Abstract
Extracellular matrix (ECM) remodeling by metalloproteinases is crucial during development. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin type I motifs) enzymes are secreted, multi-domain matrix-associated zinc metalloendopeptidases that have diverse roles in tissue morphogenesis and patho-physiological remodeling. The human family includes 19 members. In this study we identified the 19 members of the ADAMTS family in Xenopus laevis and Xenopus tropicalis. Gene identification and a phylogenetic study revealed strong conservation of the ADAMTS family and contributed to a better annotation of the Xenopus genomes. Expression of the entire ADAMTS family was studied from early stages to tadpole stages of Xenopus, and detailed analysis of ADAMTS9 revealed expression in many structures during organogenesis such as neural crest (NC) derivative tissues, the pronephros and the pancreas. Versican, a matrix component substrate of ADAMTS9 shows a similar expression pattern suggesting a role of ADAMTS9 in the remodeling of the ECM in these structures by degradation of versican.
Keywords: ADAMTS9, Xenopus laevis, Xenopus tropicalis, ECM, Versican
1. Introduction
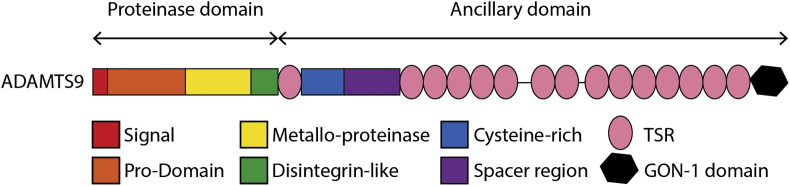
The A Disintegrin and Metalloproteinase with Thrombospondin type-1 motifs (ADAMTS) family are extracellular, secreted enzymes that have diverse functions in development and disease. Nineteen members are present in mammalian genomes. The evolutionary history of the 19 mammalian ADAMTS genes has been marked by duplication and retrotransposition events giving rise to different subfamilies, in association with the organism's complexity, creating neofunctionalization. All ADAMTS proteins have the same basic domain organisation defined by a proteinase domain and an ancillary domain (Fig. 1) (Kelwick et al., 2015). The N-terminal region comprises an amino (N)-terminal signal peptide followed by the proteinase domain which includes a pro-domain and a catalytic domain containing the metalloproteinase domain and the disintegrin-like domain. The basic ancillary domain has a central Thrombospondin type 1 Sequence Repeat (TSR), a cysteine-rich domain and a spacer region. The differences in domain organisation and function define eight sub-groups of ADAMTSs: the hyalectanases/aggrecanases (ADAMTS1, 4, 5, 8, 9, 15 and 20), the procollagen N-propeptidases (ADAMTS2, 3 and 14), the von-Willebrand Factor (vWF) cleaving protease (ADAMTS13), the Cartilage Oligomeric Matrix Protein (COMP) proteinases (ADAMTS7 and 12) and the “orphan” (ADAMTS6, 10, 16, 18,17 and 19). The C-terminal region of the ancillary domain is the most variable between ADAMTSs, except in ADAMTS4 the spacer domain is followed by additional TSR domains. The ADAMTS9, 20 pair contains 14 additional TSRs and a gonadal (GON) domain in C-terminal. ADAMTS13 has two CUB (complement C1r/C1s, Uegf (epidermal growth factor-related sea urchin protein), BMP-1 (bone morphogenetic protein-1)) domains in addition to 7 additional TSRs. Several ADAMTSs (ADAMTS2, 3, 6, 7, 10, 12, 14, 16, 17, 18 and 19) contain a PLAC (protease and lacunin) domain. In the pro-collagen N-propeptidases (ADAMTS2, 3 and 14) sub-group the PLAC domain is embedded within a region unique to these enzymes, while in all others the PLAC modules mark the C-terminus of the proteins. In ADAMTS7 and 12, a mucin/proteoglycan domain is interposed between the 7 additional TSRs, precisely between TSR4 and TSR5 (Fig. 1) (Kelwick et al., 2015).
Fig. 1.
Molecular structure of ADAMTS9. The basic ancillary domain has a central TSR, a cysteine-rich domain and a spacer region. The carboxy (C)-terminal region of the ancillary domain is the most variable between the ADAMTSs containing one to fourteen additional Thrombospondin type 1 Sequence Repeat (TSR) motifs and specific domains defining different sub-groups. Names of the different domains are indicated below the diagrams.
The largest subgroup of the ADAMTS family is the aggrecanases/hyalectanases comprising ADAMTS1, 4, 5, 8, 9, 15 and 20. These enzymes are so-called because they are able to cleave members of the hyalectan (also known as lectican) family of hyaluronan-binding chondroitin sulfate proteoglycan (CSPG) extracellular proteins, which include aggrecan, versican, brevican and neurocan (Stanton et al., 2011). Hyalectanases play diverse roles during development. Adamts9 gene-trap mutant (Gt) mice, where ADAMTS9 is restricted to the cell surface due to the insertion of the human Bcl2α (a mitochondrial membrane protein) at the C-terminus, developed normally up to gastrulation but then showed increasing developmental defects suggesting different functions for ADAMTS9 dependent on whether it is localised to the cell surface or secreted (Nandadasa et al., 2015). It has been shown that ADAMTS9, 20 and 5 have non-redundant and cooperative roles in interdigital web regression by degrading versican (Dubail et al., 2014) (McCulloch et al., 2009b). ADAMTS5 and 9 are essential for heart development by cleaving versican (Dupuis et al., 2011) (Kern et al., 2010). ADAMTS20 mutant mice (belted, bt) have a distinctive coat with a white belt in the lumbar region suggesting the role of ADAMTS20 in the development of skin pigmentation (Rao et al., 2003). This phenotype is stronger when they are also heterozygous for an Adamts9 null allele suggesting cooperation between ADAMTS9 and 20 for melanoblast survival (Silver et al., 2008) (Rao et al., 2003). The double mutated mice (Adamts9+/−; bt/bt) have a cleft palate associated with decreased versican cleavage and reduced cell proliferation in the palatal shelves suggesting that versican proteolysis is necessary for cell proliferation in the fusing palate (Enomoto et al., 2010).
The development and the morphology of most organs are conserved between Xenopus and mammals, which makes it a good model to study developmental processes such as extracellular matrix (ECM) remodelling by the ADAMTS family. Very little is known about the ADAMTS family in Xenopus. The 19 ADAMTS genes were identified in Xenopus tropicalis and ADAMTS1 was shown to play a role in Xenopus laevis development at blastula to gastrula stage as a negative regulator of FGF by its C-terminal region, independent to its protease activity (Suga et al., 2006). X. laevis and X. tropicalis diverged from each other around 48 million years ago (Ma) before the divergence of the two diploid species that gave rise to the X. laevis subgenomes L and S, which occurred around 34 Ma (Session et al., 2016). Analysis of transposable elements (TE) or ‘jumping genes’ specific for each subgenome provided an evolutionary view of the two diploid ancestral species. These two species are still unknown. Specific TEs on each subgenome were active until 18 Ma suggesting that the allotetraploidization happened around 17 Ma. The X. laevis genome is composed of two homoeologous subgenomes called L and S for long and short, respectively (Session et al., 2016). The subgenome S is shorter due to more deletions than L. They have similar chromosome sizes to the X. tropicalis genome, suggesting they have ancestral chromosome organisation. In X. laevis eight out of the nine sets of chromosome pairs (S and L) have an orthologous chromosome in X. tropicalis and the ninth set of chromosome pairs is the result of a fusion of chromosomes 9 and 10 found in X. tropicalis. No inter-chromosomal rearrangements are observed in these two amphibian species (Session et al., 2016).
In this work, we show by a phylogenetic study that the ADAMTS family is conserved between Xenopus, human and mouse suggesting possible conservation of their functions. In addition identified ADAMTS genes in this study were not yet annotated and/or allocated on chromosomes in the current genome version. By analyses of the position of their homologues in X. laevis subgenomes and their orthologues in X. tropicalis, they were assigned to their corresponding chromosome contributing to improvement of Xenopus genomes assembly and annotation. We further show the spatio-temporal expression profiles of ADAMTS9 in X. tropicalis and X. laevis, showing them to be comparable to each other and to ADAMTS9 in mouse, reinforcing the possible conserved function between these species (Jungers et al., 2005).
2. Results
2.1. ADAMTS genes identification and phylogenetic study in Xenopus laevis and Xenopus tropicalis
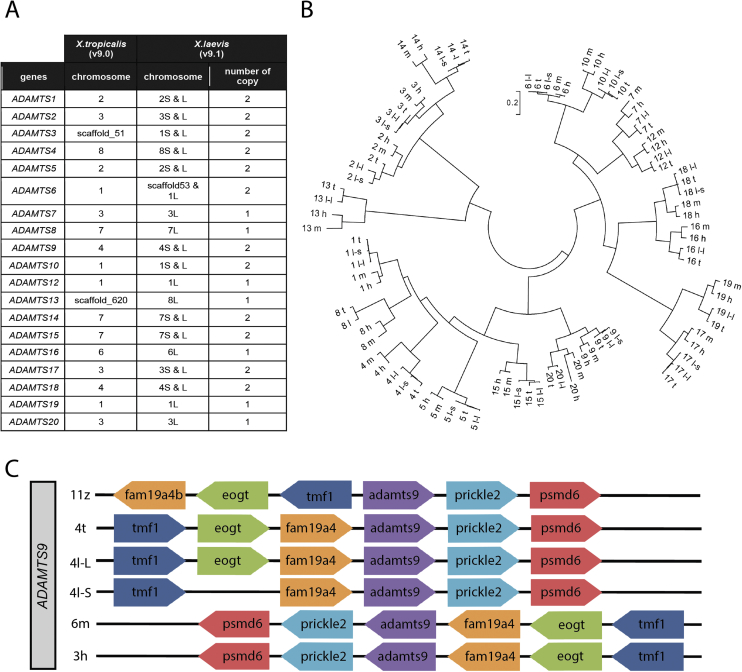
Protein sequences of the 19 human and mouse ADAMTSs were used as a reference to identify the ADAMTS genes in X. laevis and X. tropicalis using the UCSC Genome Browser. Genes with the highest percentage of similarity were selected. All 19 ADAMTS genes were present in both X. tropicalis and X. laevis genomes including seven singletons. ADAMTS7, ADAMTS8, ADAMTS12, ADAMTS13, ADAMTS16, ADAMTS19 and ADAMTS20 have only one copy in the X. laevis genome located on the L chromosome. The chromosomal position of ADAMTS genes is conserved between X. tropicalis and X. laevis confirming that no inter-chromosomal rearrangements happened in these species. X. tropicalis ADAMTS3 and ADAMTS13 and X. laevis ADAMTS6S are not allocated on chromosomes in the genome assembly. However, by analysis of their position on the set of chromosome pairs in X. laevis subgenomes and on the orthologous chromosome in X. tropicalis their positions are on chromosomes 1, 8 and 1S respectively (Fig. 2A).
Fig. 2.
(A) Chromosomal location of ADAMTS genes in Xenopus laevis and Xenopus tropicalis. Chromosome number followed by an L for the longer and an S for the shorter indicate X. laevis gene location on the two subgenomes. Scaffold number is indicated for the genes without a chromosomal location. (B) Evolutionary relationships of the ADAMTS proteins. The evolutionary history was inferred by using the Maximum Likelihood method based on the JTT matrix-based model (Jones et al., 1992). The tree with the highest log likelihood (−21183.6512) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbour-Join and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 88 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 355 positions in the final dataset. ADAMTS proteins are indicated by a number followed by a single letter code indicating the species: for example, ADAMTS1 t represents 1 from Xenopus tropicalis; 2 l-l and 2 l-s represent ADAMTS2 from Xenopus laevis subgenome L and subgenome S respectively; 3 h is ADAMTS3 from Homo sapiens; 4 m is ADAMTS4 from Mus musculus. (C) Synteny of ADAMTS9 in zebrafish, X. laevis subgenomes L and S, X. tropicalis, Mus musculus and Homo sapiens genomes. Chromosome number is indicated on the left followed by a z for zebrafish, an l with L and S for the longer and shorter X. laevis subgenomes, respectively, a t for X. tropicalis, a m for M.musculus and a h for H.sapiens.
X. laevis and X. tropicalis ADAMTSs were compared to ADAMTSs of Mus musculus and Homo sapiens by phylogenetic study using Maximum Likelihood method to determine their relations. Each X. laevis and X. tropicalis ADAMTS form a cluster on the same branch of the phylogenetic tree as the ADAMTS orthologues in H. sapiens and M. musculus with little divergences showed by the length of the branches suggesting possible conservation of their functions (Fig. 2B).
It has been shown that more gene deletions occurred on the X.laevis subgenome S than on the subgenome L, explaining the shorter size of the S subgenome compare to L and the X. tropicalis genome (Session et al., 2016). Synteny analysis was used to look at these events around the ADAMTS9 gene. Locations and orientations of neighbour genes were compared between the three genomes. In order to carry out the synteny analysis, genes close to the gene of interest were selected. Due to the lack of annotation and/or assembly of scaffolds into chromosomes in the X. laevis (v9.1) and X. tropicalis (v9.0) genomes, neighbour genes were selected based on the criteria that they had to be annotated on the three genomes, even though they might not be the closest gene to the ADAMTS gene of interest. X. laevis subgenome L and X. tropicalis genomes have been shown to be more similar to each other than the X. laevis subgenome S. The synteny study of the three copies of ADAMTS9 genes on these genomes shows that they have their chromosomal organisation conserved between X. tropicalis and X. laevis subgenome L but not with subgenome S due to loss of genes in the subgenome S (Fig. 2C). Comparison to the synteny maps of equivalent region in zebrafish, mouse and human show a closer conservation between Xenopus and mouse and human than to zebrafish.
2.2. ADAMTS family expression in Xenopus tropicalis and Xenopus laevis
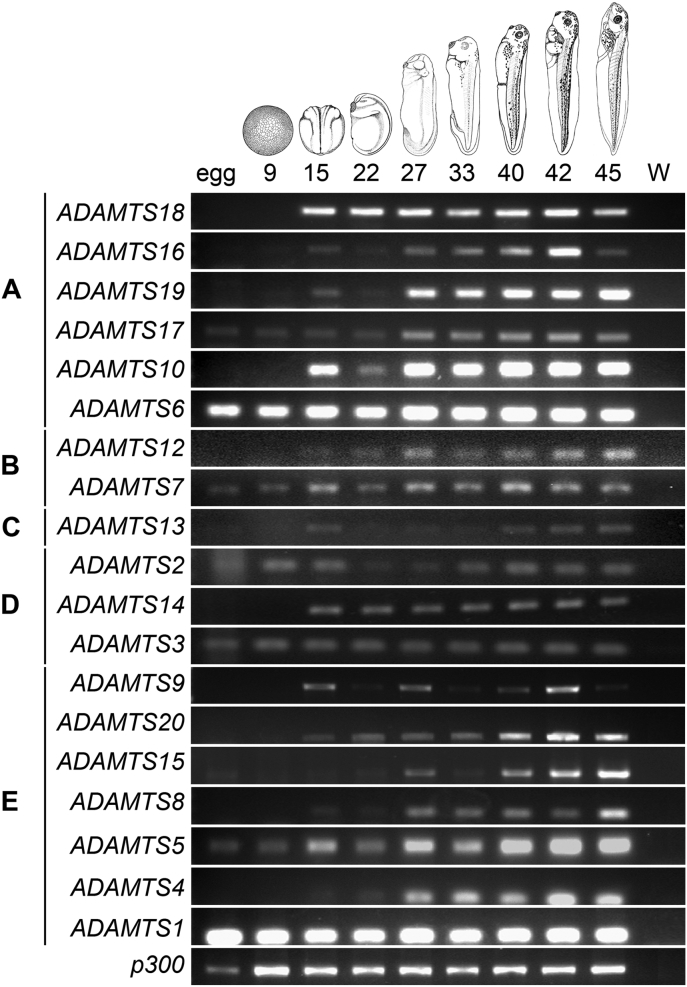
To look at the expression profiles of the ADAMTS family in Xenopus tropicalis during development the RNA of different whole embryos (n = 10) at key stages of development such as unfertilised egg, blastula (stage 9), neurula (stage 15), organogenesis at early tailbud (stage 22), tailbud (stage 27) and late tailbud (stage 33), and organogenesis at tadpole (stages 40, 42 and 45) was extracted (Fig. 3). cDNA synthesis was then carried out and the expression profiles established by RT-PCR. The same number of PCR cycles (35 cycles) was used for all ADAMTSs genes; p300 was used as a loading control and H20 as a negative control. The signal intensity was different for the members of the ADAMTS family. ADAMTS1, ADAMTS6 and ADAMTS10 were the most strongly expressed whereas ADAMTS2 and ADAMTS13 were expressed at lower levels throughout development (Fig. 3). The maternal expression was measured at the unfertilised egg stage as the embryonic transcription only begins at the mid blastula transition (stage 8). Only ADAMTS17, ADAMTS6, ADAMTS7, ADAMTS2, ADAMTS3, ADAMTS5 and ADAMTS1 had maternal expression (Fig. 3). The expression of the ADAMTSs is thus highly dynamic across X. tropicalis developmental stages (Fig. 3).
Fig. 3.
Expression of the ADAMTS genes family during Xenopus tropicalis development.
Analysis by RT-PCR of mRNA from egg to stage 45. Xenopus stages are indicated at the top according to (Nieuwkoop and Faber, 1994) (NF) and gene names are indicated on the left. W: water (H20), is a negative control and p300 is a loading control. ADAMTS genes are organised by clade as follow, (A) orphan, (B) COMP proteinases, (C) vWFCP, (D) Procollagen N-propeptidase and (E) Hyalectanases.
The focus for the rest of this study is on the hyalectanases family composed of ADAMTS1, ADAMTS4, ADAMTS5, ADAMTS8, ADAMTS9, ADAMTS15 and ADAMTS20, during Xenopus development. Hyalectans, a subfamily of proteoglycans, have been shown to be essential for development (Brunet et al., 2012) and the different members of this family, aggrecan, versican, neurocan and brevican, can be cleaved by the hyalectanases family of ADAMTSs (Stanton et al., 2011).
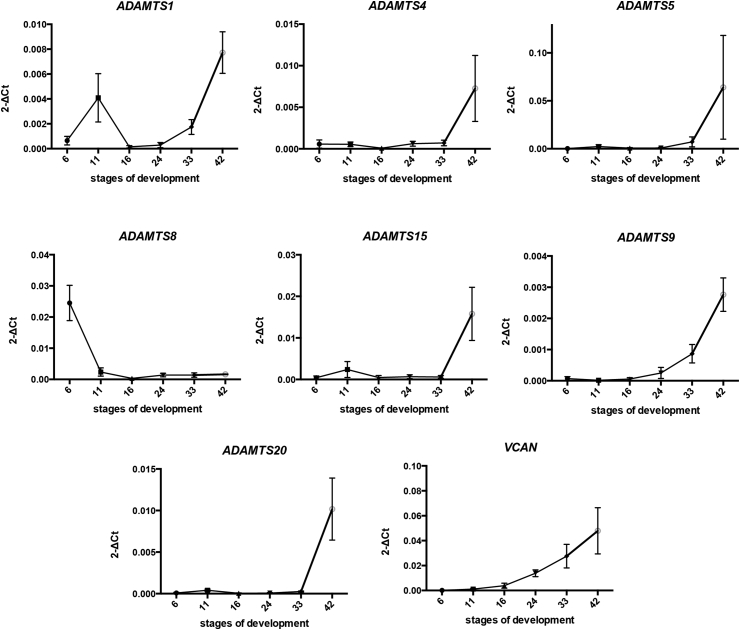
As RT-PCR is only a semi-quantitative method, quantitative RT-PCR (qRT-PCR) was used in order to look at the quantitative expression profiles of the hyalectanases during Xenopus laevis development. RNA from key developmental stages such as blastula (stage 6), gastrula (stage 11), neurula (stage 16), organogenesis at early tailbud (stage 24), late tailbud (stage 33) and tadpole (stage 42) was extracted. cDNAs synthesised from the RNA of these samples were used for qRT-PCR for the hyalectanases gene family and for versican (VCAN) one of their substrates (Fig. 4). Ct data from qRT-PCR were collected using 7500 Software v2.3 (Applied Biosystems) and analysed using Excel (Microsoft). ΔCt (gene Ct—odc Ct) were obtained from Ct values of each gene for normalization and then ΔCt values were converted to relative gene expression using the 2-ΔCt method. The 2-ΔCt method was used to determine the individual expression profiles using Graph Pad Prism 6 software (Fig. 4). The expression was low at early stages and high at late organogenesis stages (stage 42) for nearly all hyalectanase family members with the exception of ADAMTS1 that showed a peak of higher expression at stage 11 (gastrula) and ADAMTS8 that showed a peak of expression at stage 6 (blastula) suggesting a maternal expression (Fig. 4). The gene expression results for the hyalectanases gene family obtained by RT-PCR in Xenopus tropicalis (Fig. 3) showed similar higher expression at late stages of development compared to early stages as observed by qRT-PCR in Xenopus laevis (Fig. 4). Interestingly, ADAMTS9 and its substrate, VCAN, showed the same expression profile with an expression increase at neurula (stage 16) to late organogenesis (stage 42) (Fig. 4).
Fig. 4.
Expression profiles of the hyalectanases family and VCAN during Xenopus laevis development.
Analysis by qRT-PCR of mRNA from stage 6 to stage 42 embryos normalized to odc as a loading control. The experiment was done three independent times. Each graph represents the expression profile of a single gene; the numbers on the vertical axis represents the normalized expression value and on the horizontal axis represents stages of development according to Nieuwkoop and Faber (1994) (mean with SEM), n = 3.
2.3. ADAMTS9 expression in Xenopus tropicalis and Xenopus laevis
RT-PCR analysis in X. tropicalis and X. laevis show that ADAMTS9 is not expressed in early stages before neurula and early tailbud stages (Fig. 5, Fig. 6). Indeed ADAMTS9 starts to be expressed at stage 16 in X. laevis and stage 15 in X.tropicalis. ADAMTS9S in X.laevis starts to be expressed very weakly at stage 6 and goes up from stage 16. The strongest expression of ADAMTS9 in X. laevis is found during organogenesis at late tailbud and tadpole stages (from stage 24 to stage 45) whereas in X. tropicalis ADAMTS9 is more expressed at specific stages during organogenesis such as stage 27 and 42. ADAMTS9S expression in X. laevis is weaker than ADAMTS9L expression except at specific stages during organogenesis (stage 33, 40 and 42) (Fig. 5, Fig. 6A).
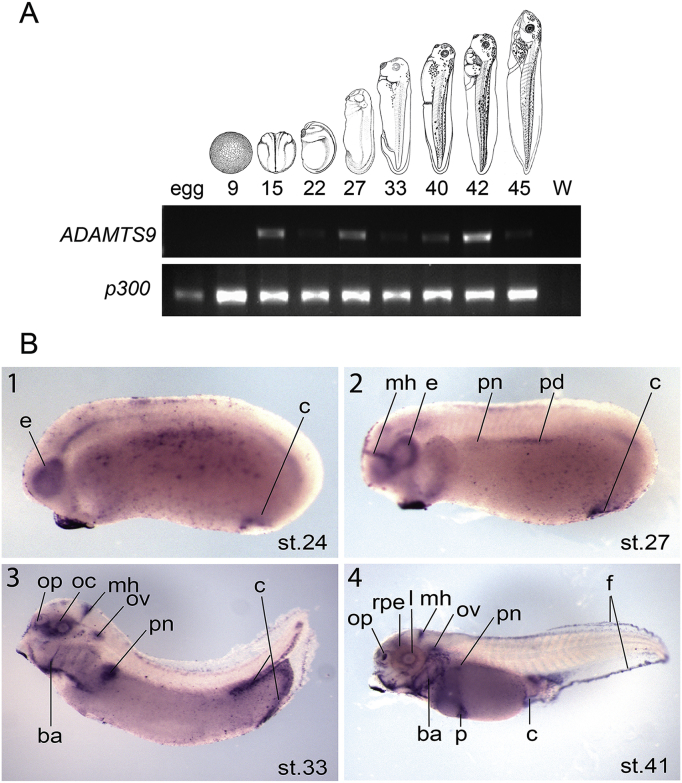
Fig. 5.
Expression of ADAMTS9 during Xenopus tropicalis development.
(A) Analysis by RT-PCR of mRNA from egg to stage 45. Xenopus stages are indicated at the top according to (Nieuwkoop and Faber, 1994) (NF) and gene names are indicated on the left. W: water (H20), is a negative control and p300 is a loading control. (B) Analysis by WISH of ADAMTS9 expression. Developmental stages are according to (Nieuwkoop and Faber, 1994). Lateral views of embryos with anterior on the left and posterior on the right. (1) At stage 24 the expression is in the eye (e) and in the cloaca (c). (2) At stage 27 the expression is in the eye, in the midbrain hindbrain boundary (mh), in the pronephros (pn) and in the pronephric duct (pd) and in the cloaca (c). (3) At stage 33 the expression is in the olfactory placode (op), in the optic cup (oc), in the midbrain hindbrain boundary, in the otic vesicle (ov), in the pronephros, in the branchial arches (ba) and in the cloaca. (4) At stage 41 the expression is also in the lens (l), in the retinal pigment epithelium (rpe) in the pancreas (p) and in the fin (f).
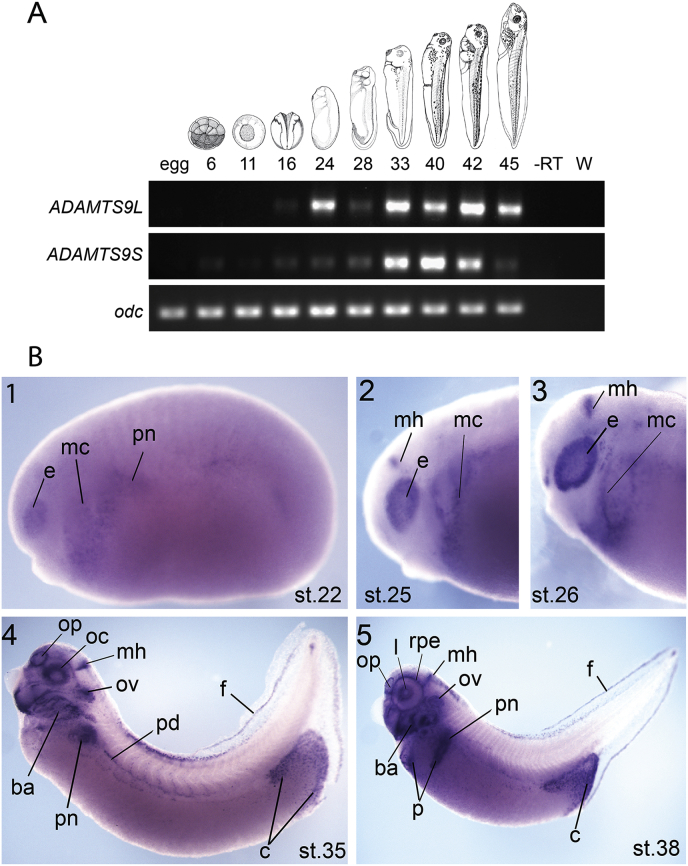
Fig. 6.
Expression of ADAMTS9 during Xenopus laevis development.
(A) Analysis by RT-PCR of mRNA from egg to stage 45. Xenopus laevis stages are indicated at the top according to (Nieuwkoop and Faber, 1994) (NF) and gene names are indicated on the left. odc is a loading control. -RT is a negative control of cDNA synthesis reaction made without adding the Reverse Transcriptase enzyme and W: water (H20), is a negative control of the PCR reaction without cDNA template. (B) Analysis by WISH of ADAMTS9 expression. Developmental stages are according to Nieuwkoop and Faber, (1994). Lateral views of embryos with anterior on the left and posterior on the right. (1) At stage 22 the expression is in the eye (e), in migrating cells (mc) and in the pronephric anlage (pn). (2, 3) At stage 25 and 26 the expression is in the eye, in migrating cells (mc) and in the midbrain hindbrain boundary (mh). (4, 5) At stage 35 and 38 the expression is in the olfactory placode (op), in the optic cup (oc), in the midbrain hindbrain boundary, in the otic vesicle (ov), in the pronephros (pn), in the pronephric duct (pd), in the branchial arches (ba), in the fin (f) and cloaca (c). (5) At stage 38 the expression is also in the lens (l), in the retinal pigment epithelium (rpe), the fin and in the pancreas (p).
We next looked at spatial expression using wholemount in situ hybridisation (Fig. 5, Fig. 6B). In X. tropicalis ADAMTS9 expression at early tailbud stages (stages 24) was mostly found in the developing eye and in the cloaca (Fig. 5B-1). At tailbud stages (stages 27), expression was observed in the eye, in the midbrain-hindbrain boundary, in the pronephros and in the pronephric duct and in the cloaca (Fig. 5B-2). At stage 33 ADAMTS9 expression was seen in a number of developing organs and structures including the pronephros, the pronephric duct, the olfactory placode, the optic cup, the otic vesicle, the branchial arches and the cloaca (Fig. 5B-3). At tadpole stages (stages 41) the eye is more defined and the ADAMTS9 expression was seen to be more specific to the lens and the retinal pigment epithelium. Expression was also observed in the pancreas and in the fin (Fig. 5B-4).
In X. laevis ADAMTS9 expression at early tailbud stages (stages 22) was mostly seen in the developing eye, in migrating cells localised in the anterior of the embryo possibly corresponding to the heart field and in the pronephric anlage (Fig. 6B-1). At tailbud stages (stages 25 and 26) expression was observed in the eye, in the midbrain hindbrain boundary, in migrating cells localised on the anterior side of the embryo (Fig. 6B–2 and 3) and in the cloaca as observed in Fig. 5B-3 (data not shown). At stage 35 ADAMTS9 expression was seen in developing organs and structures such as the pronephros, the pronephric duct, the olfactory placode, the optic cup, the otic vesicle, the branchial arches, the fin and the cloaca (Fig. 6B-4). At tadpole stages (stages 38) the eye structure was more defined and the ADAMTS9 expression was specific to the lens and the retinal pigment epithelium, the expression was also seen in the pancreas, in the fin and in the cloaca (Fig. 6B-5).
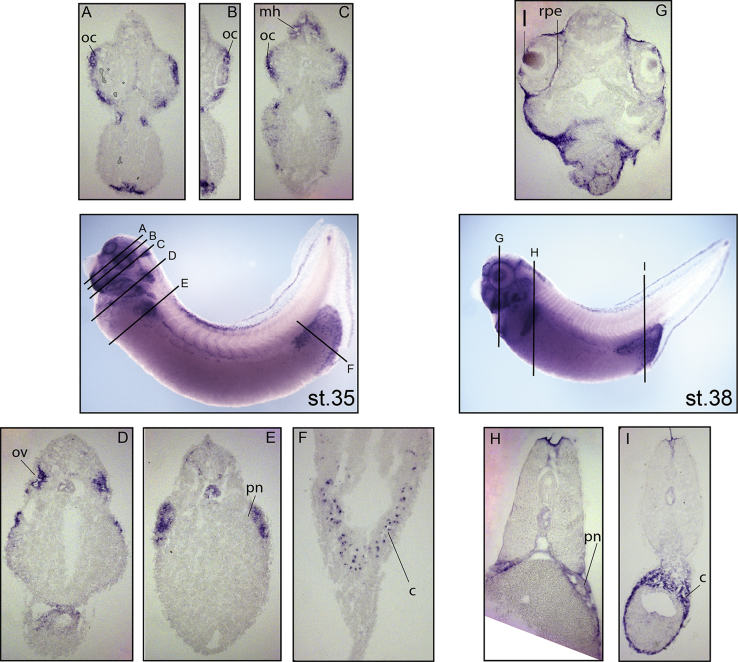
To look in more detail at the ADAMTS9 expression profiles the head and the trunk of late X. laevis tailbud and tadpole embryos (stages 35 and 38) were sectioned (Fig. 7). In the head, expression was found in the optic cup, midbrain hindbrain boundary, in the otic vesicle at stage 35 (Fig. 7–A-D), in the lens and the retinal pigment epithelium at stage 38 (Fig. 7-G). In the trunk the expression was mostly found in the pronephric anlage at stage 35 and in the pronephros at stage 38 (Fig. 7–E and H) (Lienkamp et al., 2012).
Fig. 7.
Sectioned Xenopus laevis embryos after WISH for ADAMTS9. Developmental stages are according to (Nieuwkoop and Faber, 1994). In each panel lines and numbers indicate the level of the sections. Embryos are shown with anterior on the right and sections with dorsal at the top. At stage 35, (A, B, C) the expression in in the head the expression is in the optic cup (oc), in the midbrain hindbrain boundary (mh) and (D) in the otic vesicle (ov); in the trunk (E, F) the expression is in the pronephros (pn) and in the cloaca (c). At stage 38, (G) in the head the expression is in the lens (l) and in the retinal pigment epithelium (rpe); in the trunk (H, I) the expression is in the pronephros (pn) and in the cloaca (c).
3. Discussion
Xenopus diverged from mammals some 360 million years ago and has been shown to be a good animal model relative to human as they are both tetrapod, their genomes show conserved synteny and their organ development and function are highly similar (Wheeler and Brandli, 2009). Xenopus is a good model to study ECM remodeling during embryonic development because it can be seen externally unlike in mammals where embryonic development is in utero. This advantage allows a good visualisation of the phenotypes due to the disruption of a gene, by gain or loss of function experiments, and can help to define the timing of the apparition of these phenotypes.
19 ADAMTS genes are found in the Xenopus laevis subgenome L and Xenopus tropicalis, the same as in mammals, whereas only twelve are present in the subgenome S due to a loss of copy for seven ADAMTSs (ADAMTS7, ADAMTS8, ADAMTS12, ADAMTS13, ADAMTS16, ADAMTS19 and ADAMTS20). Loss of these copies can be explained by neofunctionalization and/or regulation of the level of expression required during development in a stage and tissue dependant manner to adapt to environmental changes (gene dosage) (Otto, 2007; Suzuki et al., 2016). X. tropicalis and X. laevis ADAMTS chromosomal position is conserved, thus confirming the absence of interchromosomal rearrangements. It is known that 92% of the genes are conserved between X. tropicalis and the subgenome L of X. laevis when only 68% are conserved between X. tropicalis and the subgenome S of X. laevis (Session et al., 2016). The Xenopus ADAMTS gene family have the following proportions: 100% are present in X. tropicalis and X. laevis subgenome L but only 63% are present in X. laevis subgenome S. Some of the ADAMTS genes used in this study are not located on chromosomes or have not been annotated thus these data can help improve the genome assembly by allocating ADAMTS genes into the correct chromosome (Watanabe et al., 2016). The phylogenetic study shows that the ADAMTS family is conserved between Xenopus, human and mouse suggesting possible conservation of their functions.
The expression profile of the ADAMTS family in Xenopus embryonic development showed that all the members of the ADAMTS family are expressed in Xenopus tropicalis, visualised by RT-PCR, and have dynamic profiles showing variation in time and intensity of expression from egg to tadpole stages (stage 45). The hyalectanases family of ADAMTSs were the focus for the rest of the study as their substrate, the hyalectan/lectican family of chondroitin sulfate proteoglycans, are known to be widely expressed in the extracellular matrices during development (Brunet et al., 2012). To have a more precise view of the expression profiles of ADAMTS1, ADAMTS4, ADAMTS5, ADAMTS8, ADAMTS9, ADAMTS15, ADAMTS20 and VCAN during Xenopus laevis development qRT-PCR was carried out from blastula (stage 6) to tadpole (stage 42) stages. High-resolution RNA-seq time course, showing transcripts per embryo, during Xenopus tropicalis embryonic development was published in open access in 2016 (Owens et al., 2016) allowing the comparison of published profiles of the hyalectanase family with the data obtained by qRT-PCR. The expression profiles were conserved for all of them (ADAMTS4, ADAMTS5, ADAMTS15, ADAMTS9 and ADAMTS20) except for ADAMTS8 that showed a high expression at stage 6 (maternal expression) in Xenopus laevis but not in Xenopus tropicalis and ADAMTS1 that showed a peak of expression during gastrulation (stage 11). It has been shown that ADAMTS1 plays a role in Xenopus laevis development at blastula to gastrula stage as a negative regulator of FGF by its C-terminal region, independent to its protease activity (Suga et al., 2006).
ADAMTS9, a member of the hyalectanases family, was the focus of the study as its expression was found very similar to the expression of its substrate versican by RT-PCR during Xenopus development suggesting a role of ADAMTS9 in the remodeling of this ECM component. ADAMTS9 spatio-temporal expression profiles, assessed by RT-PCR and WISH, are similar in Xenopus tropicalis and Xenopus laevis from unfertilised egg to tadpole stages. ADAMTS9 is highly expressed from early tailbud in the eye, the midbrain hindbrain boundary, and the migrating cells along the branchial arches (possibly neural crest cells). At tadpole stages ADAMTS9 expression was seen in the retinal pigment epithelium, in the branchial arches, in the pronephros, in the pronephric duct and in the pancreas. ADAMTS9 expression in the brain, the craniofacial structures, the kidney and the pancreas are similar for mouse and Xenopus (Jungers et al., 2005). Mice homozygous for a mutation in ADAMTS9 gene, leading to a knockout, do not survive past gastrulation (E7.5). However conditional and heterozygous mice for ADAMTS9 mutation survive and show soft tissue syndactyly (STS), when two or more digits are fused together and cardiac defects, respectively (Dubail et al., 2014; Kern et al., 2010).
It has been shown that VCAN, a substrate of ADAMTS9, was expressed in Xenopus laevis at early stages during gastrulation and neurulation, and at tailbud and tadpole stages the expression was found in the migrating neural crest, in the branchial arches, heart and pronephros (Casini et al., 2008). The co expression of ADAMTS9 and its substrate VCAN in the pronephros, pronephric duct, migrating neural crest and their derivative structures, such as the branchial arches, suggest a role of ADAMTS9 in the extracellular matrix remodeling during the development of these structures by cleavage of versican (Stanton et al., 2011; Somerville et al., 2003). The cleavage of versican at the Glutamic acid (GLU) 441- Alanine (ALA) 442 position in the GAGβ domain generates a fragment containing the G1 domain and exposing the neo-epitode DPEAAE at the C-terminal position. This shorter versican fragment is called ‘versikine’. These smaller fragments of proteoglycans generated after proteolysis by the hyalectanses can have a biological function different from the intact form such as promoting apoptosis during interdigital web regression by decreasing the threshold of BMP required to induce apoptosis (McCulloch et al., 2009a). It has been shown by in vivo and in vitro study that versican is a nonpermissive matrix for NC migration leading them in the correct migratory direction by creating restrictive boundaries. Correct confinement is essential for collective NC migration during Xenopus development (Szabo et al., 2016). V0 and V1 are the most abundant versican isoforms found in neural crest cells (Dutt et al., 2006). Our expression analysis suggests a role of the hyalectanases family of ADAMTSs, such as ADAMTS9, in NC migration by potentially remodeling of the ECM by degradation of versican. In addition when we carried out chemical screens looking for compounds that affect pigment cell/melanophore development we identified a compound (NSC 84093) which affected melanophore migration by inhibiting metalloproteinase activity thus highlighting the importance of matrix remodeling (Tomlinson et al., 2009a, 2009b). MMP-2 and MMP-14 were identified as potential targets of this compound, however knock-down of these genes with morpholinos did not give rise to the same phenotype raising the hypothesis than other metalloproteinases might be involved in melanophore migration such as the ADAMTSs (Tomlinson et al. (2009a). Our results suggest that ADAMTS9 could be a good candidate. The same profile of expression of VCAN has been shown in mouse kidney development but the amount of cleaved versican, at the specific ADAMTSs cleavage site, was not affected by the knockout of ADAMTS1 and ADAMTS4 (Boerboom et al., 2011), suggesting the role of other members of the hyalectanases family, which could possibly include ADAMTS9.
ECM remodeling by ADAMTS9 modifying matrix dynamics can also have an effect on signaling pathways such as Shh and PDGFRb during umbilical cord vascular growth (Nandadasa et al., 2014). ADAMTS9 expression is found in the midbrain hindbrain boundary (MHB) from tailbud stages (Fig. 5, Fig. 6) whereas its substrate VCAN is not expressed in this area (Casini et al., 2008). FGF8 is known to be expressed in the MHB and to be essential for MHB development (Fletcher et al., 2006). ADAMTS1 has been shown to negatively regulate FGF independently to its catalytic activity (Suga et al., 2006). ADAMTS9 could play a role in the regulation of FGF signaling in the MHB development independently of its protease activity.
In order to test these hypothesis loss of function experiments using morpholinos or Crispr/Cas technology will have to be done in Xenopus embryos (Tomlinson et al., 2008; Guo et al., 2014).
In summary our work show that all the ADAMTSs members are expressed during both Xenopus laevis and Xenopus tropicalis development with different dynamic of expression during embryogenesis. In particular ADAMTS9 presents an interesting expression pattern, similar to its potential substrate versican suggesting a role either in matrix remodeling by removal of a versican scaffold and/or generation of cleavage products such as versikine.
3.1. Experimental procedures
All experiments were performed in compliance with the relevant laws and institutional guidelines at the University of East Anglia. The research has been approved by the ethics committee of the University of East Anglia.
3.2. Data source of ADAMTS gene sequences
The ADAMTS genes reported by (Brocker et al., 2009) were retrieved from Genbank (http://www.ncbi.nlm.nih.gov). With these genes as queries, the Basic Local Alignment Search Tool (BLAST) program was used to search in National Center for Biotechnology Information (NCBI) and in The UniProt Knowledgebase (UniProtKB) against M.musculus and H.sapiens genome assemblies. Orthologs of known human ADAMTS genes in Xenopus were identified by using BLAT sequence-based searches of the Xenopus genomes hosted at The Francis Crick Institute (UCSC Genome Bioinformatics Site). The version xenTro9.1 assembly was used for Xenopus tropicalis and xenLae2 assembly was used for Xenopus laevis. Nucleotide sequences were translated to a protein sequence using ExPASy - Translate tool.
3.3. Alignment and phylogenetic analysis
Multiple sequence alignments and phylogenetic trees were constructed using the amino acid residues of the full length genes and were generated using MEGA (Molecular Evolutionary Genetics Analysis) version 6 software (Tamura et al., 2013).
3.4. Reverse transcriptase-polymerase chain reaction (RT-PCR) and quantitative RT-PCR (qRT-PCR)
Xenopus laevis embryos were obtained as previously described (Harrison et al., 2004). Total RNA from embryos at the indicated stages was extracted using TRIzol® (15596-026, Life technologies, UK) and DNAse treated. Reverse transcription was performed using SuperScript® II reverse transcriptase (18064-014, Invitrogen, CA, USA), with 1 μg total RNA and random primers (C1181, Promega, Southampton, UK).
The amplification of templates was performed using a thermocycler. Total PCR reaction was 25 μl containing 10–50 ng of template cDNA, 0.2 μM of each forward and reverse primer, 1× of BioMix™ (2× reaction mix containing ultra-stableTaq DNA polymerase, Bioline). An initial denaturation step of 95 °C for 3 min was followed by a denaturation of 1 min at 95 °C. The annealing step was carried out at an annealing temperature calculated by subtracting 5 °C from the primer melting temperature, for 1 min. This was followed by 1 min of extension step at 72 °C (according to the expected size of the PCR product, 30s/500bp). 25–35 cycles (depending on the level of expression of the gene of interest) of denaturation, annealing and extension were carried out. Amplified products were fractionated in 1% (w/v) agarose Tris/Borate/EDTA (TBE 1×: 45 nM Tris-Borate, 1 mM EDTA, pH8.0) gel electrophoresis with 0.0001% (v/v) of 10 mg/ml ethidium bromide and visualised under UV light using a UV transilluminator (BIO-RAD).
Sequences and terms of use of each primer set for RT-PCR in Xenopus tropicalis are indicated below. The ADAMTS9 primers were used to make the in situ probe.
| Xenopus tropicalis gene name | Tm (°C) | Sense primer | Anti-sense primer | Reference |
|---|---|---|---|---|
| ADAMTS1 | 59.2 | CTTTTCTTGCCCCGGACTTC | CTCGGTAGAAGAAGGCTCCC | PRIMER3/ SIGMA |
| ADAMTS4 | 59.2 | GACCCCAGTTTTCTCTCCGA | TGCAGAACCCCAACAAGAGA | PRIMER3/ SIGMA |
| ADAMTS5 | 59.1 | TACGCGGATGGGAAGAAGTT | TAATGGGCATGCTTGACTGC | PRIMER3/ SIGMA |
| ADAMTS8 | 58.9 | GACCTTGCGATTTACTGCACT | CACCCTGAACACCTTTGCAT | PRIMER3/ SIGMA |
| ADAMTS15 | 59.2 | CTATGCACTTCCCTGGCTCT | TATTCCTCCCCTTGGTAGCC | PRIMER3/ SIGMA |
| ADAMTS9 | 62 | TTAGCAGTGGTCCATGATGAA | TTCCCGGCTCACATTCG | PRIMER3/ SIGMA |
| ADAMTS20 | 59 | GGGCTTGTCTGTCATCTCT | AGGTGTGGTTTGTTGTGCTC | PRIMER3/ SIGMA |
| ADAMTS2 | 58.9 | AGTTCCGGACAGTGAAGTGT | TGAGACCAAGGCCCAATTCT | PRIMER3/ SIGMA |
| ADAMTS3 | 59.1 | AAAGCTGTGGAAGTTCTGGC | CAGACCATGCCCCTGTTTTC | PRIMER3/ SIGMA |
| ADAMTS14 | 59.1 | AGCCAAAACCAATACGCAGA | TGGTGTCCGATTACAGGGTC | PRIMER3/ SIGMA |
| ADAMTS13 | 59 | CAATGGGGTGGTACTGGAGT | GGGTTCCAGGTCCATGTACA | PRIMER3/ SIGMA |
| ADAMTS7 | 59 | GGAGATGGTGGACAAGGGAA | GTGCCTCTGACTGGGTACTT | PRIMER3/ SIGMA |
| ADAMTS12 | 58.8 | ATGAGCCGTGTGATTCCTCT | TGGTAAGAAGATGCGCCTCT | PRIMER3/ SIGMA |
| ADAMTS6 | 58.9 | CCTTGTCATGGCTTCATCGG | ACTCCTCCTCCTCCTCAAGT | PRIMER3/ SIGMA |
| ADAMTS10 | 59 | GAGGTCTGGACTGGAAGCAT | CTCCAAGTGTTGACGTTGCA | PRIMER3/ SIGMA |
| ADAMTS16 | 58.3 | ACCAAGGAAGATTCAAACGATCA | TGTCAACACGTAGGTAGTAATGT | PRIMER3/ SIGMA |
| ADAMTS18 | 58.6 | GAGATCAGCGCGAAGTTCAA | GGAAACCTGACATGCCATCA | PRIMER3/ SIGMA |
| ADAMTS17 | 58.9 | ACAGAGGAGAGGGACCAAAC | TCCCTGGTTTTCTCCTCCAC | PRIMER3/ SIGMA |
| ADAMTS19 | 59.2 | CAACCAACGCATCATCTCCC | GAAGATCCTCTCCACTCCCG | PRIMER3/ SIGMA |
| Xenopus laevis gene name | Tm (°C) | Sense primer | Anti-sense primer | Reference |
|---|---|---|---|---|
| ADAMTS9S | 60 | CACCCAAGAGCCAAGTTCCTAGACA | AGTGTGCTCTGTCCTGTCAT | PRIMER3/ SIGMA |
| ADAMTS9L | 60 | CACCGTGAATGCTGAATCCCGACC | TGTCATTCACTTGGCCACTG | PRIMER3/ SIGMA |
|
ADAMTS9 (probe) |
58 | TTAGCAGTGGTCCACGATGAA | AGGAACACATACTCCATATCTG | PRIMER3/ SIGMA |
PCR products of ADAMTS9 in Xenopus laevis and Xenopus tropicalis were cloned into pGEMTeasy vector, sequenced and used as a template to generate sense and antisense in situ probes.
Quantitative RT-PCR was carried out as previously described (Hatch et al., 2016).
Sequences and terms of use of each primer set for RT-qPCR in Xenopus laevis are indicated below.
| Xenopus laevis gene name | Tm (°C) | Sense primer | Anti-sense primer | Reference |
|---|---|---|---|---|
| ADAMTS1 | 57 | CAGGAGGCACGAGGAAGAA | ATGAGGGTGAGGAGATAATGTTTC | Primerdesign |
| ADAMTS4 | 56.6 | CTATCGCCGCTATCAACTACAA | TGAGTCCTCCACCTTCCAAG | Primerdesign |
| ADAMTS5 | 57 | GGGCAAGGTGGGCTACAT | CTGAAGTGGGGAGACAACAAC | Primerdesign |
| ADAMTS8 | 56.4 | TTTAGTTCCTGATGATGCTTTTCTT | GCTGCCAGTGGTTCCATAC | Primerdesign |
| ADAMTS15 | 56.7 | AATCCAATCAACATTGTCGTTGTG | TCAGTGTCATGGCGGCATT | Primerdesign |
| ADAMTS9 | 56.4 | GCCCGACTGGAATACAATGAT | ATGTTTTGCGTTTTACTGAAGAGA | Primerdesign |
| ADAMTS20 | 56.8 | TTAGAACCACTGATGAAACCTGAT | AATGTTTGACTTTGTGCTTGTAGA | Primerdesign |
| VCAN | 56.8 | TCATTATCTGGAAGAGTGAATCTGC | CATCTCTGCTCTGGACAATCTTG | Primerdesign |
3.5. Wholemount in situ hybridizations
Wholemount in situ hybridisation was carried out as previously described (Harrison et al., 2004). Sense and antisense probes were synthesised for ADAMTS9 in Xenopus tropicalis and Xenopus laevis. The ADAMTS9 probe in Xenopus laevis was designed to detect both copies of the gene in order to obtain a stronger signal.
3.6. Cryosectioning
Embryos were sectioned as previously described (Hatch et al., 2016).
Funding
This work was supported by a BBSRC grant (BB/I022252) to GW and by the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7 under REA grant agreement number 607142 (DevCom) to ID.
Conflict of interest
None.
Acknowledgements
We would like to thank members of the Münsterberg and Wheeler labs past and present for their help and support during this project. ID would also like to thank the members of the DevCom ITN especially Laurent Coen and Marta Marin Barba and for all their support.
References
- Boerboom D., Lafond J.F., Zheng X., Lapointe E., Mittaz L., Boyer A., Pritchard M.A., DeMayo F.J., Mort J.S., Drolet R., Richards J.S. Partially redundant functions of Adamts1 and Adamts4 in the perinatal development of the renal medulla. Dev. Dynam. 2011;240:1806–1814. doi: 10.1002/dvdy.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker C.N., Vasiliou V., Nebert D.W. Evolutionary divergence and functions of the ADAM and ADAMTS gene families. Hum. Genom. 2009;4:43–55. doi: 10.1186/1479-7364-4-1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet F., Kintakas C., Smith A.D., McCulloch D.R. The function of the hyalectan class of proteoglycans and their binding partners during vertebrate development. Adv. Space Biol. Med. 2012;52 [Google Scholar]
- Casini P., Ori M., Avenoso A., D'Ascola A., Traina P., Mattina W., Perris R., Campo G.M., Calatroni A., Nardi I., Campo S. Identification and gene expression of versican during early development of Xenopus. Int. J. Dev. Biol. 2008;52:993–998. doi: 10.1387/ijdb.082582pc. [DOI] [PubMed] [Google Scholar]
- Dubail J., Aramaki-Hattori N., Bader H.L., Nelson C.M., Katebi N., Matuska B., Olsen B.R., Apte S.S. A new Adamts9 conditional mouse allele identifies its non-redundant role in interdigital web regression. Genesis. 2014;52:702–712. doi: 10.1002/dvg.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L.E., McCulloch D.R., McGarity J.D., Bahan A., Wessels A., Weber D., Diminich A.M., Nelson C.M., Apte S.S., Kern C.B. Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev. Biol. 2011;357:152–164. doi: 10.1016/j.ydbio.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S., Kleber M., Matasci M., Sommer L., Zimmermann D.R. Versican V0 and V1 guide migratory neural crest cells. J. Biol. Chem. 2006;281:12123–12131. doi: 10.1074/jbc.M510834200. [DOI] [PubMed] [Google Scholar]
- Enomoto H., Nelson C.M., Somerville R.P., Mielke K., Dixon L.J., Powell K., Apte S.S. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development. 2010;137:4029–4038. doi: 10.1242/dev.050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R.B., Baker J.C., Harland R.M. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Guo X., Zhang T., Hu Z., Zhang Y., Shi Z., Wang Q., Cui Y., Wang F., Zhao H., Chen Y. Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development. 2014;141:707–714. doi: 10.1242/dev.099853. [DOI] [PubMed] [Google Scholar]
- Harrison M., Abu-Elmagd M., Grocott T., Yates C., Gavrilovic J., Wheeler G.N. Matrix metalloproteinase genes in Xenopus development. Dev. Dynam. 2004;231:214–220. doi: 10.1002/dvdy.20113. [DOI] [PubMed] [Google Scholar]
- Hatch V.L., Marin-Barba M., Moxon S., Ford C.T., Ward N.J., Tomlinson M.L., Desanlis I., Hendry A.E., Hontelez S., van Kruijsbergen I., Veenstra G.J., Munsterberg A.E., Wheeler G.N. The positive transcriptional elongation factor (P-TEFb) is required for neural crest specification. Dev. Biol. 2016;416:361–372. doi: 10.1016/j.ydbio.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Jones D.T., Taylor W.R., Thornton J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Jungers K.A., Le Goff C., Somerville R.P., Apte S.S. Adamts9 is widely expressed during mouse embryo development. Gene Expr. Patterns. 2005;5:609–617. doi: 10.1016/j.modgep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kelwick R., Desanlis I., Wheeler G.N., Edwards D.R. The ADAMTS (a disintegrin and metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015;16:113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern C.B., Wessels A., McGarity J., Dixon L.J., Alston E., Argraves W.S., Geeting D., Nelson C.M., Menick D.R., Apte S.S. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010;29:304–316. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienkamp S.S., Liu K., Karner C.M., Carroll T.J., Ronneberger O., Wallingford J.B., Walz G. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat. Genet. 2012;44:1382–1387. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch D.R., Le Goff C., Bhatt S., Dixon L.J., Sandy J.D., Apte S.S. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr. Patterns. 2009;9:314–323. doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch D.R., Nelson C.M., Dixon L.J., Silver D.L., Wylie J.D., Lindner V., Sasaki T., Cooley M.A., Argraves W.S., Apte S.S. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev. Cell. 2009;17:687–698. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandadasa S., Nelson C.M., Apte S.S. ADAMTS9-mediated extracellular matrix dynamics regulates umbilical cord vascular smooth muscle differentiation and rotation. Cell Rep. 2015;11:1519–1528. doi: 10.1016/j.celrep.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandadasa S., Foulcer S., Apte S.S. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014;35:34–41. doi: 10.1016/j.matbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop P.D., Faber J. Garlan Publishing; New York: 1994. Normal table of Xenopus laevis (Daudin) [Google Scholar]
- Otto S.P. The evolutionary consequences of polyploidy. Cell. 2007;131:452–462. doi: 10.1016/j.cell.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Owens N.D., Blitz I.L., Lane M.A., Patrushev I., Overton J.D., Gilchrist M.J., Cho K.W., Khokha M.K. Measuring absolute RNA copy numbers at high temporal resolution reveals transcriptome kinetics in development. Cell Rep. 2016;14:632–647. doi: 10.1016/j.celrep.2015.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C., Foernzler D., Loftus S.K., Liu S., McPherson J.D., Jungers K.A., Apte S.S., Pavan W.J., Beier D.R. A defect in a novel ADAMTS family member is the cause of the belted white-spotting mutation. Development. 2003;130:4665–4672. doi: 10.1242/dev.00668. [DOI] [PubMed] [Google Scholar]
- Session A.M., Uno Y., Kwon T., Chapman J.A., Toyoda A., Takahashi S., Fukui A., Hikosaka A., Suzuki A., Kondo M., van Heeringen S.J., Quigley I., Heinz S., Ogino H., Ochi H., Hellsten U., Lyons J.B., Simakov O., Putnam N., Stites J., Kuroki Y., Tanaka T., Michiue T., Watanabe M., Bogdanovic O., Lister R., Georgiou G., Paranjpe S.S., van Kruijsbergen I., Shu S., Carlson J., Kinoshita T., Ohta Y., Mawaribuchi S., Jenkins J., Grimwood J., Schmutz J., Mitros T., Mozaffari S.V., Suzuki Y., Haramoto Y., Yamamoto T.S., Takagi C., Heald R., Miller K., Haudenschild C., Kitzman J., Nakayama T., Izutsu Y., Robert J., Fortriede J., Burns K., Lotay V., Karimi K., Yasuoka Y., Dichmann D.S., Flajnik M.F., Houston D.W., Shendure J., DuPasquier L., Vize P.D., Zorn A.M., Ito M., Marcotte E.M., Wallingford J.B., Ito Y., Asashima M., Ueno N., Matsuda Y., Veenstra G.J., Fujiyama A., Harland R.M., Taira M., Rokhsar D.S. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538:336–343. doi: 10.1038/nature19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D.L., Hou L., Somerville R., Young M.E., Apte S.S., Pavan W.J. The secreted metalloprotease ADAMTS20 is required for melanoblast survival. PLoS Genet. 2008;4:e1000003. doi: 10.1371/journal.pgen.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville R.P., Longpre J.M., Jungers K.A., Engle J.M., Ross M., Evanko S., Wight T.N., Leduc R., Apte S.S. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J. Biol. Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Stanton H., Melrose J., Little C.B., Fosang A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta. 2011;1812:1616–1629. doi: 10.1016/j.bbadis.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Suga A., Hikasa H., Taira M. Xenopus ADAMTS1 negatively modulates FGF signaling independent of its metalloprotease activity. Dev. Biol. 2006;295:26–39. doi: 10.1016/j.ydbio.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Suzuki K.T., Suzuki M., Shigeta M., Fortriede J.D., Takahashi S., Mawaribuchi S., Yamamoto T., Taira M., Fukui A. Clustered Xenopus keratin genes: A genomic, transcriptomic, and proteomic analysis. Dev. Biol. 2016;426(2):384–392. doi: 10.1016/j.ydbio.2016.10.018. [DOI] [PubMed] [Google Scholar]
- Szabo A., Melchionda M., Nastasi G., Woods M.L., Campo S., Perris R., Mayor R. In vivo confinement promotes collective migration of neural crest cells. J. Cell Biol. 2016;213:543–555. doi: 10.1083/jcb.201602083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson M.L., Garcia-Morales C., Abu-Elmagd M., Wheeler G.N. Three matrix metalloproteinases are required in vivo for macrophage migration during embryonic development. Mech. Dev. 2008;125:1059–1070. doi: 10.1016/j.mod.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Tomlinson M.L., Guan P., Morris R.J., Fidock M.D., Rejzek M., Garcia-Morales C., Field R.A., Wheeler G.N. A chemical genomic approach identifies matrix metalloproteinases as playing an essential and specific role in Xenopus melanophore migration. Chem. Biol. 2009;16:93–104. doi: 10.1016/j.chembiol.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Tomlinson M.L., Rejzek M., Fidock M., Field R.A., Wheeler G.N. Chemical genomics identifies compounds affecting Xenopus laevis pigment cell development. Mol. Biosyst. 2009;5:376–384. doi: 10.1039/b818695b. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Yasuoka Y., Mawaribuchi S., Kuretani A., Ito M., Kondo M., Ochi H., Ogino H., Fukui A., Taira M., Kinoshita T. Conservatism and variability of gene expression profiles among homeologous transcription factors in Xenopus laevis. Dev. Biol. 2016;426:301–324. doi: 10.1016/j.ydbio.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Wheeler G.N., Brandli A.W. Simple vertebrate models for chemical genetics and drug discovery screens: lessons from zebrafish and Xenopus. Dev. Dynam. 2009;238:1287–1308. doi: 10.1002/dvdy.21967. [DOI] [PubMed] [Google Scholar]