Abstract
BACKGROUND
After the introduction of the Rome IV criteria for the diagnosis of irritable bowel syndrome (IBS), studies on the clinical significance of the new criteria in the settings of a large study has been scarce.
Objective: Herein we used the infrastructures provided by one the largest cohort studies in Iran to evaluate the epidemiological features related to IBS.
METHODS
A total of 9264 participants, were enrolled in the initial registry. Diagnosis of IBS was done using the Rome IV criteria. Individuals with IBS were compared with a control group. Since the study included a large sample size of patients, we used the penalized smoothly clipped absolute deviation (SCAD) regression analysis to construct a model for the evaluation of factors associated with IBS.
RESULTS
Overall, data of 9163 participants entered the final analysis. In total, 1067 (11.6%) individuals were diagnosed with IBS, among which 57 (5.3%) were diarrhea dominant (IBS-D), 380 (35.6%) were constipation dominant (IBS-C), and 630 (59%) did not mention having any of the two (IBS-U). In the regression model, back pain/arthralgia (OR: 1.98, 95% CI: 1.65 - 2.40), insomnia (OR: 1.65, 95% CI: 1.40 - 1.93), depression (OR: 1.64, 95% CI: 1.38 - 1.95), female sex (OR: 1.58, 95% CI: 1.27 - 1.96), anxiety (OR: 1.43, 95% CI: 1.21 - 1.69), and being married (OR: 1.23, 95% CI: 1.03 - 1.48), were associated with higher rates of IBS. We found that IBS prevalence displays a peak at the age of 41 years for both men and women.
CONCLUSION
The present study provides a background for follow-up studies to be conducted in order to evaluate causality between IBS and some major diseases such as liver disease. We also found that opium use, although not statistically significant, in addition to sex, education, back/joint pain, depression, insomnia, anxiety, and marital status might be a contributing factor in IBS.
Keywords: Irritable bowel syndrome, Rome IV, Risk factor, Cohort, Iran, Oral health, Opium , Liver disease
INTRODUCTION
Irritable bowel syndrome or commonly known as IBS is the most common functional gastrointestinal disease, with an estimated prevalence of 10% to 20% in different regions worldwide.1 These rates have been reported to be lower in Asian countries compared with western countries.2 However, prevalence rates are expected to rise in Eastern countries as life style and socioeconomic factors are rapidly changing.3 The disease puts a great load on worldwide health care systems and constitutes 25% of all outpatient visits to gastroenterologists.4,5
The presentation of symptoms in IBS has a relapsing-remitting nature. No structural abnormality has been defined that would explain the symptoms of the disease, therefore it is considered a functional disease.6 Multiple factors have been related to IBS pathogenesis including changes in motility and microbiota of the gastrointestinal system, infectious factors, food and dietary sensitivity, and inflammation; however the etiology of the disease still remains unknown.7
Multiple studies have been conducted worldwide to evaluate factors associated with IBS. Besides the differences in definition of IBS, different methodology, and sampling techniques between studies, most of the literature on IBS has been in the settings of cross-sectional studies with limited resources and have included multiple confounding factors, which have resulted in varying and even contradicting findings regarding the risk factors of IBS.1 Moreover, recently the ROME group introduced the Rome IV criteria for the diagnosis of IBS, and accordingly the definition of IBS has undergone changes,8 however to date no large study has evaluated IBS considering the new diagnostic criteria.
Herein we proposed to take advantage of the settings provided by one of the largest cohort registries in Iran to evaluate epidemiological features related to IBS considering the Rome IV criteria in controlled settings and to provide the basis for future follow-up evaluations.
MATERIALS AND METHODS
Study settings
This study is part of one of the largest ongoing cohort registries in Southern Iran, termed as the Pars Cohort Study (PCS). The study is conducted among the residents of Valashahr district, Fars, Iran, which has more than 40000 inhabitants. All participants older than 40 years are included in the cohort registry. The main goal of the registry is to evaluate non-communicable diseases in the Iranian population. A total of 9264 participants were enrolled in the initial registry. The PCS questionnaire includes more than 180 variables and includes information on baseline characteristics, life style, disease history, family history, and medication history. In addition a food frequency questionnaire is filled in for each participant. Specifics regarding the PCS protocol have been described elsewhere.9
Study protocol and design
In this study, participants were evaluated based on their gastrointestinal symptoms. The PCS questionnaire on gastrointestinal symptoms included 19 questions, which were categorized as upper gastrointestinal symptoms, and lower gastrointestinal symptoms. This section included questions regarding acid reflux (frequency, severity and etc.), defecation (frequency, texture, associated symptoms such as pain, flatus, weight loss, and blood), and abdominal pain (duration and relieving factors). Diagnosis of IBS was done using the New Rome IV criteria.8 Those individuals who had missing data regarding gastrointestinal symptoms were excluded from the study.
The participants diagnosed as having IBS based on the ROME criteria were compared with individuals without IBS. Based on expert opinion specific factors were extracted from the questionnaires and compared between the two groups.
The two groups were compared regarding age, sex, body mass index (BMI), self-reported diseases such as: hypertension, diabetes, obstructive lung disease, stroke, myocardial infarction, renal failure or chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), liver disease, anxiety, depression, insomnia, back pain, arthralgia, history of previous cancer, history of ever using cigarettes, current use of cigarettes, use of tobacco product other than cigarettes, history of opioid use, physical activity, indexes of oral health such as number of decayed and missing teeth, number of normal teeth, and use of dentures.
The participants were also compared regarding specific socioeconomic factors such as status of family household (or number of individuals in the household), number of bedrooms, house area size, and status of home ownership.
Definition of variables
In the new Rome IV diagnostic criteria for gastrointestinal disorders, IBS is defined as abdominal pain with a frequency of at least 1 day per week for the last three months, which is associated with two or more of the following: symptoms related to defecation, symptoms associated with changes in defecation frequency, and symptoms associated with changes in stool form 8 with the onset at least 6 months before diagnosis.
Ethnicity of the participants was categorized in three main groups of those from Fars, those from a Tork background, and others. Educational level was classified as less than 5 years, 6-8 years, between 9 and 12 years, and more than 12 years of education. Physical activity was defined using the metabolic equivalent of task (MET) score.10 For evaluation of dental health status, we evaluated the number of decayed, missing, normal teeth, and the use of dentures for each patient separately.
Within the cohort protocol, individuals were also questioned regarding their form of stool and based on the questionnaire, individuals could select from three options of diarrhea dominancy, constipation dominancy, and normal stool (without diarrhea or constipation), which we used to define sub-types of IBS as IBS-D, IBS-C, and IBS-unclassified or IBS-U according to the ROME criteria.
Statistical analysis
For descriptive statistics and univariate analysis the SPSS® software for windows®, version 18, (SPSS Inc., Chicago, IL, USA) was used. For comparison of quantitative variables between the two groups (patients with IBS and patients without IBS), the independent t test and for comparison of qualitative variables between the two groups the Chi-square and the Fisher’s exact tests were used.
As the study included a large sample size of patients from a cohort registry, we used the penalized smoothly clipped absolute deviation (SCAD) regression analysis, which is one the most advanced methods of selecting variables when faced with a high number of variables.11 In this method, variables are introduced to the SCAD model, by which variable selection and evaluation are done simultaneously. The SCAD regression was performed using the ncvreg package12 in the R-3.3.1® software for windows.
For a better evaluation of findings, chronic diseases were categorized separately into a different group, and individuals were evaluated based on having any number of chronic diseases as one, two, three, and more than four chronic diseases. Chronic diseases included diabetes, hypertension, COPD, cardiovascular events including myocardial infarction or stroke, and CKD. Having liver disease, cancer, and back pain/arthralgia in addition to psychological diseases were evaluated separately according to expert opinion.
RESULTS
Overall, the data of 9163 participants entered the final analysis. A total of 1067 (11.6%) individuals were diagnosed with IBS. Of the individuals with IBS, 57 (5.3%) were diarrhea dominant (IBS-D), 380 (35.6%) were constipation dominant (IBS-C), and 630 (59%) did not mention having either diarrhea or constipation and were classified as IBS-unclassified (IBS-U).
Comparison of individuals with IBS and those without IBS showed that those with IBS had higher rates of female sex (69.8% vs. 51.7%; p < 0.001), were mostly involved in other types of relationships (other than single or married; p = 0.005), were mostly illiterate (62.2% vs. 47.2%; p < 0.001), had higher rates of self-reported three and more than three chronic diseases (p < 0.001), had higher rates of liver disease (3.7% vs. 2.3%; p = 0.003), anxiety (47.4% vs. 27.3%; p < 0.001), depression (36.5% vs. 17.1%; p < 0.001), insomnia (35.1% vs. 17.3%; p < 0.001), back pain/arthralgia (84.5% vs. 65.6%; p < 0.001), history of tobacco use (43% vs. 37.6%; p < 0.001), and higher rates of denture use (p = 0.001). Those with IBS were older (53.7 ± 9.6 vs. 52.5 ± 9.7 years old; p < 0.001), had higher physical activity (10.5 ± 3.2 vs. 10±3.3; p < 0.001), and more missing teeth (16.7 ± 10.6 vs. 15 ± 10.6; p < 0.001). However, those with IBS had lower rates of smoking in their medical history (14.2% vs. 21.6%; p < 0.001), lower rates of current smoking (10.2% vs. 14.5%; p < 0.001), less bedrooms in their house (1 ± 1 vs. 1.1 ± 1.3; p = 0.001), less house area size (116 ± 39 m2 vs. 123.5 ± 42.7 m2), and less normal teeth (10.7 ± 9.3 vs. 12.4 ± 9.8; p < 0.001) (Table 1).
Table 1. Baseline and clinical characteristics of patients with and without IBS.* .
| Variables | Sub group | IBS (n = 1067) | Non-IBS (n = 8096) | P value |
| Sex - no. (%) | Male | 322 (30.2) | 3914 (48.3) | < 0.001 |
| Female | 745 (69.8) | 4182 (51.7) | ||
| Ethnicity - no. (%) | Fars | 563 (52.8) | 4607 (56.9) | 0.03 |
| Turk | 442 (41.4) | 3103 (38.3) | ||
| Other | 62 (5.8) | 386 (4.8) | ||
| Marital status - no. (%) | Single | 144 (2.9) | 888 (3.2) | 0.005 |
| Married | 923 (86.5) | 7208 (89.0) | ||
| Educational level - yrs | Illiterate | 664 (62.2) | 3818 (47.2) | < 0.001 |
| < 5 | 282 (26.4) | 2423 (29.9) | ||
| 6-8 | 61(5.7) | 908 (11.2) | ||
| 9-12 | 46 (4.3) | 683 (8.3) | ||
| > 12 | 14 (1.3) | 264 (3.3) | ||
| Home ownership - no. (%) | Owner | 1023 (95.9) | 7770 (96.0) | 0.99 |
| Rent | 28 (2.6) | 208 (2.6) | ||
| Other | 16 (1.5) | 118 (1.5) | ||
| Chronic disease - no. (%) | one disease | 41 (3.8) | 221 (2.7) | < 0.001 |
| Two diseases | 659 (61.8) | 5797 (71.6) | ||
| Three diseases | 238 (22.3) | 1521 (18.8) | ||
| Four or more diseases | 129 (12.1) | 557 (6.9) | ||
| Liver disease - no. (%) | No | 1027 (96.3) | 7913 (97.9) | 0.003 |
| Yes | 40 (3.7) | 183 (2.3) | ||
| Anxiety disorder - no. (%) | No | 561 (52.6) | 5885 (72.7) | < 0.001 |
| Yes | 506 (47.4) | 2211 (27.3) | ||
| Depression - no. (%) | No | 678 (63.5) | 6713 (82.9) | < 0.001 |
| Yes | 389 (36.5) | 1383 (17.1) | ||
| Insomnia - no. (%) | No | 692 (64.9) | 6692 (82.7) | < 0.001 |
| Yes | 375 (35.1) | 1404 (17.3) | ||
| Back/joint pain - no. (%) | No | 165 (15.5) | 2781 (34.4) | < 0.001 |
| Yes | 902 (84.5) | 5315 (65.6) | ||
| Cancer - no. (%) | No | 1055 (98.9) | 8002 (98.8) | 0.92 |
| Yes | 12 (1.1) | 94 (1.2) | ||
| Cigarette ever used - no. (%) | No | 915 (85.8) | 6349 (78.4) | < 0.001 |
| Yes | 152 (14.2) | 1747 (21.6) | ||
| Cigarette current use - no. (%) | No | 958 (89.8) | 6919 (85.5) | < 0.001 |
| Yes | 109 (10.2) | 1177 (14.5) | ||
| Tobacco ever used - no. (%) | No | 608 (57.0) | 5055 (62.4) | 0.001 |
| Yes | 459 (43.0) | 3041 (37.6) | ||
| Opiate ever used - no (%) | No | 990 (92.8) | 7405(91.5) | 0.14 |
| Yes | 77 (7.2) | 691 (8.5) | ||
| Dentures use - no. (%) | No | 719 (67.4) | 5898 (72.9) | 0.001 |
| Lower or upper set | 58 (5.4) | 381 (4.7) | ||
| Lower and upper set | 290 (27.2) | 1817 (22.4) | ||
| Age - yrs | 53.7 ± 9.6 | 52.5 ± 9.7 | < 0.001 | |
| BMI - Kg/m2 | 25.8 ± 4.8 | 25.9 ± 4.7 | 0.68 | |
| Variables | Sub group | IBS (n = 1067) | Non-IBS (n = 8096) | P value |
| Family household members | 4.9 ± 2.0 | 4.9 ± 1.9 | 0.67 | |
| Home no. of rooms | 1.0 ± 1.0 | 1.1 ± 1.3 | 0.15 | |
| House area - m2 | 116.9 ± 39 | 123.5 ± 42.7 | < 0.001 | |
| Physical activity† | 10.5 ± 3.2 | 10.0 ± 3.3 | < 0.001 | |
| Decayed teeth | 3.4 ± 4.0 | 3.4 ± 3.9 | 0.88 | |
| Missing teeth | 16.7 ± 10.6 | 15.0 ± 10.6 | < 0.001 | |
| Normal teeth | 10.7 ± 9.3 | 12.4 ± 9.8 | < 0.001 |
IBS: irritable bowel syndrome, BMI: body mass index
*All plus-minus values are means and standard deviations. Data are presented as frequency and percentage unless stated otherwise.
†Physical activity was measured using the metabolic equivalent of task score.
Using the SCAD regression analysis showed that among all variables, back pain/arthralgia (OR: 1.98, 95% CI: 1.65 - 2.40), insomnia (OR: 1.65, 95% CI: 1.40 - 1.93), depression (OR: 1.64, 95% CI: 1.65 - 2.40), female sex (OR: 1.58, 95% CI: 1.27 - 1.96), anxiety (OR: 1.43, 95% CI: 1.21 - 1.69), liver disease (OR: 1.30, 95% CI: 0.87 - 1.92), ethnicity of Tork and other (compared with being from Fars) (OR: 1.12, 95% CI: 0.96 - 1.31 and OR: 1.30, 95% CI: 0.92 - 1.82, respectively), marital status as married (OR: 1.21, 95% CI: 1.03 - 1.48), history of opioid use (OR: 1.11, 95% CI: 0.80 - 1.52), physical activity (OR: 1.03, 95% CI: 1.00 - 1.05), family household members (OR:1.01, 95% CI: 0.97 - 1.04), use of dentures (lower or upper set) (OR: 1.33, 95% CI: 1.65 - 2.40), educational level, history of chronic illness, positive history of tobacco use (OR: 0.97, 95% CI: 0.85 - 1.10), and BMI (OR: 0.98, 95% CI: 0.85 - 1.10) were representative in the final model. However, only back/joint pain, insomnia, depression, anxiety, female sex, and being married were significantly associated with higher rates of IBS (Table 2).
Table 2. T Penalized smoothly clipped absolute deviation regression analysis for the evaluation of IBS associated risk factors.* .
| Variables | Sub group | Coefficient | OR | 95% CI |
| Back/joint pain | No | - | 1 | - |
| Yes | 0.69 | 1.99 | 1.65-2.40 | |
| Insomnia | No | - | 1 | - |
| Yes | 0.50 | 1.65 | 1.40-1.93 | |
| Depression | No | - | 1 | - |
| Yes | 0.50 | 1.64 | 1.38-1.95 | |
| Sex | Male | - | 1 | - |
| Female | 0.46 | 1.58 | 1.27-1.96 | |
| Education | Illiterate | - | 1 | - |
| <5 | -0.17 | 0.84 | 0.67-1.05 | |
| 6-8 | -0.38 | 0.68 | 0.45-1.03 | |
| 9-12 | -0.38 | 0.68 | 0.43-1.07 | |
| >12 | -0.42 | 0.66 | 0.31-1.37 | |
| Anxiety | No | - | 1 | - |
| Yes | 0.36 | 1.43 | 1.21-1.69 | |
| History of chronic Disease | 1 | - | 1 | - |
| 2 | -0.35 | 0.71 | 0.47-1.04 | |
| 3 | -0.27 | 0.76 | 0.51-1.13 | |
| >3 | 0.00 | 1.00 | 0.67-1.47 | |
| Dentures | No denture | - | 1 | - |
| Lower or upper set | 0.29 | 1.33 | 0.95-1.87 | |
| Lower and upper set | 0.00 | 1.00 | 0.79-1.25 | |
| Liver disease | No | - | 1 | - |
| Yes | 0.26 | 1.30 | 0.87-1.92 | |
| Ethnicity | Fars | - | 1 | - |
| Tork | 0.12 | 1.12 | 0.96-1.31 | |
| Other | 0.26 | 1.30 | 0.92-1.82 | |
| Marital status | Single | - | 1 | - |
| Married | 0.21 | 1.23 | 1.03-1.48 | |
| History of opiate use | No | - | 1 | - |
| Yes | 0.10 | 1.11 | 0.80-1.52 | |
| History of tobacco use | No | - | 1 | - |
| Yes | -0.03 | 0.97 | 0.85-1.10 | |
| Physical Activity | 0.03 | 1.03 | 1.00-1.05 | |
| Body mass index | -0.02 | 0.98 | 0.96-0.99 | |
| Family household | 0.01 | 1.01 | 0.97-1.04 |
SE: Standard error, OR: odds ratio, IBS: irritable bowel syndrome
*Those with an odds ratio of 1 were considered base for comparison
When we separately evaluated the effects of age on IBS prevalence, we found that IBS prevalence seemed to display a peak at the age of 41 years for both men and women separately. This is shown in figure 1 and supplement 1.
Fig.1.
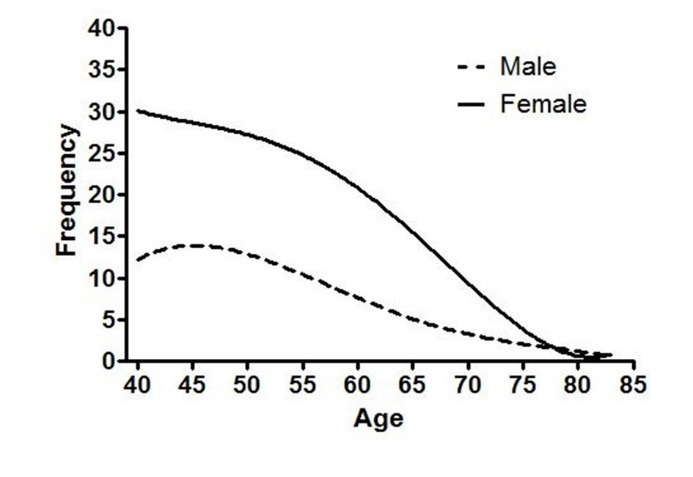
frequency of IBS according to age
Supplement 1. Pattern of changes in frequency of IBS according to age.
| Age | Males | Females | ||
| Frequency | Percent | Frequency | Percent | |
| 40 | 4 | 1.2 | 26 | 3.5 |
| 41 | 21 | 6.5 | 35 | 4.7 |
| 42 | 12 | 3.7 | 32 | 4.3 |
| 43 | 19 | 5.9 | 26 | 3.5 |
| 44 | 16 | 5.0 | 25 | 3.4 |
| 45 | 15 | 4.7 | 28 | 3.8 |
| 46 | 12 | 3.7 | 32 | 4.3 |
| 47 | 10 | 3.1 | 30 | 4.0 |
| 48 | 14 | 4.3 | 27 | 3.6 |
| 49 | 8 | 2.5 | 27 | 3.6 |
| 50 | 13 | 4.0 | 33 | 4.4 |
| 51 | 9 | 2.8 | 28 | 3.8 |
| 52 | 12 | 3.7 | 25 | 3.4 |
| 53 | 16 | 5.0 | 24 | 3.2 |
| 54 | 11 | 3.4 | 26 | 3.5 |
| 55 | 12 | 3.7 | 24 | 3.2 |
| 56 | 8 | 2.5 | 24 | 3.2 |
| 57 | 7 | 2.2 | 14 | 1.9 |
| 58 | 11 | 3.4 | 16 | 2.1 |
| 59 | 10 | 3.1 | 20 | 2.7 |
| 60 | 12 | 3.7 | 21 | 2.8 |
| 61 | 5 | 1.6 | 20 | 2.7 |
| 62 | 8 | 2.5 | 31 | 4.2 |
| 63 | 8 | 2.5 | 25 | 3.4 |
| 64 | 3 | .9 | 18 | 2.4 |
| 65 | 6 | 1.9 | 24 | 3.2 |
| 66 | 4 | 1.2 | 10 | 1.3 |
| 67 | 7 | 2.2 | 7 | 0.9 |
| 68 | 1 | 0.3 | 11 | 1.5 |
| 70 | 2 | 0.6 | 9 | 1.2 |
| 71 | 2 | 0.6 | 8 | 1.1 |
| 72 | 2 | 0.6 | 5 | 0.7 |
| 73 | 1 | 0.3 | 2 | 0.3 |
| 74 | 1 | 0.3 | 7 | 0.9 |
| 75 | 7 | 2.2 | 11 | 1.5 |
| 76 | 5 | 1.6 | 7 | 0.9 |
| 77 | 3 | 0.9 | 1 | 0.1 |
| 79 | 2 | 0.6 | 1 | 0.1 |
| 80 | 1 | 0.3 | 1 | 0.1 |
| 83 | 1 | 0.3 | 1 | 0.1 |
| 91 | 1 | 0.3 | 3 | 0.4 |
| Total | 322 | 100 | 745 | 100 |
DISCUSSION
We evaluated the prevalence of IBS and its associated factors in the setting of one of the largest cohort registries in Iran. We found that back/joint pain, insomnia, depression, female sex, anxiety, and being married, were associated with higher rates of IBS. To the best of our knowledge, this is the largest study to evaluate IBS related factors based on the New Rome IV criteria in the region and is one of the most comprehensive studies evaluating IBS risk factors in literature.
The prevalence reported in our study (11.6%) is similar to that reported as the pooled international estimate of IBS (11.2%; 95% CI: 9.8 - 12.8).13 Our estimated prevalence was similar to the highest rates reported in Singapore,14 Israel,13 China,15 Germany,16 Australia,17 Pakistan,18 and Canada19 (11%, 11.4%, 11.5%, 12.5%, 13%, 13.3%, and 13.5%, respectively).
In a study conducted in Shiraz,20 Iran, in 2004, a similar prevalence (10.9%) was reported. This study that included 1978 individuals used the Rome II criteria for diagnosis of IBS. In one of the largest studies conducted in Isfahan, Iran, Keshteli and colleagues21 evaluated 4763 seemingly healthy adults who were non-academic staff of Isfahan Medical University. Using the Rome III criteria, they found a prevalence of 21.5% for IBS, which was associated with female sex (OR = 1.49, 95% CI: 1.27 - 1.76) and being married (OR = 1.27, 95% CI: 1.03 - 1.57). These results were similar to our results as we also found female sex and being married to be associated with IBS and did not find age to be associated with IBS. We studied a rural population as part of a large cohort registry, and our results were similar to that of the mentioned studies.
Our finding regarding the higher risks of IBS in women compared with men (OR: 1.58) is very similar to that of international reports 1 as women are considered to be at higher risks of developing IBS (OR: 1.67).
Higher age has been documented to be associated with lower prevalence of IBS in multiple studies.13 Furthermore, age of 50 years old has been reported in some studies to be a turning point, as IBS prevalence is 25% less in those over 50 years old 22 and milder symptoms have been reported among those over 50 years old.23 In our study for the first time, we documented a different age cut-off at which IBS prevalence undergoes change. According to our primary analysis an age between 40 to 45 years is a changing point at which IBS prevalence peaks and decreases.
Some special forms of exercise such as yoga and bicycle riding have been shown to decrease the severity of symptoms in patients with IBS.24,25 However, whether these activities decrease the prevalence of the disease is still unknown and our results did not support the notion that physical activity (not any specific exercise) is associated with risk of IBS.
The prevalence of psychiatric disorders in patients with IBS is considered to be higher compared with the normal population. In an epidemiological study by Mykletun and co-workers26 the association of anxiety and depressive disorders were evaluated and compared between individuals with IBS, individuals with hypertension, and individuals with diabetes. They found that patients with IBS had a significantly higher rate of current mood or anxiety disorder compared with the patients with diabetes or hypertension (27.5% vs. 13.6% and 14.4%, respectively; p < 0.001; OR = 2.62). These individuals had a lifetime odds ratio of 2.08 (95% CI: 1.34 - 3.24) for acquiring mood disorders and 1.86 (95% CI: 1.08 - 3.21) for anxiety disorders in their study. In a study in 2014, Grzesiak and colleagues27 evaluated the lifetime prevalence of anxiety and depressive disorders among patients with IBS and patients with gastric reflux using the ICD-10 criteria. They found that anxiety disorders existed in about 47% of the patients with IBS. Other studies have also documented anxiety to be an exacerbating factor for IBS symptoms.28 In an epidemiological study among 3429 individuals in Korea,29 using the Rome II criteria the prevalence of IBS was reported as 10.9%. They evaluated the relationship between insomnia and IBS. They found that individuals with insomnia, after adjusting for confounding factors, had a 1.81 (95% CI: 1.48 - 2.29) higher chance of having IBS. This was similar to our finding, as we found insomnia to be a strong risk factor for IBS (OR: 1.77). Our study also supported previous literature as we found insomnia, depression, and anxiety to be associated with IBS.
In a cross-sectional study among 340 nurses in China,30 the association between psychological disorders and IBS was assessed. Using a regression analysis they found that alcohol consumption, physical exercise, and the general symptom index (which is an index for severity of psychological distress) were associated with IBS. They did not find education, marital status, age, and sex to be associated with IBS. In our study using the SCAD regression analysis, we did not find age to be associated with IBS; however we found female sex and being married to be associated with IBS. Our study included the general population and their study included nurses, which is not applicable to the general population.
Perhaps one of the most interesting findings in our study, although not statistically significant, was the association between history of opium inhalation and IBS (OR: 1.11). From another perspective, current focus is on the use of opium agonists such as eluxadoline in diarrhea dominant IBS.31,32 According to our results, individuals with a history of opium consumption may be at risk of developing IBS in the future. This could be explained as opioids alter gut motility and the autonomous system of the gut,33 which maybe, if supported by future studies, causing IBS symptoms in long term among these individuals.
Studies evaluating socioeconomic status (SES) have been contradicting as some studies have shown IBS to be associated with lower SES,34 while others have supported the theory that IBS is a disease of the industrialized world and is seen in higher socioeconomic classes.35 We found IBS to be associated with one specific socioeconomic index, which was marital status, as being married was a risk factors for IBS.
Another interesting factor that was evaluated in our study was the relationship between oral health, and IBS. In a recent study, Fourie and others36 evaluated oral microbiome among patients with IBS and compared it with healthy controls. They found that Desulfobacterium and Prevotella species, Dialister invisus, and Mycoplasma hominis, were associated with IBS. In addition, sulfate reducing bacteria like the Desulfobacterium species have been associated with periodontal diseases in previous literature.37 Inhere we did not find a significant relationship between oral health indices and IBS.
Although we did not find an association between BMI and IBS in our final model, in a large cohort study from Israel in 2015, the researchers found underweight individuals to be at higher risks of developing IBS compared with obese individuals (hazard ratio: 1.28). They also found overweight individuals to have the lowest risk of developing IBS compared with normal weight, underweight, and obese individuals.38
In a study published in 2016,39 significant increases in hepatic enzymes including alanine aminotransferase (ALT), alkaline phosphatase (ALP) and gamma glutamyl transferase (γ-GT) were reported among individuals with IBS compared with a control group. In this study patients with IBS were diagnosed using the ROME III criteria, and when they stratified patients according to obesity, this difference did no longer exist. Furthermore, in their multivariate model they found elevated ALT to be significantly associated with IBS (OR: 2.3, 95% CI: 1.044 - 5.066). This was further addressed in a review by Scalera and colleagues,40 in which they evaluated whether metabolic diseases related to the liver such as nonalcoholic fatty liver disease (NAFLD) are associated and causing symptoms related to the gastrointestinal system (more commonly as IBS). In our study although liver diseases did contribute to our final model, its association with IBS remained to be statistically insignificant.
Literature, as stated previously, shows IBS to be associated with psychological disorders. Whether serotonin is indirectly affecting the prevalence of systemic diseases (similar to that seen with psychosomatic disorders) among patients with IBS or if central dysregulations through the brain-gut interactions [as the central nervous system has a significant effect on blood flow, secretion, and motility41] is affecting the prevalence of systemic diseases, still remains an unanswered question.
This study was conducted using data from one of the largest cohort registries in Southern Iran, and is by far the largest study to evaluate IBS within the region. In this study we used a positive diagnostic approach for IBS and those individuals who had other associating symptoms such as weight loss were not excluded from the IBS group, although these individuals were few according to our primary analysis. The cohort registry included more than 180 variables, for which we used expert opinion to select variables (regarding their association with IBS) to be included in the present study.
This study was not without limitations. For disease assessment we used self-reported data from individuals, which may not be as precise as clinical diagnosis and tests; however self-reported data are somewhat reliable, especially with systemic diseases (such as cardiac, renal, and etc.), as individuals would not claim to have a systemic disease unless they have been previously diagnosed and visited by a physician. Another important issue relates to the fact that cross-sectional studies like ours, merely show an association between IBS and different diseases and conditions, and do not explain if IBS was the cause or an effect originating from these diseases. For example, a prospective-longitudinal study in Taiwan, suggested that IBS was perhaps a cause to depression and sleep disorders.42 We used the ROME criteria to diagnose individuals with IBS and accordingly, the ROME criteria considers a positive diagnosis and does not exclude individuals with alarming signs such as weight loss, bleeding, or anemia and perhaps a group of our patients might have some other forms of systemic diseases, which presented as IBS such as celiac disease, and acid reflux disease. However, as literature has showed only a small percentage of individuals with alarming signs eventually present with serious illnesses such as colon cancer43,44 and in a large scale study like the present study, this group of individuals are probably too small to affect final analysis. Moreover, our control group was merely selected from individuals who did not have IBS (according to the ROME criteria), and these individuals may not have been a healthy control. Although, in our final multivariate model all associational relationships were adjusted for. To the best of the authors’ knowledge, this is among the first studies to evaluate epidemiological features of IBS using the Rome IV criteria in the settings of a large cohort registry. Using the new Rome criteria, the reported prevalence rates of the disease decreases compared to when using the Rome III criteria, as in the new criteria, abdominal pain is considered the leading symptom among patients with IBS and abdominal discomfort has been omitted from the definition. Compared with the previous definition of IBS, the Rome IV mainly diagnoses individuals with more severe gastrointestinal symptoms, more associated comorbidities such as psychological disorders, and lower quality of life,45 which would render different results regarding the factors associated with IBS.
In conclusion the present study provides a background for follow-up studies to be conducted in order to evaluate causality between IBS and some major diseases such as liver disease. We also found that opium use, although not statistically significant, in addition to sex, education, back/joint pain, depression, insomnia, anxiety, and marital status might be a contributing factor in IBS.
Ethical consideration
The study protocol followed the guidelines reported in the declaration of Helsinki and was in accordance with the ethical standards of the institutional research committees of Shiraz and Tehran Universities of Medical Sciences. The study protocol was further approved by the Institutional Review Board of Shiraz University of Medical Sciences (ethical code # 1396.s134). All participants gave their informed and written consent to enter the study.
Acknowledgments
The authors would like to thank all personnel who helped to establish this cohort registry and participants who patiently took part in this study. This study was registered as a dissertation of Master of Public Health (MPH) courses by Dr. Peyman Arasteh.
Please cite this paper as:
Arasteh P, Maharlouei N, Eghbali SS, Amini M, Lankarani KB, Malekzadeh R. A Comprehensive Look at Irritable Bowel Syndrome and its Associated Factors Considering the Rome IV Criteria: A Penalized Smoothly Clipped Absolute Deviation Regression Approach in the Pars Cohort Study. Middle East J Dig Dis 2018;10:149-159. doi: 10.15171/mejdd.2018.104.
Footnotes
Funding This study was part of an ongoing cohort registry under the supervision of the Ministry of Health and was funded by Shiraz University of Medical Sciences (Grant # 910210).
ETHICAL APPROVAL There is nothing to be declared.
CONFLICT OF INTEREST The authors declare no conflict of interest related to this work.
References
- 1.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gwee KA, Bak YT, Ghoshal UC, Gonlachanvit S, Lee OY, Fock KM. et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189–205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]
- 3.Gwee KA. Irritable bowel syndrome in developing countries-a disorder of civilization or colonization? Neurogastroenterol Motil. 2005;17:317–24. doi: 10.1111/j.1365-2982.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:1023–34. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 5.Butt AS, Salih M, Jafri W, Yakoob J, Wasay M, Hamid S. Irritable bowel syndrome and psychiatric disorders in Pakistan: a case control study. Gastroenterol Res Pract. 2012;2012:291452. doi: 10.1155/2012/291452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Irritable bowel syndrome: a 10-yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol. 2008;103:1229–39. doi: 10.1111/j.1572-0241.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 7.Occhipinti K, Smith JW. Irritable bowel syndrome: a review and update. Clin Colon Rectal Surg. 2012;25:46–52. doi: 10.1055/s-0032-1301759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD. et al. Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Gandomkar A, Poustchi H, Moini M, Moghadami M, Imanieh H, Fattahi MR. et al. Pars cohort study of non-communicable diseases in Iran: protocol and preliminary results. Int J Public Health. 2017;62:397–406. doi: 10.1007/s00038-016-0848-2. [DOI] [PubMed] [Google Scholar]
- 10.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF. et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Fan J, Li R. Variable selection via nonconcave penalized likelihood and its oracle properties. J Am Statist Assoc. 2001;96:1348–60. doi: 10.1198/016214501753382273. [DOI] [Google Scholar]
- 12.Breheny P. ncvreg: Regularization Paths for SCAD-and MCP-Penalized Regression Models. R package version. 2013;2:6–0. [Google Scholar]
- 13.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21 e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Gwee KA, Wee S, Wong ML, Png DJ. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an asian urban community. Am J Gastroenterol. 2004;99:924–31. doi: 10.1111/j.1572-0241.2004.04161.x. [DOI] [PubMed] [Google Scholar]
- 15.Xiong LS, Chen MH, Chen HX, Xu AG, Wang WA, Hu PJ. A population-based epidemiologic study of irritable bowel syndrome in South China: stratified randomized study by cluster sampling. Aliment Pharmacol Ther. 2004;19:1217–24. doi: 10.1111/j.1365-2036.2004.01939.x. [DOI] [PubMed] [Google Scholar]
- 16.Icks A, Haastert B, Enck P, Rathmann W, Giani G. Prevalence of functional bowel disorders and related health care seeking: a population-based study. Z Gastroenterol. 2002;40:177–83. doi: 10.1055/s-2002-22324. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Boyce PM, Jones M. Predictors of health care seeking for irritable bowel syndrome: a population based study. Gut. 1997;41:394–8. doi: 10.1136/gut.41.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husain N, Chaudhry IB, Jafri F, Niaz SK, Tomenson B, Creed F. A population-based study of irritable bowel syndrome in a non-Western population. Neurogastroenterol Motil. 2008;20:1022–9. doi: 10.1111/j.1365-2982.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 19.Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–35. doi: 10.1023/A:1013208713670. [DOI] [PubMed] [Google Scholar]
- 20.Khademolhosseini F, Mehrabani D, Nejabat M, Beheshti M, Heydari ST, Mirahmadizadeh A. et al. Irritable bowel syndrome in adults over 35 years in Shiraz, southern Iran: prevalence and associated factors. J Res Med Sci. 2011;16:200–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Keshteli AH, Dehestani B, Daghaghzadeh H, Adibi P. Epidemiological features of irritable bowel syndrome and its subtypes among Iranian adults. Ann Gastroenterol. 2015;28:253–8. [PMC free article] [PubMed] [Google Scholar]
- 22. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712-21.e4. [DOI] [PubMed]
- 23.Tang YR, Yang WW, Liang ML, Xu XY, Wang MF, Lin L. Age-related symptom and life quality changes in women with irritable bowel syndrome. World J Gastroenterol. 2012;18:7175–83. doi: 10.3748/wjg.v18.i48.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuttner L, Chambers CT, Hardial J, Israel DM, Jacobson K, Evans K. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Res Manag. 2006;11:217–23. doi: 10.1155/2006/731628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Tilburg MA, Palsson OS, Levy RL, Feld AD, Turner MJ, Drossman DA. et al. Complementary and alternative medicine use and cost in functional bowel disorders: a six month prospective study in a large HMO. BMC Complement Altern Med. 2008;8:46. doi: 10.1186/1472-6882-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mykletun A, Jacka F, Williams L, Pasco J, Henry M, Nicholson GC. et al. Prevalence of mood and anxiety disorder in self reported irritable bowel syndrome (IBS) An epidemiological population based study of women. BMC Gastroenterol. 2010;10:88. doi: 10.1186/1471-230X-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grzesiak M, Beszlej JA, Mulak A, Szechinski M, Szewczuk-Boguslawska M, Waszczuk E. et al. The lifetime prevalence of anxiety disorders among patients with irritable bowel syndrome. Adv Clin Exp Med. 2014;23:987–92. doi: 10.17219/acem/37356. [DOI] [PubMed] [Google Scholar]
- 28.Jerndal P, Ringstrom G, Agerforz P, Karpefors M, Akkermans LM, Bayati A. et al. Gastrointestinal-specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22:646–e179. doi: 10.1111/j.1365-2982.2010.01493.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee SK, Yoon DW, Lee S, Kim J, Choi KM, Shin C. The association between irritable bowel syndrome and the coexistence of depression and insomnia. J Psychosom Res. 2017;93:1–5. doi: 10.1016/j.jpsychores.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Xiao QF, Zhang YL, Yao SK. A cross-sectional study of irritable bowel syndrome in nurses in China: prevalence and associated psychological and lifestyle factors. J Zhejiang Univ Sci B. 2014;15:590–7. doi: 10.1631/jzus.B1300159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cash BD, Lacy BE, Schoenfeld PS, Dove LS, Covington PS. Safety of Eluxadoline in Patients with Irritable Bowel Syndrome with Diarrhea. Am J Gastroenterol. 2017;112:365–74. doi: 10.1038/ajg.2016.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keating GM. Eluxadoline: A Review in Diarrhoea-Predominant Irritable Bowel Syndrome. Drugs. 2017;77:1009–1016. doi: 10.1007/s40265-017-0756-7. [DOI] [PubMed] [Google Scholar]
- 33.Lacy BE. Emerging treatments in neurogastroenterology: eluxadoline–a new therapeutic option for diarrhea-predominant IBS. Neurogastroenterol Motil. 2016;28:26–35. doi: 10.1111/nmo.12716. [DOI] [PubMed] [Google Scholar]
- 34.Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P. Consortium for the European Review of Social Determinants of Health and the Health Divide. . WHO European review of social determinants of health and the health divide. Lancet. 2012;380:1011–29. doi: 10.1016/S0140-6736(12)61228-8. [DOI] [PubMed] [Google Scholar]
- 35.Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am. 2005;34:189–204. doi: 10.1016/j.gtc.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Fourie NH, Wang D, Abey SK, Sherwin LB, Joseph PV, Rahim-Williams B. et al. The microbiome of the oral mucosa in irritable bowel syndrome. Gut Microbes. 2016;7:286–301. doi: 10.1080/19490976.2016.1162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heggendorn FL, Souza Gonçalves L, Dias EP, Silva Junior A, Galvão MM, Lutterbach MT. Detection of sulphate-reducing bacteria in human saliva. Acta Odontol Scand. 2013;71:1458–63. doi: 10.3109/00016357.2013.770163. [DOI] [PubMed] [Google Scholar]
- 38.Carter D, Beer-Gabel M, Tzur D, Levy G, Derazne E, Novis B. et al. Predictive factors for the diagnosis of irritable bowel syndrome in a large cohort of 440,822 young adults. J Clin Gastroenterol. 2015;49:300–5. doi: 10.1097/MCG.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Kim KN, Kim KM, Joo NS. Irritable Bowel Syndrome May Be Associated with Elevated Alanine Aminotransferase and Metabolic Syndrome. Yonsei Med J. 2016;57:146–52. doi: 10.3349/ymj.2016.57.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scalera A, Di Minno MN, Tarantino G. What does irritable bowel syndrome share with non-alcoholic fatty liver disease? World J Gastroenterol. 2013;19:5402–20. doi: 10.3748/wjg.v19.i33.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–9. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YT, Hu LY, Shen CC, Huang MW, Tsai SJ, Yang AC. et al. Risk of psychiatric disorders following irritable bowel syndrome: a nationwide population-based cohort study. PloS One. 2015;10:e0133283. doi: 10.1371/journal.pone.0133283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talley NJ. Evolution of the Diagnosis of Functional Gut Disorders: Is an Objective Positive Diagnostic Approach Within Reach? Clin Transl Gastroenterol. 2015;6:e104. doi: 10.1038/ctg.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammer J, Eslick GD, Howell SC, Altiparmak E, Talley NJ. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut. 2004;53:666–72. doi: 10.1136/gut.2003.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vork L, Weerts Z, Mujagic Z, Kruimel JW, Hesselink MAM, Muris JWM. et al. Rome III vs Rome IV criteria for irritable bowel syndrome: A comparison of clinical characteristics in a large cohort study. Neurogastroenterol Motil. 2018:30. doi: 10.1111/nmo.13189. [DOI] [PubMed] [Google Scholar]


