Abstract
Objective
To assess the usefulness of modified esophagogastroduodenoscopy (EGD) for the detection of second primary malignancies of the esophagus or hypopharynx in patients with oral squamous cell carcinoma and determine the association between the oral lesion subsite and esophageal or hypopharyngeal lesion occurrence.
Study Design
Retrospective review.
Methods
In total, 166 patients with oral squamous cell carcinoma without any established symptoms of esophageal or hypopharyngeal squamous cell carcinoma underwent modified EGD based on the Valsalva maneuver and U‐turn method, image‐enhanced endoscopy, and chromoendoscopy using Lugol's iodine for diagnosis. All suspected lesions were biopsied to determine the clinical stages and duplication rates. Odds ratios for the occurrence of duplicate lesions according to the oral lesion subsite were determined.
Results
In total, 37 esophageal and 16 hypopharyngeal lesions were detected. According to the Union for International Cancer Control/American Joint Committee on Cancer classification (2009), 75.7% and 5.4% esophageal lesions were classified as stage IA and IB, respectively, and 50% and 18.8% hypopharyngeal lesions as stage II and stage I, respectively. Approximately 59.1% and 50% esophageal and hypopharyngeal lesions, respectively, were successfully treated by endoscopic resection. Oral lesions involving the floor of the mouth were more frequently accompanied by second primary malignancies of the esophagus or hypopharynx.
Conclusions
Modified EGD is an effective noninvasive technique for early diagnosis and treatment of second primary malignancies of the esophagus and hypopharynx in patients with oral squamous cell carcinoma. In particular, patients with floor of the mouth lesions need close monitoring for hypopharyngeal and esophageal lesions.
Level of Evidence
3b.
Keywords: Field cancerization, head and neck cancer, esophageal cancer, oral cancer, squamous cell carcinoma, hypopharyngeal cancer
INTRODUCTION
Oral cancer is one of the common cancers worldwide, and it accounted for approximately 145 000 deaths in 2012. The most common pathological type is squamous cell carcinoma (SCC).1 The theory of “field cancerization” advocated by Slaughter et al. suggests that multiple primary cancers simultaneously or metachronously occur with a high frequency, particularly in the head and neck region and the esophagus, in individuals from similar fields and with similar backgrounds and risk factors. For instance, the oral cavity, laryngopharynx, and esophagus are characterized by mucosa lined by squamous epithelium, and according to the theory of field cancerization, oral SCC (OSCC) is often accompanied by second primary malignancies (SPMs) of the esophagus (esophageal SCC [ESCC]) or hypopharynx (hypopharyngeal SCC [HSCC]).2, 3, 4 Common risk factors for SCC in the head and neck region and esophagus include male sex, alcohol, and tobacco use.5, 6 Moreover, genetic defects resulting in the lack of the enzyme acetaldehyde dehydrogenase 2 (ALDH2) are recognized as risk factors for SCC. The accumulation of acetaldehyde, which should be metabolized by ALDH2, has carcinogenic effects in several organs, including the esophagus and organs in the head and neck region, including the oral cavity and hypopharynx.7, 8
The prognosis of patients is often regulated by the occurrence of SPMs.9 In particular, ESCC and HSCC often require highly invasive treatment, including surgery with reconstruction, chemotherapy, or radiotherapy. Moreover, these modalities often cause severe complications that result in critical conditions.10, 11 Early detection at lower stages generally results in a better prognosis and necessitates less invasive treatment; thus, the patient's quality of life is maintained.10, 12, 13 However, an appropriate screening system for esophageal cancer or head and neck cancer has not been established.14 Recently, some articles proposed that the concept of field cancerization can be utilized for the estimation of SPMs in patients with primary cancer.15, 16, 17 According to previous studies, when a patient has ESCC or HSCC, there is a strong possibility of the presence of another SCC.18, 19, 20, 21 In particular, the incidences of ESCC, HSCC, and OSCC are strongly interrelated; the presence of one is associated with an increased risk of the other.22, 23 Based on this theory, we routinely screen patients with OSCC for the detection of ESCC or HSCC using esophagogastroduodenoscopy (EGD). The aim of the present study was to assess the usefulness of modified EGD for the detection of ESCC or HSCC in patients with OSCC and determine the association between the subsite of the oral lesion and the occurrence of ESCC or HSCC.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 166 patients who were diagnosed with OSCC at Tokyo Medical and Dental University (TMDU) Dental Hospital and referred to the Department of Gastrointestinal Surgery at TMDU Medical Hospital, where they underwent EGD for screening of the head and neck region and upper gastrointestinal tract, between August 2009 and May 2015. The inclusion criteria for our study were as follows: no other simultaneous cancers and no symptoms associated with ESCC or HSCC. We could not obtain complete data regarding habitual alcohol consumption and smoking for all patients, so these data were excluded from our analysis. Endoscopy was performed using the EG 530NW (Fuji Film, Tokyo, Japan) endoscope inserted via the transnasal route without sedation. For screening of the head and neck region, the oral cavity was observed first, followed by examination of the pharynx and larynx via the transnasal approach.24 The procedure included the Valsalva maneuver, which involved inflation of the cheek to open the hypopharyngeal region (Fig. 1),25, 26 and the U‐turn method with maximum upward angle of the scope in the oropharyngeal region.27 The esophagus, stomach, and duodenum were observed using routine assessment methods.28, 29 In all cases, computed virtual chromoendoscopy for the detection of esophageal lesions involved a sequential approach involving Flexible Spectral Imaging Color Enhancement (FICE) and Lugol staining, which has been previously reported in detail.20, 30
Figure 1.
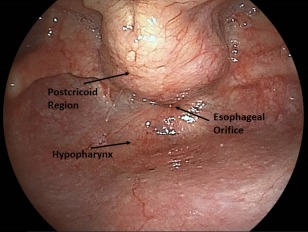
Representative endoscopy images of a healthy patient, acquired using modified esophagogastroduodenoscopy based on the Valsalva maneuver.
All suspicious lesions, including tumors, Lugol‐voiding lesions (LVLs) measuring > 5 mm in diameter, and abnormal lesions detected by FICE, in the esophagus and hypopharynx were biopsied for histologically confirmed diagnosis. All obtained specimens were histologically evaluated by experienced pathologists, and cancerous lesions were classified according to the 7th American Joint Committee on Cancer/Union for International Cancer Control TNM staging system.31
All endoscopic data and pathological diagnoses were recorded in an electronic database known as NEXUS (Fuji‐Film Co., Ltd). Based on these findings, we evaluated the frequency of duplicate cancers in the esophagus or hypopharynx. In addition, we determined the odds ratios (ORs) for the occurrence of ESCC or HSCC according to the subsite of the oral lesion.
All patients provided written informed consent. The study protocol and the informed consent procedure were in accordance with the tenets of the Helsinki Declaration and approved by the Clinical Research Center of Tokyo Medical and Dental University Hospital (number, M2015‐517).
Statistical Analysis
All tests were two‐tailed, and the level of significance was set at P < .05. All analyses were performed using EZR on R commander, version 1.31 (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
ORs and 95% confidence intervals (CIs) for the occurrence of ESCC or HSCC according to the subsite of the oral lesion were determined and assessed using univariate modeling. All tests were two‐tailed, and a P‐value of < .05 was considered statistically significant.
RESULTS
Frequency of ESCC and HSCC in OSCC Patients
The baseline characteristics of patients in the current study are summarized in Table 1. In total, 166 patients with OSCC were assessed, including 91 with tongue SCC (54.8%), 38 with gingival SCC (22.9%), 17 with floor of the mouth SCC (10.2%), 13 with buccal SCC (7.8%), and two with palatal SCC (1.2%). Five patients exhibited multiple subsites of involvement: tongue and floor of the mouth in three (1.8%), tongue and buccal mucosa in one (0.6%), and tongue and gingiva in one (1.8%).
Table 1.
Characteristics of Patients With Oral Squamous Cell Carcinoma Included in the Present Study
| Total N = 166 | ||
|---|---|---|
| Number | Range as % | |
| Sex (male) | 121 | (72.9) |
| Median age, years | 68 | (32‐89) |
| Anatomical location of the tumor site | ||
| Tongue | 91 | (54.8) |
| Mandibular gingiva | 38 | (22.9) |
| Floor of the mouth | 17 | (10.2) |
| Buccal mucosa | 13 | (7.8) |
| Upper jaw | 2 | (1.2) |
| Tongue and floor of the mouth | 3 | (1.8) |
| Tongue and buccal mucosa | 1 | (0.6) |
| Tongue and mandibular gingiva | 1 | (0.6) |
| Frequency of duplicate cancers | ||
| ESCC | 37 | (22.3) |
| Hypopharyngeal SCC | 16 | (9.6) |
| Gastric cancer (Adenocarcinoma) | 0 | (0) |
ESCC = esophageal squamous cell carcinoma; OSCC = oral cavity squamous cell carcinoma.
ESCC and HSCC were detected in 37 (22.3%) and 16 (9.6%) patients, respectively. All lesions in the esophagus and hypopharynx were entirely SCCs.
Clinical Staging and Treatment of ESCC and HSCC
According to the UICC/AJCC classification of 2009, ESCC was classified as stage IA in 28 patients (75.7%), stage IB in two patients (5.4%), stage IIB in one patient (2.7%), stage IIIA in one patient (2.7%), stage IIIC in two patients (5.4%), stage IV in one patient (2.7%), and unidentified in two patients (5.4%). With regard to treatment, 22 patients (59.5%) were treated by endoscopic resection, six (16.2%) with surgery, and nine (24.3%) with chemoradiotherapy (Table 2).
Table 2.
Clinical Staging and Treatment of Esophageal Squamous Cell Carcinoma in Patients With Oral Squamous Cell Carcinoma
| Total N = 37 (%) | ||
|---|---|---|
| Clinical Stages | ||
| Stage IA | 28 | (75.7) |
| Stage IB | 2 | (5.4) |
| Stage IIB | 1 | (2.7) |
| Stage IIIA | 1 | (2.7) |
| Stage IIIC | 2 | (5.4) |
| Stage IV | 1 | (2.7) |
| Unknown | 2 | (5.4) |
| Treatment Methods | ||
| Endoscopic resection | 22 | (59.1) |
| Esophagectomy | 6 | (22.7) |
| Chemoradiotherapy | 9 | (18.2) |
ESCC = esophageal squamous cell carcinoma; OSCC = oral cavity squamous cell carcinoma.
HSCC was classified as stage I in three patients (18.8%), Stage II in eight patients (50.0%), Stage III in two patients (12.5%), and Stage IVA in three patients (18.8%). Eight patients (50.0%) were treated by endoscopic resection, four (25.0%) with chemoradiotherapy, and two (12.5%) with chemotherapy; the remaining two patients (12.5%) were untreated (Table 3).
Table 3.
Clinical Staging and Treatment of Hypopharyngeal Squamous Cell Carcinoma in Patients With Oral Squamous Cell Carcinoma
| Total N = 16 (%) | ||
|---|---|---|
| Clinical Stages | ||
| Stage I | 3 | (18.8) |
| Stage II | 8 | (50) |
| Stage III | 2 | (12.5) |
| Stage IVA | 3 | (18.8) |
| Treated Methods | ||
| Endoscopic resection | 8 | (50) |
| Chemoradiotherapy | 4 | (25) |
| Chemotherapy alone | 2 | (12.5) |
| Untreated | 2 | (12.5) |
ESCC = esophageal squamous cell carcinoma; SCC = squamous cell carcinoma.
Occurrence of ESCC and HSCC According to the Subsite of the Oral Lesion
Univariate analyses revealed an increased risk of ESCC (OR, 3.44; 95% CI, 1.13–10.09; Table 4) and HSCC (OR, 4.09; 95% CI, 0.96–14.89; Table 5) in patients with OSCC involving the floor of the mouth.
Table 4.
Odds Ratios for the Occurrence of Esophageal Squamous Cell Carcinoma According to the Subsite of Oral Squamous Cell Carcinoma
| ESCC | ||||
|---|---|---|---|---|
| ESCC (%) | OR | (95% CI) | P value | |
| Anatomical subsites | ||||
| Tongue N = 96 | 21 (21.8) | 0.94 | 0.42–2.13 | .634 |
| Mandibular gingiva N = 39 | 5 (12.8) | 0.43 | 0.12–0.26 | .076 |
| Floor of the mouth N = 20 | 9 (45) | 3.44 | 1.13–10.09 | .013 |
| Buccal mucosa N = 14 | 3 (21.4) | 0.94 | 0.16–3.86 | .619 |
| Upper jaw N = 2 | 1 (50) | 3.55 | 0.04–280.56 | .397 |
CI = confidence intervals; ESCC = esophageal squamous cell carcinoma; SCC = squamous cell carcinoma.
Table 5.
Odds Ratios for the Occurrence of Pharyngeal Squamous Cell Carcinoma According to the Subsite of Oral Squamous Cell Carcinoma
| Hypopharyngeal SCC | ||||
|---|---|---|---|---|
| ESCC (%) | OR | (95% CI) | P value | |
| Anatomical Subsites | ||||
| Tongue N = 96 | 10 (10.4) | 1.24 | 0.38–4.37 | .454 |
| Mandibular gingiva N = 39 | 1 (2.5) | 0.19 | 0.004–1.37 | .071 |
| Floor of the mouth N = 20 | 5 (25) | 4.09 | 0.96–14.89 | .027 |
| Buccal mucosa N = 14 | 2 (14.2) | 1.64 | 0.16–8.59 | .4 |
| Upper jaw N = 2 | 1 (50) | 9.93 | 0.11–779.04 | .184 |
CI = Confidence intervals; ESCC = Esophageal squamous cell carcinoma; SCC = Squamous cell carcinoma.
DISCUSSION
In support of the theory of “field cancerization,” the results of the present study showed that a large proportion of patients with OSCC had another SCC lesion in the esophagus or hypopharynx. In particular, OSCC involving the floor of the mouth was more frequently accompanied by duplicate cancers or SPMs of the esophagus or hypopharynx compared with OSCC involving other subsites, even though the floor of the mouth was a less commonly involved subsite compared with the tongue and gingiva. The factors influencing the high rate of SPMs in patients with OSCC involving the floor of the mouth remain unclear, and we do not have similar findings from previous reports to substantiate our findings. Nevertheless, this can serve as an important clue during endoscopic screening for ESCC and HSCC in patients with OSCC involving the floor of the mouth.
The rates of detected duplicate cancers in the present study were higher than those obtained in previous similar studies.18, 19, 20, 21 These high rates suggest that the theory of “field cancerization” can be utilized for more efficient screening and detection of ESCC and HSCC. To the best of our knowledge, this is the first study where ESCCs and HSCCs were diagnosed in such early stages using modified EGD in patients with OSCC. Approximately 80% of the detected ESCCs were Stage I lesions, while 70% of the detected HSCCs were Stage I or II lesions. Furthermore, more than half the ESCC and HSCC lesions were successfully treated by endoscopic resection. Early‐stage and smaller lesions are associated with a more satisfactory prognosis and a lower risk of metastasis.10, 32 Superficial ESCC can be treated by local, less‐invasive treatment methods such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Recently, endoscopy‐assisted mucosal resection of the pharynx or larynx, technically known as endoscopic laryngo‐pharyngeal surgery (ELPS),33 has been proposed as a novel treatment for early‐stage HSCC. These minimally invasive modalities will be able to replace conventional therapy, surgery, or chemoradiotherapy, although the early detection of ESCC and HSCC is an inevitable indication for their application.
The satisfactory outcomes in our study may have resulted from the assessment using modified EGD at our facility. We routinely adopt additional procedure in each EGD; computed virtual chromoendoscopy with Lugol staining involved a sequential approach using image‐enhanced endoscopy for ESCC, and Valsalva maneuver and U‐turn method for HSCC. Although these extra steps require further techniques and extra time compared with ordinary examinations, they should be particularly introduced for high‐risk patients with SCCs, such as those with a history of another SCC or habitual factors. The lack of ALDH2 is also a significant risk factor for SCC, because it leads to the accumulation of acetaldehyde, which has been proven to be carcinogenic for various organs. This genetic disorder is characterized by symptoms such as flushing of the face, palpitations, and nausea during alcohol consumption; this is collectively known as the flushing phenomenon. The combined use of a questionnaire regarding the flushing phenomenon and precise EGD examinations will result in a higher sensitivity for the detecting of SCC lesions.
The present study has some limitations. First, although most patients with OSCC were referred for EGD screening from the Department of Oral Surgery, the referral rate was not 100%, and this bias could be one reason for the better detection rates. Second, this was a retrospective study with a small sample size. Larger prospective studies are necessary to confirm the genuine benefits of EGD screening for the early detection and treatment of SPMs in patients with OSCC, such as an improvement in the long‐term survival rates and a decrease in the mortality rate.
CONCLUSION
In conclusion, our results suggest that the use of modified EGD is useful for the screening and early detection of ESCCs or HSCCs as SPMs in patients with OSCC. In particular, patients with OSCC involving the floor of the mouth are more likely to exhibit SPMs and should be closely monitored. The early detection of ESCC and HSCC will permit the use of minimally invasive treatments such as EMR. However, this was a preliminary study, and further prospective case–control studies with larger sample sizes are necessary to improvise this novel diagnostic strategy and establish it as one of the standard procedures for the early detection of ESCC and HSCC as SPMs in patients with OSCC.
ACKNOWLEDGMENTS
We would like to thank Editage (http://www.editage.com) for English language editing and Publication Support.
BIBLIOGRAPHY
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:359–386. [DOI] [PubMed] [Google Scholar]
- 2. Slaughter P, Southwick W, Smejkal W. ‘Field cancerization’ in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953;6:963–967. [DOI] [PubMed] [Google Scholar]
- 3. Muto M, Nakane M, Hitomi Y, et al. Association between aldehyde dehydrogenase gene polymorphisms and the phenomenon of field cancerization in patients with head and neck cancer. Carcinogenesis 2002;23:1759–1765. [DOI] [PubMed] [Google Scholar]
- 4. Tortora GJ, Derrickson B. Principles of Anatomy & Physiology. 14th ed. New York: John Wiley & Sons, Inc.; 2014. [Google Scholar]
- 5. Szymanska K, Hung RJ, Wunsch‐Filho V, et al. Alcohol and tobacco, and the risk of cancers of the upper aerodigestive tract in Latin America: a case‐control study. Cancer Causes Control 2011;22:1037–1046. [DOI] [PubMed] [Google Scholar]
- 6. Guha N, Boffetta P, Filho VW, et al. Original contribution oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case‐control studies. Am J Epidemiol 2007;166:1159–1173. [DOI] [PubMed] [Google Scholar]
- 7. Cui R, Kamatani Y, Takahashi A, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology 2009;137:1768–1775. [DOI] [PubMed] [Google Scholar]
- 8. Yokoyama A, Watanabe H, Fukuda H, et al. Multiple cancers associated with esophageal and oropharyngolaryngeal squamous cell carcinoma and the Aldehyde dehydrogenase‐2 genotype in male Japanese drinkers. Cancer Epidemiol Biomarkers Prev 2002;11:895–900. [PubMed] [Google Scholar]
- 9. Lim H, Kim DH, Jung HY, et al. Clinical significance of early detection of esophageal cancer in patients with head and neck cancer. Gut Liver 2015;9:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013;381:400–412. [DOI] [PubMed] [Google Scholar]
- 11. Chen AY, Zhu J, Fedewa S. Temporal trends in oropharyngeal cancer treatment and survival: 1998–2009. Laryngoscope 2014;124:131–138. [DOI] [PubMed] [Google Scholar]
- 12. Konda VJA, Ferguson MK. Esophageal resection for high‐grade dysplasia and intramucosal carcinoma: when and how? World J Gastroenterol 2010;16:3786–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang CC, Kelly J, August M, Donoff B. Early carcinoma of the oral cavity: a conservative approach with radiation therapy. J Oral Maxillofac Surg 1995;53:687–690. [DOI] [PubMed] [Google Scholar]
- 14. Chung C, Lee Y, Wang C, et al. Secondary Prevention of esophageal squamous cell carcinoma in areas where smoking, alcohol, and betel quid chewing are prevalent. J Formos Med Assoc 2010;109:408–421. [DOI] [PubMed] [Google Scholar]
- 15. Hung SH, Tsai MC, Liu TC, Lin HC, Chung SD. Routine endoscopy for esophageal cancer is suggestive for patients with oral, oropharyngeal and hypopharyngeal cancer. PLoS One 2013; 8(8):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris LGT, Sikora AG, Hayes RB, Patel SG, Ganly I. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control 2011;22:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung C‐S, Liao L‐J, Lo W‐C, et al. Risk factors for second primary neoplasia of esophagus in newly diagnosed head and neck cancer patients: a case‐control study. BMC Gastroenterol 2013;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shiozaki H, Tahara H, Kobayashi K, Yano H, et al. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer 1990;66:2068–2071. [DOI] [PubMed] [Google Scholar]
- 19. Tincani AJ, Brandalise N, Altemani A, et al. Diagnosis of superficial esophageal cancer and dysplasia using endoscopic screening with a 2% lugol dye solution in patients with head and neck cancer. Head Neck 2000;22:170–174. [DOI] [PubMed] [Google Scholar]
- 20. Lee C, Chang C, Lee Y, et al. Narrow‐band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy 2010;42:613–619. [DOI] [PubMed] [Google Scholar]
- 21. Fukuda F, Yasumoto M. The relation between an esophageal cancer and associated cancers in adjacent organs. Cancer 1995;76:101–105. [DOI] [PubMed] [Google Scholar]
- 22. Boute P, Page C, Biet A, Cuvelier P, Strunski V, Chevalier D. Epidemiology, prognosis and treatment of simultaneous squamous cell carcinomas of the oral cavity and hypopharynx. Eur Ann Otorhinolaryngol Head Neck Dis 2014;131:283–287. [DOI] [PubMed] [Google Scholar]
- 23. Roh J‐L, Baek S, Jung JH, Choi S‐H, Nam SY, Kim SY. Second cancer incidence, risk factor, and specific mortality in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 2013;149:579–586. [DOI] [PubMed] [Google Scholar]
- 24. Huang YC, Lee YC, Tseng PH, et al. Regular screening of esophageal cancer for 248 newly diagnosed hypopharyngeal squamous cell carcinoma by unsedated transnasal esophagogastroduodenoscopy. Oral Oncol 2016;55:55–60. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka T, Niwa Y, Tajika M. Prospective evaluation of a transnasal endoscopy utilizing flexible spectral imaging color enhancement (FICE) with the valsalva maneuver for detecting pharyngeal and esophageal cancer. Hepatogastroenterology 2014;61:1627–1634. [PubMed] [Google Scholar]
- 26. Murono S, Tsuji A, Endo K, Kondo S. Evaluation of modified Killian's method: a technique to expose the hypopharyngeal space. Laryngoscope 2014;124:2526–2530. [DOI] [PubMed] [Google Scholar]
- 27. Kawada K, Okada T, Sugimoto T, Kishimoto S, Kawano T. Intraoropharyngeal U‐turn method using transnasal esophagogastroduodenoscopy. Endoscopy 2014;46:137–138. [DOI] [PubMed] [Google Scholar]
- 28. Early DS, Ben‐Menachem T, Decker GA, et al. Appropriate use of GI endoscopy. Gastrointest Endosc 2012;75:1127–1131. [DOI] [PubMed] [Google Scholar]
- 29. Lee S‐H, Park Y‐K, Cho S‐M, Kang J‐K, Lee D‐J. Technical skills and training of upper gastrointestinal endoscopy for new beginners. World J Gastroenterol 2015;21:759–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Urabe Y, Hiyama T, Tanaka S, et al. Metachronous multiple esophageal squamous cell carcinomas and Lugol‐voiding lesions after endoscopic mucosal resection. Endoscopy 2009;41:304–309. [DOI] [PubMed] [Google Scholar]
- 31. Edge A, Byrd S, Compton DR, Fritz CC, Greene AG, Trotti FL. AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 32. de Almeida JR, Li R, Magnuson JS, et al. Oncologic outcomes after transoral robotic surgery: a multi‐institutional study. JAMA Otolaryngol Head Neck Surg 2015;5820:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tateya I, Muto M, Morita S. Endoscopic laryngo‐pharyngeal surgery for superficial laryngo‐ pharyngeal cancer. Surg Endosc 2016;30:323–329. [DOI] [PubMed] [Google Scholar]


