has the potential to undergo tolerance. Tolerance is the reduction of the potency of the AED with repeated administration. Tolerance to BDZs has been attributed to the desensitization of the γ‐aminobutyric acid (GABA) receptors with chronic dosing necessitating a dose increase. It is not clear whether all patients treated with CLB would develop tolerance and if it is dose or duration dependent. In a recent post hoc analysis of data of a phase 3 clinical trial in patients with Lennox‐Gastaut syndrome, the majority of the patients treated with CLB did not experience tolerance over a 2‐year period of follow‐up, suggesting that tolerance rates could be overestimated.20 In the setting of SE, there is no evidence to support or refute the occurrence of tolerance to CLB. However, there is a possibility of lack of response to BZDs in patients with prolonged SE. This has been attributed to the internalization of the synaptic GABAA receptors, the BDZs target. Despite that, CLB might have contributed to seizure remission in almost half of the reported patients. The possible retention of CLB efficacy in some of those patients could be because those patients did not have reduced response to BDZs or a result of the unique 1,5‐benzodiazepine structure of CLB. In an in vitro study characterizing the activity of CLB on various GABAA receptor subtypes, CLB and its metabolite nor‐clobazam (nor‐CLB) have been found to have similar activities on GABA receptor subtypes compared to clonazepam, an example of the classic 1,4 BDZ. On the other hand, CLB and its metabolite had higher efficacy on potentiating the signaling of the α6β2δ receptor, an example of the extrasynaptic GABAA receptors, compared to clonazepam.21 Those receptor subtypes are believed to remain intact in the setting of SE and could be a potential target for RSE management.22 The clinical significance of this differential pharmacology is not known but it could potentially contribute, at least in part, to the efficacy of CLB in causing remission in a select group of patients with SE. Further studies are needed.
Safety of clobazam in status epilepticus
The long‐term efficacy and safety of CLB therapy has been established in other epilepsy syndromes such as Lennox‐Gastaut syndrome.23 Common adverse effects of CLB therapy typically include upper respiratory tract infections, somnolence, sedation, dizziness, drooling, and ataxia.23 In the case series by Tinuper et al.,18 a single loading dose of CLB was associated with no significant sedation or hemodynamic instability in patients with SE. On the other hand, Sivakumar et al.4 have reported sedation in about one‐third of the patients treated with CLB, resulting in discontinuation at discharge. Based on the available evidence, it is not feasible to determine the safety of CLB in SE. In addition, it is not clear that the reported tolerability to CLB could be attributed to the lack of potency in SE population. SE is associated with significant morbidity and mortality, and many patients who achieve remission are left with severe motor, language, and other cognitive deficits. When evaluating patients in a retrospective manner, it can be challenging to attribute an adverse effect explicitly to an intervention itself. One should look carefully at the time course of the administered intervention in relation to the onset of the adverse effect. Furthermore, CLB‐treated patients were generally sicker: had more complex, longer duration, and refractory SE compared to those not treated with CLB (confounding by indication). Prospective studies are needed to fully establish whether CLB is safe in the setting of SE.
Dosing of clobazam in status epilepticus
Optimal treatment of SE entails consideration of several different factors including patient specific factors, such as underlying diagnosis, previously unsuccessful treatment attempts, and comorbidities. Current evidence to guide optimal treatment of SE remains limited, and is most often guided by clinical experience and clinician preference. In this review, dosing information, where available, was collected and reported (Tables 1 and 2). Due to the small sample size of the included studies, it is difficult to compare CLB dose with its efficacy. However, the median dosage of CLB in patients with SE was similar among studies. In general, the suggested CLB maintenance dose in SE is 20 mg/day titrated to response with a dose range of 10–60 mg/day.3, 4, 12, 24 In all the studies that have reported the route of administration, CLB was given orally. However, it is not clear if it was given by mouth or through enteral feeding tube. Generally, patients with a reduced level of consciousness should receive CLB via the enteral feeding tube.4 Furthermore, case series suggested an initial single loading dose of 60–70 mg, which resulted in remission within 2 h of CLB administration.18, 19 However, there is not enough evidence from CLB studies in epilepsy to support high loading doses. This needs to be tested further in prospective studies.
This review is limited by the nature of the included studies. The current available evidence was based only on retrospective studies with small sample sizes and case reports that are at risk of bias inherent to those study designs. In addition, the studies were heterogeneous, representing different patient populations with various etiologies, employed different definitions for CLB response and RSE, and included patients who were exposed to CLB at different times throughout their illness, making direct comparison difficult. In addition, it is not clear if concomitant AEDs have been discontinued with the introduction of CLB, as this could be an added confounder to CLB efficacy. Therefore, in conclusion, the current systematic review of literature suggests that there is not sufficient evidence to determine the safety and efficacy of CLB in the setting of SE. However, the current limited evidence combined with the unique characteristics of CLB suggest that the agent might be considered as an add‐on option in SE with suggested dosage range of 10–60 mg/day. Prospective studies of CLB in SE are needed to fully establish its role in the management of SE.
Funding
There is no funding associated with this work.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgment
The authors of the manuscript would like to acknowledge Janice Kung (librarian) for her guidance in the literature search process for this review.
Biography
Dr. Sherif Hanafy Mahmoud is a clinical assistant professor at the Faculty of Pharmacy, University of Alberta.

References
- 1. Gauthier AC, Mattson RH. Clobazam: a safe, efficacious, and newly rediscovered therapeutic for epilepsy. CNS Neurosci Ther 2015;21:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robertson MM. Current status of the 1,4‐ and 1,5‐benzodiazepines in the treatment of epilepsy: the place of clobazam. Epilepsia 1986;27(Suppl 1):S27–S41. [DOI] [PubMed] [Google Scholar]
- 3. Madzar D, Geyer A, Knappe RU, et al. Effects of clobazam for treatment of refractory status epilepticus. BMC Neurol 2016;16:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sivakumar S, Ibrahim M, Parker D Jr, et al. Clobazam: an effective add‐on therapy in refractory status epilepticus. Epilepsia 2015;56:e83–e89. [DOI] [PubMed] [Google Scholar]
- 5. Rupp W, Badian M, Christ O, et al. Pharmacokinetics of single and multiple doses of clobazam in humans. Br J Clin Pharmacol 1979;7(Suppl 1):51S–57S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus–Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56:1515–1523. [DOI] [PubMed] [Google Scholar]
- 7. Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 8. Glauser T, Shinnar S, Gloss D, et al. Evidence‐based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 11. Rossetti AO, Lowenstein DH. Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol 2011;10:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swisher C, Bethea J, Pineda O, et al. Clobazam as add‐on therapy for patients with refractory nonconvulsive seizures or nonconvulsive status epilepticus. Neurocrit Care 2017;27(2 Suppl 1):S337. [Google Scholar]
- 13. Lu YT, Hsu CW, Tsai WC, et al. Status epilepticus associated with pregnancy: a cohort study. Epilepsy Behav 2016;59:92–97. [DOI] [PubMed] [Google Scholar]
- 14. Holzer FJ, Rossetti AO, Heritier‐Barras AC, et al. Antibody‐mediated status epilepticus: a retrospective multicenter survey. Eur Neurol 2012;68:310–317. [DOI] [PubMed] [Google Scholar]
- 15. Mameniskiene R, Bast T, Bentes C, et al. Clinical course and variability of non‐Rasmussen, nonstroke motor and sensory epilepsia partialis continua: a European survey and analysis of 65 cases. Epilepsia 2011;52:1168–1176. [DOI] [PubMed] [Google Scholar]
- 16. Kravljanac R, Djuric M, Jankovic B, et al. Etiology, clinical course and response to the treatment of status epilepticus in children: a 16‐year single‐center experience based on 602 episodes of status epilepticus. Eur J Paediatr Neurol 2015;19:584–590. [DOI] [PubMed] [Google Scholar]
- 17. Manning DJ, Rosenbloom L. Non‐convulsive status epilepticus. Arch Dis Child 1987;62:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tinuper P, Aguglia U, Gastaut H. Use of clobazam in certain forms of status epilepticus and in startle‐induced epileptic seizures. Epilepsia 1986;27(Suppl 1):S18–S26. [DOI] [PubMed] [Google Scholar]
- 19. Corman C, Guberman A, Benavente O. Clobazam in partial status epilepticus. Seizure 1998;7:243–247. [DOI] [PubMed] [Google Scholar]
- 20. Gidal BE, Wechsler RT, Sankar R, et al. Deconstructing tolerance with clobazam: post hoc analyses from an open‐label extension study. Neurology 2016;87:1806–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hammer H, Ebert B, Jensen HS, et al. Functional characterization of the 1,5‐benzodiazepine clobazam and its major active metabolite N‐desmethylclobazam at human GABA(A) receptors expressed in Xenopus laevis oocytes. PLoS One 2015;10:e0120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Storustovu SI, Ebert B. Pharmacological characterization of agonists at delta‐containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther 2006;316:1351–1359. [DOI] [PubMed] [Google Scholar]
- 23. Ng Y‐T, Conry J, Paolicchi J, et al. Long‐term safety and efficacy of clobazam for Lennox‐Gastaut syndrome: interim results of an open‐label extension study. Epilepsy Behav 2012;25:687–694. [DOI] [PubMed] [Google Scholar]
- 24. Mutis JA, Rodriguez JH, Nava‐Mesa MO. Rapidly progressive cognitive impairment with neuropsychiatric symptoms as the initial manifestation of status epilepticus. Epilepsy Behav Case Rep 2017;7:20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gastaut H, Tinuper P, Aguglia U, et al. [Treatment of certain forms of status epilepticus by means of a single oral dose of clobazam]. Rev Electroencephalogr Neurophysiol Clin 1984;14:203–206. [DOI] [PubMed] [Google Scholar]
- 26. Sawicka K, Cooley R, Hunter G. New onset refractory status epilepticus (NORSE) lasting 110 days resulting in a positive outcome. Neurology 2016;86(Suppl 1):P3.198.Abstract. [Google Scholar]
- 27. Tran TPY, Leduc K, Savard M, et al. Acute porphyria presenting as epilepsia partialis continua. Case Rep Neurol 2013;5:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reuber M, Evans J, Bamford JM. Topiramate in drug‐resistant complex partial status epilepticus [2]. Eur J Neurol 2002;9:111–112. [DOI] [PubMed] [Google Scholar]
- 29. Murchison JT, Sellar RJ, Steers AJW. Status epilepticus presenting as progressive dysphasia. Neuroradiology 1995;37:438–439. [DOI] [PubMed] [Google Scholar]


