Summary
Objective
Treatment with carbamazepine (CBZ), a potent enzyme inducer, is known to affect the lipid profile, steroid, and vitamin D metabolism. Consequently, it has been postulated that patients on CBZ should be switched to noninducing antiepileptic drugs (AEDs). However, little is known about the seizure outcome following a CBZ switch in seizure‐free patients. We aimed to address this issue using a controlled observational study design.
Methods
Fifty‐eight patients taking CBZ for focal epilepsy were assessed for discontinuing CBZ treatment due to concerns of long‐term adverse‐effects; 34 discontinued its therapy and 24 continued with CBZ. Six‐month seizure freedom was the primary end point. Furthermore, serum samples (total cholesterol (TC), low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), triglycerides, sex hormone–binding globulin (SHBG), free testosterone, and 25‐hydroxyvitamin D levels from before and at least 3 months after discontinuation or continuation were obtained from all patients.
Results
Seizure‐free patients had a 5‐fold elevated odds of seizure recurrence if CBZ was discontinued (95% confidence interval [CI 0.51–49.3; p = 0.17). A significant decrease in serum levels of TC, LDL, HDL, and SHBG as well as a significant increase in that of free testosterone were found in the discontinuation group compared with those who continued CBZ. Nonsignificant changes in triglycerides and vitamin D levels were detected.
Significance
Discontinuation of CBZ in seizure‐free patients seems to carry a moderate, but legitimate, risk of relapse. Conversely, our results indicate that CBZ might have unfavorable effects on serum levels of TC, LDL, HDL, SHBG, and free testosterone.
Keywords: Antiepileptic drugs, Treatment transition, Seizures
Key Points.
There has been speculation about whether patients being treated with inducing AEDs should be switched to noninducing AEDs
A significant decrease in serum levels of TC, LDL, HDL, and SHBG and a significant increase in free testosterone levels were found in the CBZ discontinuation group compared with those who continued with CBZ
Discontinuation of CBZ in seizure‐free patients seems to carry a moderate, but legitimate risk of relapse
With regard to the potential of chronic adverse‐effects related to EI, the practice of switching patients from CBZ to a noninducing AED might be worthy of consideration
Epilepsy often requires long‐term, even lifelong, treatment with antiepileptic drugs (AEDs). The target of epilepsy therapy is always seizure freedom with as few adverse effects as possible. Carbamazepine (CBZ) is considered as a drug of choice in focal epilepsy,1 although a range of well‐tolerated and effective AEDs have become available during the past 2 decades. In addition to epilepsy, CBZ is used widely in the treatment of neuralgic pain syndromes, migraine, and some psychiatric indications.
CBZ is a potent inducer of the cytochrome P450 (CYP450) enzyme system.2 In addition to their well‐known effects on drug metabolism, CYP450 enzymes are also involved in endogenous metabolic pathways,3 and can influence bone biochemistry, gonadal steroids, and cholesterol synthesis.4, 5, 6, 7 Second‐generation AEDs have weak or nonexistent inducing properties.8 To date, a reduction in total cholesterol levels has been documented after switching from inducing AEDs (CBZ or phenytoin) to lamotrigine, levetiracetam, oxcarbazepine, topiramate, or zonisamide.9, 10, 11, 12 One major concern is that AEDs could contribute to the pandemic of vascular disease.13 Accordingly, it has been proposed that CBZ should not be regarded as first‐line therapy in newly diagnosed epilepsy.14
The impact of these potential deleterious effects of long‐term EI in those patients currently receiving CBZ therapy is unclear, and this constitutes a significant issue in current clinical management. If metabolic complications are detected, they might be treated, but switching to a newer AED may be a better option. Such alterations are often considered and implemented in clinical practice with the appreciation that the switch also carries a risk of relapse. In addition, there are a number of clinical situations requiring a switch from CBZ to a newer AED (for example, pharmacotherapy for cancer). Consequently, it has been postulated that patients being treated with EI AEDs should be switched to noninducing AEDs.15
Here, we provide practical information related to discontinuation of CBZ due to concerns about the long‐term effects of enzyme induction (EI) from 3 different viewpoints: (1) the patient′s perspective, (2) seizure outcome, and (3) laboratory parameters (lipids, sex hormone‐binding globulin [SHBG], testosterone, and vitamin D). The main objective was to clarify the importance of these data on individual parameters, when deciding whether to continue CBZ treatment.
Materials and methods
Patients with focal epilepsy (aged ≥18 years) treated in Tampere University Hospital were identified from the hospital patient registry on 31 December 2014 using International Classification of Diseases (ICD‐10) diagnostic codes for focal and unclassifiable focal epilepsy (G40.1X, G40.2X and G40.9). In addition, patients from Seinäjoki Central Hospital and Vaasa Central Hospital were identified. We included patients who were currently being treated with CBZ (monotherapy or polytherapy) and whose treating epileptologist had been considering whether to discontinue CBZ due to concerns about the long‐term effects linked with EI. This situation was defined as the baseline. Those who were switched from CBZ to some other AED due to unsatisfactory seizure control, adverse events, drug interactions, or any reason other than the possible long‐term consequences of enzyme induction, were excluded. The discontinuation group consisted of 2 subgroups: (1) conversion from CBZ to a newer AED or (2) slow withdrawal of CBZ without conversion to any other AED. Patients who preferred to continue on CBZ were designated as a control group for comparison with those undergoing CBZ discontinuation. The decisions concerning discontinuation were made purely on clinical grounds. In all cases, an individual therapeutic plan was devised by the treating epileptologist for the patient′s benefit. The rate of initiation of therapy with the new AED and tapering off from CBZ were pursued at the discretion of the treating epileptologist and were individualized for each patient. The minimum target dose for the new AED was 800 mg daily for eslicarbazepine acetate, 200 mg daily for lacosamide, 1,000 mg daily for levetiracetam, and 900 mg for gabapentin. We retrospectively reviewed patient backgrounds, comorbidities, current and previous AED use, and seizure frequency from the patient records. Refractory epilepsy was qualified as having persistent seizures after trials with at least 2 AEDs at maximally tolerated doses (sequentially or in combination therapy). The use of other potent‐inducing AEDs (phenytoin, primidone, and phenobarbital) is extremely limited in our district and therefore was not included in the current study.
In our institutions, patients who are established on EI AEDs are regularly screened for associated long‐term problems including osteoporosis, hyperlipidemia, and sexual dysfunction. As a part of this process, we measure the following serum variables: SHBG, free testosterone for male patients, 25‐hydroxyvitamin D, total cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), and triglycerides. Among the women with epilepsy, sex hormone profiles are not routinely measured due to complicated interpretations with respect to the menstrual cycle. In the case of CBZ discontinuation, control blood samples were obtained at least 3 months after the last dose of CBZ in order to observe the possible improvement in laboratory parameters after de‐induction. A 3‐month timespan was designated to ensure sufficient time for complete de‐induction of the CYP450 system. In case of CBZ continuation, blood samples were controlled regularly as a part of the clinical routine. The design of the study entailed each patient having blood drawn after a minimum of 12 hours of fasting on 2 occasions: first at baseline, while taking CBZ; a second time at 3 months after the last dose of CBZ.
Given the drug interactions between CBZ and statins, the lipid analyses were performed with exclusion of the statin users.
Seizure outcome was established for 12 months before and for the 6 months after baseline. We defined seizure outcome as either (1) having no seizures for 6 months, which signified that the patient was seizure‐free; or (2) having experienced a seizure on a therapeutic dose of their AED during the 6‐month period, which indicated the patient was not seizure‐free. Isolated auras were not considered in this assessment. We also excluded seizures occurring during the titration period of the new AED.
Descriptive statistics (means and ranges [min to max] or frequencies and proportions) were utilized to summarize patients' characteristics at baseline. Changes in serum samples were calculated as the difference between the end of the follow‐up and baseline and were reported as means and standard deviations. We combined 5 serum samples (HDL, LDL, triglycerides, SHBG, and vitamin D) to evaluate their overall significance in patients. To combine values from these 5 different scaled variables, we first standardized each of them, that is, rescaled them to have a mean of zero and a standard deviation one. The value of the standardized variable (also called z‐score) represents its distance from the mean in standard deviation units. For example, a standardized value of −0.5 indicates that the value is half a standard deviation below the mean. Thus, a positive value of the sum of 5 standardized variables (z‐sum) indicates that the value is above the mean of the z‐sum. Before summation, we changed the sign for variables LDL, triglycerides, and SHBG in order to code all 5 variables in same order, that is, large positive value indicates a “better situation” for all variables.
Chi‐square test was used to analyze differences between groups and categorical variables, this being based on the assumption of normality; Student's t‐test or Mann‐Whitney U‐test was used for continuous variables. Binary logistic regression analysis was used to estimate the association between groups and seizure recurrence at 6 months after baseline. No essential changes in the estimate of group variable were found after adjustments for age, gender, and the number of prior AEDs and therefore only results from unadjusted models were reported. After fitting logistic regression models, predicted probabilities for seizure recurrence 6 months after baseline were calculated by converting to odds.
All analyses were conducted using Stata statistical software version 13.1 (StataCorp, College Station, Texas, U.S.A.). For all statistical tests, p‐value <0.05 was considered statistically significant.
This was an observational, noninterventional retrospective study, which does not require ethics committee approval according to Finnish Law on Research. Access to patient records was based on a decision made by the Head of Science Centre, Tampere University Hospital Research and Innovation Services, Science Center.
Results
Of the 58 patients participating in the study, 24 decided to continue with CBZ, 10 were withdrawn (7 of them seizure‐free) from CBZ without switching to some other AED, and 24 (13 of them seizure‐free) were converted from CBZ to some other AED (8 to eslicarbazepine acetate, 8 to lacosamide, 7 to levetiracetam, and one to gabapentin). The main reasons affecting individual decisions to continue with CBZ were as follows; 11 were anxious that there would be seizure‐relapse, 7 were afraid of losing their job and/or driver′s license should a seizure occur, 2 were not concerned about the long‐term effects of EI, with the final 4 subjects deciding for reasons best to known themselves.
The baseline data for all patients appear in Table 1. There were significant differences between the groups with respect to seizure freedom and mean free testosterone levels. Recurrent seizures were more frequent, and the testosterone level was lower in the discontinuation group at baseline compared to continuation group. The time between the first and second blood samplings in these patients ranged from 91 to 334 days (mean 141 days).
Table 1.
Baseline characteristics of the study patients
| CBZ status at baseline | p‐value | ||
|---|---|---|---|
| Continuation | Discontinuation | ||
| N | 24 | 34 | |
| Age in years (range) | 52.6 (25–69) | 49.1 (29–78) | 0.29a |
| Female (%) | 13 (54.2) | 19 (55.9) | 0.90b |
| Statin use (%) | 2 (8.3) | 5 (14.7) | 0.46b |
| Number of prior AEDs (%) | 0.11b | ||
| 0 | 10 (41.7) | 13 (38.2) | |
| 1–2 | 9 (37.5) | 6 (17.7) | |
| 3+ | 5 (20.8) | 15 (44.1) | |
| Refractory (%) | 9 (37.5) | 15 (44.1) | 0.61b |
| Number of concomitant AEDs (%) | 0.62b | ||
| 0 | 12 (50.0) | 15 (44.1) | |
| 1 | 7 (29.2) | 14 (41.2) | |
| 2–3 | 5 (20.8) | 5 (14.7) | |
| Duration of epilepsy, years (range) | 34.4 (2–53) | 30.3 (1–62) | 0.32a |
| Duration of CBZ, years (range) | 29.0 (2–48) | 23.7 (1–49) | 0.14a |
| Daily dose of CBZ, mg (range) | 810 (400–1500) | 750 (400–1600) | 0.47a |
| Seizure free (%) | 21 (87.5) | 20 (58.8) | 0.018b |
| Total cholesterol, mm (SD) | 5.7 (1.2) | 5.9 (1.3) | 0.59a |
| HDL, mm (SD) | 1.8 (0.6) | 2.0 (0.6) | 0.25a |
| LDL, mm (SD) | 3.6 (1.0) | 3.7 (1.0) | 0.72a |
| Triglyceride, mm (SD) | 1.3 (0.8) | 1.1 (0.6) | 0.65c |
| SHBG, nm (SD) | 115.5 (87.1) | 100.4 (48.2) | 0.97c |
| Free testosterone, pm (SD)d | 212.8 (73.8) | 156.9 (57.5) | 0.046c |
| Vitamin D, nm (SD) | 80.2 (34.4) | 78.1 (35.7) | 0.83a |
| z‐score sume | −0.36 (2.66) | 0.25 (2.01) | 0.32a |
AEDs, antiepileptic drugs; CBZ, carbamazepine; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SD, standard deviation, SHBG, sex hormone–binding globulin.
Student's t‐test.
Chi‐square test.
Mann‐Whitney U‐test.
n = 26 (men only).
The sum of 5 variables (z‐sum) was calculated so that the greater value of z‐sum corresponds to better total situation.
Compared to those who continued the CBZ treatment, patients in the CBZ discontinuation group exhibited significant decreases in serum total cholesterol (16%), HDL (11%), LDL (18%), and SHBG (18%) concentrations (Table 2.) In men, the free testosterone level was significantly increased (39%) in the CBZ discontinuation group compared to those who continued with CBZ medication. There were no significant changes in the serum concentrations of triglyceride and vitamin D.
Table 2.
Laboratory data after baseline (sampling 2) and comparison of laboratory parameters between sampling one and sampling two. Statin users were excluded from the lipid analyses
| CBZ status at baseline | Change from sampling 1 to sampling 2 | |||||
|---|---|---|---|---|---|---|
| Continue (n = 24) | Discontinue (n = 34) | p‐value | Continue (n = 24) | Discontinue (n = 34) | p‐value | |
| TC, mm (SD)c | 5.8 (1.2) | 5.1 (1.0) | 0.033a | 0.05 (0.51) | −0.84 (0.78) | <0.001a |
| HDL, mm (SD)c | 1.9 (0.7) | 1.8 (0.5) | 0.70a | 0.02 (0.16) | −0.21 (0.34) | 0.004a |
| LDL, mm (SD)c | 3.5 (1.1) | 3.1 (0.9) | 0.18a | −0.05 (0.64) | −0.64 (0.90) | 0.006a |
| Triglyceride, mm (SD)c | 1.18 (0.75) | 0.94 (0.40) | 0.41b | −0.06 (0.32) | −0.14 (0.42) | 0.13b |
| SHBG, nm (SD) | 124.7 (88.8) | 82.1 (40.3) | 0.042b | 9.1 (18.0) | −18.4 (39.9) | <0.001b |
| FT, pm (SD)d | 212.9 (80.2) | 218.1 (93.6) | 0.92b | 0.09 (22.5) | 61.2 (91.8) | 0.017b |
| Vitamin D, nm (SD) | 80.3 (31.3) | 81.4 (27.2) | 0.89a | 0.1 (19.2) | 3.3 (22.4) | 0.58a |
| z‐score sume | −0.84 (2.84) | 0.59 (2.06) | 0.030a | −0.48 (1.65) | 0.34 (1.61) | 0.064a |
FT, free testosterone; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SD, standard deviation; SHBG, sex hormone–binding globulin; TC, total cholesterol.
Student's t‐test.
Mann‐Whitney U‐test.
n = 51 (statin users excluded).
n = 26 (men only).
The sum of 5 variables (z‐sum) was calculated so that the greater value of z‐sum corresponds to better total situation.
Eslicarbazepine acetate might itself have some impact on lipids, or perhaps other serologic markers. Therefore, we re‐analyzed the data regarding all the serologic markers after exclusion of those who were switched to eslicarbazepine acetate. The results remained unchanged after exclusion (data not shown). Two patients had a sporadic seizure during the titration period of the new drug. In contrast, none had withdrawal seizures due to tapering of the CBZ medication. Data from these seizures are not included here. Table 3 demonstrates unadjusted odds ratios for the various subgroups relative to their seizure status at baseline. The logistic regression model was adjusted for covariates (age, gender, and number of prior AEDs), but this did not produce results that differed markedly from those obtained with the unadjusted model (data not shown). Table 4 presents probabilities for seizure recurrence 6 months after baseline in the various subgroups of previously seizure‐free patients. Probabilities are based on calculations made from the results of the logistic regression model (Table 3).
Table 3.
Odds of seizure recurrence 6 months after baseline, among various subgroups relative to seizure status at baseline (seizure‐free or not seizure‐free)
| Comparison | Odds ratio | 95% CI | p‐value |
|---|---|---|---|
| Seizure‐free patients at baseline, CBZ discontinue versus continue (n = 41) | 5.00 | 0.51–49.3 | 0.17 |
| Seizure‐free patients at baseline, CBZ withdrawal versus continue (n = 28) | 8.00 | 0.60–106.9 | 0.12 |
| Seizure‐free patients at baseline, CBZ switch to other AED versus continue (n = 34) | 3.64 | 0.30–44.8 | 0.31 |
AED, antiepileptic drug; CBZ, carbamazepine; CI, confidence interval.
Table 4.
Probabilities for seizure recurrence 6 months after baseline among various subgroups relative to seizure status at baseline (seizure‐free or not‐seizure‐free)
| Comparison | Seizure recurrence probabilities | Difference |
|---|---|---|
| Seizure‐free patients at baseline, CBZ discontinue versus continue (n = 41) | 20.0% versus 4.8% | 15.2 PP |
| Seizure‐free patients at baseline, CBZ withdrawal versus continue (n = 28) | 28.6% versus 4.8% | 23.8 PP |
| Seizure‐free patients at baseline, CBZ switch to other AED versus continue (n = 34) | 15.4% versus 4.8% | 10.6 PP |
AED, antiepileptic drug; CBZ, carbamazepine; PP, percentage point.
Discussion
The major feature of current study is the systematic assessment on the seizure outcome of CBZ discontinuation in various subgroups. In addition, we show here that CBZ discontinuation produces a broad spectrum of significant changes in serum concentrations of total cholesterol, HDL, LDL, SHBG, and testosterone, whereas previous studies have focused on single metabolic pathways. Despite the relatively modest sample size, the significance of the findings attests to the robust nature of the effect. The overall consequence of these changes would be expected to result in a considerable decline in the risk for ischemic vascular disease and sexual dysfunction in men.
In the current study, approximately 40% of the patients were ultimately not willing to discontinue CBZ even though they had initially expressed acquiescence toward an evaluation of metabolic side‐effects related to CBZ. Most patients had been seizure‐free with CBZ for decades without experiencing any noticeable side effects. On the other hand, aging is the most important risk factor for vascular events. However, a significant proportion of patients decided to continue CBZ despite their appreciation of the possible risks related to the long‐term effects of EI. The importance of good communication between patient and treating physician is underscored in these situations.
There were some differences in the baseline characteristics of the study patients relative to CBZ status at baseline (continuation vs discontinuation). The proportion of seizure‐free patients was significantly higher in those who continued CBZ and who were anxious of the possibility of the re‐appearance of seizures in these currently seizure‐free subjects. The percentage of free testosterone was lower in the CBZ discontinuation group, suggesting that especially men with low testosterone levels might be more willing to end CBZ medication in comparison with their counterparts with normal testosterone levels.
The inclusion of a control group in our study has added an important methodologic feature missing from all previous outcome investigations, with 2 exceptions done by the same group.16, 17 However, in the study by Wang et al.,16 all switched patients were initially taking phenytoin or CBZ, whereas the controls were being treated with a diverse group of 10 different AEDs. We compared the patients who discontinued CBZ to those taking CBZ who continued taking CBZ.
One of the major features of the current study is of practical significance because we were able to calculate probabilities for seizure recurrence among various subgroups (Table 4). The recurrence rate after CBZ withdrawal resulted in an approximately 24 percentage point incremental additional risk of seizure recurrence. Similarly, converting CBZ to another AED in seizure‐free patients resulted in an 11 percentage point additional risk of recurrent seizures, which is in line with recent study.17 We also calculated the odds of seizure recurrence in seizure‐free patients, but the absolute differences might be more interesting from the clinician's perspective.
Most of the epidemiological data demonstrate that patients with epilepsy have an increased risk of developing cardiovascular and cerebrovascular disease compared to the general population.18, 19, 20, 21, 22 In addition, carotid media intima thickness is significantly increased in patients with epilepsy, particularly among those taking CBZ.23 Furthermore, Sillanpää et al.24 conducted a population‐based cohort study and demonstrated that there was a striking increase in magnetic resonance imaging (MRI) abnormalities related to cerebrovascular disease in patients with epilepsy compared to healthy controls at the age of 45 years. Following an extensive meta‐analysis, Lossius et al.25 concluded that a decrease in LDL of 0.51 mm, similar to the decline detected in our patients, could reduce overall mortality by 5 percentage points and cardiovascular mortality by 11%.26 Based on the above findings, atherosclerotic vascular disease appears to be a genuine risk in the epileptic population. However, contradictory data do exist.27, 28, 29, 30
We observed that there was a decrease in serum concentrations of total cholesterol, HDL, and LDL following discontinuation of CBZ. Furthermore, there was a small, statistically insignificant, decline in triglyceride levels seen after CBZ discontinuation. These results are rather similar to those seen when patients were either switched from CBZ to some other AED or CBZ was withdrawn.9, 10, 18, 25 The declines in HDL are likely to be more than compensated by the negative effects on pro‐atherogenic markers.11 Some variability was seen in the CBZ continuation group, presumably reflecting the inherent fluctuations in these parameters.
Most statins are extensively metabolized by the CYP system and there would be expected to reduce serum levels of these drugs in the presence of an enzyme inducer.15, 18 However, patients taking statins had been excluded from all previous studies investigating this topic. In the current study, some of the patients receiving statins displayed moderate declines in the lipid profile, others exhibited rather major declines, for example, 4.5 mm total cholesterol or 3.7 mm LDL (Fig. 1). Although the number of patients with statins was low and caution is necessary when considering the clinical implications of this finding, one might speculate that patients receiving statin therapy should avoid CBZ treatment.
Figure 1.
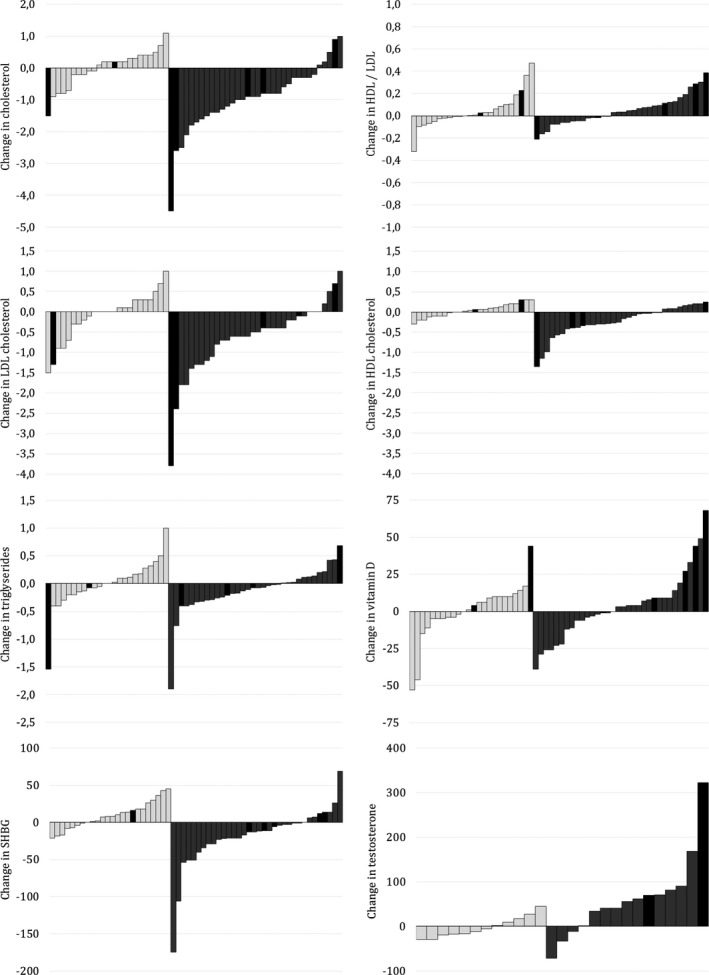
Change in laboratory parameters in patients who continued with CBZ and in those with CBZ discontinuation. Each bar shows the absolute change in the laboratory parameter between the first and second samplings for individual subjects, with 24 patients who continued CBZ shown in white on the left, and the 34 patients who discontinued CBZ in gray on the right. A black bar indicates a statin user.
We detected a significant decrease in SHBG levels in both genders following CBZ discontinuation. In addition, there was a significant increase in the free testosterone level in men after CBZ discontinuation. This corresponds well with clinical experience that some men enjoy improvements in sexual function after CBZ discontinuation. Unfortunately, standardized sexual function questionnaires are not routinely used in our institutions, so it was not possible to investigate whether the improvements in serum concentrations of SHBG and free testosterone correlated with recovery from sexual dysfunction. However, biochemical abnormalities are clearly evident in men, and these might have a real impact on patient well‐being. The design of our study meant that we were not able to assess the sex hormone profiles of females as it is not convenient in clinical practice to request the patient to come to the clinic to provide a blood sample at a fixed time point relative to her menstrual cycle.
Unexpectedly, discontinuation of CBZ, however, was not associated with any increase in serum vitamin D concentrations. Several factors might be speculated to account for this phenomenon. First, extensive variability was also seen in the CBZ continuation group in vitamin D levels, which might be related to seasonal changes of sun exposure with respect to the time of year. Second, vitamin D supplements are prescription‐free and widely used in Finland. In the northern hemisphere at latitudes greater than around 40°N (north of Barcelona), sunlight is not strong enough to trigger the synthesis of vitamin D in the skin from October to March. Anecdotally, vitamin D levels seemed to be elevated particularly in those patients with statin co‐medication in the CBZ discontinuation group (Fig. 1), which might indicate that statins may increase vitamin D concentrations.31
As far as we are aware, no previous study has reported such a comprehensive laboratory panel related to enzyme‐inducing (or EI) AEDs. This pattern of laboratory tests was found useful in helping the clinician to estimate the overall effects of EI in the individual patient. Furthermore, the numeric parameters may highlight the long‐term consequences of EI to the patient in a more concrete manner and assist the patient to decide for her/himself whether to continue with CBZ therapy.
Some points must be kept in mind when drawing conclusions from our study. This was a retrospective study, not a prospective randomized controlled trial, complicating the comparability of patients in the different groups. However, this clinical question could not be adequately answered in a double‐blind randomized study. One potential ascertainment bias might have affected the results: those who switched may have already had some notion that they had a problem (e.g., with bones or cholesterol), or a family history of problems, to make them concerned. Moreover, because the seizure data was gathered only from the previous year, we were unable to eliminate the possibility of remitting‐relapsing pattern, which is believed to be present in up to 16% of patients with epilepsy, that is, the patients fluctuate between periods of seizure freedom and recurrence.32 Sample size might be represented as a limitation for seizure outcomes, but our results are similar to the prior 2 studies,16, 17 suggesting that this is not truly an issue. Furthermore, we were unable to study patients starting to receive CBZ therapy because this is not compatible with our daily clinical practice. Finally, we did not account for other factors that might influence the changes in laboratory parameters, such as body weight, diet, exercise, or smoking habits.
In conclusion, we believe that the clinical implications of the current study are considerable for the general health of patients with epilepsy. With regard to the potential for chronic adverse effects related to EI, the practice of switching CBZ patients to noninducing AED might be worth consideration. CBZ is responsible for an elevation in the lipid profile, alterations in male reproductive function, and potential drug interactions (especially with statins). Therefore, the use of CBZ is problematic, particularly in poststroke epilepsy and in patients with an increased risk of vascular disease. On the other hand, one cannot state that all CBZ should be switched to another non‐EI AED; for example, if the patient has achieved seizure‐freedom but is subsequently found to have side effects on important metabolic pathways, there is nonetheless a real risk of seizure recurrence if he/she should be switched to some other AED. All of these challenges might be avoided by assigning the appropriate drug in the first place with respect to the latest expert opinion on treatment of epilepsy, which compared to earlier surveys, highlights a move away from CBZ as the drug of choice.33
Conflict of interest and sources of funding
Jussi Mäkinen has received support for travel congresses from Biogen‐Idec, Boehringer‐Ingelheim, and Eisai; received speaker honoraria from Boehringer‐Ingelheim; received research funding from Finnish Brain Foundation sr, Finnish Cultural Foundation, Pirkanmaa Regional Fund, Finnish Epilepsy Association, Finnish Norwegian Medical Foundation, and Orion Research Foundation sr; and participated in an advisory board for Eisai.
Sirpa Rainesalo has received speaker honoraria from FennoMedical, Orion Pharma, UCB, and received support for travel to congresses from Abbvie and UCB.
Jukka Saarinen has participated in scientific advisory boards for AstraZeneca, Bayer, Biogen‐Idec, Sanofi‐Genzyme, and Shire; has received funding for travel from Abbvie, Bayer, Biogen‐Idec, Sanofi‐Genzyme, Medtronic, Merck, Roche, Shire, and Teva; has received speaker honoraria from Biogen‐Idec, Boehringer Ingelheim, Novartis, Sanofi‐Genzyme, Orion, and Shire; and has received research support from Sanofi‐Genzyme and Bayer.
Jukka Peltola has participated in clinical trials for Eisai, UCB, and Bial; received research grants from Eisai, Medtronic, UCB, and Cyberonics; received speaker honoraria from Cyberonics, Eisai, Medtronic, Orion Pharma, and UCB; received support for travel congresses from Cyberonics, Eisai, Medtronic, and UCB; and participated in advisory boards for Cyberonics, Eisai, Medtronic, UCB, and Pfizer.
The remaining authors have no conflicts of interest.
Acknowledgments
All authors meet the International Committee of Medical Journals Editors (ICMJE) criteria for authorship and have given final approval for the manuscript to be published. The authors confirm that they have read the Journal′s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Biography
Jussi Mäkinen, postdoctoral researcher in the Department of Neurology, University of Tampere, neurologist at Lapland Central Hospital, Finland.

References
- 1. Karceski S, Morrell MJ, Carpenter D. Treatment of epilepsy in adults: expert opinion. Epilepsy Behav 2005;7:S1–S67. [DOI] [PubMed] [Google Scholar]
- 2. Patsalos PN, Duncan JS, Shorvon SD. Effect of the removal of individual antiepileptic drugs on antipyrine kinetics, in patients taking polytherapy. Br J Clin Pharmacol 1988;26:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet 2002;360:1155–1162. [DOI] [PubMed] [Google Scholar]
- 4. Herzog AG, Drislane FW, Schomer DL, et al. Differential effects of antiepileptic drugs on sexual function and hormones in men with epilepsy. Neurology 2005;65:1016–1020. [DOI] [PubMed] [Google Scholar]
- 5. Pack AM, Morrell MJ, Marcus R, et al. Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol 2005;57:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mintzer S, Boppana P, Toguri J, et al. Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia 2006;47:510–515. [DOI] [PubMed] [Google Scholar]
- 7. Nikolaos T, Stylianos G, Chryssoula N, et al. The effect of long‐term antiepileptic treatment on serum cholesterol (TC, HDL, LDL) and triglyceride levels in adult epileptic patients on monotherapy. Med Sci Monit 2004;10:MT50–MT52. [PubMed] [Google Scholar]
- 8. Anderson GD. Pharmacogenetics and enzyme induction/inhibition properties of antiepileptic drugs. Neurology 2004;63:S3–S8. [DOI] [PubMed] [Google Scholar]
- 9. Isojärvi J, Pakarinen AJ, Lukkarinen O, et al. Liver enzyme induction and serum lipid levels after replacement of carbamazepine with oxcarbazepine. Epilepsia 1994;35:1217–1220. [DOI] [PubMed] [Google Scholar]
- 10. Mintzer S, Skidmore CT, Abidin CJ, et al. Effects of antiepileptic drugs on lipids, homocysteine, and C‐reactive protein. Ann Neurol 2009;65:448–456. [DOI] [PubMed] [Google Scholar]
- 11. Mintzer S, Skidmore CT, Rankin SJ, et al. Conversion from enzyme‐inducing antiepileptic drugs to topiramate: effects on lipids and c‐reactive protein. Epilepsy Res 2012;98:88–93. [DOI] [PubMed] [Google Scholar]
- 12. Mintzer S, Miller R, Shah K, et al. Long‐term effect of antiepileptic drug switch on serum lipids and C‐reactive protein. Epilepsy Behav 2016;58:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beaglehole R, Bonita R, Horton R, et al. Priority actions for the non‐communicable disease crisis. Lancet 2011;377:1438–1447. [DOI] [PubMed] [Google Scholar]
- 14. Mintzer S, Mattson RT. Should enzyme‐inducing antiepileptic drugs be considered first‐line agents? Epilepsia 2009;50:S42–S50. [DOI] [PubMed] [Google Scholar]
- 15. Brodie MJ, Mintzer S, Pack AM, et al. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 2013;54:11–27. [DOI] [PubMed] [Google Scholar]
- 16. Wang SP, Mintzer S, Skidmore CT, et al. Seizure recurrence and remission after switching antiepileptic drugs. Epilepsia 2013;54:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finamore JM, Sperling MR, Zhan T, et al. Seizure outcome after switching antiepileptic drugs: a matched, prospective study. Epilepsia 2016;57:1294–1300. [DOI] [PubMed] [Google Scholar]
- 18. Candrilli SD, Manjunath R, Davis KL, et al. The association between antiepileptic drug and HMG‐CoA reductase inhibitor co‐medication and cholesterol management in patients with epilepsy. Epilepsy Res 2010;91:260–266. [DOI] [PubMed] [Google Scholar]
- 19. Annegers JF, Hauser WA, Shirts SB. Heart disease mortality and morbidity in patients with epilepsy. Epilepsia 1984;25:699–704. [DOI] [PubMed] [Google Scholar]
- 20. Nilsson L, Tomson T, Farahmand BY, et al. Cause‐specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia 1997;38:1062–1068. [DOI] [PubMed] [Google Scholar]
- 21. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia 2004;45:1613–1622. [DOI] [PubMed] [Google Scholar]
- 22. Jansky I, Hallqvist J, Tomson T, et al. Increased risk and worse prognosis of myocardial infarction in patients with prior hospitalization for epilepsy – The Stockholm Heart Epidemiology Program. Brain 2009;132:2798–2804. [DOI] [PubMed] [Google Scholar]
- 23. Hamed SA, Hamed EA, Hamdy R, et al. Vascular risk factors and oxidative stress as independent predictors of asymptomatic atherosclerosis in adult patients with epilepsy. Epilepsy Res 2007;74:183–192. [DOI] [PubMed] [Google Scholar]
- 24. Sillanpää M, Anttinen A, Rinne JO, et al. Childhood‐onset epilepsy five decades later. A prospective population‐based cohort study. Epilepsia 2015;56:1774–1783. [DOI] [PubMed] [Google Scholar]
- 25. Lossius MI, Nakken KO, Mowinckel P, et al. Favorable change of lipid profile after carbamazepine withdrawal. Acta Neurol Scand 2016;134:219–223. [DOI] [PubMed] [Google Scholar]
- 26. Cholesterol Treatment Trialists′ (CTT) Collaboration . Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomized trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luoma PV, Sotaniemi EA, Arranto AJ. Serum LDL cholesterol, the LDL/HDL cholesterol ratio and liver microsomal enzyme induction evaluated by antipyrine kinetics. Scand J Clin Lab Invest 1983;43:671–675. [PubMed] [Google Scholar]
- 28. Luoma PV, Sotaniemi EA, Pelkonen RO, et al. Serum low‐density lipoprotein and high‐density lipoprotein cholesterol, and liver size in subjects on drugs inducing hepatic microsomal enzymes. Eur J Clin Pharmacol 1985;28:615–618. [DOI] [PubMed] [Google Scholar]
- 29. Sudhop T, Bauer J, Elger CE, et al. Increased high‐density lipoprotein cholesterol in patients with epilepsy treated with carbamazepine: a gender‐related study. Epilepsia 1999;40:480–484. [DOI] [PubMed] [Google Scholar]
- 30. Nakken KO, Kornstad S. Do males 30‐50 years of age with chronic epilepsy and on long‐term anticonvulsant medication have lower‐than‐expected risk of developing coronary heart disease? Epilepsia 1998;39:326–330. [DOI] [PubMed] [Google Scholar]
- 31. Mazidi M, Rezaie P, Vatanparast H, et al. Effect of statins on serum vitamin D concentrations: a systematic review and meta‐analysis. Eur J Clin Invest 2017;47:93–101. [DOI] [PubMed] [Google Scholar]
- 32. Brodie MJ, Barry SJE, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012;78:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shih JJ, Whitlock JB, Chimato N, et al. Epilepsy treatment in adults and adolescents: expert opinion, 2016. Epilepsy Behav 2017;69:186–222. [DOI] [PubMed] [Google Scholar]


