Abstract
E-cadherin is conventionally considered to be a good prognostic marker in cancer. The loss of E-cadherin is one of the key hallmarks of epithelial-to-mesenchymal transition, a biological process that promotes cancer cell invasiveness and metastasis. Recent evidence has cast doubt on the importance of epithelial-to-mesenchymal transition in metastasis. The availability of protein-level data in the Cancer Genome Atlas allows for the quantitative analysis of protein and prognosis. The prognostic values of E-cadherin and β-catenin were revisited across 19 cancer types, and high E-cadherin was found to correlate with good prognosis in most cancers. Conversely, higher E-cadherin and β-catenin correlated with shorter survival in invasive breast carcinoma. Stratifying breast cancers by histologic subtype revealed that the poor prognosis of E-cadherin and β-catenin proteins was characteristic of infiltrating ductal, but not lobular, carcinomas. To further corroborate the protein findings and examine cellular localization, immunohistochemistry was used for E-cadherin and β-catenin in 163 breast patient samples from the Iowa cohort. Most previous studies showing that reduced or absent E-cadherin and β-catenin was inversely associated with tumor stages in ductal carcinomas were confirmed. Taken together, these results lead us to question the prognostic values of E-cadherin and β-catenin in ductal carcinomas and indicate a complicated role of E-cadherin and β-catenin in breast cancer progression.
E-cadherin is a structural component of adherens junctions, linking the actin cytoskeleton to adjacent cells forming epithelial tissues.1 Throughout carcinomas (cancers of epithelial origin), E-cadherin expression has been inversely correlated with tumor stage, pathologic stage, and prognosis.2, 3, 4, 5, 6, 7 Intuitively, the loss of E-cadherin in carcinomas is thought to encourage invasion and metastasis via loss of cell-to-cell interactions.8, 9 The loss of E-cadherin has been used as a hallmark for epithelial-to-mesenchymal transition (EMT) genetic reprogramming that changes the epithelial characteristics of cancer cells.10 However, recent studies have called into question whether cancer cells require the loss of E-cadherin or EMT to invade and metastasize.11, 12, 13 Moreover, several studies have suggested the clustering of tumor cells via adherens or adherens-like junctions may facilitate metastasis.14
Catenins, including α-catenin, β-catenin, and p120-catenin, are intracellular components of the adherens junction. Catenins provide structural support as part of the adaptor complex that attaches the actin cytoskeleton to E-cadherin. In addition, β-catenin is a transcriptional coactivator in the WNT signaling pathway with established roles in embryogenesis, stem cell regulation, carcinogenesis, and EMT.15 E-cadherin is believed to sequester β-catenin to the plasma membrane at a 1:1 ratio.16, 17 With the presence of E-cadherin, structural β-catenin is prevented from participating in WNT ligand–mediated signaling.18 However, it is unknown whether deficiency in E-cadherin is sufficient to drive β-catenin activation because the loss of E-cadherin is often accompanied by coloss of β-catenin in breast cancer.19, 20, 21
Breast cancer is one of the few cancer types for which E-cadherin and β-catenin have been investigated for diagnostic, prognostic, and mechanistic value, with inconsistent and sometimes contrasting conclusions.7, 22, 23, 24, 25 Invasive breast cancer is divided into two major subgroups on the basis of histologic and molecular traits, including infiltrating ductal carcinoma (IDC), with 70% to 80% of total incidence, and infiltrating lobular carcinoma (ILC), with 10% to 15% of total incidence.26 Clear distinction in prognosis on the basis of ILC versus IDC has been disputed, with groups finding no difference,27 ILC having better prognosis,28, 29, 30 and ILC having worse prognosis.29, 31 Recent investigation by The Cancer Genome Atlas (TCGA) network has demonstrated distinct molecular subtypes in ILC that differ in overall and disease-free survival, which may account for a portion of the mixed results.32
A defining feature of many ILC lesions is the loss of E-cadherin staining via immunohistochemistry (IHC). This loss of E-cadherin is a result of truncating mutations in E-cadherin gene CDH1 seen in up to 50% of ILC or epigenetic silencing of CDH1 in up to 41% of ILC.33, 34, 35 Concomitant with CDH1 mutations is the loss of 16q, the location of CDH1; this is thought to be an early event in the development of ILC.32 In contrast, loss of E-cadherin in IDC has been considered a precursor step to invasion and metastasis, with controversial associations with both higher grade and pathologic stage.7, 24, 36, 37 Interestingly, IDC lymph node and distant metastases can be positive for E-cadherin staining, suggesting a reexpression of E-cadherin.7, 38 Although the literature has indicated a poor prognostic association of reduced or loss of E-cadherin in IDC, several reports have found no association between E-cadherin status and tumor stage, lymph node status, presence of metastatic lesion, or recurrence-free survival.22, 23, 24 In a nuclear-grade–controlled breast cancer cohort of 470 specimens, the expression levels of E-cadherin and catenins were directly associated with shorter patient survival,25 casting some doubt on the commonly believed good prognostic indication of E-cadherin and catenins in breast cancer.
As a central node between cell adhesion and stem-like/mesenchymal signaling, the adherens junction has been investigated for both prognosis and mechanistic underpinnings in carcinomas. Herein, we reexamined the relationship between E-cadherin and β-catenin in 19 cancer types, with a focus on their prognostic values using computational and immunohistochemical approaches. We found a general agreement of E-cadherin and β-catenin as good prognosis markers in most cancers, with breast cancer and kidney papillary cell carcinoma as outliers.
Materials and Methods
TCGA Data Analysis
Publically available replicate-based normalized reverse-phase protein array (RPPA) data from the Cancer TCGA Pan-Cancer Analysis data sets were downloaded from the MD Anderson The Cancer Protein Atlas.39 Clinical data matrices were downloaded from the University of California, Santa Cruz, Cancer Browser.40 A total of 5144 samples contained both RPPA and clinical information and were subdivided by cancer type. Replicate-based normalized protein levels of E-cadherin and β-catenin were compared across the Pan-Cancer data sets using Z-score scaled protein level. Survival analysis was performed using R packages: survival (version 2.41-3), survMisc (version 0.5.4), and survminer (version 0.4.0) (The R Project, https://cran.r-project.org). Graphical display of hazard ratio summary across the Pan-Cancer cohorts used –log10(P value) and Cox proportional hazard ratio, with size of points based on the –log10(P value). Survival analysis for CDH1 used the optimal cutoff function available through survMisc for TCGA data, Kaplan-Meier (KM) Plotter cohort,41 and the The Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) patient cohort.42 The heat map for the invasive breast carcinoma (BRCA) cohort of TCGA data set was made using the heatmap.3 function in R. β-Catenin/E-cadherin ratio was calculated as a quotient of the two factors with mean centralization of natural log transformation.
Tumor Samples
Formalin-fixed, paraffin-embedded cancer samples from breast cancer excisions were obtained from the surgical pathology archives of the University of Iowa Hospitals and Clinics. Samples were derived from two cohorts: consecutive cases of excised estrogen receptor (ER)− breast cancer with sufficient tissue to put in tissue microarrays and a smaller group of consecutive excised ER+ breast cancer. Pathologic data recorded from patient pathology reports included the following: tumor size, T stage, lymph node status, and ER/progesterone receptor/human epidermal growth factor receptor 2 (HER2) biomarker status. Survival data were available for 30 of the 163 patients. ER and progesterone receptor (IHC) and HER2 (IHC and/or fluorescence in situ hybridization) status had been assessed under the context of patient care.
Tissue Microarray Construction and IHC
Tissue microarrays were constructed using the Manual Tissue Arrayer MTA-1 (Beecher Instruments, Sun Prairie, WI), with tumors arrayed in triplicate 1-mm cores. IHC was performed on 4-μm–thick tissue sections on a Dako Autostainer Link 48 (Dako, Carpinteria, CA) after deparaffinization, rehydration, and heat-induced epitope retrieval with Tris/EDTA Target Retrieval Solution (pH 9) on the Dako PT Link. Mouse monoclonal antibodies to β-catenin (clone β-catenin-1; 1:500; 15-minute primary and secondary antibody incubations; Dako) and E-cadherin (clone NCH-38; 1:50; 15-minute primary and secondary antibody incubations; Dako) were used. Specific signal was visualized with the polymer-based Dako EnVision Flex System, with 3,3′-diaminobenzidine as the chromogen. Immunostained slides were counterstained with Harris hematoxylin (Leica Biosystems, Buffalo Grove, IL) and coverslipped. The positive control tissues for β-catenin and E-cadherin were solid pseudopapillary neoplasm and breast cancer, respectively.
Immunohistochemical Scoring
IHC was scored by two pathologists (K.C. and A.B.) at a double-headed light microscope. For β-catenin and E-cadherin, one of the following three patterns was assigned: intact membranous (crisp complete staining readily observable at ×20 to ×40 magnification), reduced membranous (weaker incomplete staining), and absent (complete absence of cell membrane staining). β-Catenin was additionally assessed for nuclear staining.
Statistical Analysis and Visualization
Hazard ratios and P values were calculated using the Cox proportional hazard function in R, comparing the upper with the lower quartile of the indicated proteins. Significance testing for sampling distribution was conducted using the χ2 test. Correlations were calculated with the psych R package (version 1.7.8) with the Spearman rank-based approach, which is more robust for outliers. Analyses and graphical plotting were performed using RStudio and the ggplot2 R package (version 2.2.1) and the ggridges (version 0.4.1) for the density-based histogram plots or ridge plots.
Graphical summary of the data sources, data analysis, and results is provided in Supplemental Figure S1.
Results
β-Catenin and E-Cadherin Protein Correlation and Predictive Ability across Cancers
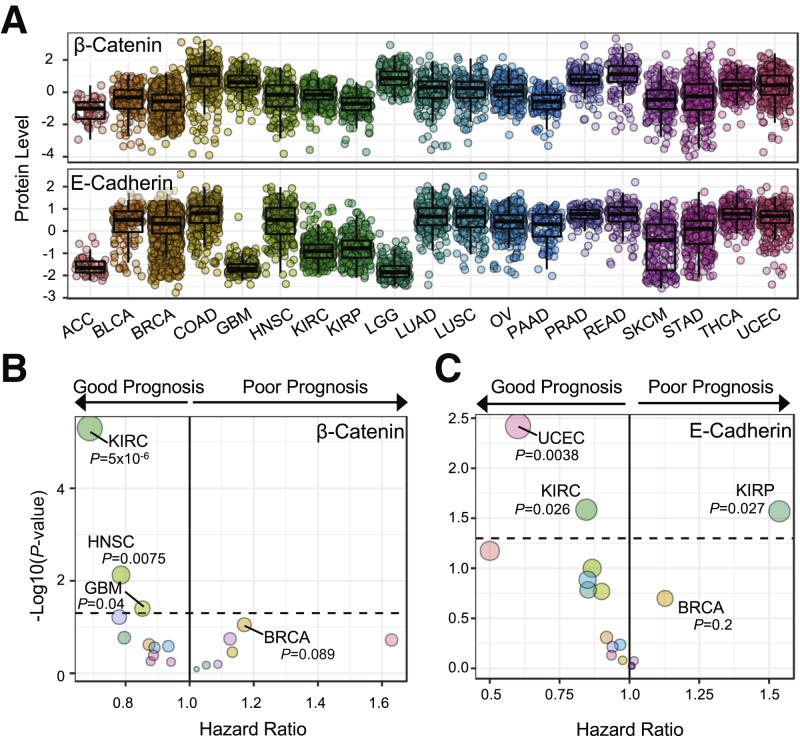
With the recent availability of the RPPA data for TCGA samples developed by MD Anderson, the protein level of β-catenin and E-cadherin was examined in 19 cancer types (Figure 1A). Across these TCGA cancer cohorts, a consistent positive correlation was found between β-catenin and E-cadherin protein level, with Spearman ρ >0.5 in 15 of 19 cancer types. Low correlations between β-catenin and E-cadherin were observed in adrenocortical carcinomas (ρ = −0.321, P = 0.0297), glioblastoma multiforme (ρ = 0.199, P = 0.00470), kidney clear cell carcinoma (KIRC; ρ = 0.275, P = 3.97e-09), and low-grade glioma (ρ = −0.144, P = 0.0213). The highest versus lowest quartile of samples in each cancer type for β-catenin and E-cadherin was compared using the Cox regression analysis. β-Catenin exhibited a range of hazard ratios, as either a good or a poor prognostic indicator, dependent on the cancer type (Figure 1B). However, with a significance threshold of P ≤ 0.05, only three cancer types with β-catenin as a good prognostic indicator met the criteria: KIRC [hazard ratio (HR), 0.688], head and neck squamous cell carcinoma (HR, 0.786), and glioblastoma multiforme (HR, 0.853). In contrast, high E-cadherin correlated with good prognosis in 12 of the 19 cancer types, with two cancer cohorts reaching significance threshold of P ≤ 0.05 (Figure 1C). Notably, E-cadherin was a poor prognosis marker in kidney papillary cell carcinoma (HR, 1.537; P = 0.027) and in BRCA (HR, 1.127; P = 0.2) cohorts of TCGA data sets (Figure 1C). Despite being below the significance threshold of P ≤ 0.05, breast cancer was studied because it is a large cohort of patient specimens with a variety of histologic and subtypic designations that may be obscuring the prognostic prediction of E-cadherin or β-catenin.
Figure 1.
Protein level and prognostic value of β-catenin and E-cadherin across 19 cancer types. A: Z-score protein level of β-catenin and E-cadherin in The Cancer Genome Atlas Pan-Cancer cohort (P < 0.001 for β-catenin and E-cadherin). B: Summary of Cox proportional hazard regression comparing the upper quartile with the lowest quartile of β-catenin protein level. Dotted line indicates P = 0.05. C: Summary of Cox proportional hazard regression comparing the upper quartile with the lowest quartile of E-cadherin protein level. Dotted line indicates P = 0.05. Size of points is on a relative scale on the basis of –log10(P value). n = 46 [A, adrenocortical carcinoma (ACC)]; n = 127 [A, bladder urothelial carcinoma (BLCA)]; n = 820 [A–C, breast invasive carcinoma (BRCA)]; n = 326 [A, colon adenocarcinoma (COAD)]; n = 201 [A and B, glioblastoma multiforme (GBM)]; n = 203 (A and B, head and neck squamous cell carcinoma (HNSC)]; n = 444 [A–C, kidney clear cell carcinoma (KIRC)]; n = 207 [A, kidney papillary cell carcinoma (KIRP) and skin cutaneous melanoma (SKCM), and C, KIRP]; n = 257 [A, lower-grade glioma (LGG)]; n = 233 [A, lung adenocarcinoma (LUAD)]; n = 192 [A, lung squamous cell carcinoma (LUSC)]; n = 408 [A, ovarian serous cystadenocarcinoma (OV)]; n = 105 [A, pancreatic adenocarcinoma (PAAD)]; n = 164 [A, prostate adenocarcinoma (PRAD)]; n = 127 [A, rectum adenocarcinoma (READ)]; n = 299 [A, stomach adenocarcinoma (STAD)]; n = 374 [A, thyroid carcinoma (THCA)]; n = 404 [A and C, uterine corpus endometrial carcinoma (UCEC)].
β-Catenin and E-Cadherin Protein in Histologic Subtypes of Breast Cancer
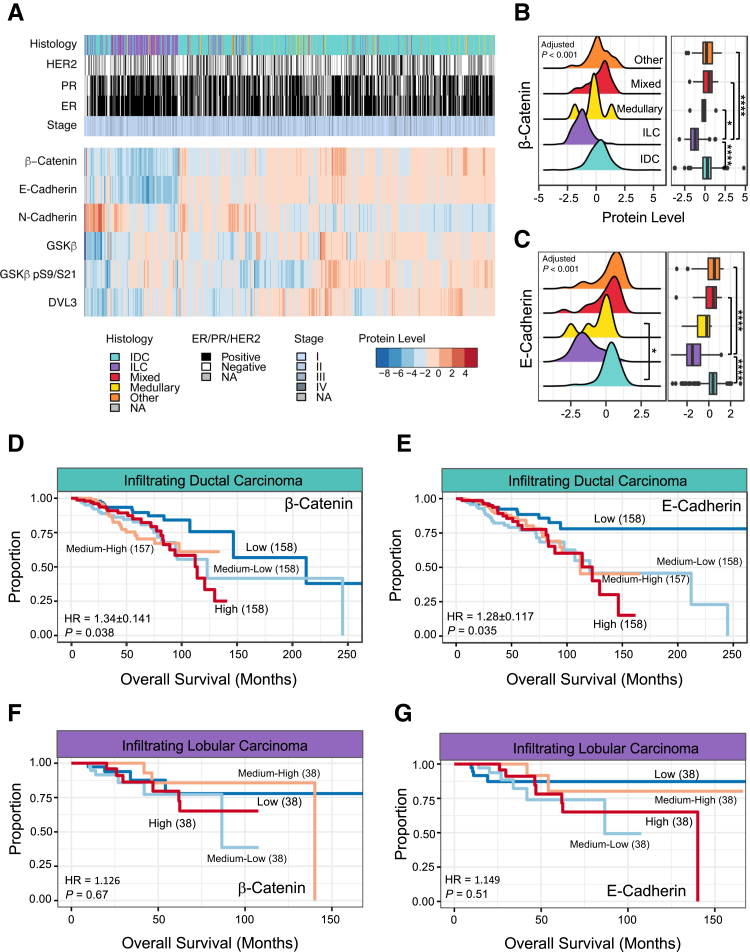
Previous literature has reported the loss of E-cadherin as a diagnostic marker of ILC, together with loss of catenins.33, 34, 35, 43 Using unsupervised clustering of β-catenin, E-cadherin, and other components involved in the maintenance or signaling of the adherens junction, it was found that the ILC clustered distinctly compared with other histologic diagnoses (Figure 2A). The ILC cluster was defined by significant decreases in β-catenin (Figure 2, A and B) and E-cadherin (Figure 2, A and C). Conversely, IDC varied in β-catenin and E-cadherin protein levels and significantly correlated with each other (Spearman ρ = 0.64, P < 1e-15). In IDC, E-cadherin inversely correlated with N-cadherin, a marker of EMT (ρ = −0.340, P = 1.33e-15) compared with ILC (ρ = 0.301, P = 0.0108). E-cadherin–to–N-cadherin switching has been previously seen as a stage- and grade-dependent phenomenon in IDC.44 After subsetting the IDC patient samples, Cox regression analysis demonstrated β-catenin (Figure 2D) and E-cadherin (Figure 2E) proteins as poor prognostic indicators. In contrast, neither β-catenin (HR, 1.126; P = 0.67) (Figure 2F) nor E-cadherin (HR, 1.149; P = 0.51) (Figure 2G) was predictive for prognosis in ILC patients.
Figure 2.
The dynamics of β-catenin and E-cadherin in breast invasive carcinoma. A: Heat map of the clinical and pathologic features of The Cancer Genome Atlas invasive breast carcinoma (BRCA) cohort. Samples across the BRCA cohort underwent unsupervised clustering on the basis of the indicated protein levels. B: Density-based histogram and corresponding boxplot of β-catenin protein level by histologic diagnosis. Adjusted P < 0.001. C: Density-based histogram and corresponding boxplot of E-cadherin protein level by histologic diagnosis. Adjusted P < 0.001. D: Kaplan-Meier curve using β-catenin protein level to split infiltrating ductal carcinoma (IDC) samples into quartiles, using Cox proportional hazard regression comparing the upper quartile with the lowest quartile. E: Kaplan-Meier curve using E-cadherin protein level to split IDC samples into quartiles, using Cox proportional hazard regression comparing the upper quartile with the lowest quartile. F: Kaplan-Meier curve using β-catenin protein level to split infiltrating lobular carcinoma (ILC) samples into quartiles, using Cox proportional hazard regression comparing the upper quartile with the lowest quartile. G: Kaplan-Meier curve using E-cadherin protein level to split ILC samples into quartiles, using Cox proportional hazard regression comparing the upper quartile with the lowest quartile. n = 873 (A); n = 631 (B, IDC); n = 152 (B, ILC); n = 6 (B, medullary carcinoma); n = 23 (B, mixed histology); n = 60 (B, other). ∗P < 0.05, ∗∗∗∗P < 0.0001. DVL, segment polarity protein dishevelled homolog 3; ER, estrogen receptor; GSK, glycogen synthase kinase; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; NA, not applicable; PR, progesterone receptor.
To further corroborate the finding of E-cadherin as a poor prognostic indicator in IDC, mRNA-based data sets were queried. In TCGA BRCA cohort, the correlation between CDH1 mRNA and E-cadherin was first examined, and Spearman was found to be ρ = 0.53 (Supplemental Figure S2A), indicating a significant positive correlation between RNA and protein levels. Similar to the protein survival analysis, CDH1 mRNA expression was a poor prognosis indicator across all IDC in TCGA BRCA cohort (N = 772; HR, 1.94) (Supplemental Figure S2B). We observed the same trend in the second (N = 3951; HR, 1.37; KM Plotter) (Supplemental Figure S2C) and third (N = 1500; HR, 1.32; METABRIC) (Supplemental Figure S2D) breast cancer cohorts, consisting of normalized microarray data.41, 42 Similar to the protein data, CDH1 mRNA was not predictive of prognosis in ILC patients (Supplemental Figure S2D), which may be complicated by low RNA and protein expression in ILC. There was no significant correlation between CTNNB1 mRNA and β-catenin protein (ρ = 0.092, data not shown), discounting the validity of using mRNA as a predicative marker for breast cancer.
Immunohistological Evaluation of β-Catenin and E-Cadherin in IDC
The poor prognostic indication of E-cadherin in the IDC samples led us to examine β-catenin and E-cadherin status using IDC patient specimens from the University of Iowa Hospital and Clinics. Tissue arrays were constructed, including 220 specimens with known clinical information. Of the initial cohort, 163 of 220 cases were further analyzed for IHC staining. This reduction from 220 to 163 was because of incomplete clinical information or fragment and absent cores in the tissue microarray (Table 1). Samples were scored on the basis of the localization of β-catenin or E-cadherin and the strength of the IHC staining (Figure 3). Of the 163 cases, there were four staining patterns for β-catenin: 47 samples showed membranous β-catenin (Figure 3A), 94 samples had a reduced pattern (Figure 3C), 20 samples were absent (Figure 3E), and 2 samples displayed a component of nuclear β-catenin staining (Figure 3G). Both samples with nuclear staining for β-catenin had corresponding strong membranous E-cadherin staining (Figure 3H). In contrast, there were three patterns of E-cadherin staining: 81 cases had an intact membranous E-cadherin (Figure 3B), 71 cases had reduced E-cadherin (Figure 3D), and 11 cases lacked staining (Figure 3F).
Table 1.
Summary of IHC TMA Patient Cohort
| Parameter | Cohort | Total |
|---|---|---|
| Tumor stage | ||
| Tis | 4 | 163 |
| T1 | 73 | |
| T2 | 61 | |
| T3 | 9 | |
| T4 | 9 | |
| NA | 7 | |
| Node stage | ||
| N0 | 68 | 163 |
| N1 | 37 | |
| N2 | 15 | |
| N3 | 17 | |
| NX | 11 | |
| NA | 15 | |
| Biomarker | ||
| ER | 16 | 108 |
| HER2 | 28 | |
| TNBC | 64 | |
| Histologic diagnosis | ||
| DCIS | 9 | 163 |
| IDC | 137 | |
| ILC | 7 | |
| Medullary | 2 | |
| Metaplastic | 1 | |
| Mucinous | 3 | |
| Papillary | 1 | |
| NA | 3 | |
DCIS, ductal carcinoma in situ; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IDC, infiltrating ductal carcinoma; IHC, immunohistochemistry; ILC, infiltrating lobular carcinoma; NA, not available; Tis, in situ; TMA, tissue microarray; TNBC, triple-negative breast cancer.
Figure 3.
Immunohistochemical (IHC) staining results for β-catenin and E-cadherin in breast cancer samples. Representative IHC staining patterns that are observed: membranous staining for both β-catenin (A) and E-cadherin (B); reduced membranous expression for both β-catenin (C) and E-cadherin (D); absence of staining for both β-catenin (E) and E-cadherin (F); and membranous and nuclear staining of β-catenin (G) with strong E-cadherin membranous staining (H). Arrows indicate cells with nuclear staining of β-catenin. Original magnification, ×400 (A–H).
β-Catenin and E-Cadherin Status in IDC by Clinical Subtype
β-Catenin and E-cadherin staining patterns were assessed, according to breast cancer biomarker status or clinical subtype [ie, ER+, ER−/HER2+ (referred to herein as HER2+), or triple-negative breast cancer (TNBC)]. Of the 163 samples analyzed, 108 could be assigned as ER, HER2+, or TNBC (Table 2). The E-cadherin IHC expression patterns showed higher proportions of intact membranous E-cadherin staining in ER+ tumors and a high percentage of reduced IHC expression in TNBC (P = 0.0011). In addition, HER2+ tumors had intact staining pattern of E-cadherin. The same significant association was not seen with β-catenin and biomarker status (P = 0.439). Notably, HER2+ samples had the least pronounced correlation between β-catenin and E-cadherin, with 78.5% of samples with intact membranous E-cadherin and only 25% of samples with membranous β-catenin staining (Table 2).
Table 2.
Summary of IHC Staining Observed for E-Cadherin and β-Catenin on the Basis of 108 of the 163 Samples Designated as TNBC, Triple Negative, ER, or HER2+
| Diagnosis | Samples | E-cadherin |
β-Catenin |
||||
|---|---|---|---|---|---|---|---|
| Membranous | Reduced | Absent | Membranous | Reduced | Absent | ||
| TNBC | 64 | 24 | 33 | 5 | 18 | 42 | 5 |
| ER+ | 16 | 10 | 5 | 1 | 6 | 7 | 3 |
| HER2+ | 28 | 22 | 5 | 1 | 7 | 18 | 3 |
| P = 0.011 | P = 0.439 | ||||||
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; TNBC, triple-negative breast cancer.
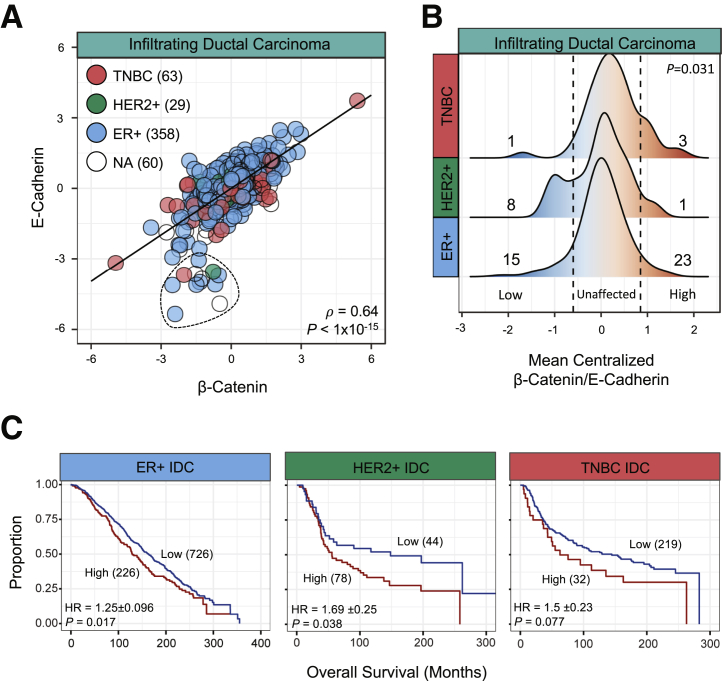
Comparing the IHC results, the RPPA data were used for IDC samples, and they were classified into clinical subtypes on the basis of IHC confirmation of ER+ (n = 358), HER+ (n = 29), or TNBC (n = 63). To examine the relationship of β-catenin and E-cadherin in the context of the adherens junction, the correlation between the two proteins across IDC samples was first examined (ρ = 0.64) (Figure 4A). Across IDC, 22 samples were designated as outliers (Figure 4A), with E-cadherin protein level <−3 and a β-catenin level >−3. There was no significant difference in these samples by biomarker, tumor stage, nodal stage, or survival compared with the rest of the IDC cohort. Using the mean-centralized ratio of β-catenin/E-cadherin, a method of identifying outliers in either β-catenin or E-cadherin protein level, density-based histograms were produced to examine subgroup-specific differences in the relationship (Figure 4B). Similar to the IHC results, HER2+ samples had 8 of 29 samples with β-catenin/E-cadherin ratio below the identified cutoff, indicating a relative decrease in β-catenin in HER2+ samples. In contrast, ER+ patient samples had a normal distribution of β-catenin/E-cadherin ratio. TNBC samples had a relative increase in β-catenin/E-cadherin ratio that is inconsistent with the IHC results that found a greater reduction in β-catenin compared with E-cadherin in TNBC (Table 2). However, this higher level of β-catenin is consistent with literature that suggests β-catenin can drive TNBC and is a poor prognostic indicator for TNBC patients.45 Survival analysis could not be performed using TCGA BRCA cohort because of the limited size and high degree of censored samples in the HER2/TNBC subgroups. Alternatively, the METABRIC IDC samples were used, separating the samples by IHC-confirmed clinical subtype. Despite the difference in β-catenin–to–E-cadherin dynamics, CDH1 was a poor prognostic indicator in ER+ and HER2+ IDC (Figure 4C). The highest CDH1 levels in TNBC were associated with poor prognosis, with a P value slightly higher than the P < 0.05 cutoff (Figure 4C). Similarly, in the KM Plotter data set, CDH1 was a poor prognostic indicator for recurrence-free survival in ER+ (HR, 1.46; P = 0.044), HER2+ (HR, 3.48; P = 7.6e-6), and TNBC (HR, 1.62; P = 0.033) breast cancers in the KM Plotter data set (Supplemental Figure S3).
Figure 4.
β-Catenin and E-cadherin protein in infiltrating ductal carcinoma (IDC) samples on the basis of immunohistochemical designation. Outliers, defined as E-cadherin <−3 and β-catenin >−3, circled withdotted line. A: Scatterplot of β-catenin and E-cadherin protein across IDC samples. B: Density histogram of mean-centralized quotient of β-catenin and E-cadherin. Cutoff values of −0.65 (low, left-bound dotted line) and 0.8 (high, right-bound dotted line) were used on the basis of distinct subpopulations. χ2 Tests were performed comparing low, high, and unaffected population distributions. C: Kaplan-Meier curve from the estrogen receptor (ER)+ (blue), human epidermal growth factor receptor 2 (HER2)+ (green), and triple-negative breast cancer (TNBC; red) samples in the METABRIC cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression. NA, not applicable.
β-Catenin and E-Cadherin Status in IDC by Stage
Using IHC staining, the trend in β-catenin and E-cadherin staining patterns was next investigated by the American Joint Committee on Cancer tumor and node stage (Table 3).46 Both β-catenin and E-cadherin demonstrated statistically significant negative correlation with tumor stage. In late-stage tumors (T2 through T4), β-catenin and E-cadherin were significantly reduced or absent compared with the T0 and T1 stages. β-Catenin and E-cadherin status was less predictive of lymph node involvement (Table 3). However, the characteristics between the sample distribution of β-catenin and E-cadherin by lymph node status varied. E-cadherin had relative equal sample distribution between each node status, with reduced E-cadherin seen in 40% to 45.5% of samples in each node stage. Conversely, β-catenin had bimodal distribution of reduced staining, with peaks at N0 (62.3%) and N2-3 (80%) stages.
Table 3.
Summary of IHC Staining Observed for β-Catenin and E-Cadherin Staining on the Basis of Available Tumor Stage and Nodal Status Information
| Target | Parameter | n | Membranous | Reduced | Absent | P value |
|---|---|---|---|---|---|---|
| β-Catenin | Tumor stage | 144 | 0.0258 | |||
| Tis | 2 | 1 | 1 | |||
| T1 | 26 | 36 | 5 | |||
| T2 | 11 | 37 | 7 | |||
| T3 + T4 | 3 | 12 | 3 | |||
| Nodal status | 133 | 0.0834 | ||||
| N0 | 21 | 43 | 5 | |||
| N1 | 11 | 17 | 5 | |||
| N2 | 3 | 12 | 0 | |||
| N3 | 4 | 12 | 0 | |||
| E-cadherin | Tumor stage | 153 | 0.00541 | |||
| Tis | 3 | 0 | 0 | |||
| T1 | 44 | 26 | 3 | |||
| T2 | 22 | 33 | 3 | |||
| T3 + T4 | 6 | 8 | 5 | |||
| Nodal status | 135 | 0.955 | ||||
| N0 | 33 | 31 | 4 | |||
| N1 | 17 | 16 | 2 | |||
| N2 | 8 | 6 | 1 | |||
| N3 | 8 | 8 | 1 |
IHC, immunohistochemistry; Tis, in situ.
Discussion
Using protein-level data, a large-scale proteomic analysis of 5144 patient samples was performed across 19 cancer types for β-catenin and E-cadherin. A high correlation was found between β-catenin and E-cadherin across most cancers (Figure 1A), suggesting the two proteins likely function at the level of the adherens junction. β-Catenin and E-cadherin did not highly correlate in neural tumors, which do not express E-cadherin in normal tissues, adrenocortical tumors, and renal papillary cell carcinoma. Using this large data set, the prognostic value of β-catenin and E-cadherin was determined (Figure 1, B and C). Several observations countervailed previous IHC reports. For example, despite having a modest correlation between β-catenin and E-cadherin, KIRC tumors demonstrated good prognostic value for both factors (Figure 1, B and C). This finding is refuted in the literature, with the absence of E-cadherin in KIRC being reported as a poor prognostic indicator, and the reduced-to-negative IHC staining of E-cadherin can be used to differentiate KIRC from renal chromophobe tumors.47, 48
Similar to KIRC, E-cadherin in breast carcinoma has been used as both a differentiating marker and an indicator of poor prognosis.24, 33, 34, 36, 37 Stratifying TCGA BRCA cohort by histologic diagnosis, E-cadherin protein level was found to predict worse overall survival in IDC, but not in ILC. Likewise, in TCGA BRCA cohort, an amalgamated patient cohort from KM Plotter,41 and the METABRIC breast cancer patient cohort,42 the highest levels of RNA for CDH1 were found to be predictive of poor overall or recurrence-free survival (Supplemental Figure S2). The poor prognostic indication of CDH1 was also seen after stratifying IDC samples into ER+ and HER+ subgroups (Figure 4C and Supplemental Figure S3). With only survival information available for 30 of the 163 patients from the IHC cohort, the prognostic implications could not be addressed. However, the IHC findings are similar to several studies finding a decrease in E-cadherin predictive of tumor stage in IDC.22, 23
This discordance between immunohistochemical and multiomic analyses is not uncommon and has been the center of several concerted efforts to find greater agreement between the modalities to improve patient diagnostics and treatment.49, 50 Analogously, large-scale meta-analyses of the predictive value of E-cadherin immunohistochemistry have been conducted in several cancers.51, 52, 53, 54, 55, 56 In general, these meta-analyses found loss of E-cadherin as a poor prognostic indicator for overall and recurrence/progression-free survival, with the exception of colorectal cancer.55 The coalescing of IHC-based studies into meta-analysis relies on correcting for differing methods of the immunohistology and is semiquantitative. Conversely, RPPA quantifies >200 proteins using validated antibodies via a micro-to-nano scale dot blot system.57 Several limitations exist for RPPA in comparison to IHC in tumor pathology, centering on the loss of spatial distribution of the epitope and the sampling position effects, similar to RNA quantification.58 In a similar avenue to sample positioning, impurity of the sample for tumor cells can have biased effects. Within TCGA cohort, tumor cellular purity for most cohorts ranges from 75% to 90%, with notable lower levels (>50%) in purity for lung adenocarcinoma, stomach adenocarcinoma, and pancreatic adenocarcinoma cohorts.59, 60
Immunohistochemical- and RNA-based subtyping of IDC is an established and growing component in patient management. Subtype-specific patterns in β-catenin and E-cadherin have previously been found for E-cadherin staining, with maintained E-cadherin in luminal/ER+ tumors24 and decreased E-cadherin in TNBC.61 This appears to be consistent with the normal mammary duct, with the luminal compartment staining positive for E-cadherin, whereas the basal compartment has a variable staining pattern for E-cadherin.44 Beyond subtypic trends in E-cadherin, a negative correlation was found between E-cadherin and N-cadherin in IDC samples (Figure 2A). Cadherin switching, from E-cadherin to N-cadherin, appears to not only mark post-EMT tumor cells, but leads to N-cadherin–mediated cellular motility.62, 63 No coherent explanation was forthcoming for the poor survival at the highest levels of E-cadherin mRNA or protein across IDC samples or across clinical subtypes.
In contrast, β-catenin signaling has been reported as a poor prognostic marker, independent of subtype, and drives TNBC tumors.45, 64, 65, 66 Despite the reports of β-catenin in promoting progression, positive nuclear localization of β-catenin in IDC and ILC via IHC has been consistently uncommon.45, 67, 68 This trend was recapitulated in the IHC cohort, with only 2 of 163 patients positive for nuclear β-catenin, 1 from the TNBC and the ER+ subtypes. The strong protein correlation between β-catenin and E-cadherin, as well as the discoordination between β-catenin protein and mRNA, suggests that structural β-catenin, rather than WNT-activated nuclear β-catenin, drives the prognostic value in IDC (Figure 2D). Subtype-specific difference of β-catenin/E-cadherin ratio was not noticed (Figure 4B), indicating a more complex interaction between the two proteins at structural or signaling levels. For example, a relative reduction was found in β-catenin compared with E-cadherin in HER2+ samples by IHC (Table 2) and in the RPPA data (Figure 4B). A recent article from Tung et al69 found that monoallelic loss of Ctnnb1, the gene that encodes β-catenin, drives an increase in HER2+ murine tumorigenic capacity. Monoallelic loss of Ctnnb1 drove multiplicity in the HER2+ mice and appeared to reduce differentiation by histologic assessment, with most tumors described as poorly differentiated acini.69
In conclusion, the proteomic analysis revealed both β-catenin and E-cadherin are poor prognostic indicators in infiltrating ductal carcinoma. Similarly, across TCGA BRCA data set and multiple patient cohorts, CDH1 gene expression was a poor prognosis indicator. This poor prognostic trend of CDH1 was maintained across ER+, HER2+, and TNBC samples. The presence of β-catenin and E-cadherin in IDC patient samples was dependent on clinical subtype and stage, which may be a partial explanation for the difference in patient outcomes. Further assessment of E-cadherin as a predictive marker in breast cancer is warranted, with pointed appraisal between emergent proteomic modalities and immunohistochemistry.
Acknowledgments
The results herein are in whole or in part based on data generated by The Cancer Genome Atlas Research Network. W.Z. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supported by NIH grants R01 CA200673 (W.Z.), F30 CA206255 (N.B.), and T32 AI007260 (R.K.). This work benefited from an Oberley Award, Cancer and Aging Award, and National Cancer Institute of the NIH award P30CA086862.
N.B. and K.C. contributed equally to this work.
Disclosures: None declared.
Current address of K.C., Henry Ford Hospital, Detroit, MI.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.05.003.
Contributor Information
Andrew Bellizzi, Email: andrew-bellizzi@uiowa.edu.
Weizhou Zhang, Email: weizhou-zhang@uiowa.edu.
Supplemental Data
Graphical summary of results and workflow of the article, starting with survival analysis of β-catenin and E-cadherin across 5441 samples in the Pan-Cancer The Cancer Genome Atlas (TCGA; 1). Further focus on breast invasive cancer (BRCA) found increased β-catenin and E-cadherin as poor prognostic indicators in infiltrating ductal carcinoma (IDC), mirroring survival analysis of CDH1 in 6465 patient samples. This led to the further investigation of β-catenin and E-cadherin staining and localization using tissue microarrays (2) and further characterization of β-catenin versus E-cadherin dynamics and survival using bioinformatics (3). ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; KM, Kaplan-Meier; TNBC, triple-negative breast cancer.
A: Scatterplot of E-cadherin and CDH1 across the invasive breast carcinoma (BRCA) The Cancer Genome Atlas cohort. B: Kaplan-Meier curve from the BRCA cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression. C: Kaplan-Meier curve from the Kaplan-Meier Plotter cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression. D: Kaplan-Meier curve from the METABRIC cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression and separated by histologic diagnosis of either infiltrating ductal carcinoma (IDC; left panel) or infiltrating lobular carcinoma (ILC; right panel). HR, hazard ratio.
Kaplan-Meier curve from the estrogen receptor (ER)+ (A), human epidermal growth factor receptor 2 (HER2)+/ER− (B), and triple-negative breast cancer (TNBC; C) samples in the Kaplan-Meier Plotter cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression. HR, hazard ratio.
References
- 1.Ozawa M., Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer B., Johnson J.P., Leitl F., Jauch K.W., Heiss M.M., Schildberg F.W., Birchmeier W., Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–1695. [PubMed] [Google Scholar]
- 3.Dorudi S., Sheffield J.P., Poulsom R., Northover J.M., Hart I.R. E-cadherin expression in colorectal cancer: an immunocytochemical and in situ hybridization study. Am J Pathol. 1993;142:981–986. [PMC free article] [PubMed] [Google Scholar]
- 4.Bremnes R.M., Veve R., Hirsch F.R., Franklin W.A. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36:115–124. doi: 10.1016/s0169-5002(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 5.Bringuier P.P., Umbas R., Schaafsma H.E., Karthaus H.F., Debruyne F.M., Schalken J.A. Decreased E-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res. 1993;53:3241–3245. [PubMed] [Google Scholar]
- 6.Umbas R., Isaacs W.B., Bringuier P.P., Schaafsma H.E., Karthaus H.F., Oosterhof G.O., Debruyne F.M., Schalken J.A. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54:3929–3933. [PubMed] [Google Scholar]
- 7.Oka H., Shiozaki H., Kobayashi K., Inoue M., Tahara H., Kobayashi T., Takatsuka Y., Matsuyoshi N., Hirano S., Takeichi M., Mori T. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53:1696–1701. [PubMed] [Google Scholar]
- 8.Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 9.Berx G., Van Roy F. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 2001;3:289–293. doi: 10.1186/bcr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Fischer K.R., Durrans A., Lee S., Sheng J., Li F., Wong S.T., Choi H., El Rayes T., Ryu S., Troeger J., Schwabe R.F., Vahdat L.T., Altorki N.K., Mittal V., Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavert N., Vivanti A., Hazin J., Brabletz T., Ben-Ze'ev A. L1-mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol Cancer Res. 2011;9:14–24. doi: 10.1158/1541-7786.MCR-10-0406. [DOI] [PubMed] [Google Scholar]
- 13.Tsai J.H., Donaher J.L., Murphy D.A., Chau S., Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aceto N., Bardia A., Miyamoto D.T., Donaldson M.C., Wittner B.S., Spencer J.A., Yu M., Pely A., Engstrom A., Zhu H., Brannigan B.W., Kapur R., Stott S.L., Shioda T., Ramaswamy S., Ting D.T., Lin C.P., Toner M., Haber D.A., Maheswaran S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 16.Aberle H., Butz S., Stappert J., Weissig H., Kemler R., Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(Pt 12):3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 17.Hinck L., Nathke I.S., Papkoff J., Nelson W.J. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson W.J., Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida R., Kimura N., Harada Y., Ohuchi N. The loss of E-cadherin, alpha- and beta-catenin expression is associated with metastasis and poor prognosis in invasive breast cancer. Int J Oncol. 2001;18:513–520. [PubMed] [Google Scholar]
- 20.Zschiesche W., Schonborn I., Behrens J., Herrenknecht K., Hartveit F., Lilleng P., Birchmeier W. Expression of E-cadherin and catenins in invasive mammary carcinomas. Anticancer Res. 1997;17:561–567. [PubMed] [Google Scholar]
- 21.Dillon D.A., D'Aquila T., Reynolds A.B., Fearon E.R., Rimm D.L. The expression of p120ctn protein in breast cancer is independent of alpha- and beta-catenin and E-cadherin. Am J Pathol. 1998;152:75–82. [PMC free article] [PubMed] [Google Scholar]
- 22.Brzozowska A., Sodolski T., Duma D., Mazurkiewicz T., Mazurkiewicz M. Evaluation of prognostic parameters of E-cadherin status in breast cancer treatment. Ann Agric Environ Med. 2012;19:541–546. [PubMed] [Google Scholar]
- 23.Nakagawa M., Bando Y., Nagao T., Morimoto M., Takai C., Ohnishi T., Honda J., Moriya T., Izumi K., Takahashi M., Sasa M., Tangoku A. Expression of p53, Ki-67, E-cadherin, N-cadherin and TOP2A in triple-negative breast cancer. Anticancer Res. 2011;31:2389–2393. [PubMed] [Google Scholar]
- 24.Siitonen S.M., Kononen J.T., Helin H.J., Rantala I.S., Holli K.A., Isola J.J. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol. 1996;105:394–402. doi: 10.1093/ajcp/105.4.394. [DOI] [PubMed] [Google Scholar]
- 25.Gillett C.E., Miles D.W., Ryder K., Skilton D., Liebman R.D., Springall R.J., Barnes D.M., Hanby A.M. Retention of the expression of E-cadherin and catenins is associated with shorter survival in grade III ductal carcinoma of the breast. J Pathol. 2001;193:433–441. doi: 10.1002/path.831. [DOI] [PubMed] [Google Scholar]
- 26.Li C.I., Anderson B.O., Daling J.R., Moe R.E. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 27.Arpino G., Bardou V.J., Clark G.M., Elledge R.M. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149–R156. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toikkanen S., Pylkkanen L., Joensuu H. Invasive lobular carcinoma of the breast has better short- and long-term survival than invasive ductal carcinoma. Br J Cancer. 1997;76:1234–1240. doi: 10.1038/bjc.1997.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi Y., Ishiguro J., Kotani H., Hisada T., Ichikawa M., Gondo N., Yoshimura A., Kondo N., Hattori M., Sawaki M., Fujita T., Kikumori T., Yatabe Y., Kodera Y., Iwata H. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer. 2016;16:248. doi: 10.1186/s12885-016-2275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C.I., Moe R.E., Daling J.R. Risk of mortality by histologic type of breast cancer among women aged 50 to 79 years. Arch Intern Med. 2003;163:2149–2153. doi: 10.1001/archinte.163.18.2149. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z., Yang J., Li S., Lv M., Shen Y., Wang B., Li P., Yi M., Zhao X., Zhang L., Wang L., Yang J. Invasive lobular carcinoma of the breast: a special histological type compared with invasive ductal carcinoma. PLoS One. 2017;12:e0182397. doi: 10.1371/journal.pone.0182397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciriello G., Gatza M.L., Beck A.H., Wilkerson M.D., Rhie S.K., Pastore A., Zhang H., McLellan M., Yau C., Kandoth C., Bowlby R., Shen H., Hayat S., Fieldhouse R., Lester S.C., Tse G.M., Factor R.E., Collins L.C., Allison K.H., Chen Y.Y., Jensen K., Johnson N.B., Oesterreich S., Mills G.B., Cherniack A.D., Robertson G., Benz C., Sander C., Laird P.W., Hoadley K.A., King T.A., Network T.R., Perou C.M. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Leeuw W.J., Berx G., Vos C.B., Peterse J.L., Van de Vijver M.J., Litvinov S., Van Roy F., Cornelisse C.J., Cleton-Jansen A.M. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997;183:404–411. doi: 10.1002/(SICI)1096-9896(199712)183:4<404::AID-PATH1148>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Berx G., Cleton-Jansen A.M., Strumane K., de Leeuw W.J., Nollet F., van Roy F., Cornelisse C. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13:1919–1925. [PubMed] [Google Scholar]
- 35.Sarrio D., Moreno-Bueno G., Hardisson D., Sanchez-Estevez C., Guo M., Herman J.G., Gamallo C., Esteller M., Palacios J. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer. 2003;106:208–215. doi: 10.1002/ijc.11197. [DOI] [PubMed] [Google Scholar]
- 36.Kowalski P.J., Rubin M.A., Kleer C.G. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217–R222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez M.A., Pinder S.E., Wencyk P.M., Bell J.A., Elston C.W., Nicholson R.I., Robertson J.F., Blamey R.W., Ellis I.O. An immunohistochemical examination of the expression of E-cadherin, alpha- and beta/gamma-catenins, and alpha2- and beta1-integrins in invasive breast cancer. J Pathol. 1999;187:523–529. doi: 10.1002/(SICI)1096-9896(199904)187:5<523::AID-PATH296>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Bukholm I.K., Nesland J.M., Karesen R., Jacobsen U., Borresen-Dale A.L. E-cadherin and alpha-, beta-, and gamma-catenin protein expression in relation to metastasis in human breast carcinoma. J Pathol. 1998;185:262–266. doi: 10.1002/(SICI)1096-9896(199807)185:3<262::AID-PATH97>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 39.Li J., Lu Y., Akbani R., Ju Z., Roebuck P.L., Liu W., Yang J.Y., Broom B.M., Verhaak R.G., Kane D.W., Wakefield C., Weinstein J.N., Mills G.B., Liang H. TCPA: a resource for cancer functional proteomics data. Nat Methods. 2013;10:1046–1047. doi: 10.1038/nmeth.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cline M.S., Craft B., Swatloski T., Goldman M., Ma S., Haussler D., Zhu J. Exploring TCGA Pan-Cancer data at the UCSC Cancer Genomics Browser. Sci Rep. 2013;3:2652. doi: 10.1038/srep02652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyorffy B., Lanczky A., Eklund A.C., Denkert C., Budczies J., Li Q., Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 42.Curtis C., Shah S.P., Chin S.F., Turashvili G., Rueda O.M., Dunning M.J., Speed D., Lynch A.G., Samarajiwa S., Yuan Y., Graf S., Ha G., Haffari G., Bashashati A., Russell R., McKinney S., Group M., Langerod A., Green A., Provenzano E., Wishart G., Pinder S., Watson P., Markowetz F., Murphy L., Ellis I., Purushotham A., Borresen-Dale A.L., Brenton J.D., Tavare S., Caldas C., Aparicio S. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dabbs D.J., Bhargava R., Chivukula M. Lobular versus ductal breast neoplasms: the diagnostic utility of p120 catenin. Am J Surg Pathol. 2007;31:427–437. doi: 10.1097/01.pas.0000213386.63160.3f. [DOI] [PubMed] [Google Scholar]
- 44.ElMoneim H.M., Zaghloul N.M. Expression of E-cadherin, N-cadherin and snail and their correlation with clinicopathological variants: an immunohistochemical study of 132 invasive ductal breast carcinomas in Egypt. Clinics (Sao Paulo) 2011;66:1765–1771. doi: 10.1590/S1807-59322011001000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geyer F.C., Lacroix-Triki M., Savage K., Arnedos M., Lambros M.B., MacKay A., Natrajan R., Reis-Filho J.S. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 46.Giuliano A.E., Connolly J.L., Edge S.B., Mittendorf E.A., Rugo H.S., Solin L.J., Weaver D.L., Winchester D.J., Hortobagyi G.N. Breast cancer: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 47.Tan P.H., Cheng L., Rioux-Leclercq N., Merino M.J., Netto G., Reuter V.E., Shen S.S., Grignon D.J., Montironi R., Egevad L., Srigley J.R., Delahunt B., Moch H., Panel I.R.T. Renal tumors: diagnostic and prognostic biomarkers. Am J Surg Pathol. 2013;37:1518–1531. doi: 10.1097/PAS.0b013e318299f12e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katagiri A., Watanabe R., Tomita Y. E-cadherin expression in renal cell cancer and its significance in metastasis and survival. Br J Cancer. 1995;71:376–379. doi: 10.1038/bjc.1995.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uhlen M., Zhang C., Lee S., Sjostedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z., Edfors F., Sanli K., von Feilitzen K., Oksvold P., Lundberg E., Hober S., Nilsson P., Mattsson J., Schwenk J.M., Brunnstrom H., Glimelius B., Sjoblom T., Edqvist P.H., Djureinovic D., Micke P., Lindskog C., Mardinoglu A., Ponten F. A pathology atlas of the human cancer transcriptome. Science. 2017;357 doi: 10.1126/science.aan2507. pii: eaan2507. [DOI] [PubMed] [Google Scholar]
- 50.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A. Proteomics: tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 51.Xing X., Tang Y.B., Yuan G., Wang Y., Wang J., Yang Y., Chen M. The prognostic value of E-cadherin in gastric cancer: a meta-analysis. Int J Cancer. 2013;132:2589–2596. doi: 10.1002/ijc.27947. [DOI] [PubMed] [Google Scholar]
- 52.Chen J., Zhao J., Ma R., Lin H., Liang X., Cai X. Prognostic significance of E-cadherin expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e103952. doi: 10.1371/journal.pone.0103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie Y., Li P., Gao Y., Gu L., Chen L., Fan Y., Zhang F., Zhang X. Reduced E-cadherin expression is correlated with poor prognosis in patients with bladder cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:62489–62499. doi: 10.18632/oncotarget.19934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z., Yin S., Zhang L., Liu W., Chen B. Prognostic value of reduced E-cadherin expression in breast cancer: a meta-analysis. Oncotarget. 2017;8:16445–16455. doi: 10.18632/oncotarget.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He X., Chen Z., Jia M., Zhao X. Downregulated E-cadherin expression indicates worse prognosis in Asian patients with colorectal cancer: evidence from meta-analysis. PLoS One. 2013;8:e70858. doi: 10.1371/journal.pone.0070858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gould Rothberg B.E., Bracken M.B. E-cadherin immunohistochemical expression as a prognostic factor in infiltrating ductal carcinoma of the breast: a systematic review and meta-analysis. Breast Cancer Res Treat. 2006;100:139–148. doi: 10.1007/s10549-006-9248-2. [DOI] [PubMed] [Google Scholar]
- 57.Tibes R., Qiu Y., Lu Y., Hennessy B., Andreeff M., Mills G.B., Kornblau S.M. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 58.Sottoriva A., Spiteri I., Piccirillo S.G., Touloumis A., Collins V.P., Marioni J.C., Curtis C., Watts C., Tavare S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aran D., Sirota M., Butte A.J. Systematic pan-cancer analysis of tumour purity. Nat Commun. 2015;6:8971. doi: 10.1038/ncomms9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng X., Zhang N., Wu H.J., Wu H. Estimating and accounting for tumor purity in the analysis of DNA methylation data from cancer studies. Genome Biol. 2017;18:17. doi: 10.1186/s13059-016-1143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rakha E.A., El-Sayed M.E., Green A.R., Lee A.H., Robertson J.F., Ellis I.O. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 62.Shamir E.R., Ewald A.J. Adhesion in mammary development: novel roles for E-cadherin in individual and collective cell migration. Curr Top Dev Biol. 2015;112:353–382. doi: 10.1016/bs.ctdb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nieman M.T., Prudoff R.S., Johnson K.R., Wheelock M.J. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez-Knowles E., Zardawi S.J., McNeil C.M., Millar E.K., Crea P., Musgrove E.A., Sutherland R.L., O'Toole S.A. Cytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2010;19:301–309. doi: 10.1158/1055-9965.EPI-09-0741. [DOI] [PubMed] [Google Scholar]
- 65.Dolled-Filhart M., McCabe A., Giltnane J., Cregger M., Camp R.L., Rimm D.L. Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res. 2006;66:5487–5494. doi: 10.1158/0008-5472.CAN-06-0100. [DOI] [PubMed] [Google Scholar]
- 66.Lin S.Y., Xia W., Wang J.C., Kwong K.Y., Spohn B., Wen Y., Pestell R.G., Hung M.C. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klemm F., Bleckmann A., Siam L., Chuang H.N., Rietkotter E., Behme D., Schulz M., Schaffrinski M., Schindler S., Trumper L., Kramer F., Beissbarth T., Stadelmann C., Binder C., Pukrop T. beta-Catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis. 2011;32:434–442. doi: 10.1093/carcin/bgq269. [DOI] [PubMed] [Google Scholar]
- 68.Khramtsov A.I., Khramtsova G.F., Tretiakova M., Huo D., Olopade O.I., Goss K.H. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tung B., Schade B., Cardiff R.D., Aina O.H., Sanguin-Gendreau V., Muller W.J. beta-Catenin haploinsufficiency promotes mammary tumorigenesis in an ErbB2-positive basal breast cancer model. Proc Natl Acad Sci U S A. 2017;114:E707–E716. doi: 10.1073/pnas.1610383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphical summary of results and workflow of the article, starting with survival analysis of β-catenin and E-cadherin across 5441 samples in the Pan-Cancer The Cancer Genome Atlas (TCGA; 1). Further focus on breast invasive cancer (BRCA) found increased β-catenin and E-cadherin as poor prognostic indicators in infiltrating ductal carcinoma (IDC), mirroring survival analysis of CDH1 in 6465 patient samples. This led to the further investigation of β-catenin and E-cadherin staining and localization using tissue microarrays (2) and further characterization of β-catenin versus E-cadherin dynamics and survival using bioinformatics (3). ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; KM, Kaplan-Meier; TNBC, triple-negative breast cancer.
A: Scatterplot of E-cadherin and CDH1 across the invasive breast carcinoma (BRCA) The Cancer Genome Atlas cohort. B: Kaplan-Meier curve from the BRCA cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression. C: Kaplan-Meier curve from the Kaplan-Meier Plotter cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression. D: Kaplan-Meier curve from the METABRIC cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression and separated by histologic diagnosis of either infiltrating ductal carcinoma (IDC; left panel) or infiltrating lobular carcinoma (ILC; right panel). HR, hazard ratio.
Kaplan-Meier curve from the estrogen receptor (ER)+ (A), human epidermal growth factor receptor 2 (HER2)+/ER− (B), and triple-negative breast cancer (TNBC; C) samples in the Kaplan-Meier Plotter cohort using CDH1 levels split by auto-cutoff for high versus low samples, using Cox proportional hazard regression. HR, hazard ratio.