Abstract
Background
Acute laryngospasm sufficient to cause obstructive apnea is a medical emergency that can be difficult to manage within the very short time available for establishing an airway. We have presented substantial evidence that laryngospasm‐based obstructive apnea is the cause of sudden death in epilepsy, and airway management is particularly challenging during seizure activity.
Objective
We sought to determine if the transtracheal delivery of a bolus of oxygen or room air below the level of an obstruction to inflate the lungs could be an effective method to prolong the time available for responders seeking to establish a stable airway, and, if so, what could be learned about optimization of delivery parameters from a rat model.
Methods
Rats were fitted with a t‐shaped tracheal tube for controlling access to air and for measuring airway pressures. After respiratory arrest from simulated laryngospasm, bolus transtracheal lung inflation with a volume of gas equivalent to half the vital capacity was delivered to the closed respiratory system as the only resuscitation step.
Results
Bolus lung inflation was sufficient for resuscitation, improving cardiac function and re‐establishing adequate oxygen status to support life. Inflation steps could be repeated and survival times were approximately 3 times that of non‐inflated lungs.
Conclusion
The properties and consequences of bolus lung inflation are described as a foundation for procedures or devices that can be useful in cases of severe laryngospasm and other cases of upper airway obstruction.
Level of Evidence
3
Keywords: Laryngospasm, obstructive apnea, emergency airway
INTRODUCTION
Apnea from acute airway obstruction is a medical emergency that can be difficult to manage depending on the nature of the obstruction and the short time between occlusion onset and cardiac arrest. Laryngospasm has been suspected during seizure activity in patients based on stridor or intensive inspiratory effort1 and postictal observations of the airway. (eg, when attempting to place an endotracheal tube).2
We have demonstrated in a rat model that seizure activity induces laryngospasm and that laryngospasm can be sufficient to cause obstructive apnea, rapid desaturation, and death.3 The hypoxemia and decreased cardiac output during obstructive apnea cause the seizure to abort, but the laryngospasm can persist beyond the termination of the seizure to the point of respiratory arrest, followed ultimately by cardiac arrest.3, 4 The profound forces of vocal fold contractions during seizure‐induced laryngospasm were demonstrated by: 1) direct visualization of an active vocal fold crossing the midline when it was unopposed by a paralyzed vocal fold, and 2) the complete absence of their usual abduction during attempts to gasp in the presence of an obstructed airway.3 The MORTality in Epilepsy Monitoring Unit Study (MORTEMUS) study identified a consistent sequence of events in epilepsy patients beginning with a generalized tonic clonic seizure and ending in death, including the presence of our biomarkers indicating obstructive apnea.5
The age‐old emergency response teaching in managing the airway of a patient that has a generalized tonic clonic seizure is to allow the seizure to terminate on its own prior to any interventions. The rationale has always been for the protection of the responder and the patient, either of whom could sustain injury in trying to manipulate the oral cavity of a patient having a generalized seizure.6 Obstructive apnea due to seizure‐induced laryngospasm will result in agonal gasp attempts, which can mimic convulsive movements or successful breaths. Eventually, the degree of laryngospasm will diminish as the seizure abates and will permit lung inflation by bag valve mask (based on animal experiments where these events are directly observed3 and the resuscitation experience reported in the MORTEMUS study5. By waiting so long, however, the patient is put a much greater risk of lasting damage from extended hypoxia.
We hypothesized that transtracheal delivery of a single bolus of oxygen or room air below the level of an obstruction to inflate the lungs could be an effective method to prolong the time available for responders seeking to establish a stable airway. Here we report on an approach involving the transtracheal delivery of a bolus of oxygen or room air below the level of an obstruction to inflate the lungs and the optimization of delivery parameters from a rat model. We also discuss the advantages of this method in relation to transtracheal insufflation or jet ventilation.
METHODS
All procedures were approved by an Animal Care and Use Committee and conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals. Adult male Sprague‐Dawley albino rats (180–340 g; Harlan, Chicago, IL) were housed in AAALAC‐accredited facilities and maintained on a 12‐hour light:dark cycle with a temperature of 23˚C and humidity of 55%, monitored daily, and had unrestricted access to water and food.
Urethane (1.5 g/kg ip) was used for anesthesia. Temperature was monitored with a rectal thermometer and maintained with an isothermal heating pad (Deltaphase, Braintree Scientific, Braintree, MA).
We studied 42 episodes of airway occlusion with and without bolus lung inflation with oxygen, room air, or nitrogen gas in 21 rats (randomized such that 7 animals received bolus inflation with oxygen or room air, 7 received bolus inflation with nitrogen, and 7 received no inflation treatment). Group sizes were based on expected effect sizes from prior work.3 In each experiment, the impact of controlled airway occlusion on cardiovascular function were assessed with electrocardiography (ECG) and pulse oximetry, and these same measures were used to evaluate the impact of transtracheal bolus lung inflation.
Monitoring and Recording
ECG recordings
Limb‐lead ECG was recorded using copper strips coated with conductive gel wrapped around forelimbs and tail. Signals were amplified and filtered to pass 1 Hz to 1 kHz and digitized at 2 kHz. Rate was calculated from the number of beats per unit time. Rhythm was assessed by reviewing P waves and associated QRS complexes for variations in wave shape, beat‐to‐beat intervals, and atrial‐ventricular coupling.
Pulse oximetry
Arterial oxygen saturation was measured by using a clip sensor on the thigh (TDR‐43C, Med Associates, St. Albans, VT) coupled to a pulse oximeter (CANL‐425SV‐A, Med Assoc.). The raw pulsatile waveform of the pulse oximeter was digitized (2 kHz) with other signals. Mean oxygen saturation values were recorded at 10‐second intervals. The photoplethysmogram was used to evaluate changes in intrathoracic pressure, cardiac filling, and blood delivery to the periphery.7 In this context, we interpreted the magnitude of the waveform as a measure of cardiovascular performance.
Inspiratory airway pressures
A pressure transducer (CyQ, Columbus Instruments, Columbus, OH) was connected to the sidearm of a T‐shaped tracheal tube (see below). Inspiratory pressures during complete airway occlusion were measured relative to those observed during baseline breathing. Signals were digitized along with other signals recorded at the same time.
Other Manipulations and Implants
Tracheal implants
A T‐shaped tracheal tube was placed through an incision between cartilaginous rings of the trachea after the trachea was exposed in the neck and the recurrent laryngeal nerve was dissected free bilaterally to maintain laryngeal motor function for observation by laryngoscopy. Dissections were verified as successful by direct laryngoscopy. Vocal fold movement was thus preserved, but all airflow was through the tracheal T‐tube.
Controlled airway occlusion
Laryngospasm sufficient to produce obstructive apnea was simulated with controlled airway occlusion. A tracheal implant with a sidearm was used as follows. The straight path served breathing or could be occluded. A pressure transducer on the tracheal tube sidearm recorded forces developed during either normal breathing with the tracheal tube open to the atmosphere or during complete closure of the open port with an airtight cap. In prior work, we showed that during occlusion, respiratory effort exerted to inspire progressively increased with each attempt until effort ceased and this point of respiratory arrest was associated with acute left ventricular dilatation, hypokinesis, and asystole 3.
Lung inflation
We inflated lungs with oxygen, room air, or nitrogen in volumes of 5 ml at atmospheric pressure, or 10 ml in a subset of cases, delivered into the “closed” respiratory system. Oxygen and room air were tested separately, but had similar actions on resuscitation, and data were pooled. Inflation with nitrogen was used to assess the effects of airway pressure in the absence of improving systemic oxygen levels. The inflation volume of 5 ml at atmospheric pressure was based on rat tidal volume (about 1.5 ml)3, 8 and vital capacity (about 12 ml) data.8
Experimental Design
After tracheal implant and configuration of physiological monitoring, each animal was subjected to a period of controlled airway occlusion and the point of respiratory arrest was noted based on the cessation of inspiratory attempts. Each animal was then given a bolus of gas for lung inflation, the identity of the gas based on random assignment. Some measures taken up to the point of lung inflation were pooled across all animals. Measures taken after lung inflation were sorted based on the gas delivered (detailed in the Results).
Data Analysis and Statistics
Data were streamed directly to disk and analyzed offline. Analyses were done with Spike 2 software (Cambridge Electronic Design, Ltd., Cambridge, UK). Data are reported as means ± standard deviation in the text and figures unless otherwise noted. Statistics were computed with Prism 6 software (GraphPad Software, La Jolla, CA). A P < .05 was predefined, after appropriate post‐hoc correction for multiple comparisons where appropriate, to be statistically significant.
RESULTS
Basic Properties of Airway Occlusion
From the onset of occlusion, respiratory effort that was required to inspire increased progressively, then ceased, as reported previously,3 in less than 1 minute (mean = 50.0 ± 9.7 seconds, N = 42 events in 21 animals). Arterial oxygen saturation at the point of respiratory arrest was 36.6 ± 13.3 percent (N = 28 occlusion episodes; see also Nakase et al.3. These features are illustrated in Figure 1 with the points A (pre‐occlusion baseline), B (15 seconds after the onset of airway occlusion), C (the point of respiratory arrest), and D (10 seconds after the point of respiratory arrest). As the tracheal pressure during each inspiration attempt increased, the photoplethysmogram (pulse ox AC) became completely dominated by the negative pressures, with large negative transients likely due to blood redistributing centrally associated with the inspiratory attempts and a post‐attempt positive phase likely due to blood being released to the periphery (best seen approaching point C in Figs. 1 and 2).
Figure 1.
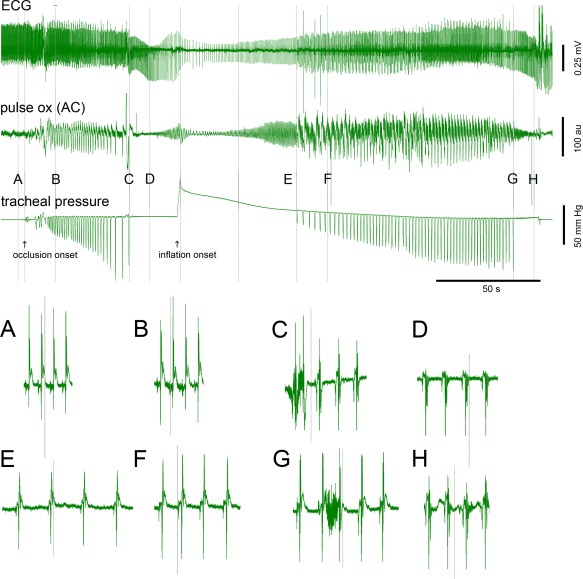
Illustration of overall experiment, sequence of milestones, and ECG detail for resuscitation by bolus lung inflation after respiratory arrest.
Top illustrates the overall experiment and sequence of milestones. ECG (top sweep), photoplethysmography (middle sweep), and tracheal pressure (bottom sweep) for a controlled airway occlusion followed by bolus lung inflation. The onset of airway occlusion and the onset of lung inflation are marked by arrows below the tracheal pressure trace. Bottom A–H illustrate details of ECG morphology and heart rate at the points labeled above the tracheal pressure trace and marked with vertical lines. Note the development of bradyarrhythmia and significant ST segment changes from A to D (A: pre‐occlusion baseline, B: 15 seconds post occlusion onset, C: point of respiratory arrest, and D: 10 seconds post RA). Occlusion onset, inflation peak pressure, and the midpoint between the inflation peak and the first inspiratory attempt are marked with unlabeled vertical lines. Note 1) the recovery of cardiac performance as evidenced by the improvements in ECG amplitude and ST morphology (E, F) and rate (from the minimum rate shortly after inflation onset), and 2) improvements in systemic oxygen delivery as evidenced by the photoplethysmography leading up to point E. Calibrations are shown on the figure.
Labeled points (A–H) correspond to the times used for summary plots in Figure 3.
Figure 2.
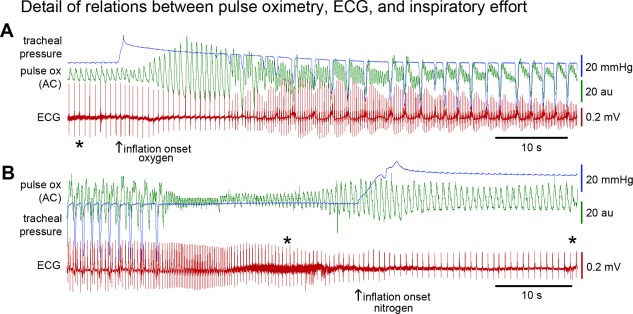
Detail of relations between ECG, photoplethysmography, and airway pressure.
Top (A) shows the recovery of cardiac function after bolus lung inflation with oxygen. Signals are allowed to partially overlap to facilitate comparison of temporal details. Tracheal pressure is shown in blue, photoplethysmography (pulse ox—AC) is shown in green, and ECG is shown in red. Photoplethysmography signal during pre‐inflation respiratory arrest is due only to weak cardiac contractility. Improved cardiac performance is seen after lung inflation and before onset of attempts to inspire. During effort to inspire, each inspiratory attempt (negative pressure transients) is associated with a decreased pulse ox signal, and the cessation of inspiratory effort is associated with an increase in pulse ox signal. Bottom (B) shows an example of inflation with nitrogen. The nitrogen example shows that the inflation improves blood delivery to the periphery, but this does not improve the ECG. Artifacts of handling the animal resulted in increased noise on the ECG recordings and are marked with asterisks (below the ECG in A, above the ECG in B). Calibrations are shown on the figure.
The core metrics in response to airway occlusion are illustrated in Figures 1 and 2, and summarized for the entire study in Figure 3. For panel A of Figure 3, F(1.634, 66.98) = 67.69, P < .0001, changes in RR interval were smallest between baseline versus 15 seconds after occlusion onset (P = .014) and became larger at later time intervals (with P < .0001; Dunnett's multiple comparisons test). The red trendline shown in panel A is also statistically significant (P < .0001). Note, too, that the respiratory changes (eg, Fig. 3D) occur earlier in time than the cardiac changes (eg, Fig. 3B; see also Nakase et al.3.
Figure 3.
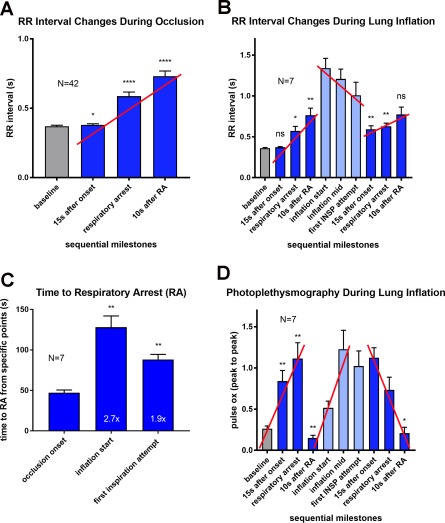
Summary of ECG and photoplethysmography changes during airway occlusion and resuscitation by bolus lung inflation.
A. Changes in RR interval during airway occlusion up to and beyond the point of respiratory arrest in all animals. Time points selected for comparison to prior studies (eg, Nakase et al.3 and to simplify analyses of variance. Trendlines based on these selected time points approximate trendlines derived from the complete data set (difference in slopes < 20%, R2 > 0.9; red trendlines in all panels indicate significant trends: P < .0001). B. Changes in RR interval duration during occlusion and bolus lung inflation with oxygen. For the first 4 bars, F(1.331, 7.987) = 17.9, P = .0020, P < .13, .03, .01 Dunnett's multiple comparison; test for trend P < .0001). During lung inflation, F(2.312, 13.87) = 10.06, P = .0015, P < .01, .01, and .06 for comparisons with the inflation start (Dunnett's multiple comparisons). C. Comparison of times to respiratory arrest (RA) from onset of occlusion to first arrest, onset of lung inflation to second arrest, and first inspiratory attempt after lung inflation to second arrest. D. Photoplethysmography changes during occlusion and bolus lung inflation. For the first 4 bars, F(1.122, 6.735) = 26.49, P = .0013, P < .01, .01, .01, Dunnett's multiple comparison; test for trend showing impact of large negative intrathoracic pressures on peak‐to‐peak photoplethysmogram amplitude P < .0001). In the center, light blue bars highlight the large photoplethysmogram amplitudes in the absence of any effort to inspire indicating a basis for resuscitation when lung inflation is the only manipulation (F [1.77, 10.62] = 6.37, P = .0172). During lung inflation, photoplethysmogram remains elevated until after respiratory arrest due to the added impact of inspiratory attempts on blood flow in the periphery (F[2.133, 12.8] = 10.12, P = .0021, P = .92, .45, and .03 for comparisons with the inflation start).
Basic Properties of Bolus Lung Inflation
Our goal was to assess whether a single bolus of oxygen (N = 7 animals, 3 of which were also tested with room air) to effectively pressurize the respiratory system would be effective for cardiopulmonary resuscitation without any other intervention. Seven other animals received nitrogen for lung inflation. The initial occlusion step was the same for all 14 animals. Some parameters measured before gas delivery could be pooled across all animals, irrespective of gas. Once the gas was delivered, the total pool was segregated based on the gases. Each animal was allowed to reach the point of respiratory arrest, and lung inflation (with oxygen, room air, or nitrogen) was initiated at approximately 20 seconds after the point of respiratory arrest (range 11–31 seconds; mean = 25.1 ± 5.0 seconds; N = 14 animals). The minimum oxygen saturation levels reached between the point of respiratory arrest and the start of lung inflation were 20.2 ± 15.5 % (N = 14 animals).
Bolus lung inflation with oxygen or room air, with no other intervention, restored cardiovascular performance for significant times. As illustrated in Figures 1, 2, and 3, lung inflation with oxygen or room air reversed ECG ST segment changes and improved perfusion as evidenced by photoplethysmography. The time from the start of lung inflation to the first oxygen saturation value over 90% was 27.3 ± 13.7 seconds (saturation was 97.1 ± 3.5%). The time from inflation onset to the peak heart rate was 69.0 ± 14.3 seconds.
ECG and photoplethysmography showed continuous decline in the nitrogen‐treated animals, equivalent to leaving the airway occluded without any resuscitation effort (see example in Fig. 2.).
Respiratory drive was minimal while the airway remained closed and pressurized for nearly one minute, even as cardiac performance continued to improve (Figs. 1 and 2). At about one minute, with the airway still completely occluded, clear evidence of inspiratory effort was visible on the airway pressure sensor. The pattern of inspiratory effort was different from that observed in the initial occlusion. Typically, the inspiratory attempt rate steadily decreased and the inspiratory effort increased from occlusion onset to the point of respiratory arrest. In the post‐inflation period, the inspiratory attempt rate typically started at a low rate, increased as the occlusion continued, and then decreased again at the approach to a second respiratory arrest. The time to the point of respiratory arrest from the point of bolus lung inflation averaged 128.0 ± 36.9 seconds (compared with the time from occlusion onset to the point of respiratory arrest in the initial occlusion (47.2 ± 8.8 seconds) or the time from first respiratory attempt during lung inflation to the point of respiratory arrest (88.1 ± 17.1 seconds; see Fig. 3C). Comparing the time to respiratory arrest from inflation onset or from the first inspiratory attempt to the time to respiratory arrest from occlusion onset (Fig. 3C) showed significant benefit of lung inflation (F[1.024, 6.143] = 24.51, P = .0023; P = .005 for both comparisons, Dunnett's multiple comparisons).
Airway pressure resulting from the gas bolus declined over time (Fig. 4). We sought to estimate the changes in airway pressure as a function of time and irrespective of the gas delivered. Peak pressures averaged 34.2 ± 8.2 mm Hg (N = 16 events, all gasses included) and declined to 19.1 ± 5.8 mmHg by 20 seconds after the peak and 14.1 ± 2.8 by 50 seconds. The best fit curve was based on two exponentials with short (about 1–2 s) and long (about 30 s), suggesting that the short time constant may be related to lung compliance and the longer time constant related to redistribution of blood. Estimates of lung compliance from the resulting airway pressure were consistent with published values for rat lung (0.1 ml per cm H20, comparable to the value previously reported of 0.08 ml per cm H2O9).
Figure 4.
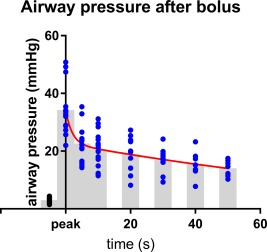
Decay of airway pressure after bolus inflation.
Tracheal pressures were measured from the continuous records at the peak pressure after inflation and at 5, 10, 20, 30, 40, and 50 seconds after the peak. Data from nitrogen inflations were pooled with oxygen data and were essential for evaluation of pressures at times ≥30 seconds. Sixteen events, including 7 nitrogen inflations were used to study the decay of airway pressure over time. N's at each time point were as follows: pre‐inflation “baseline” (16), peak (16), 5 s post‐peak (16), 10 s (15), 20 S (13), 30 S (10), 40 (8), and 50 s (7). Individual points are shown together with gray bars representing means at each time and the best fit curve (two‐phase exponential decay; R2 = 0.545; red). The fitted equation was Y = 28.1e(−0.07x) + 11.6e(−0.72x)‐5.4 with time constants of 2.0 and 135.5 seconds. The change in volume of +5 ml with a resulting change in pressure of 35 mm Hg (about 50 cm H2O) gives a resulting lung compliance of 0.1 ml per cm H2O, comparable to the value previously reported of 0.08 ml per cm H2O9.
With volumes of 5 or 10 ml for inflation, there was never evidence of airway or lung trauma, which would have caused blood or bloody secretions in the airway and possibly pneumothorax.
Repeated Bolus Injections
Bolus lung inflation could be repeatedly used for resuscitation. Figure 5 shows an example where lung inflation was used to restore cardiorespiratory function, the animal was allowed to experience respiratory arrest a second time, and then bolus lung inflation was repeated for resuscitation (N = 3 animals). Responses to a second bolus lung inflation closely resembled responses to the first bolus lung inflation, indicating that lung inflation approach could be used repeatedly without loss of effect.
Figure 5.
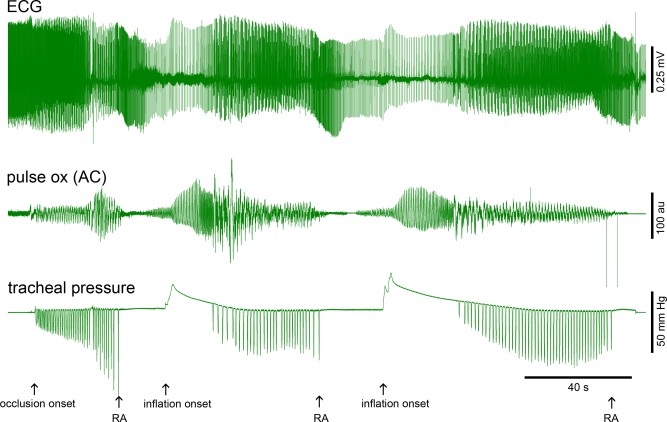
Illustration of repeated use of bolus lung inflation to prolong resuscitation times and two inflation volumes.
See Figure 1 and the text for additional details. Original occlusion onset, three points of respiratory arrest (RA), and two points of inflation onset are marked with arrows beneath the tracheal pressure trace. The first inflation volume was 5 ml of room air. The second was 10 ml of room air. The tracheal pressure traces were well fit with two‐exponential curves from the inflation peak to the point of first inspiration attempt. R2 for the first inflation was 0.9983 and R2 for the second inflation was 0.9989. Time constants were 1.0 and 17.1 seconds for the first inflation and 0.7 and 27.9 seconds for the second inflation. ECG changes are seen only at slow sweep speeds here, but can be seen at higher temporal resolution in Figures 1 and 2. Calibrations are shown on the figure.
DISCUSSION
Acute upper airway obstruction (eg, laryngospasm) can be difficult to manage because of the very short times necessary for desaturation, respiratory arrest, and cardiac arrest. We demonstrate that bolus lung inflation with oxygen or room air can significantly restore cardiac performance during airway occlusion, even after the point of respiratory arrest. Further, lung inflation increased the time window available for establishing a stable airway by nearly three‐fold in response to a single inflation step, and inflation steps could be repeated for longer periods of support. Whereas this method is potentially useful in cases of severe laryngospasm, it will have equal utility in other cases of upper airway obstruction. We envision an approach where a single bolus of oxygen or air is delivered to prolong the time available for stabilization of the airway with removal of the injection hardware immediately after the airway is stabilized.
Utility of Bolus Lung Inflation
Our long‐term goal is to prevent sudden death in epilepsy. Given the narrow time window that exists for successful cardiopulmonary resuscitation between the natural diminution of seizure‐induced laryngospasm and cardiac arrest from hypoxemia (eg, Ryvlin et al.5, bolus lung inflation may be a simple, effective intervention. Resuscitation with only bolus lung inflation was unexpected, given the poor cardiovascular performance at this point (eg, Nakase et al.3. Clearly, the approach has promise even if many questions remain with regard to bolus delivery, maintenance of system pressure, and actual outcomes.
Comparison with Transtracheal Insufflation and Jet Ventilation
Transtracheal insufflation and jet ventilation are related options for introducing oxygen to stabilize an oxygen supply in a variety of settings, including emergencies, intensive care, and surgeries where airway management is or becomes problematic (see also other variants reviewed elsewhere10, 11.
In transtracheal insufflation, oxygen or room air is delivered via cannula at a steady flow of approximately 2 liters/minute directly into the trachea with a separate transtracheal port or an open airway providing the exhaust port.12, 13, 14, 15, 16, 17, 18 Its key utility has been as a continuous source of oxygen as an adjunct to surgical procedures involving the upper airway, an adjunct to mechanical ventilation, or as an alternate method to achieve oxygenation during cardiopulmonary resuscitation.
Jet ventilation19, 20, 21, 22, 23, 24, 25, 26 has been used more commonly in cases of upper airway obstruction to increase the time available for intubation. Gas is delivered at high pulse rates with low tidal volumes or low pulse rates with larger tidal volumes via rigid cannula that can be associated with a bronchoscope or laryngoscope, via cricothyroidotomy cannula, or introduced into a tracheal tube. Jet ventilation devices can deliver nearly 1 liter per second through a 14 gauge needle at 30 psi (2 atmospheres) and 1.25 liters per second at 50 psi (3.4 atm),25 and do so for as long as they are active. The main concern is that the continuous use of high driving pressures can easily traumatize the airway or cause pneumothorax.26
Our lung inflation method produced long‐duration, but modest peak intratracheal pressures of less than 50 mmHg (less than 0.1 additional atmospheres), and showed no evidence of trauma to the airway or lungs. The single inflation step yields rapid reoxygenation and has the added benefit of improving blood flow to the periphery.
In the case of a “leaky” airway, for example due to incomplete upper airway obstruction, repeated application of bolus lung inflation thus resembles the insufflation methods described above. Creating a pressurized closed system with a mask in cases of partial upper airway obstruction is equivalent to continuous positive airway pressure (CPAP) devices, but such a configuration creates an obstruction for manipulating the upper airway.
What remains from a utilization standpoint is the optimization of methods for lung inflation and when it should be performed for the best outcomes. This animal model will allow many of the key details to be evaluated. Clearly, bolus lung inflation as an approach to resuscitation has the potential for timely, trouble‐free application that can save lives.
LIMITATIONS
The major limitations of this study relate to scaling the intervention from a rat model to humans. A goal of this study on rats was the evaluation of the basic physiological consequences of lung inflation before the very different job of designing appropriate delivery devices began. The volume of gas delivered in the rat model and its benefit for resuscitation requires a proportional volume of gas delivered quickly in human subjects. The human trachea is about 10 times the diameter of the rat trachea, but a functional emergency gas delivery system for humans is expected to be practically limited at its entry into the trachea to 1 to 2 mm in diameter. Gas flow rates need to be considerably higher, as discussed above, but design details at the injection site will be critical for minimizing airway trauma and maximizing ease of use. These data from a rat model on respiratory and cardiovascular physiology during bolus lung inflation establish the key physiological targets to be achieved in the design and testing of delivery methods in large animals models (eg, sheep, pigs) with the ultimate goal of human use.
CONCLUSIONS
A proof‐of‐concept that bolus lung inflation can resuscitate an animal past the point of respiratory arrest is demonstrated in a rat model. Our results establish key metrics and expectations with regard to the development of methods that can be used for emergency resuscitation in cases of upper airway occlusion.
ACKNOWLEDGMENTS
We are grateful for the continuous support of Dr. Kiyomi Koizumi for our work. This work was supported by university and philanthropic contributions. Sophia Villiere received a stipend for a portion of her effort from an NIH RISE grant (R25‐GM105553).
Conflict of Interest: None of the authors (SV, KN, RK, HA, JL, KS, JS, ML, DW, or MS) have any conflicts of interest to declare.
Financial support: This work was supported by university and philanthropic contributions. Sophia Villiere received a stipend for a portion of her effort from an NIH RISE grant (R25‐GM105553).
BIBLIOGRAPHY
- 1. Amir J, Ashkenazi S, Schonfeld T, Weitz R, Nitzan M. Laryngospasm as a single manifestation of epilepsy. Arch Dis Child 1983;58:151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tavee J, Morris H, 3rd . Severe postictal laryngospasm as a potential mechanism for sudden unexpected death in epilepsy: a near‐miss in an EMU. Epilepsia 2008;49:2113–2117. [DOI] [PubMed] [Google Scholar]
- 3. Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: Defining pathophysiology for sudden death in a rat model. Epilepsy Res 2016;128:126–139. [DOI] [PubMed] [Google Scholar]
- 4. Stewart M, Kollmar R, Nakase K, et al. Obstructive apnea due to laryngospasm links ictal to postictal events in SUDEP cases and offers practical biomarkers for review of past cases and prevention of new ones. Epilepsia 2017;58:e87–e90. [DOI] [PubMed] [Google Scholar]
- 5. Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 6. Web‐Based Integrated 2010 & 2015 American Heart Association and American Red Cross Guidelines for First Aid. Available at: https://eccguidelines.heart.org/index.php/circulation/aha-red-cross-first-aid-guidelines/part-15-first-aid/. last accessed 4/11/2018.
- 7. Rusch TL, Sankar R, Scharf JE. Signal processing methods for pulse oximetry. Comput Biol Med 1996;26:143–159. [DOI] [PubMed] [Google Scholar]
- 8. Whitehead GS, Kimmel EC, Reboulet JE, Still KR; Naval Health Research Center . Wright‐Patterson Afb Oh Toxicology D. Pulmonary Function in Normal Rats. Available at: http://handle.dtic.mil/100.2/ADA367865. last accessed 4/11/2018.
- 9. Guerrero T, Castillo R, Sanders K, Price R, Komaki R, Cody D. Novel method to calculate pulmonary compliance images in rodents from computed tomography acquired at constant pressures. Phys Med Biol 2006;51:1101–1112. [DOI] [PubMed] [Google Scholar]
- 10. Boccio E, Gujral R, Cassara M, et al. Combining transtracheal catheter oxygenation and needle‐based Seldinger cricothyrotomy into a single, sequential procedure. Am J Emerg Med 2015;33:708–712. [DOI] [PubMed] [Google Scholar]
- 11. Almeida EP, Almeida AC, Almeida FF, Montessi J, Gomes CA, Ferreira LE. Transtracheal puncture: a forgotten procedure. Braz J Med Biol Res 2015;48:725–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Black IH, Janus SA, Grathwohl KW. Low‐flow transtracheal rescue insufflation of oxygen after profound desaturation. J Trauma 2005;59:344–349. [DOI] [PubMed] [Google Scholar]
- 13. Ayoub IM, Brown DJ, Gazmuri RJ. Transtracheal oxygenation : an alternative to endotracheal intubation during cardiac arrest. Chest 2001;120:1663–1670. [DOI] [PubMed] [Google Scholar]
- 14. Siddiqui FM, Campbell S, Ie S, Biscardi F, Rubio E. Three decades of transtracheal oxygen therapy: A review of the associated complications with an illustrative case presentation. Lung India 2017;34:448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnas GM, Smalley AJ, Miller J, Park SG, Delaney PA, Mackenzie CF. Efficacy of several modes of continuous‐flow insufflation for resuscitation of a canine model of acute respiratory arrest. Ann Emerg Med 1996;27:617–624. [DOI] [PubMed] [Google Scholar]
- 16. Mackenzie CF, Barnas G, Nesbitt S. Tracheal insufflation of oxygen at low flow: capabilities and limitations. Anesth Analg 1990;71:684–690. [DOI] [PubMed] [Google Scholar]
- 17. Mackenzie CF, Barnas GM, Smalley J, Moorman R, Baptiste J. Low‐flow endobronchial insufflation with air for 2 hours of apnea provides ventilation adequate for survival. Anesth Analg 1990;71:279–284. [DOI] [PubMed] [Google Scholar]
- 18. Branditz FK, Kern KB, Campbell SC. Continuous transtracheal oxygen delivery during cardiopulmonary resuscitation. An alternative method of ventilation in a canine model. Chest 1989;95:441–448. [DOI] [PubMed] [Google Scholar]
- 19. Evans KL, Keene MH, Bristow AS. High‐frequency jet ventilation—a review of its role in laryngology. J Laryngol Otol 1994;108:23–25. [DOI] [PubMed] [Google Scholar]
- 20. Bourgain JL, Desruenne E, Fischler M, Ravussin P. Safety and jet ventilation. Br J Anaesth 2008;101:573; author reply 574. [DOI] [PubMed] [Google Scholar]
- 21. Bourgain JL, Desruennes E, Fischler M, Ravussin P. Transtracheal high frequency jet ventilation for endoscopic airway surgery: a multicentre study. Br J Anaesth 2001;87:870–875. [DOI] [PubMed] [Google Scholar]
- 22. Desruennes E, Bourgain JL, Mamelle G, Luboinski B. Airway obstruction and high‐frequency jet ventilation during laryngoscopy. Ann Otol Rhinol Laryngol 1991;100:922–927. [DOI] [PubMed] [Google Scholar]
- 23. Beydon L, Bourgain JL, Benlabed M, Bourgain L. Pulmonary volume measurements during high‐frequency jet ventilation in anesthetized man. Crit Care Med 1990;18:1102–1106. [DOI] [PubMed] [Google Scholar]
- 24. Benhamou D, Bourgain JL, Rouby JJ, Viars P. High‐frequency jet ventilation vs continuous positive airway pressure for postoperative respiratory support. Chest 1984;85:733–738. [DOI] [PubMed] [Google Scholar]
- 25. Doi T, Miyashita T, Furuya R, Sato H, Takaki S, Goto T. Percutaneous transtracheal jet ventilation with various upper airway obstruction. Biomed Res Int 2015;2015:454807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duggan LV, Ballantyne SB, Law JA, Morris IR, Murphy MF, Griesdale DE. Transtracheal jet ventilation in the ‘can't intubate can't oxygenate’ emergency: a systematic review. Br J Anaesth 2016;117(Suppl 1):i28–i38. [DOI] [PubMed] [Google Scholar]


